Abstract
The application of a topical triple-antibiotic ointment (containing neomycin, polymyxin, and bacitracin) eradicated resident bacteria through 25 layers of the stratum corneum and prevented repopulation of bacteria overnight but not at 1 week. Through 15 layers, mupirocin had some effect, whereas a double-antibiotic ointment had none. The reservoir of resident bacteria in the sebaceous glands is not affected by a topical antibiotic.
Coagulase-negative staphylococci (CNS) are resident bacterial flora of normal human skin. CNS in the stratum corneum (SC) arise from a reservoir in the sebaceous glands in the dermis, whereas CNS on the skin surface derive from many sources (1). In previous work (2), topical antimicrobials eradicated bacteria on the surface but not in the SC, except for triple-antibiotic ointment (TAO; containing neomycin, polymyxin, and bacitracin), which eradicated organisms in the SC and prevented overnight repopulation with CNS. TAO may have prevented overnight repopulation by eradicating bacteria in the dermal reservoir (sebaceous glands) or by binding to cells in the SC to kill organisms entering the area from the reservoir.
The present study was designed to determine which component of TAO was responsible for the eradication of organisms in the SC and whether TAO acted in the sebaceous glands or bound to cells in the SC to prevent overnight repopulation. Because the SC regenerates approximately once a week, antimicrobials bound to cells in this layer should slough off within that time (3).
Fifty healthy adults who had not used antibiotics for 2 weeks were recruited from the university community. Subjects signed informed-consent statements approved by the Human Investigation Committee and received compensation for their time. Six skin sites on each subject were matched across the midline so that an active treatment could be administered on one side and a placebo could be administered on the other to account for variations in density of resident skin bacteria. The methods used for the culture, sampling, and removal of layers of the SC by tape-stripping were previously described (1, 2). In brief, contact plates (BBL Rodac; Becton Dickinson and Company, Sparks, Md.) containing Trypticase soy agar were applied to the skin through a hole the size of a contact plate in a template of flexible clear plastic. The edges of the template were marked on the skin to allow repeated sampling of the site. The template was wiped with 70% ethanol between samplings. The plates were incubated aerobically at 35°C, and colonies were counted at 24 and 48 h. The method described by Pinkus (4) was used to sample the SC beneath the surface. Three-inch-wide cellophane tape (Scotch; 3M, St. Paul, Minn.) was applied to the skin and stripped off to remove a layer of keratinized epithelium. A new tape surface was applied immediately and stripped off to remove another layer. The area was recultured (with the template as a guide) after sequential strippings as noted below.
A different ointment was applied to each of six sites: (i) for site A, TAO containing 5 mg of neomycin, 5,000 U of polymyxin, and 400 U of bacitracin per g (Neosporin; Pfizer, Inc., New York, N.Y.); (ii) for site B, the petrolatum vehicle base for site A containing no additives; (iii) for site C, double-antibiotic ointment (DAO) containing 10,000 U of polymyxin and 500U of bacitracin per g (Polysporin; Pfizer); (iv) for site D, the vehicle for site A containing proprietary additives (tocopherol acetate, sodium pyruvate, or fatty acids; cocoa butter, cottonseed oil, and olive oil) but no antibiotics; (v) for site E, 2% mupirocin ointment (Bactroban; SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.); and (vi) for site F, petroleum jelly (Vaseline; Chesebrough-Ponds USA Co., Greenwich, Conn.).
The skin of each adult was washed with Ivory bar soap, rinsed with water, and air dried before a pretreatment sample was obtained. A strip of ointment the length of the distal phalanx of the technician's index finger was applied with a sterile, gloved finger before the site was covered with sterile gauze. After 6 h, a posttreatment surface sample was obtained; then, tape-stripping of 5, 15, and 25 layers of the SC was done to obtain samples at those depths (termed 5-, 15-, and 25-layer samples). Each skin site was covered with sterile gauze overnight before the overnight surface and overnight 5-layer samples were obtained. Treated sites were then left uncovered. After 1 week, sites A, C, and D were washed with soap, rinsed, and air dried. Skin orientation marks were used as a guide for template placement, and surface, 15-layer, and 25-layer samples were obtained at 1 week.
Geometric mean titers (GMTs) of the number of CFU of bacteria per contact plate sample were calculated after log transformation; a measurement of 0 CFU per plate was assigned a value of −0.1 log10. GMT differences were compared by one-way analysis of variance, including the Tukey-Kramer multiple-comparison test. Differences in proportions were analyzed by Fisher's exact test.
Three hundred sites were studied. Bacteria on untreated skin surfaces ranged from 0 to 1,102 colonies; one site had no colonies. After treatment, 6 to 10% of the three placebo sites had no detectable bacteria (Table 1). There was no appreciable change in the proportion of placebo-treated sites with no bacteria (range, 0 to 16%) after the removal of successive layers of the SC and/or after overnight coverage.
TABLE 1.
Effect of ointment treatment for 6 h on skin of 50 healthy adults
| Sample | % of sites with eradication of bacteria after treatment witha:
|
|||||
|---|---|---|---|---|---|---|
| Antibiotic ointment
|
Placebo ointment
|
|||||
| Triple (A) | Double (C) | Mupirocin (E) | Base (B) | Vehicle (D) | Petroleum jelly (F) | |
| Pretreatment | 2 | 0 | 0 | 0 | 0 | 0 |
| After 6 h of treatment | ||||||
| Surface | 96* | 14 | 40* | 6 | 10 | 8 |
| 5 layers | 90* | 22* | 28* | 12 | 4 | 0 |
| 15 layers | 84* | 14 | 26* | 2 | 8 | 4 |
| 25 layers | 62* | 4 | 14 | 0 | 14 | 8 |
| After overnight treatment | ||||||
| Surface | 72* | 8 | 9 | 12 | 16 | 0 |
| 5 layers | 60* | 12 | 18 | 6 | 8 | 6 |
| At 1 wk posttreatment | ||||||
| Surface | 0 | 0 | 0 | |||
| 15 layers | 0 | 0 | 0 | |||
| 25 layers | 0 | 0 | 2 | |||
Eradication (0 CFU) of aerobic bacteria was determined at sites on the skin surface and in the SC. Each antibiotic-containing ointment was paired across the midline with a placebo ointment (i.e., A and B, C and D, E and F). *, P ≤ 0.01 versus placebo.
Treatment with TAO eradicated bacteria from 48 of 50 sites (P < 0.0001 compared to results with placebo at sites B). Bacteria were also eradicated through 25 layers and after overnight coverage at the majority of sites. Except for the five-layer samples, the results for the DAO-treated sites did not differ from the results for placebo-treated sites (Table 1). Mupirocin ointment eradicated bacteria at 40% of the surface sites sampled, at 28% of the 5-layer sites sampled, and at 26% of the 15-layer sites sampled (P < 0.01compared to results with placebo for all three). Thereafter, the results at mupirocin-treated sites were not different from the results at sites F, treated with placebo.
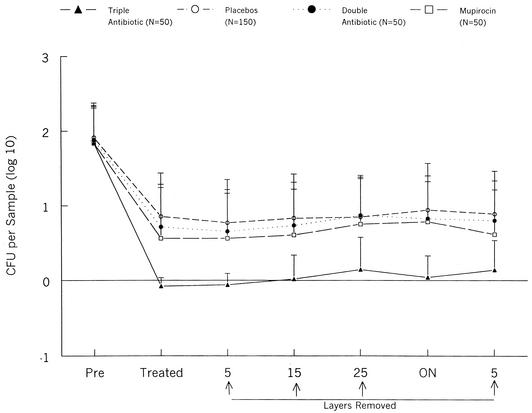
The titers measured at the three placebo-treated sites were combined for analysis of the GMTs (Fig. 1). All treatments significantly reduced the mean number of colonies at the surface. The GMTs at placebo-treated sites remained constant as successive layers were removed and after overnight coverage. The GMTs at the sites receiving DAO or mupirocin were similar to the GMTs for the sites receiving placebos, whereas the GMTs of the TAO-treated sites were lower than those of both the placebo-treated sites and those treated with other antimicrobials.
FIG. 1.
GMTs (+ standard deviation) of aerobic bacteria at treated skin sites. ON, overnight.
Three sites (A, C, and D) were recultured 1 week after treatment. Bacteria were found at all sites through 25 layers (Table 1), and the GMTs for these sites were similar (data not shown). The results for TAO-treated sites resembled those for DAO- and placebo-treated sites.
TAO eradicated aerobic resident bacteria in the SC of human skin. DAO was not effective, a result implying that the neomycin in TAO is critical for the eradication of CNS. Although TAO prevented overnight repopulation of the SC with resident bacteria, it had no residual effect 1 week later, indicating that bacteria in the sebaceous glands were not killed. This finding suggests that TAO prevents overnight repopulation by binding to cells in the SC. Cells with bound neomycin were sloughed within a week (3), resulting in no residual bacterial killing.
The neomycin in TAO kills bacteria in the SC but does not reach the sebaceous glands from which resident bacteria arise. Preoperative treatment of skin sites with TAO may not prevent intraoperative contamination of an incision through the skin with CNS if the incision is made through a sebaceous gland.
Acknowledgments
This work was supported by Pfizer Consumer Healthcare, Morris Plains, N.J.
We thank Peter Van Zile for his perseverance.
REFERENCES
- 1.Brown, E., R. P. Wenzel, and J. O. Hendley. 1989. Exploration of the microbial anatomy of normal human skin by using plasmid profiles of coagulase-negative staphylococci: search for the reservoir of resident skin flora. J. Infect. Dis. 160:644-650. [DOI] [PubMed] [Google Scholar]
- 2.Hendley, J. O., and K. M. Ashe. 1991. Effect of topical antimicrobial treatment on aerobic bacteria in the stratum corneum on human skin. Antimicrob. Agents Chemother. 35:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirton, V., and D. Munro-Ashman. 1965. Contact dermatitis from neomycin and framycetin. Lancet i:138-139. [DOI] [PubMed]
- 4.Pinkus, H. 1951. Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping. J. Investig. Dermatol. 16:383-386. [DOI] [PubMed] [Google Scholar]