Abstract
Sixteen (1.5%) of the 1,043 clinical macrolide-resistant Streptococcus pneumoniae isolates collected and analyzed in the 1999-2000 PROTEKT (Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin) study have resistance mechanisms other than rRNA methylation or efflux. We have determined the macrolide resistance mechanisms in all 16 isolates by sequencing the L4 and L22 riboprotein genes, plus relevant segments of the four genes for 23S rRNA, and the expression of mutant rRNAs was analyzed by primer extension. Isolates from Canada (n = 4), Japan (n = 3), and Australia (n = 1) were found to have an A2059G mutation in all four 23S rRNA alleles. The Japanese isolates additionally had a G95D mutation in riboprotein L22; all of these originated from the same collection center and were clonal. Three of the Canadian isolates were also clonal; the rest were not genetically related. Four German isolates had A2059G in one, two, and three 23S rRNA alleles and A2058G in two 23S rRNA alleles, respectively. An isolate from the United States had C2611G in three 23S rRNA alleles, one isolate from Poland had A2058G in three 23S rRNA alleles, one isolate from Turkey had A2058G in four 23S rRNA alleles, and one isolate from Canada had A2059G in two 23S rRNA alleles. Erythromycin and clindamycin resistance gradually increased with the number of A2059G alleles, whereas going from one to two mutant alleles caused sharp rises in the azithromycin, roxithromycin, and rokitamycin MICs. Comparisons of mutation dosage with rRNA expression indicates that not all alleles are equally expressed. Despite their high levels of macrolide resistance, all 16 isolates remained susceptible to the ketolide telithromycin (MICs, 0.015 to 0.25 μg/ml).
Macrolide, lincosamide, and streptogramin B (MLSB) resistance in Streptococcus pneumoniae occurs either by modification of the drug-binding site or by active efflux of the drug. Target modification is usually the result of dimethylation of the adenine residue at position 2058 on the 23S rRNA by a methylase enzyme (30). In S. pneumoniae, Erm(B) [encoded by the erm(B) gene] is the enzyme mostly responsible (7, 30), although, more rarely, a methylase encoded by the erm(A) subclass erm(TR) gene is implicated (7, 23).
In vitro studies have demonstrated that target modification can also be achieved via mutations in domains II and V of 23S rRNA and in the genes encoding riboproteins L4 and L22 and can confer macrolide, lincosamide, streptogramin, and ketolide resistance (2, 24). Although previous reports are rare, such mutations have been found in MLSB-resistant clinical isolates (3, 14, 25). However, until now there have been no studies on the prevalence and epidemiology of these types of mutations in clinical isolates on a worldwide scale.
PROTEKT (Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin) is a longitudinal, global, multicenter surveillance study of respiratory tract pathogens. We screened all macrolide-resistant S. pneumoniae isolates from the PROTEKT 1999-2000 study for the common efflux and methylase genes associated with macrolide resistance to determine the global distribution of these mechanisms. Among the 1,043 isolates screened, 16 (1.5%) isolates repeatedly tested negative for these genes while they remained resistant to macrolides (7). In the study described here we determined the mechanisms of resistance in these isolates.
(Preliminary data were presented in abstract form at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 2001.)
MATERIALS AND METHODS
Bacterial isolates and control strains.
The 16 macrolide-resistant isolates of S. pneumoniae were obtained from the PROTEKT 1999-2000 surveillance study. These isolates were repeatedly negative for macrolide resistance mechanisms by two previously described PCR-based methods (6, 22) with five single-colony subcultures. Before sequencing, macrolide resistance was confirmed in these isolates by MIC determination by the National Committee for Clinical and Laboratory Standards (NCCLS) broth microdilution methodology (12) and comparison of the results with the initial MIC results. NCCLS breakpoints were used to determine susceptibility status (13). The reference strain S. pneumoniae R6 was analyzed in parallel to provide a control for all the sequencing procedures.
PCR amplification of 23S rRNA gene alleles and L4 and L22 riboprotein genes. (i) Isolate preparation.
All isolates were subcultured from storage (a −70°C freezer or plates stored at 4°C) onto horse blood agar and incubated overnight at 36°C in 5 to 6% CO2. An aliquot (100 μl) of RNase- and DNase-free H2O (Sigma, Poole, United Kingdom) was added to each of the 96 wells of a MicroAmp plate (Applied Biosystems, Warrington, United Kingdom). For each isolate, a confluent area of growth was sampled by using a 1-μl plastic loop, and the sample was transferred to a well in the MicroAmp plate. The plate was incubated at 95°C for 8 min in a PE 9700 thermocycler (Applied Biosystems) and then placed in a Jouan C4.12 centrifuge (Jouan Ltd., Ilkeston, United Kingdom) at 2,290 × g for 5 min. The resultant supernatant was used for further analysis.
(ii) Amplification of the four S. pneumoniae 23S rRNA alleles.
Amplification of the four S. pneumoniae 23S rRNA alleles was performed by using a modification of a previously published method (24). Each sample (5 μl) was transferred to a PCR master mixture containing 2.5 U of Platinum Taq (Invitrogen Ltd., Paisley, United Kingdom), 1× Platinum Taq PCR buffer, 200 μmol of each deoxynucleoside triphosphate (dNTP; dATP, dCTP, dGTP, and dTTP), 2.5 mmol of MgCl2, 25 pmol of the forward primer (primer DF23F, whose sequence is common to all four alleles), and 25 pmol of the reverse primer (whose sequence is specific for each allele) in a final reaction volume of 50 μl. The primer sequences used are shown in Table 1. This amplification was performed four times (each time with a different reverse primer) to amplify each of the 23S rRNA gene alleles.
TABLE 1.
Primers used for 23S and riboprotein amplification and sequencing
| PCR and primer designation | Sequence 5′ to 3′ | Amplicon size |
|---|---|---|
| Primary allele PCR | ||
| DF23F | GTTAATAAGGGCGCACGGTG | |
| 4DS18R | GCCAGCTGAGCTACACCGCC | 4 kb |
| 4DS23R | TACACACTCACATATCTCTG | 4 kb |
| 4DS30R | TTTTACCACTAAACTACACC | 4 kb |
| 4DS91R | TACCAACTGAGCTATGGCCGG | 4 kb |
| Nested PCR | ||
| 23S 2058 region | ||
| DF-SPN-23S-F1 | AGCGAAGGTATGAATTGAAGC | 279 bp |
| DF-SPN-23S-R1 | AACTGGCGTCCCGATCTC | |
| 23S 2611 region | ||
| DF-SPN-23S-F2 | GGTTTGGCACCTCGATGTCG | 276 bp |
| DF-SPN-23S-R2 | TCACACTTAGATGCTTTCAGC | |
| 23S 2505 region | ||
| DF-SPN-23S-F1 | AGCGAAGGTATGAATTGAAGC | 892 bp |
| DF-SPN-23S-R2 | TCACACTTAGATGCTTTCAGC | |
| 23S 752 region | ||
| DFDOM2F | GGCGAGTTACGTTATGATGC | 289 bp |
| DFDOM2R | GCTCTACCTCCAAGAATCTC | |
| L4 and L22 hotspots | ||
| DF-L22-F | GAACTCAGCTGTAGCTAACGC | 176 bp |
| DF-L22-R | TTCTGCAACAGCTACAGTGATG | |
| DF-L4-F | AGCGATGCAGTATTTGGTATCG | 238 bp |
| DF-L4-R | GCCGTATGAACGTGGAGTTG |
Three sets of parameters were necessary for successful amplification: (i) for reverse primers 4DS18R and 4DS23R, 94°C for 2 min; 92°C for 30 s, 55°C for 30 s, and 70°C for 5 min for 15 cycles; 92°C for 30 s, 55°C for 30 s, and 70°C 5 min and 15 s for 18 cycles; and 70°C for 10 min; (ii) for primer 4DS30R, 94°C for 2 min; 92°C for 30 s, 50°C for 30 s, and 70°C for 5 min for 15 cycles; 92°C for 30 s, 50°C for 30 s, and 70°C for 5 min and 15 s for 18 cycles; and 70°C for 10 min; and (iii) for primer 4DS91R, 94°C for 2 min; 92°C for 30 s, 46°C for 30 s, and 68°C for 5 min for 15 cycles; 92°C for 30 s, 46°C for 30 s, and 68°C for 5 min and 15 s for 18 cycles; and 70°C for 10 min. All amplifications of rRNA and riboprotein genes were performed in a PE 9700 thermocycler.
(iii) Nested PCR of 23S rRNA loci involved in macrolide resistance.
Each of the 4-kb products from the amplifications described above was used as a DNA template to amplify the 23S rRNA loci known to be involved in macrolide resistance. The areas around 23S rRNA gene nucleotides 2058, 2611, and 2505 in domain V and nucleotide 752 in domain II (Escherichia coli numbering) were amplified by using the primers listed in Table 1. The DNA template (10 μl) was added to a master mixture containing 2.0 U of Platinum Taq, 1× Platinum Taq PCR buffer, 200 μmol of each dNTP (dATP, dCTP, dGTP, and dTTP), 1.5 mmol of MgCl2, and 25 pmol of the forward reverse primer specific for each region in a final reaction volume of 50 μl. The following cycling parameters were used for all amplifications: 94°C for 2 min and then 94°C for 30 s, 54°C for 30 s, and 72°C for 40 s for 25 cycles, followed by 72°C for 7 min.
(iv) Amplification of L4 and L22 loci.
The DNA template (10 μl) was added to a master mixture containing 2.0 U of Platinum Taq, 1× Platinum Taq PCR buffer, 200 μmol of each dNTP (dATP, dCTP, dGTP, and dTTP), 1.5 mmol MgCl2, and 25 pmol of the forward reverse primer specific for each region in a final reaction volume of 50 μl. The following cycling parameters were used for all amplifications: 94°C for 2 min and then 94°C for 30 s, 52°C for 30 s, and 72°C for 40 s for 35 cycles, finishing with 72°C for 7 min.
(v) Sequencing of PCR products.
PCR products were prepared for cycle sequencing by using shrimp alkaline phosphatase (SAP; Amersham Pharmacia, Little Chalfont, United Kingdom) and exonuclease I (Exo I; Amersham Pharmacia) treatment. Briefly, 5 μl of each PCR product was added to 5 μl of the reaction mixture (1 U of SAP and 1 U of Exo I in DNase- and RNase-free H2O), and the mixture was incubated at 37°C for 60 min and then at 75°C for 15 min to inactivate the enzymes. Each sample was then diluted 1 in 5 by adding 40 μl of DNase- and RNase-free H2O. The primers used for sequencing were the same as those used for product amplification (Table 1). Each diluted SAP- and Exo I-treated product (5 μl) was added to 15 μl of a reaction mixture containing 1 μl of Ready Reaction Mix (Applied Biosystems), 4 μl of 5× sequencing buffer (Applied Biosystems), 9.5 μl of RNase- and DNase-free sterile distilled H2O (Sigma), and 3.2 pmol of each target-specific forward and reverse primer. Cycling parameters were 25 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min.
All sequencing was performed on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). Sequence analysis was performed by using the DNASTAR analysis program (DNASTAR, Madison, Wis.).
(vi) Primer extension analysis of 23S rRNA.
For each isolate, total RNA was extracted from the culture at the logarithmic phase by using a High Pure RNA Isolation kit (Roche Diagnostics, Lewes, United Kingdom). Primer extension analysis was performed with the RNA as described previously (9, 21). Briefly, RNA was extended with avian myeloblastosis virus reverse transcriptase and combinations of dNTPs and dideoxynucleoside triphosphates (ddNTPs) from 5′ 32P-end-labeled primers hybridizing adjacent to the mutated nucleotide site. The extension products were run on 6% polyacrylamide-7 M urea gels alongside the sequencing reactions performed with an unmodified rRNA template (RNA from S. pneumoniae strain R6). The gels were autoradiographed and quantified by scanning with a Storm 840 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). We screened all rRNA samples for dimethylation of position 2058 using a reverse transcription technique that has been described previously (19, 28). The absence of an extension stop before A2058 when ddNTPs are omitted from the reaction mixture indicates the lack of dimethylation.
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis was carried out by using SmaI digestion, as described previously (4).
MLST.
Multilocus sequence typing (MLST) was carried out as described previously (5). Briefly, genomic DNA was prepared from each isolate, and internal fragments of about 450 bp were amplified from seven housekeeping genes: aroE (shikimate dehydrogenase), gdh (glucose-6-phosphate dehydrogenase), gki (glucose kinase), recP (transketolase), spi (signal peptidase I), xpt (xanthine phosphoribosyltransferase), and ddl (d-alanine-d-alanine ligase). Both strands of these fragments were then sequenced (as described above), and the sequences were compared with those included in the MLST database (www.mlst.net). Each isolate was ascribed to a known or novel sequence type.
Serotyping.
Isolates were serotyped with antisera from the Statens Serum Institute (Copenhagen, Denmark).
RESULTS
Determination of resistance mechanisms.
Dimethylation at adenine residue 2058 of the 23S rRNA was absent from all 16 isolates, which excludes the possibility that a variant of an Erm methylase (16), other than those for which we screened, is responsible for macrolide resistance. Most isolates demonstrated high-level macrolide resistance and were neither penicillin resistant nor multiresistant. All 16 isolates were highly susceptible to the ketolide telithromycin (MIC range, 0.015 to 0.25 μg/ml; mode MIC, 0.03 μg/ml) (Table 2).
TABLE 2.
Antimicrobial activities of the agents tested against 16 S. pneumoniae isolates from the PROTEKT 1999-2000 study with ribosomal mutations as the cause of macrolide resistance
| Isolate | Origin | MIC (μg/ml)a
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLI | Macrolides
|
TEL | QUI | DAL | Q-D | LZD | TET | SXT | LVX | PEN | ||||||
| ERYb | CLRb | ROXb | AZMc | KITd | ||||||||||||
| P1008006 | United States | 0.5 | >64 | 32 | >32 | 8 | 0.5 | 0.015 | 128 | 32 | 4 | 1 | 0.25 | 0.25 | 0.5 | 0.015 |
| P1024010 | Canada | 1 | >64 | >32 | >32 | >64 | 16 | 0.03 | 128 | 4 | 1 | 2 | 0.25 | 0.5 | 1 | 0.015 |
| P1024019 | Canada | 0.5 | 32 | 8 | 32 | >64 | 16 | 0.015 | 128 | 8 | 1 | 2 | 0.5 | 0.25 | 1 | 0.015 |
| P1025005 | Canada | 2 | >64 | >32 | >32 | >64 | 16 | 0.03 | 128 | 4 | 1 | 2 | 0.25 | 32 | 16 | 0.015 |
| P1025009 | Canada | 2 | >64 | >32 | >32 | >64 | 32 | 0.03 | 64 | 4 | 1 | 2 | 0.25 | 32 | 8 | 0.015 |
| P1025028 | Canada | 1 | >64 | >32 | >32 | >64 | 16 | 0.03 | >128 | 4 | 1 | 2 | 1 | 16 | 16 | 0.015 |
| P1080014 | Japan | 1 | 64 | 32 | >32 | >64 | 32 | 0.06 | 128 | 16 | 4 | 2 | 0.5 | 2 | 4 | 0.015 |
| P1080019 | Japan | 1 | 64 | 32 | >32 | >64 | 32 | 0.06 | 64 | 8 | 2 | 1 | 0.25 | 1 | 2 | 0.03 |
| P1080026 | Japan | 1 | >64 | >32 | >32 | >64 | 32 | 0.06 | 64 | 16 | 2 | 1 | 0.5 | 1 | 4 | 0.015 |
| P1091027 | Australia | 0.5 | 16 | 4 | >32 | >64 | >32 | 0.06 | >128 | 8 | 2 | 1 | 0.25 | 0.12 | 1 | 0.015 |
| P1521024 | Germany | 1 | 64 | 16 | >32 | >64 | 16 | 0.015 | >128 | 8 | 1 | 1 | 0.25 | 4 | 0.5 | 0.25 |
| P1523030 | Germany | 0.25 | 8 | 2 | >32 | >64 | 32 | 0.015 | >128 | 4 | 0.5 | 1 | 0.25 | 0.5 | 0.5 | 0.03 |
| P1526033 | Germany | 8 | >64 | >32 | >32 | >64 | 1 | 0.12 | >128 | 16 | 1 | 2 | 32 | 4 | 1 | 0.5 |
| P1526059 | Germany | 0.12 | 1 | 1 | 2 | 0.5 | 0.5 | 0.015 | >128 | 4 | 0.5 | 1 | 0.25 | 0.25 | 1 | 0.015 |
| P1640193 | Poland | 1 | 64 | >32 | >32 | >64 | 1 | 0.03 | 64 | 8 | 1 | 2 | 32 | 0.25 | 1 | 0.015 |
| P1660008 | Turkey | 8 | >64 | >32 | >32 | >64 | 1 | 0.25 | 16 | 8 | 0.5 | 2 | 0.25 | 32 | 2 | 2 |
| ATCC 49619 | 0.12 | <0.12 | 0.12 | 0.25 | <0.12 | 64 | 2 | 1 | 2 | |||||||
Abbreviations: CLI, clindamycin; ERY, erythromycin A; CLR, clarithromycin; ROX, roxithromycin; AZM, azithromycin; KIT, rokitamycin; TEL, telithromycin; QUI, quinupristin; DAL, dalfopristin; Q-D, quinupristin-dalfopristin; LZD, linezolid; TET, tetracycline; SXT, co-trimoxazole; LVX, levofloxacin; PEN, penicillin G.
Fourteen membered.
Fifteen membered.
Sixteen membered.
With the exception of one isolate from the United States with a C2611G mutation in three of the four alleles, all the strains contained mutations at 23S rRNA position 2058 or 2059 (Table 3). The most prevalent mutation found was A2059G, which occurred in 12 of the isolates (Table 3 and Fig. 1). Examples of the occurrence of this mutation in one, two, three, or four of the 23S rRNA alleles were found among the isolates. Although difficult to interpret due to a nonisogenic background, a mutation dosage effect was generally apparent, with macrolide MICs increasing with the number of alleles mutated (Table 4). The MICs of azithromycin, rokitamycin, and roxithromycin changed markedly from ≤2 to ≥32 μg/ml with the change from one to two mutated alleles, while the MICs of erythromycin A and clarithromycin rose gradually with the increasing occurrence of the A2059G mutation in one to four alleles (Table 4). The MICs of telithromycin, clindamycin, quinupristin, dalfopristin, quinupristin-dalfopristin, and linezolid all appeared to be stable regardless of the number of alleles with the A2059G mutation (Table 4).
TABLE 3.
Ribosomal mutations found in 16 macrolide-resistant S. pneumoniae isolates from the PROTEKT 1999-2000 study
| Isolate | Origin | Gene amplification and sequencing analysis
|
Primer extension analyses
|
|||
|---|---|---|---|---|---|---|
| 23S rRNA mutation (no. of alleles) | Mutant allele(s) | L4 and L22 gene(s) | Mutations in rRNAa | Proportion (%) of total rRNA that is mutantb | ||
| P1008006 | United States | C2611G (3) | 18, 23, 30 | Wc | C2611G | 75.8 |
| P1024010 | Canada | A2059G (4) | 18, 23, 30, 91 | W | A2059G | 97.8 |
| P1024019 | Canada | A2059G (2) | 18, 30 | W | A2059G | 78.4 |
| P1025005 | Canada | A2059G (4) | 18, 23, 30, 91 | W | A2059G | 99.5d |
| P1025009 | Canada | A2059G (4) | 18, 23, 30, 91 | W | A2059G | 101.4 |
| P1025028 | Canada | A2059G (4) | 18, 23, 30, 91 | W | A2059G | 98.4 |
| P1080014 | Japan | A2059G (4) | 18, 23, 30, 91 | L22 G95D | A2059G | 96.9 |
| P1080019 | Japan | A2059G (4) | 18, 23, 30, 91 | L22 G95D | A2059G | 99.9 |
| P1080026 | Japan | A2059G (4) | 18, 23, 30, 91 | L22 G95D | A2059G | 96.8 |
| P1091027 | Australia | A2059G (4) | 18, 23, 30, 91 | W | A2059G | 98.4 |
| P1521024 | Germany | A2059G (3) | 18, 30, 91 | W | A2059G | 55.2 |
| P1523030 | Germany | A2059G (2) | 30, 91 | W | A2059G | 45.7 |
| P1526033 | Germany | A2058G (2) | 30, 91 | W | A2058G | 33.2 |
| P1526059 | Germany | A2059G (1) | 18 | W | A2059G | 14.1 |
| P1640193 | Poland | A2058G (3) | 18, 23, 91 | W | A2058G | 61.6 |
| P1660008 | Turkey | A2058G (4) | All | W | A2058G | 95.8 |
These were the only mutations found after sequencing of domain V of the rRNA at about nucleotides 2058 and 2611.
Values for the expression of mutant rRNAs are the means of at least three measurements; standard deviations are within 3% in all cases.
W, the wild type sequence was found in these genes.
Strains in which all four rRNA alleles are mutated must have 100% mutant rRNA in their ribosomes, so the deviations from this value in the homozygous strains reflects the experimental error in primer extension analysis.
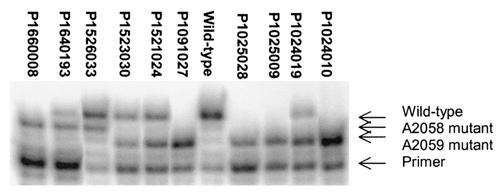
FIG. 1.
Primer extension analysis of strains with mutations at nucleotides 2059 and 2058 (listed in Table 3). Comparisons of band intensities revealed the proportions of mutant rRNA being expressed in each strain.
TABLE 4.
Mutation dosage responses in S. pneumoniae isolates with A2059G mutations
| Isolate | Origin | No. of A2059G alleles | MIC (μg/ml)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLI | ERY | CLR | ROX | AZM | KIT | TEL | DAL | QUI | Q-D | LZD | |||
| P1526059 | Germany | 1 | 0.12 | 1 | 1 | 2 | 0.5 | 0.5 | 0.015 | >128 | 4 | 0.5 | 1 |
| P1523030 | Germany | 2 | 0.25 | 8 | 2 | >32 | >64 | 32 | 0.015 | >128 | 4 | 0.5 | 1 |
| P1024019 | Canada | 2 | 0.5 | 32 | 8 | 32 | >64 | 16 | 0.015 | 128 | 8 | 1 | 2 |
| P1521024 | Germany | 3 | 1 | 64 | 16 | >32 | >64 | 16 | 0.015 | >128 | 8 | 1 | 1 |
| P1024010 | Canada | 4 | 1 | >64 | >32 | >32 | >64 | 16 | 0.03 | 128 | 4 | 1 | 2 |
| P1025005 | Canada | 4 | 2 | >64 | >32 | >32 | >64 | 16 | 0.03 | 128 | 4 | 1 | 2 |
| P1080014 | Japan | 4 | 1 | 64 | 32 | >32 | >64 | 32 | 0.06 | 128 | 16 | 4 | 2 |
| P1091027 | Australia | 4 | 0.5 | 16 | 4 | >32 | >64 | >32 | 0.06 | >128 | 8 | 2 | 1 |
Abbreviations: CLI, clindamycin; ERY, erythromycin A; CLR, clarithromycin; ROX, roxithromycin; AZM, azithromycin; KIT, rokitamycin; TEL, telithromycin; DAL, dalfopristin; QUI, quinupristin; Q-D, quinupristin-dalfopristin; LZD, linezolid.
The correlation between the number of mutant alleles and macrolide resistance was not perfect in all cases, however, and the Australian isolate (with four A2059G alleles) was more sensitive to certain drugs than might be expected. A similar effect was seen with clindamycin resistance in the case of the mutants with the A2058G mutations, in which strains with either two or four mutant alleles had higher levels of resistance (MICs, 8 μg/ml) than the strain with three mutated alleles (MIC, 1 μg/ml). The reason for this is not known and does not appear to be linked to the levels of mutant gene expression (see below and Table 3). The A2058G mutation was found in only three isolates (isolate P1526033 from Germany with two mutant alleles, isolate P1640193 from Poland with three mutant alleles, and isolate P1660008 from Turkey with four mutant alleles).
Three isolates from the same center in Japan combined the A2059G mutation (in all four alleles) with a G95D amino acid substitution in the L22 riboprotein. These were shown to be clonal by PFGE, MLST, and serotyping (Table 5). Three isolates from a center in Canada were also found to be genetically identical. Two isolates from another Canadian center were not clonally related to this group or each other. Apart from the six isolates of the two clones, the isolates were not genetically related (Table 5).
TABLE 5.
Epidemiological characteristics of 16 S. pneumoniae isolates from the PROTEKT 1999-2000 study with ribosomal mutations as the cause of macrolide resistance
| Isolate | Origin | MLST no.
|
Sequence type | Sero- type | PFGE type | Strain historya | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aroE | gdh | gki | recP | spi | xpt | ddl | ||||||
| P1008006 | United States | 7 | 11 | 10 | 1 | 6 | 58 | 14 | New | 9V | A | Eight isolates from the United Kingdom and Canada, 1994-2000, of which seven were tested: six isolates were ERY; serotypes 9V, 19F, and 14; the seventh isolate (Canada) was ERYr |
| P1024010 | Canada | 18 | 5 | 4 | 1 | 1 | 27 | 8 | New | 19F | B | No previous isolates in database |
| P1024019 | Canada | 1 | 10 | 4 | 1 | 9 | 3 | 8 | 43 | 19F | C | Two isolates from the United Kingdom and Finland, 1995 and 1996; only one was tested (ERYs); both isolates were serotype 19F |
| P1025005 | Canada | 1 | 8 | 1 | 2 | 6 | 4 | 6 | 33 | 23F | D | One isolate (ST33) from The Netherlands, ERYs; seven SLV isolates of ST33 from the United States, Denmark, Germany, The Netherlands, England, and Finland; among five SLV isolates tested; three were ERYs and two were ERYr and were both from the United States; serotypes 23, 23B, and 23F |
| P1025009 | Canada | 1 | 8 | 1 | 2 | 6 | 4 | 6 | 33 | 23F | D | |
| P1025028 | Canada | 1 | 8 | 1 | 2 | 6 | 4 | 6 | 33 | 23F | D | |
| P1080014 | Japan | 5 | 5 | 6 | 1 | 9 | 14 | 14 | SLVb 547 | 34 | E | One isolate from the United States; one isolate from Finland, 1994; ERY susceptibility was not provided; serotype 34 |
| P1080019 | Japan | 5 | 5 | 6 | 1 | 9 | 14 | 14 | SLV 547 | 34 | E | |
| P1080026 | Japan | 5 | 5 | 6 | 1 | 9 | 14 | 14 | SLV 547 | 34 | E | |
| P1091027 | Australia | 10 | 5 | 4 | 5 | 13 | 10 | 18 | 205 | 4 | F | Nine isolates from Sweden, the United Kingdom, Denmark, Australia, Canada, 1995-1999; all nine isolates were ERYs; eight of the nine were serotype 4 (one was not typed) |
| P1521024 | Germany | 7 | 13 | 8 | 6 | 15 | 3 | 8 | SLV 339 | 19F | G | One isolate from Norway, 1998; ERYs; serotype 19 |
| P1523030 | Germany | 1 | 9 | 53 | 1 | 10 | 15 | 18 | New | 34 | H | No isolates in database |
| P1526033 | Germany | 2 | 19 | 2 | 17 | 15 | 22 | 14 | New | 19A | I | No isolates in database |
| P1526059 | Germany | 2 | 9 | 15 | 11 | 16 | 3 | 14 | New | 8 | J | No isolates in database |
| P1640193 | Poland | 7 | 5 | 1 | 1 | 13 | 2 | 14 | SLV 440 | 23F | K | One isolate from England, 2000; ERY susceptibility was not provided; serotype 23F |
| P1660008 | Turkey | 7 | 11 | 10 | 1 | 6 | 58 | 1 | SLV 156 | 14 | L | Forty-one isolates from Spain, the United Kingdom, Poland, Norway, Czech Republic, The Netherlands, France, Uruguay, Taiwan, and Canada, 1988-1998; 19 of the 25 isolates whose susceptibilities were tested were ERYs; serotypes 9V, 14, and 19F; major PENr Spanish clone, Spain 9V-3, and variants |
Information obtained from www.mlst.net. Includes single-locus variants. Abbreviations: ERYs, erythromycin susceptible (MIC, ≤0.25 μg/ml) (note that susceptibility results were not available for some isolates); ERYr, erythromycin resistant (MIC, ≥1 μg/ml); PENr, penicillin resistant (MIC, ≥2 μg/ml); ST, sequence type.
SLV = single-locus variant.
Levels of expression of mutant rRNAs.
rRNAs were extracted from all isolates and analyzed by primer extension with reverse transcriptase (Fig. 1; Table 3). Generally, the level of expression of mutant rRNA increased with the number of mutant alleles, although not in a linear manner. If each of the four rRNA operons were expressed to the same extent and the mutant rRNAs were incorporated into ribosomal subunits as efficiently as wild-type rRNA, it would be expected that the proportion of mutant rRNA would be 25% in a strain with one mutant allele, 50% in a strain with two mutant alleles, etc. Although this was found to be the case for isolate P1008006, which had three C2611G alleles and in which 75.8% ± 1.2% of its total rRNA possessed the mutation, this strain was an exception. All the other strains with mutations at positions 2058 and 2059 (with the exception of strain P1024019) contained levels of mutant rRNA that were lower than expected. This suggests either that the mutant alleles are transcribed to a lower degree than they are in the wild type or, more likely, that the mutant rRNAs are not as efficiently assembled into 50S particles and are more rapidly degraded. It should be noted that the rRNAs were prepared from cells grown in the absence of drug, and inclusion of a macrolide in the medium could change the ribosomal assembly of the mutant rRNA (27).
Strain P1024019 is an anomaly. Repeated PCR analyses of this strain showed that it has the A2059G mutation in only two of the rRNA operons (alleles 18 and 30), whereas the proportion of mutant rRNA (from cells grown in the absence of drug) consistently indicated that at least 75% of the operons have this mutation (Table 3). Possibly, duplication of one or more of the operons (alleles 18 and 30) has occurred in this strain. Such an event would not have been detected by the PCR screening approach used here and would effectively increase the gene dosage of the mutant alleles.
The levels of drug resistance generally increased with the proportions of mutant rRNA being expressed (Tables 3 and 4), although, as discussed above, there was not necessarily a linear correlation.
DISCUSSION
In this study we describe 16 clinical S. pneumoniae isolates with ribosomal mutations as the sole cause of macrolide resistance. One of these had a combination of a 23S rRNA mutation (A2059G) and an L22 riboprotein mutation (G95D) that has not previously been identified in a clinical isolate. The occurrence of this mutation validates the relevance of in vitro selection approaches, which have previously isolated strains with similar combinations of 23S rRNA and L22 (and L4) resistance mutations (2). Other recent studies have described ribosomal mutations in 11 clinical isolates from Finland (14) and 20 clinical isolates from Eastern Europe and North America (25); however, the study presented here is the first to investigate the incidence of these mutations from a global perspective. The overall prevalence of ribosomal mutations as a cause of macrolide resistance was found to be 1.5% among resistant isolates (16 of 1,043). Unlike previous studies of in vitro mutants (2, 24) and clinical isolates (14, 25), we found no isolates with L4 mutations as the cause of macrolide resistance.
The A2059G substitution, which occurred in from one to four rRNA alleles, was found most frequently. Isolates with the A2059G mutation are well distributed on a global scale, with isolates with this mutation found in Canada, Japan, Australia, and Germany. The gene dosage effect on MICs seen in our study match those previously described for laboratory-derived mutants (24) and clinical isolates (25), although P1526059 is the first isolate to be described with an A2059G mutation in only one allele. From these data, it would appear that different macrolides show various degrees of potency against the mutants with the A2059G mutation. The antimicrobial effects of telithromycin, clindamycin, quinupristin, dalfopristin, quinupristin-dalfopristin, and linezolid remain largely unaltered by the A2059G mutation (Table 4). It has been shown by transformation of the R6 strain that the A2059G mutation is sufficient to cause macrolide resistance (25). However, it is not known whether these mutations in clinical strains were acquired in a stepwise fashion, with acquisition related to dosage and exposure.
The quinupristin-dalfopristin MICs for three isolates of the Japanese clone with combined A2059G (in all four alleles) and L22 riboprotein G95D mutations were increased (MICs, 2 to 4 μg/ml). This may have been due to the slightly higher streptogramin A (dalfopristin) MIC of 8 to 16 μg/ml or a conformational change in riboprotein L22. Mutations occurring in the same region in L22, but at amino acids distinctly different from the amino acids at which mutations were observed here, have recently been shown to confer quinupristin-dalfopristin resistance in Staphylococcus aureus (10). It is likely that both the rRNA and riboprotein mutations contribute to the resistance phenotype, as these sites are spatially close in the 50S ribosomal subunit, although it would be necessary to test each mutation independently to confirm this relationship (1, 15, 17).
Factors other than ribosomal mutations also probably contribute to the observed MICs. This is apparent in the cases of the strains with the A2058G mutation, in which those with two or four mutant alleles had higher levels of resistance to clindamycin (MICs, 8 μg/ml) than all of the other strains, whereas the strain with three mutated alleles did not (MIC, 1 μg/ml). While the reason for this is not clear, it is not connected to the proportions of mutant rRNA expressed (Table 3). Possibly, as these strains are of different serotypes, differences in the rate of uptake or retention of the antimicrobial could be influencing the MICs.
We found one isolate (isolate P1008006 from the United States) with a C2611G mutation in three alleles of the 23S rRNA gene. This mutation (but in all four alleles) has been described in strain 6Az, a laboratory-derived mutant (24), and, more recently, in strain r1045, a clinical isolate from Finland (14). Like strains 6Az and r1045, the streptogramin A (dalfopristin) MIC for P1008006 was notably increased (MIC, 32 μg/ml, which was the highest MIC for all mutants tested). P1008006 was resistant to quinupristin-dalfopristin (MIC, 4 μg/ml). Interestingly, the quinupristin-dalfopristin MIC for r1045 was reported to have been 2 μg/ml and the dalfopristin MIC for r1045 was much lower than that for P1008006 (MICs, 8 and 32 μg/ml, respectively). Strains 6Az and r1045 were reported to have notable resistance to 14- and 15-membered macrolides but less resistance to 16-membered macrolides and susceptibility to clindamycin (14, 24). P1008006 has a pattern similar to this but is appreciably less resistant to the 15-membered macrolide azithromycin than to erythromycin A (MICs, 8 and >64 μg/ml, respectively), perhaps due to mutations in three rather than four alleles. Also, the 14-membered macrolide roxithromycin was much more active (MIC, 2 μg/ml), suggesting that subtle differences in target specificity may exist among the 14-membered macrolides. Such differences might be inferred from the crystallographic models of erythromycin, roxithromycin (17), and azithromycin (8) bound to their 50S ribosomal subunit targets. However, this point remains controversial (8).
Generally, the percentage of mutant to wild-type 23S rRNAs determined by primer extension of RNA correlated with the number of mutated alleles determined by amplification and sequencing (Table 3 and Fig. 1), with the exception of the percentage for isolate P1024019 from Canada, which is discussed above. Further investigation is needed to determine if this differential expression is due to some environmental stimulus. The 23S rRNA structure around A2058 and A2059 is critical for protein synthesis and for extrusion of the newly formed peptide through the ribosomal channel (11, 26). Mutations in this highly conserved region would be expected to have some biological cost. Interestingly, although the A2058G change is the most common mutation conferring macrolide resistance in many bacterial species (29), it was found in only 3 of the 1,043 macrolide-resistant S. pneumoniae isolates studied here. Apparently, other species are better equipped to accommodate this mutation, possibly by means of a compensatory mutation at another site that alleviates the biological cost of the A2058G mutation (29).
Telithromycin demonstrated very high levels of activity in vitro against all strains including the strain with the A2058G mutation in all four alleles, although that strain did have the lowest susceptibility (MIC, 0.25 μg/ml). This activity, along with previously demonstrated high levels of activity in vitro against S. pneumoniae strains with erm(B)-, mef(A)- and combined erm(B)- and mef(A)-mediated macrolide resistance (7), emphasizes the potential of telithromycin for the treatment of community-acquired respiratory tract infections caused by macrolide-resistant S. pneumoniae isolates.
Serotyping alone did not allow adequate discrimination to determine clonality. PFGE and MLST both produced good and comparable levels of discrimination among the isolates. MLST provided the added benefit of being able to track other identical MLST types to determine previous descriptions of the geographical locations and antimicrobial activities of isolates registered on the MLST website (www.mlst.net). The histories of the strains that we have described show that they are geographically diverse and that most have recently been tested and found to be susceptible to erythromycin (Table 5). This would suggest that acquisition of resistance caused by mutation is a relatively recent event, perhaps due to the selective pressure of increased levels of exposure to macrolides in recent years. In a large European surveillance study conducted from 1997 to 1999 (with 1,191 erythromycin A-resistant S. pneumoniae isolates), no isolates with ribosomal mutations were found (18). It will be interesting to monitor the prevalence of these mutations in future years of the PROTEKT study.
In conclusion, we show here that the global prevalence of S. pneumoniae strains in which macrolide resistance is conferred by ribosomal gene mutations is low (1.5% of 1,043 macrolide-resistant isolates) but could be on the rise. All isolates came from a global surveillance study on community-acquired respiratory infection (PROTEKT). We have determined the exact ribosomal gene mutations in all 16 isolates to define the mechanisms of macrolide resistance, which are shown here to be varied. There was evidence of the clonal spread of these mechanisms, but only within the same center and not between centers in the same country or between countries. Although the 1.5% incidence of these mechanisms is relatively low, they were completely absent from isolates analyzed in a comparable study conducted 3 years ago (18). Testing of macrolide-resistant S. pneumoniae isolates remains in progress to monitor whether there is an increase in the prevalence and spread of this form of macrolide resistance.
Acknowledgments
We are grateful to our colleagues worldwide for the supply of bacterial isolates as part of the PROTEKT study and the GR Micro PROTEKT team who performed the initial MIC determinations.
Aventis is acknowledged for its financial support of the PROTEKT study. S.D. was supported by the Danish Biotechnology Instrument Centre (DABIC), The European Commission's Fifth Framework Program (grant QLK2-CT2000-00935), and the Nucleic Acid Centre of the Danish Grundforskningsfond.
REFERENCES
- 1.Ban, N., P. Nissen, J. Hansen, P. B. Moore, and T. A. Steitz. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289:905-920. [DOI] [PubMed] [Google Scholar]
- 2.Canu, A., B. Malbruny, M. Coquemont, T. Davies, P. C. Appelbaum, and R. Leclercq. 2002. Diversity of ribosomal mutations conferring resistance to macrolides, clindamycin, streptogramin, and telithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Depardieu, F., and P. Courvalin. 2001. Mutations in 23S rRNA responsible for resistance to 16-membered macrolides and streptogramins in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Descheemaeker, P., S. Chapelle, C. Lammens, M. Hauchecorne, M. Wijdooghe, P. Vandamme, et al. 2000. Macrolide resistance and erythromycin resistance determinants among Belgian Streptococcus pyogenes and Streptococcus pneumoniae isolates. J. Antimicrob. Chemother. 45:167-173. [DOI] [PubMed] [Google Scholar]
- 5.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 6.Farrell, D. J., I. Morrissey, S. Bakker, and D. Felmingham. 2001. Detection of macrolide resistance mechanisms in Streptococcus pneumoniae and Streptococcus pyogenes using a multiplex rapid cycle PCR with microwell-format probe hybridization. J. Antimicrob. Chemother. 48:541-544. [DOI] [PubMed] [Google Scholar]
- 7.Farrell, D. J., I. Morrissey, S. Bakker, and D. Felmingham. 2002. Molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae and Streptococcus pyogenes isolated from the PROTEKT 1999-2000 study. J. Antimicrob. Chemother. 50(Suppl. S1):39-47. [DOI] [PubMed] [Google Scholar]
- 8.Hansen, J. L., J. A. Ippolito, N. Ban, P. Nissen, P. B. Moore, and T. A. Steitz. 2002. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 10:117-128. [DOI] [PubMed] [Google Scholar]
- 9.Hansen, L. H., P. Mauvais, and S. Douthwaite. 1999. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol. Microbiol. 31:623-631. [DOI] [PubMed] [Google Scholar]
- 10.Malbruny, B., A. Canu, B. Bozdogan, B. Fantin, V. Zarrouk, S. Dutka-Malen, C. Feger, and R. Leclercq. 2002. Resistance to quinupristin-dalfopristin due to mutation in L22 ribosomal protein in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2200-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatogawa, H., and K. Ito. 2002. The ribosomal exit tunnel functions as a discriminating gate. Cell 108:629-636. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical and Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 5th ed. NCCLS document M7-A5. National Committee for Clinical and Laboratory Standards, Wayne, Pa.
- 13.National Committee for Clinical and Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. NCCLS document M100-12. National Committee for Clinical and Laboratory Standards, Wayne, Pa.
- 14.Pihlajamäki, M., J. Kataja, H. Seppälä, J. Elliot, M. Leinonen, P. Huovinen, and J. Javala. 2002. Ribosomal mutations in Streptococcus pneumoniae clinical isolates. Antimicrob. Agents Chemother. 46:654-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poehlsgaard, J., and S. Douthwaite. 2002. The macrolide binding site on the bacterial ribosome. Curr. Drug Targets Infect. Disorders 2:67-78. [DOI] [PubMed] [Google Scholar]
- 16.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlünzen, F., R. Zarivach, J. Harms, A. Bashan, A. Tocilj, R. Albrecht, A. Yonath, and F. Franceschi. 2001. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413:814-821. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz, F. J., M. Perdikouli, A. Beeck, J. Verhoef, and A. C. Fluit. 2001. Molecular surveillance of macrolide, tetracycline and quinolone resistance mechanisms in 1911 clinical European Streptococcus pneumoniae isolates. Int. J. Antimicrob. Agents 18:433-436. [DOI] [PubMed] [Google Scholar]
- 19.Sigmund, C. D., M. Ettayebi, A. Borden, and E. A. Morgen. 1988. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 164:673-690. [DOI] [PubMed] [Google Scholar]
- 20.Skinner, R., E. Cundliffe, and F. J. Schmidt. 1983. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J. Biol. Chem. 258:12702-12706. [PubMed] [Google Scholar]
- 21.Stern, S., D. Moazed, and H. F. Noller. 1988. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 164:481-489. [DOI] [PubMed] [Google Scholar]
- 22.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syrogiannopoulos, G. A., I. N. Grivea, A. Tait-Kamradt, G. D. Katopodis, N. G. Beratis, J. Sutcliffe, P. C. Appelbaum, and T. A. Davies. 2001. Identification of an erm(A) erythromycin resistance methylase gene in Streptococcus pneumoniae isolated in Greece. Antimicrob. Agents Chemother. 45:342-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tait-Kamradt, A., T. Davies, M. Cronan, M. R. Jacobs, P. C. Appelbaum, and J. Sutcliffe. 2000. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob. Agents Chemother. 44:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tait-Kamradt, A., T. Davies, P. C. Appelbaum, F. Depardieu, P. Courvalin, J. Petipas, L. Wondrack, A. Walker, M. R. Jacobs, and J. Sutcliffe. 2000. Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob. Agents Chemother. 44:3395-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenson, T., and M. Ehrenberg. 2002. Regulatory nascent peptides in the ribosomal tunnel. Cell 108:591-594. [DOI] [PubMed] [Google Scholar]
- 27.Usary, J., and W. S. Champney. 2001. Erythromycin inhibition of 50S ribosomal subunit formation in Escherichia coli cells. Mol. Microbiol. 40:951-962. [DOI] [PubMed] [Google Scholar]
- 28.Vester, B., and S. Douthwaite. 1994. Domain V of 23S rRNA contains all the structural elements necessary for recognition by the ErmE methyltransferase. J. Bacteriol. 176:6999-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vester, B., and S. Douthwaite. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]