Abstract
Among respiratory tract isolates of Streptococcus pneumoniae from children, resistance to penicillins, cephalosporins, macrolides, and trimethoprim-sulfamethoxazole (SXT) increases on an annual basis. Pediatric patients who do not respond to conventional therapy for respiratory tract infections someday may be treated with fluoroquinolones. In this study, MICs of β-lactams, azithromycin, SXT, and levofloxacin were determined and interpreted by using NCCLS guidelines for isolates of S. pneumoniae (2,834 from children and 10,966 from adults), Haemophilus influenzae (629 from children and 2,281 from adults), and Moraxella catarrhalis (389 from children and 1,357 from adults) collected during the 2000-2001 and 2001-2002 respiratory illness seasons in the United States as part of the ongoing TRUST surveillance studies. Rates of resistance to penicillin, azithromycin, and SXT were ≥7.5% higher among patients ≤4 years old than among patients 5 to 10, 11 to 17, and ≥18 years old in both the 2000-2001 and the 2001-2002 respiratory illness seasons. Levofloxacin resistance was detected in 2 of 2,834 isolates (0.07%) from patients <18 years old. Levofloxacin MICs of 0.25 to 1 μg/ml accounted for 99.6, 99.5, 99.3, 99.7, 98.4, and 98.0% of isolates from patients <2, 2 to 4, 5 to 10, 11 to 17, 18 to 64, and >64 years old. Multidrug resistance was twice as common among patients ≤4 years old (25.3%) as among patients 5 to 10 years old (13.7%), 11 to 17 years old (11.9%), 18 to 64 years old (12.1%), and >64 years old (12.4%). The most common multidrug resistance phenotype in S. pneumoniae isolates for all age groups was resistance to penicillin, azithromycin, and SXT (70.3 to 76.6%). For H. influenzae and M. catarrhalis isolates from patients <2, 2 to 4, 5 to 10, 11 to 17, 18 to 64, and >64 years old, levofloxacin MICs at which 90% of the isolates were inhibited were 0.015 and 0.03 to 0.06 μg/ml, respectively, in the 2000-2001 and 2001-2002 respiratory illness seasons. In the 2000-2001 and 2001-2002 respiratory illness season surveillance studies in the United States, 99.9% of pediatric isolates of S. pneumoniae were susceptible to levofloxacin. If fluoroquinolones become a treatment option for pediatric patients, careful monitoring of fluoroquinolone susceptibilities will be increasingly important in future surveillance studies.
Streptococcus pneumoniae is the most common bacterial cause of community-acquired pneumonia, sinusitis, meningitis, and otitis media in the United States (16, 17). Otitis media and sinusitis are particularly common in children, and resistance to penicillin, cephalosporins, macrolides, tetracycline, and trimethoprim-sulfamethoxazole (SXT) is significantly higher in isolates that cause these infections in children than in the same pathogens isolated from adults (11, 25). Until the early 1990s, the management of pneumococcal respiratory tract infections was relatively straightforward, at least in the United States. Such infections were effectively treated, for the most part, with β-lactams or, for patients with allergies to β-lactams, with macrolides (11). In the United States, the last decade has witnessed remarkable decreases in the susceptibility of pneumococci to β-lactams, macrolides, and SXT, particularly in clinical isolates from children; rates of susceptibility to penicillin, erythromycin, and SXT in 2000 were reported to be 65.8, 73.8, and 64.1%, respectively (11).
Haemophilus influenzae and Moraxella catarrhalis are clinically important respiratory tract pathogens and are often isolated from children with otitis media and sinusitis and from elderly patients with acute exacerbations of chronic bronchitis (12). Ampicillin resistance arising in clinical isolates of H. influenzae through β-lactamase production (TEM-1 and ROB-1) was first reported in the 1970s (26) and increased markedly during the 1980s and early 1990s (9, 11, 25). β-Lactamase-positive M. catarrhalis isolates (BRO-1 and BRO-2) hydrolyze penicillin, ampicillin, and amoxicillin (10). Recent North American and European studies have reported a rate of β-lactamase production in clinical isolates of M. catarrhalis of >90%, with insignificant geographic variations (10, 12, 25).
Resistance to current antimicrobial empirical therapies continues to increase in pneumococci, particularly in isolates from children. Prescription of oral β-lactams and macrolides for empirical therapy in children may eventually prove unreliable, and alternative antimicrobial classes will be required for empirical and directed therapies. Fluoroquinolones have proven to be highly effective in treating respiratory tract infections in adults, making it logical to assume that these agents will be considered for treatment in children. The rationale for using fluoroquinolones in children depends on the demonstration that bacterial isolates from children are susceptible to these agents. At present there is limited published information regarding the susceptibility to levofloxacin and other fluoroquinolones of pathogens isolated from children. We examined the activity of levofloxacin against clinical isolates of S. pneumoniae, H. influenzae, and M. catarrhalis obtained from pediatric patients during the 2000-2001 and 2001-2002 respiratory illness seasons as part of the TRUST (Tracking Resistance in the United States Today) surveillance studies. The results for levofloxacin were compared to those for β-lactams, azithromycin, and SXT against clinical isolates from both children and adults.
MATERIALS AND METHODS
Respiratory tract isolates.
During the 2000-2001 and 2001-2002 respiratory illness seasons (September 2000-April 2001 and September 2001-April 2002) in the United States, the TRUST surveillance studies tested 2,834, 629, and 389 clinical isolates of S. pneumoniae, H. influenzae, and M. catarrhalis, respectively, from patients <18 years old. In addition, 10,966, 2,281, and 1,357 isolates of S. pneumoniae, H. influenzae, and M. catarrhalis, respectively, from patients ≥18 years old were tested. Isolates were prospectively collected from 281 clinical microbiology laboratories distributed across the United States. Participating laboratories served institutions with various sizes (<100 to >999 beds), patient demographics, and specialties. Isolates were considered to be etiologic agents of infections by individual laboratory algorithms and were collected without regard to patient age, gender, inpatient or outpatient status, or specimen source quotas. Each laboratory was requested to supply 35 S. pneumoniae, 7 H. influenzae, and 4 M. catarrhalis isolates in the 2000-2001 respiratory illness season and 30 S. pneumoniae, 6 H. influenzae, and 4 M. catarrhalis isolates in the 2001-2002 respiratory illness season to a central laboratory (Focus Technologies, Herndon, Va.). Duplicate isolates from the same patient were excluded, the identities of isolates were confirmed, and MICs were determined in the central laboratory. Observed alpha-hemolysis on blood agar and an optochin disk zone diameter of ≥14 mm were used to confirm isolates of S. pneumoniae; when necessary, a bile solubility test was also performed. H. influenzae isolates were confirmed by X- and V-factor requirements, and M. catarrhalis isolates were confirmed by a positive oxidase reaction and indoxyl butyrate degradation. Isolates of H. influenzae and M. catarrhalis were tested for β-lactamase production by using the chromogenic substrate nitrocefin.
The 2,834 isolates of pneumococci from pediatric patients were from the following specimen sources: upper respiratory tract (n = 846, 29.9% of isolates; nasopharynx, throat, nose, and sinus), ear culture (n = 580, 20.5%), blood culture (n = 553, 19.5%), lower respiratory tract (n = 453, 16.0%; sputum, bronchial washings, and tracheal aspirates), other sources (n = 384, 13.5%), and unknown sources (n = 18, 0.6%). S. pneumoniae isolates from adult patients were composed of 6,036 lower respiratory tract isolates (55.0%), 3,481 blood culture isolates (31.7%), 580 upper respiratory tract isolates (5.3%), 153 ear culture isolates (1.4%), 648 isolates from other sources (5.9%), and 68 isolates from unknown sources (0.6%). H. influenzae isolates from pediatric and adult patients, respectively, were composed of 123 and 1,857 lower respiratory tract isolates, 211 and 179 upper respiratory tract isolates, 117 and 12 ear culture isolates, 14 and 95 blood culture isolates, 158 and 123 isolates from other sources, and 6 and 15 isolates from unknown sources. M. catarrhalis isolates from pediatric and adult patients, respectively, were composed of 130 and 1,158 lower respiratory tract isolates, 142 and 132 upper respiratory tract isolates, 46 and 4 ear culture isolates, 8 and 19 blood culture isolates, 62 and 37 isolates from other sources, and 1 and 7 isolates from unknown sources.
Antimicrobial susceptibility testing.
Broth microdilution antimicrobial susceptibility testing was performed in the central laboratory according to NCCLS guidelines (18) by using panels manufactured by TREK Diagnostics (Westlake, Ohio). MICs were interpreted by using 2002 NCCLS recommended breakpoints (19).
MDR S. pneumoniae.
The prevalence and phenotypes of multidrug-resistant (MDR) S. pneumoniae isolates were studied by defining MDR as concurrent resistance to three or more of the following antimicrobial agents (intermediate isolates were excluded): penicillin, ceftriaxone, azithromycin, SXT, and levofloxacin. Although this definition includes two β-lactam agents, our intention in defining multidrug resistance was to use the phenotype as a marker for isolates for which therapeutic choices would be diminished. Therefore, because ceftriaxone may remain a therapeutic alternative for some penicillin-resistant strains, it was included in the definition criteria.
RESULTS
For isolates of S. pneumoniae, rates of resistance to penicillin, azithromycin, and SXT were ≥7.5% higher in patients ≤4 years of old than in patients 5 to 10, 11 to 17, 18 to 64, and >64 years old (Table 1). Rates of resistance to these agents in isolates from patients >4 years old were generally similar (Table 1). Likewise, isolates from patients 5 years old and older demonstrated rates of resistance to amoxicillin-clavulanate and ceftriaxone that were 3 to 6% lower than those of isolates from patients ≤4 years old. Two levofloxacin-resistant isolates of S. pneumoniae were detected in patients <18 years old, both in 2001-2002; one isolate was from a child 2 to 4 years old, and one isolate was from a child 5 to 10 years old. In 2000-2001 and 2001-2002, respective rates of levofloxacin resistance (MIC, ≥8 μg/ml) in patients 18 to 64 years old were 0.7 and 1.1%, and those in patients >64 years old were 1.2 and 1.3%.
TABLE 1.
Summary of MIC and MIC interpretationa data for isolates of S. pneumoniae from pediatric and adult patients in the United States in 2000-2001 and 2001-2002
| Antimicrobial agent | Patient age (yr) | 2000-2001b
|
2001-2002c
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml)
|
% of isolates that were:
|
MIC (μg/ml)
|
% of isolates that were:
|
||||||||||
| Range | 50% | 90% | S | I | R | Range | 50% | 90% | S | I | R | ||
| Penicillin | <2 | ≤0.03-8 | 0.12 | 2 | 48.5 | 23.1 | 28.3 | ≤0.03->8 | 0.12 | 4 | 45.5 | 20.5 | 34.0 |
| 2-4 | ≤0.03-8 | 0.06 | 2 | 51.0 | 22.2 | 26.8 | ≤0.03->8 | 0.06 | 4 | 53.5 | 17.5 | 28.9 | |
| 5-10 | ≤0.03-4 | ≤0.03 | 2 | 66.3 | 17.6 | 16.1 | ≤0.03-4 | ≤0.03 | 2 | 68.9 | 13.2 | 17.9 | |
| 11-17 | ≤0.03-4 | ≤0.03 | 2 | 65.8 | 14.9 | 19.3 | ≤0.03-8 | ≤0.03 | 2 | 72.0 | 13.4 | 14.7 | |
| 18-64 | ≤0.03->8 | ≤0.03 | 2 | 66.0 | 18.5 | 15.4 | ≤0.03-8 | ≤0.03 | 2 | 69.0 | 15.6 | 15.4 | |
| >64 | ≤0.03-8 | ≤0.03 | 2 | 66.9 | 17.5 | 15.6 | ≤0.03->8 | ≤0.03 | 2 | 69.8 | 14.1 | 16.0 | |
| Amoxicillin-clavulanate | <2 | ≤0.015-8 | 0.06 | 4 | 85.9 | 7.9 | 6.1 | ≤0.015->8 | 0.12 | 4 | 84.2 | 5.9 | 9.9 |
| 2-4 | ≤0.015-8 | 0.06 | 4 | 85.1 | 7.7 | 7.3 | ≤0.015-8 | 0.03 | 4 | 86.4 | 4.9 | 8.8 | |
| 5-10 | ≤0.015-8 | 0.03 | 2 | 93.8 | 4.7 | 1.6 | ≤0.015-8 | 0.03 | 2 | 93.3 | 2.9 | 3.8 | |
| 11-17 | ≤0.015-8 | 0.03 | 2 | 92.1 | 5.3 | 2.6 | ≤0.015-8 | 0.03 | 2 | 91.4 | 4.7 | 3.9 | |
| 18-64 | ≤0.015->8 | 0.03 | 2 | 93.7 | 4.1 | 2.3 | ≤0.015->8 | 0.03 | 2 | 94.4 | 2.2 | 3.4 | |
| >64 | ≤0.015->8 | 0.03 | 2 | 93.9 | 3.8 | 2.3 | ≤0.015->8 | 0.03 | 2 | 94.4 | 2.8 | 2.9 | |
| Ceftriaxone | <2 | ≤0.015-4 | 0.06 | 1 | 94.8 | 2.7 | 2.5 | ≤0.015-8 | 0.12 | 1 | 92.0 | 3.4 | 4.5 |
| 2-4 | ≤0.015-8 | 0.06 | 1 | 91.2 | 3.8 | 5.0 | ≤0.015-4 | 0.06 | 1 | 93.5 | 3.5 | 3.0 | |
| 5-10 | ≤0.015-8 | ≤0.015 | 1 | 96.9 | 1.6 | 1.6 | ≤0.015-8 | 0.03 | 1 | 95.7 | 2.6 | 1.7 | |
| 11-17 | ≤0.015-8 | 0.03 | 1 | 97.4 | 0 | 2.6 | ≤0.015-4 | 0.03 | 1 | 95.3 | 3.9 | 0.9 | |
| 18-64 | ≤0.015-8 | 0.03 | 1 | 97.6 | 1.3 | 1.1 | ≤0.015-8 | 0.03 | 1 | 96.7 | 1.9 | 1.5 | |
| >64 | ≤0.015-8 | 0.03 | 1 | 97.1 | 1.6 | 1.2 | ≤0.015-8 | 0.03 | 1 | 97.5 | 1.5 | 1.0 | |
| Azithromycin | <2 | ≤0.015->32 | 0.06 | >32 | 58.7 | 0.7 | 40.6 | ≤0.015->32 | 0.12 | >32 | 56.0 | 0.5 | 43.5 |
| 2-4 | ≤0.015->32 | 0.06 | >32 | 62.1 | 0 | 37.9 | ≤0.015->32 | 0.12 | >32 | 60.4 | 0 | 39.6 | |
| 5-10 | ≤0.015->32 | 0.06 | 8 | 76.7 | 0 | 23.3 | ≤0.015->32 | 0.06 | >32 | 72.5 | 0 | 27.5 | |
| 11-17 | 0.03->32 | 0.06 | 8 | 74.6 | 0 | 25.4 | 0.03->32 | 0.06 | 8 | 77.2 | 0.4 | 22.4 | |
| 18-64 | ≤0.015->32 | 0.06 | 8 | 74.4 | 0.5 | 25.1 | ≤0.015->32 | 0.06 | 16 | 74.6 | 0.3 | 25.1 | |
| >64 | ≤0.015->32 | 0.06 | 16 | 72.3 | 0.7 | 27.0 | ≤0.015->32 | 0.06 | 16 | 76.2 | 0.2 | 23.6 | |
| SXT | <2 | ≤0.06->4 | 1 | >4 | 47.6 | 8.2 | 44.2 | ≤0.06->4 | 1 | >4 | 48.5 | 6.7 | 44.8 |
| 2-4 | ≤0.06->4 | 0.5 | >4 | 50.2 | 8.8 | 41.0 | ≤0.06->4 | 0.25 | >4 | 56.2 | 6.9 | 36.9 | |
| 5-10 | ≤0.06->4 | 0.25 | >4 | 67.4 | 7.8 | 24.9 | ≤0.06->4 | 0.25 | >4 | 70.1 | 5.7 | 24.2 | |
| 11-17 | ≤0.06->4 | 0.25 | >4 | 66.7 | 7.9 | 25.4 | ≤0.06->4 | 0.25 | >4 | 76.7 | 2.6 | 20.7 | |
| 18-64 | ≤0.06->4 | 0.25 | >4 | 68.8 | 5.1 | 26.1 | ≤0.06->4 | 0.25 | >4 | 71.4 | 5.8 | 22.7 | |
| >64 | ≤0.06->4 | 0.25 | >4 | 67.6 | 6.0 | 26.4 | ≤0.06->4 | 0.25 | >4 | 70.5 | 6.2 | 23.3 | |
| Levofloxacin | <2 | ≤0.06-2 | 0.5 | 1 | 100 | 0 | 0 | 0.25-2 | 0.5 | 1 | 100 | 0 | 0 |
| 2-4 | ≤0.06-1 | 0.5 | 1 | 100 | 0 | 0 | ≤0.06-8 | 0.5 | 1 | 99.8 | 0 | 0.2 | |
| 5-10 | 0.12-1 | 0.5 | 1 | 100 | 0 | 0 | 0.12-8 | 1 | 1 | 99.8 | 0 | 0.2 | |
| 11-17 | 0.5-1 | 1 | 1 | 100 | 0 | 0 | 0.25-2 | 1 | 1 | 100 | 0 | 0 | |
| 18-64 | ≤0.06->8 | 0.5 | 1 | 99.2 | 0.1 | 0.7 | ≤0.06->8 | 1 | 1 | 98.8 | 0.1 | 1.1 | |
| >64 | ≤0.06->8 | 0.5 | 1 | 98.8 | 0.1 | 1.2 | ≤0.06->8 | 1 | 1 | 98.5 | 0.2 | 1.3 | |
Interpreted using NCCLS 2002 breakpoints (19). S, susceptible; I, intermediate; R, resistant.
During 2000-2001, 441 isolates were collected from patients <2 years old, 261 were collected from patients 2 to 4 years old, 193 were collected from patients 5 to 10 years old, 114 were collected from patients 11 to 17 years old, 3,042 were collected from patients 18 to 64 years old, and 2,245 were collected from patients >64 years old.
During 2001-2002, 639 isolates were collected from patients <2 years old, 536 were collected from patients 2 to 4 years old, 418 were collected from patients 5 to 10 years old, 232 were collected from patients 11 to 17 years old, 3,297 were collected from patients 18 to 64 years old, and 2,382 were collected from patients >64 years old.
Table 2 depicts levofloxacin MIC distributions by patient age group. The levofloxacin MICs at which 50% of the isolates were inhibited (MIC50s) were 0.5 μg/ml for patients <2 and 2 to 4 years old and 1 μg/ml for all other patient age groups. The levofloxacin MIC90 was 1 μg/ml for all patient age groups. The highest levofloxacin MIC noted for patients <18 years old was 8 μg/ml (two isolates).
TABLE 2.
Comparison of levofloxacin MIC distributions for S. pneumoniae isolated from patients in different age groups in the United States from 2000 to 2002
| Patient age (yr) | No. of isolates tested | No. of isolates for which the levofloxacin MIC (μg/ml) was as follows (cumulative % of isolates inhibited):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | >8 | ||
| <2 | 1,080 | 1 (0.1) | 0 (0.1) | 7 (0.7) | 621 (58.2) | 448 (99.7) | 3 (100) | |||
| 2-4 | 797 | 2 (0.3) | 1 (0.4) | 3 (0.8) | 448 (57.0) | 342 (99.9) | 0 (99.9) | 0 (99.9) | 1 (100) | |
| 5-10 | 611 | 2 (0.3) | 0 (0.3) | 287 (47.3) | 320 (99.7) | 1 (99.8) | 0 (99.8) | 1 (100) | ||
| 11-17 | 346 | 1 (0.3) | 163 (47.4) | 181 (99.7) | 1 (100) | |||||
| 18-64 | 6,339 | 10 (0.2) | 7 (0.3) | 56 (1.2) | 3,056 (49.4) | 3,126 (98.7) | 21 (99.0) | 6 (99.1) | 35 (99.7) | 22 (100) |
| >64 | 4,627 | 7 (0.2) | 4 (0.2) | 25 (0.8) | 2,210 (48.5) | 2,298 (98.2) | 19 (98.6) | 6 (98.7) | 28 (99.4) | 30 (100) |
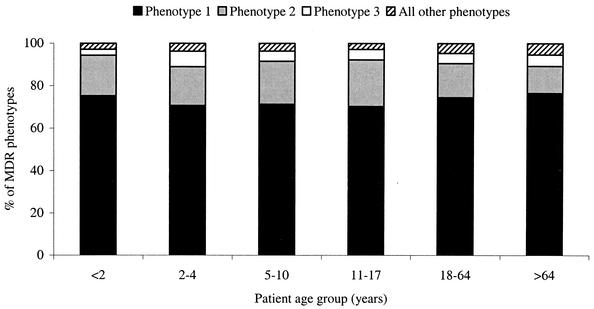
Table 3 depicts the prevalence of MDR isolates by patient age group and the most prevalent MDR phenotypes in isolates of S. pneumoniae from the 2000-2002 respiratory illness season. MDR isolates were approximately twice as common in patients ≤4 years old (24.1 to 26.1%) as in patients in any of the older age groups (11.9 to 13.7%). Concurrent resistance to penicillin, azithromycin, and SXT was the most prevalent MDR phenotype (70.3 to 76.6%), regardless of the patient age group (Fig. 1). MDR phenotypes for patients <2 and 11 to 17 years old did not involve levofloxacin. The two levofloxacin-resistant isolates from patients 2 to 4 years old (one isolate) and 5 to 10 years old (one isolate) were MDR isolates. In patients 18 to 64 and >64 years old, 3.3% (0.4% of all isolates in this age group) and 3.8% (0.5% of all isolates in this age group) of MDR phenotypes involved levofloxacin (MIC, ≥8 μg/ml).
TABLE 3.
Prevalence of MDR phenotypes in S. pneumoniae isolated from patients in different age groups in the United States from 2000 to 2002
| Patient age (yr) | No. of isolates tested | No. (%) of isolates with an MDR phenotypea | % of MDR isolates with the most prevalent MDR phenotypeb | No. (%) of MDR isolates that included levofloxacin resistance | % of all isolates that were MDR and resistant to levofloxacin |
|---|---|---|---|---|---|
| <2 | 1,080 | 283 (26.1) | 75.3 | 0 (0) | 0 |
| 2-4 | 797 | 192 (24.1) | 70.8 | 1 (0.5) | 0.1 |
| 5-10 | 611 | 84 (13.7) | 71.4 | 1 (1.2) | 0.2 |
| 11-17 | 346 | 41 (11.9) | 70.3 | 0 (0) | 0 |
| 18-64 | 6,339 | 767 (12.1) | 74.6 | 25 (3.3) | 0.4 |
| >64 | 4,627 | 573 (12.4) | 76.6 | 22 (3.8) | 0.5 |
Resistance to three or more of the following antimicrobial agents: penicillin, ceftriaxone, azithromycin, SXT, and levofloxacin (19).
The most prevalent MDR phenotype consisted of resistance to penicillin, azithromycin, and SXT. For all age groups, this phenotype included ceftriaxone-intermediate isolates five to seven times more frequently than ceftriaxone-susceptible isolates.
FIG. 1.
S. pneumoniae MDR phenotypes by patient age group in the United States in 2000-2002. Phenotype 1 was resistance to penicillin, azithromycin, and SXT; phenotype 2 was resistance to penicillin, ceftriaxone, azithromycin, and SXT; and phenotype 3 was resistance to penicillin, ceftriaxone, and SXT.
In 2000-2001, for patients <2, 2 to 4, 5 to 10, 11 to 17, 18 to 64, and >64 years old, 33.9, 39.4, 36.6, 28.6, 27.2, and 26.9% of H. influenzae isolates were β-lactamase positive, respectively. In 2001-2002, for patients <2, 2 to 4, 5 to 10, 11 to 17, 18 to 64, and >64 years old, 32.9, 42.3, 33.3, 22.0, 25.6, and 25.6% of H. influenzae isolates were β-lactamase positive, respectively. Consequently, ampicillin resistance rates were highest in patients 2 to 4, 5 to 10, and <2 years old, generally 10% higher than the resistance rates found for isolates from patients >10 years old. All isolates of H. influenzae were susceptible to ceftriaxone; 1 non-levofloxacin-susceptible isolate from a patient >64 years old in 2001-2002 and 19 non-azithromycin-susceptible isolates (9 isolates in 2000-2001 and 10 isolates in 2001-2002) were identified (Table 4). Two isolates were resistant to amoxicillin-clavulanate and were isolated from patients ≥18 years old. SXT resistance rates were lowest in patients 5 to 10 years old (7.3% in 2000-2001 and 14.6% in 2001-2002) and 11 to 17 years old (8.6% in 2000-2001 and 14.3% in 2001-2002) and similar (range, 17.6 to 22.9%) in patients <5 and ≥18 years old.
TABLE 4.
Summary of MIC and MIC interpretationa data for isolates of H. influenzae from pediatric and adult patients in the United States in 2000-2001 and 2001-2002
| Antimicrobial agent | Patient age (yr) | 2000-2001b
|
2001-2002c
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml)
|
% of isolates that were:
|
MIC (μg/ml)
|
% of isolates that were:
|
||||||||||
| Range | 50% | 90% | S | I | R | Range | 50% | 90% | S | I | R | ||
| Ampicillin | <2 | ≤0.12->8 | 1 | >8 | 65.1 | 0.9 | 33.9 | ≤0.12->8 | 0.25 | >8 | 67.8 | 0 | 32.2 |
| 2-4 | ≤0.12->8 | 0.5 | >8 | 60.6 | 1.4 | 38.0 | ≤0.12->8 | 1 | >8 | 54.3 | 1.1 | 44.6 | |
| 5-10 | ≤0.12->8 | 0.25 | >8 | 63.4 | 0 | 36.6 | ≤0.12->8 | 0.5 | >8 | 65.9 | 1.2 | 32.9 | |
| 11-17 | ≤0.12->8 | 0.25 | >8 | 71.4 | 0 | 28.6 | ≤0.12->8 | 0.25 | >8 | 82.1 | 0 | 17.9 | |
| 18-64 | ≤0.12->8 | 0.25 | >8 | 72.6 | 0.1 | 27.3 | ≤0.12->8 | 0.25 | >8 | 76.1 | 0 | 23.9 | |
| >64 | ≤0.12->8 | 0.5 | >8 | 72.7 | 0.2 | 27.1 | ≤0.12->8 | 0.25 | >8 | 74.4 | 0.9 | 24.7 | |
| Amoxicillin-clavulanate | <2 | ≤0.015-4 | 0.5 | 2 | 100 | — | 0 | 0.12-4 | 0.5 | 1 | 100 | — | 0 |
| 2-4 | 0.25-4 | 0.5 | 1 | 100 | — | 0 | 0.12-4 | 0.5 | 1 | 100 | — | 0 | |
| 5-10 | 0.12-4 | 0.5 | 1 | 100 | — | 0 | 0.12-4 | 0.5 | 1 | 100 | — | 0 | |
| 11-17 | 0.25-4 | 0.5 | 1 | 100 | — | 0 | 0.12-2 | 0.25 | 1 | 100 | — | 0 | |
| 18-64 | ≤0.015-16 | 0.5 | 1 | 99.7 | — | 0.3 | ≤0.015-4 | 0.5 | 1 | 100 | — | 0 | |
| >64 | ≤0.015-4 | 0.5 | 2 | 100 | — | 0 | ≤0.015-8 | 0.5 | 2 | 99.8 | — | 0.2 | |
| Ceftriaxone | <2 | ≤0.015-0.06 | ≤0.015 | ≤0.015 | 100 | — | — | ≤0.015-0.06 | ≤0.015 | ≤0.015 | 100 | — | — |
| 2-4 | ≤0.015-≤0.015 | ≤0.015 | ≤0.015 | 100 | — | — | ≤0.015-0.25 | ≤0.015 | ≤0.015 | 100 | — | — | |
| 5-10 | ≤0.015-0.25 | ≤0.015 | ≤0.015 | 100 | — | — | ≤0.015-0.12 | ≤0.015 | ≤0.015 | 100 | — | — | |
| 11-17 | ≤0.015-≤0.015 | ≤0.015 | ≤0.015 | 100 | — | — | ≤0.015-≤0.015 | ≤0.015 | ≤0.015 | 100 | — | — | |
| 18-64 | ≤0.015-0.25 | ≤0.015 | ≤0.015 | 100 | — | — | ≤0.015-0.12 | ≤0.015 | ≤0.015 | 100 | — | — | |
| >64 | ≤0.015-0.25 | ≤0.015 | ≤0.015 | 100 | — | — | ≤0.015-0.12 | ≤0.015 | ≤0.015 | 100 | — | — | |
| Azithromycin | <2 | ≤0.015-4 | 1 | 2 | 100 | — | — | 0.06-8 | 1 | 2 | 99.3 | — | — |
| 2-4 | 0.06-2 | 1 | 2 | 100 | — | — | 0.25-4 | 1 | 2 | 100 | — | — | |
| 5-10 | 0.5-4 | 1 | 2 | 100 | — | — | 0.25-4 | 1 | 2 | 100 | — | — | |
| 11-17 | 0.5-8 | 1 | 2 | 97.1 | — | — | 0.12-4 | 1 | 2 | 100 | — | — | |
| 18-64 | ≤0.015->32 | 1 | 2 | 98.9 | — | — | 0.12-8 | 1 | 2 | 99.6 | — | — | |
| >64 | ≤0.015-4 | 1 | 2 | 100 | — | — | 0.06->32 | 1 | 2 | 98.5 | — | — | |
| SXT | <2 | ≤0.015->4 | 0.25 | >4 | 71.6 | 5.5 | 22.9 | ≤0.015->4 | 0.12 | >4 | 72.7 | 5.6 | 21.7 |
| 2-4 | 0.03->4 | 0.12 | >4 | 76.1 | 2.8 | 21.1 | 0.03->4 | 0.12 | >4 | 75.0 | 6.5 | 18.5 | |
| 5-10 | 0.03->4 | 0.12 | 1 | 85.4 | 7.3 | 7.3 | 0.03->4 | 0.12 | 4 | 81.7 | 3.7 | 14.6 | |
| 11-17 | 0.03->4 | 0.12 | 2 | 85.7 | 5.7 | 8.6 | 0.03->4 | 0.12 | 4 | 82.1 | 3.6 | 14.3 | |
| 18-64 | ≤0.015->4 | 0.25 | >4 | 76.7 | 4.1 | 19.2 | ≤0.015->4 | 0.12 | >4 | 77.7 | 4.2 | 18.0 | |
| >64 | ≤0.015->4 | 0.25 | >4 | 74.1 | 4.1 | 21.8 | ≤0.015->4 | 0.12 | >4 | 80.2 | 2.2 | 17.6 | |
| Levofloxacin | <2 | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — |
| 2-4 | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — | |
| 5-10 | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — | 0.008-0.03 | 0.015 | 0.015 | 100 | — | — | |
| 11-17 | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — | 0.008-0.03 | 0.015 | 0.015 | 100 | — | — | |
| 18-64 | ≤0.004-0.06 | 0.015 | 0.015 | 100 | — | — | ≤0.004-0.03 | 0.015 | 0.015 | 100 | — | — | |
| >64 | ≤0.004-0.25 | 0.015 | 0.015 | 100 | — | — | ≤0.004-8 | 0.015 | 0.015 | 99.8 | — | — | |
Interpreted using NCCLS 2002 breakpoints (19). Only susceptible breakpoints are published by NCCLS for ceftriaxone, azithromycin, and levofloxacin. S, susceptible; I, intermediate; R, resistant; —, MIC criteria not defined by NCCLS (19).
During 2000-2001, 109 isolates were collected from patients <2 years old, 71 were collected from patients 2 to 4 years old, 41 were collected from patients 5 to 10 years old, 35 were collected from patients 11 to 17 years old, 729 were collected from patients 18 to 64 years old, and 532 were collected from patients >64 years old.
During 2001-2002, 143 isolates were collected from patients <2 years old, 92 were collected from patients 2 to 4 years old, 82 were collected from patients 5 to 10 years old, 56 were collected from patients 11 to 17 years old, 566 were collected from patients 18 to 64 years old, and 454 were collected from patients >64 years old.
In 2000-2001, for patients <2, 2 to 4, 5 to 10, 11 to 17, 18 to 64, and >64 years old, 97.2, 97.3, 95.5, 100, 94.0, and 91.7% of M. catarrhalis isolates were β-lactamase positive, respectively. In 2001-2002, for patients <2, 2 to 4, 5 to 10, 11 to 17, 18 to 64, and >64 years old, 98.4, 98.0, 94.2, 93.3, 92.8, and 93.1% of M. catarrhalis isolates were β-lactamase positive. The 2002 NCCLS breakpoints for Staphylococcus aureus were used to interpret the MICs for M. catarrhalis (Table 5). All isolates were susceptible to amoxicillin-clavulanate, ceftriaxone, and levofloxacin. For isolates collected in 2001-2002, one- or two-doubling-dilution increases in the MIC50s of ampicillin and amoxicillin-clavulanate were noted for patients <2 and 2 to 4 years old compared to all other age groups. The MIC50s for all agents except azithromycin and levofloxacin were generally higher for isolates collected from pediatric patients than for those collected from adult patients in both 2000-2001 and 2001-2002. No change in the MIC90s was observed for M. catarrhalis isolates when age group data were compared for amoxicillin-clavulanate, SXT, and levofloxacin.
TABLE 5.
Summary of MIC and MIC interpretationa data for isolates of M. catarrhalis from pediatric and adult patients in the United States in 2000-2001 and 2001-2002
| Antimicrobial agent | Patient age (yr) | 2000-2001b
|
2001-2002c
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml)
|
% of isolates that were:
|
MIC (μg/ml)
|
% of isolates that were:
|
||||||||||
| Range | 50% | 90% | S | I | R | Range | 50% | 90% | S | I | R | ||
| Ampicillin | <2 | ≤0.12->8 | 4 | >8 | 2.8 | 0 | 97.2 | ≤0.12->8 | 4 | >8 | 3.4 | 0 | 96.6 |
| 2-4 | ≤0.12->8 | 4 | >8 | 5.4 | 0 | 94.6 | ≤0.12->8 | 4 | >8 | 1.6 | 0 | 98.4 | |
| 5-10 | ≤0.12->8 | 2 | 8 | 4.5 | 0 | 95.5 | ≤0.12-8 | 2 | 8 | 12.8 | 0 | 87.2 | |
| 11-17 | 0.5->8 | 2 | 4 | 0 | 0 | 100 | ≤0.12->8 | 2 | >8 | 12.5 | 0 | 87.5 | |
| 18-64 | ≤0.12->8 | 4 | 8 | 13.3 | 0 | 86.7 | ≤0.12->8 | 2 | 8 | 12.8 | 0 | 87.2 | |
| >64 | ≤0.12->8 | 2 | 8 | 17.8 | 0 | 82.2 | ≤0.12->8 | 2 | 8 | 13.0 | 0 | 87.0 | |
| Amoxicillin-clavulanate | <2 | ≤0.015-0.5 | 0.25 | 0.25 | 100 | 0 | 0 | ≤0.015-0.5 | 0.25 | 0.25 | 100 | 0 | 0 |
| 2-4 | ≤0.015-0.5 | 0.25 | 0.25 | 100 | 0 | 0 | 0.03-0.5 | 0.25 | 0.25 | 100 | 0 | 0 | |
| 5-10 | ≤0.015-0.25 | 0.06 | 0.25 | 100 | 0 | 0 | ≤0.015-0.5 | 0.12 | 0.25 | 100 | 0 | 0 | |
| 11-17 | 0.03-0.5 | 0.12 | 0.25 | 100 | 0 | 0 | ≤0.015-0.25 | 0.12 | 0.25 | 100 | 0 | 0 | |
| 18-64 | ≤0.015-0.5 | 0.12 | 0.25 | 100 | 0 | 0 | ≤0.015-0.5 | 0.12 | 0.25 | 100 | 0 | 0 | |
| >64 | ≤0.015-1 | 0.12 | 0.25 | 100 | 0 | 0 | ≤0.015-0.5 | 0.12 | 0.25 | 100 | 0 | 0 | |
| Ceftriaxone | <2 | ≤0.015-2 | 0.5 | 1 | 100 | 0 | 0 | ≤0.015-2 | 0.5 | 1 | 100 | 0 | 0 |
| 2-4 | ≤0.015-2 | 0.5 | 1 | 100 | 0 | 0 | ≤0.015-2 | 0.5 | 1 | 100 | 0 | 0 | |
| 5-10 | ≤0.015-2 | 0.12 | 1 | 100 | 0 | 0 | ≤0.015-1 | 0.5 | 1 | 100 | 0 | 0 | |
| 11-17 | ≤0.015-1 | 0.12 | 0.5 | 100 | 0 | 0 | ≤0.015-1 | 0.5 | 1 | 100 | 0 | 0 | |
| 18-64 | ≤0.015-2 | 0.25 | 1 | 100 | 0 | 0 | ≤0.015-2 | 0.25 | 1 | 100 | 0 | 0 | |
| >64 | ≤0.015-2 | 0.12 | 0.5 | 100 | 0 | 0 | ≤0.015-2 | 0.25 | 1 | 100 | 0 | 0 | |
| Azithromycin | <2 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 |
| 2-4 | ≤0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | ≤0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | |
| 5-10 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 | |
| 11-17 | ≤0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 | |
| 18-64 | ≤0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 | |
| >64 | ≤0.015-4 | 0.03 | 0.03 | 99.7 | 0.3 | 0 | ≤0.015-0.06 | 0.03 | 0.03 | 100 | 0 | 0 | |
| SXT | <2 | 0.06-2 | 0.25 | 0.5 | 100 | 0 | 0 | 0.06-2 | 0.25 | 0.5 | 100 | 0 | 0 |
| 2-4 | 0.03-1 | 0.12 | 0.5 | 100 | 0 | 0 | 0.06-4 | 0.12 | 0.5 | 98.4 | 0 | 1.6 | |
| 5-10 | 0.12-4 | 0.25 | 0.5 | 95.5 | 0 | 4.5 | 0.06-0.5 | 0.12 | 0.25 | 100 | 0 | 0 | |
| 11-17 | 0.06-1 | 0.12 | 0.5 | 100 | 0 | 0 | 0.06-0.5 | 0.12 | 0.5 | 100 | 0 | 0 | |
| 18-64 | ≤0.015-2 | 0.25 | 0.5 | 100 | 0 | 0 | 0.06-1 | 0.12 | 0.25 | 100 | 0 | 0 | |
| >64 | 0.06-2 | 0.12 | 0.5 | 100 | 0 | 0 | 0.03-2 | 0.25 | 0.25 | 100 | 0 | 0 | |
| Levofloxacin | <2 | 0.015-0.12 | 0.03 | 0.03 | 100 | 0 | 0 | 0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 |
| 2-4 | 0.015-0.12 | 0.03 | 0.06 | 100 | 0 | 0 | 0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | |
| 5-10 | 0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | 0.03-0.12 | 0.03 | 0.06 | 100 | 0 | 0 | |
| 11-17 | 0.03-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | 0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | |
| 18-64 | 0.015-0.12 | 0.03 | 0.06 | 100 | 0 | 0 | 0.015-1 | 0.03 | 0.06 | 100 | 0 | 0 | |
| >64 | 0.008-0.25 | 0.03 | 0.06 | 100 | 0 | 0 | 0.015-0.06 | 0.03 | 0.06 | 100 | 0 | 0 | |
Interpreted using NCCLS 2002 breakpoints for staphylococci (19). S, susceptible; I, intermediate; R, resistant.
During 2000-2001, 72 isolates were collected from patients <2 years old, 37 were collected from patients 2 to 4 years old, 22 were collected from patients 5 to 10 years old, 14 were collected from patients 11 to 17 years old, 331 were collected from patients 18 to 64 years old, and 360 were collected from patients >64 years old.
During 2001-2002, 119 isolates were collected from patients <2 years old, 62 were collected from patients 2 to 4 years old, 47 were collected from patients 5 to 10 years old, 16 were collected from patients 11 to 17 years old, 312 were collected from patients 18 to 64 years old, and 354 were collected from patients >64 years old.
DISCUSSION
The data presented in this study indicate that rates of resistance to penicillin, azithromycin, and SXT in pneumococci were ≥7.5% higher in patients ≤4 years old than in patients 5 to 10, 11 to 17, and ≥18 years old and that MDR was twice as common in patients ≤4 years old (25.3%) as in patients 5 to 10 years old (13.7%), 11 to 17 years old (11.9%), 18 to 64 years old (12.1%), and >64 years old (12.4%). Previous studies reported similar data (2, 11, 24). In 2000 in the United States, Doern et al. (11) found that of the S. pneumoniae isolates collected from children 0 to 5 years old, 42.5% (n = 190) were penicillin intermediate or resistant and 32.9% (n = 147) were erythromycin resistant; these levels were 7 to 10% higher than those in older patients. A recent epidemiological study also reported penicillin resistance rates to be significantly higher in families with children 0 to 4 years old (L. Li and B. P. Currie, Program Abstr. 7th Int. Congr. Infect. Dis., abstr. 18.008, 1998). Thomson et al. (24) and Black et al. (2) both reported that the penicillin susceptibility of pneumococcal isolates did not affect the activity of levofloxacin; MIC90s in both studies were 1 μg/ml for penicillin-susceptible, -intermediate, and -resistant isolates (2, 24). Active Bacterial Core surveillance, an ongoing population-based surveillance system for invasive pneumococcal disease in selected areas of the United States, reported that all levofloxacin-resistant isolates in 1998 and 1999 (0.3%, 14/4,791) were from adults (16). Taken together, these data suggest that resistance to penicillin, macrolides, and SXT in isolates from young children has emerged at the same time that susceptibility to fluoroquinolones has remained close to 100%. These observations support suggestions (28) that fluoroquinolones may be useful in the empirical therapy of respiratory infections in children.
It would be assumed that in a large surveillance study, such as that conducted here and by other investigators, that several fluoroquinolone-resistant isolates of S. pneumoniae would be isolated from children, theoretically the result of clonal spread from an adult relative. The lack of such an observation may suggest the presence of a barrier to transmission between isolates infecting and colonizing adults and children or perhaps that fluoroquinolone resistance in pneumococci is still sufficiently rare for clonal spread in both adults and children and remains largely undetectable. With regard to this observation, Chiu et al. (5) recently studied 383 isolates of S. pneumoniae from children 2 to 6 years old and attending 79 day care centers or kindergartens in Hong Kong. Of these isolates, 58% were penicillin intermediate or resistant; 81, 77, and 60% were resistant to SXT, erythromycin, and clindamycin, respectively; and 39.4% were MDR. No isolate was resistant to levofloxacin (5). Given that the rate of resistance to levofloxacin was previously documented to be 5.5% in pneumococcal isolates from hospitalized patients in Hong Kong (13), concern existed that the resistant isolates would be passed from adult carriers to children and then become disseminated in day care centers and kindergartens. At present, this appears not to have happened.
The fluoroquinolone data presented in this study focused on levofloxacin. Recent observations regarding the susceptibility of pneumococci to levofloxacin indicated that the resistance of isolates from adults in the United States remained low (<1%) from 1999 to 2001 (11, 20; L. J. Kelly, C. Thornsberry, M. E. Jones, et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2109, 2001). This situation has occurred despite reports that nonsusceptibility to ciprofloxacin (MIC, ≥4 μg/ml) has increased in pneumococcal isolates from Canadian patients >64 years old (4). However, Chen et al. (4) found no isolate of S. pneumoniae for which the ciprofloxacin MIC was ≥4 μg/ml (n = 2,311) in patients <15 years old. Ciprofloxacin data from the 1997-1998 respiratory illness season in the United States supported this observation, in that the MIC did not exceed 2 μg/ml for any isolate from patients <15 years old (21).
Although fluoroquinolones are highly effective and safe therapies for adults, they have not been widely prescribed for pediatric patients principally because of laboratory studies demonstrating arthropathy in immature animals following exposure to fluoroquinolones (3, 6, 7, 27). A rather large body of information regarding fluoroquinolone use in children and adolescents for a variety of conditions has accumulated, mainly due to compassionate use in certain pediatric patient populations (1, 3, 14, 27, 29). There has been no report in the scientific literature of a definitive fluoroquinolone-associated case of arthropathy in a child despite several radiological and magnetic resonance imaging investigations (3, 8, 14, 22). Retrospective reviews and prospective studies support the view that fluoroquinolone-associated toxicity unique to children may not occur or occurs at frequencies that are extremely low (1, 3, 14, 23, 27, 29).
Given the increase in drug resistance in respiratory tract bacterial pathogens and the experience of successfully treating adults with respiratory tract infections with fluoroquinolones, it is very likely that the potential risks and benefits of using fluoroquinolones to treat children will be reassessed. If fluoroquinolones are introduced into use in pediatric populations, it will be critical to monitor susceptibility and to use these agents in a manner that minimizes risk for inducing resistance to this class. Appropriate use will depend on careful consideration of the pharmacokinetics of these agents in children as well as adherence to policies that prevent unnecessary use of these drugs in children (15). In the 2000-2001 and 2001-2002 respiratory illness seasons in the United States, two levofloxacin-resistant isolates of S. pneumoniae (0.07%, 2/2,834) and no nonsusceptible isolate of H. influenzae (n = 629) were identified in pediatric patients. Patterns of resistance to fluoroquinolones in pathogens isolated from children are anticipated to be similar to those occurring in adults. Fluoroquinolones, such as levofloxacin, have the potential to be useful in treating children with serious respiratory tract infections due to these commonly occurring pathogens.
Acknowledgments
We thank Ortho-McNeil Pharmaceutical for financial support of TRUST surveillance studies.
We are especially grateful to all of the clinical testing institutions that participate in TRUST surveillance studies and that have contributed valuable data to this investigation.
REFERENCES
- 1.Alghasham, A. A., and M. C. Nahata. 2000. Clinical use of fluoroquinolones in children. Ann. Pharmacother. 34:347-359. [DOI] [PubMed]
- 2.Black, J., E. S. Moland, S. A. Chartrand, and K. S. Thomson. 2001. Activity of oral agents against pediatric isolates of Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 39:195-197. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt, J. E., J. N. Walterspiel, and U. B. Schaad. 1997. Quinolone arthropathy in animals versus children. Clin. Infect. Dis. 25:1196-1204. [DOI] [PubMed] [Google Scholar]
- 4.Chen, D. K., A. McGeer, J. C. De Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 5.Chiu, S. S., P. L. Ho, F. K. H. Chow, K. Y. Yuen, and Y. L. Lau. 2001. Nasopharyngeal carriage of antimicrobial-resistant Streptococccus pneumoniae among young children attending 79 kindergartens and day care centers in Hong Kong. Antimicrob. Agents Chemother. 45:2765-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christ, W., T. Lehnert, and B. Uklbrich. 1988. Specific toxicologic aspects of the fluoroquinolones. Rev. Infect. Dis. 10:141-146. [DOI] [PubMed] [Google Scholar]
- 7.Dagan, R. 1995. Fluoroquinolones in paediatrics—1995. Drugs 49(Suppl. 2):92-99. [DOI] [PubMed] [Google Scholar]
- 8.Danisovicovà, A., T. Krcméryova, S. Belan, H. Kayserova, E. Kaiserova, I. Hruskovic, K. Orosova, S. Dluholucky, K. Galova, and E. Matheova. 1995. Magnetic resonance imaging in diagnosis of potential arthropathogenicity in children receiving quinolones. No evidence of quinolone-induced arthropathy. Drugs 49(Suppl. 2):492-494. [DOI] [PubMed] [Google Scholar]
- 9.Doern, G. V., A. B. Brueggemann, G. Pierce, T. Hogan, H. P. Holley, and A. Rauch. 1996. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob. Agents Chemother. 40:2884-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doern, G. V., and The Alexander Project Collaborative Group. 1996. Antimicrobial resistance among lower respiratory tract isolates of Haemophilus influenzae: results of a 1992-1993 Western Europe and USA collaborative surveillance study. J. Antimicrob. Chemother. 38(Suppl. A):59-69. [DOI] [PubMed] [Google Scholar]
- 11.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. L. Coffman, and A. B. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felmingham, D., J. Washington, and The Alexander Project Group. 1999. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens—findings of the Alexander Project 1992-1996. J. Chemother. 11:5-21. [DOI] [PubMed] [Google Scholar]
- 13.Ho, P. L., T. L. Que, D. N. Tsang, T. K. Ng, K. H. Chow, and W. H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafri, H. S., and G. H. McCracken. 1999. Fluoroquinolones in pediatrics. Drugs 58(Suppl. 2):43-48. [DOI] [PubMed] [Google Scholar]
- 15.Mandell, L. A., L. R. Peterson, R. Wise, D. Hooper, D. E. Low, U. B. Schadd, K. P. Klugman, and P. Couralin. 2002. The battle against emerging antibiotic resistance: should fluoroquinolones be used to treat children? Clin. Infect. Dis. 35:721-727. [DOI] [PubMed] [Google Scholar]
- 16.Morbidity and Mortality Wkly. Report. 2001. Resistance of Streptococcus pneumoniae to fluoroquinolones—United States, 1995-1999. Morb. Mortal. Wkly. Rep. 5:800-804. [PubMed] [Google Scholar]
- 17.Musher, D. M. 1995. Streptococcus pneumoniae, p. 1811-1826. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, Philadelphia, Pa.
- 18.National Committee for Clinical Laboratory Standards. 2002. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. Approved standard, document M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Sahm, D. F., J. A. Karlowsky, L. J. Kelly, I. A. Critchley, M. E. Jones, C. Thornsberry, Y. R. Mauriz, and J. Kahn. 2001. Need for annual surveillance of antimicrobial resistance in Streptococcus pneumoniae in the United States: 2-year longitudinal analysis. Antimicrob. Agents Chemother. 45:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahm, D. F., D. E. Peterson, I. A. Critchley, and C. Thornsberry. 2000. Analysis of ciprofloxacin activity against Streptococcus pneumoniae after 10 years of use in the United States. Antimicrob. Agents Chemother. 44:2521-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaad, U. B. 1993. Use of fluoroquinolones in pediatrics. Drugs 45(Suppl. 3):37-41. [DOI] [PubMed] [Google Scholar]
- 23.Schaad, U. B. 1994. Use of the new quinolones in pediatrics. Isr. J. Med. Sci. 30:463-468. [PubMed] [Google Scholar]
- 24.Thomson, K. S., S. A. Chartrand, C. C. Sanders, and S. L. Block. 1997. Trovafloxacin, a new fluoroquinolone with potent activity against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:478-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornsberry, C., M. E. Jones, M. L. Hickey, Y. Mauriz, J. Kahn, and D. F. Sahm. 1999. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 199-1998. J. Antimicrob. Chemother. 44:749-759. [DOI] [PubMed] [Google Scholar]
- 26.Tomeh, M. O., S. E. Starr, J. E. McGowan, Jr., P. M. Terry, and A. J. Nahamias. 1974. Ampicillin-resistant Haemophilus influenzae type B infection. J. Am. Med. Assoc. 229:295-297. [PubMed] [Google Scholar]
- 27.Warren, R. W. 1997. Rheumatologic aspects of pediatric cystic fibrosis patients treated with fluoroquinolones. Pediatr. Infect. Dis. J. 16:118-122. [DOI] [PubMed] [Google Scholar]
- 28.Yagupsky, P., O. Katz, N. Peled, and R. Dagan. 2001. In vitro activity of novel fluoroquinolones against Streptococcus pneumoniae isolated from children with acute otitis media. Chemotherapy 47:354-358. [DOI] [PubMed] [Google Scholar]
- 29.Yee, C. L., C. Duffy, P. G. Gerbino, S. Stryker, and G. J. Noel. 2002. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. Pediatr. Infect. Dis. J. 21:525-529. [DOI] [PubMed] [Google Scholar]