Abstract
To evaluate the incidence of vancomycin tolerance among Streptococcus pneumoniae isolates, we performed killing curve studies with 633 isolates. The penicillin MIC was ≥0.12 mg/liter for 481 (76%) of the isolates. All strains were susceptible to vancomycin. Killing curve studies were performed with a vancomycin concentration of 2.5 mg/liter. The Tupelo strain was used for quality control. No vancomycin-tolerant strain was detected.
Streptococcus pneumoniae is the most common bacterial cause of acute meningitis and pneumonia, for which mortality rates among treated patients are 30 and 5%, respectively (9, 10). Resistance to β-lactams, such as penicillin, represents a major problem in the treatment of pneumococcal infections. Penicillin resistance has been found among up to 50% of clinical isolates, according to reports from many parts of the world (2). In addition, an increasing amount of multidrug resistance makes the treatment of these infections even more difficult. No resistance of the pneumococcus to the glycopeptide vancomycin has yet been reported, so this drug represents the ultimate backup drug for the treatment of infections caused by multidrug-resistant pneumococci (3).
Antibiotic tolerance is the ability of bacteria to survive but not proliferate in the presence of a particular bactericidal antibiotic. This translates to increased bacterial survival and regrowth when the antibiotic is removed. Tolerance to penicillin was first recognized among clinical isolates of pneumococci 15 years ago, when it was demonstrated that five of six multidrug-resistant clinical isolates of pneumococci were also tolerant to β-lactam antibiotics (5). Recently, vancomycin-tolerant S. pneumoniae strains have been described in the laboratory (8) and in a patient with recurrent meningitis (6). This problem of tolerance to vancomycin is especially significant in those countries where the incidence of penicillin resistance among pneumococci is high, necessitating inclusion of vancomycin in the initial empirical treatment of meningitis. Tolerance to vancomycin is not detected by routine in vitro susceptibility tests, so specific studies are needed to evaluate the incidence of vancomycin tolerance among pneumococcal isolates in our region.
(This study was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy [M. Ortega et al., Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1648, 2002].)
From January 1999 to December 2001 a total of 1,033 S. pneumoniae strains were isolated from different samples submitted to the microbiology laboratory of our institution. The MICs for all strains were determined by a commercial microdilution method (Sensititre; Trek Diagnostic Systems, West Sussex, United Kingdom) with cation-adjusted Mueller-Hinton broth supplemented with 5% lysed sheep blood. The recommendations of NCCLS (7) were followed for classification of the strains as susceptible, intermediate, or resistant to the antimicrobial agents tested, including penicillin, cefotaxime, erythromycin, levofloxacin, and vancomycin. Quality control was assured by use of the following strains: S. pneumoniae ATCC 49619, Staphylococcus aureus ATCC 29213, and Enterococcus faecalis ATCC 29212. All strains were stored at −70°C in double-strength skim milk until needed.
Tolerance to vancomycin was studied by a killing curve method with Todd-Hewitt broth plus 0.5% yeast extract as the culture medium. Vancomycin was considered bactericidal when a ≥3-log10 reduction in colony counts was reached at 6 h; otherwise, the strain was considered tolerant (6). The strains tested were inoculated (final concentration, 107 to 108 CFU/ml) in 10 ml of broth without antibiotic (positive growth) or supplemented with 2.5 mg of vancomycin per liter. The test tubes were incubated at 35°C, and the numbers of CFU per milliliter were determined after 0 and 6 h. All tests were performed in duplicate. Strains that showed ≥104 CFU/ml after 6 h of incubation were reanalyzed, and the numbers of CFU per milliliter were determined after 0, 2, 4, and 6 h. The Tupelo strain (a vancomycin-tolerant strain), a gift from J. A. McCullers (6), was used for quality control.
Among the 1,033 S. pneumoniae strains isolated, 633 were selected for use in the killing curve studies. All strains (n = 233) recovered from sterile fluids (blood, cerebrospinal fluid) were included. Among the other isolates, only those for which the penicillin MIC was ≥0.12 mg/liter (n = 400) were analyzed. The strains were classified according to the penicillin MICs as follows: 152 strains were susceptible (MICs, ≤0.06 mg/liter), 270 showed intermediate susceptibilities (MICs, 0.12 to 1 mg/liter), and 211 were resistant (MICs, ≥2 mg/liter). Table 1 shows the overall rates of resistance to penicillin, cefotaxime, erythromycin, and levofloxacin over the 3-year study period. All strains were susceptible to vancomycin. For analysis purposes, the intermediate and resistant categories are considered together.
TABLE 1.
Incidence of strains with reduced susceptibilities to different antimicrobial agents during the 3 years of the study
| Yr/total no. of isolates | No. (%) of strains for which MICs were as follows:
|
|||
|---|---|---|---|---|
| Penicillin MIC, ≥0.12 μg/ml | Cefotaxime MIC, ≥1 μg/ml | Erythromycin MIC, ≥0.5 μg/ml | Levofloxacin MIC, ≥4 μg/ml | |
| 1999/361 | 170 (47) | 62 (17) | 130 (36) | 7 (2) |
| 2000/380 | 178 (46) | 61 (16) | 155 (41) | 16 (4) |
| 2001/292 | 133 (45) | 53 (18) | 115 (39) | 6 (2) |
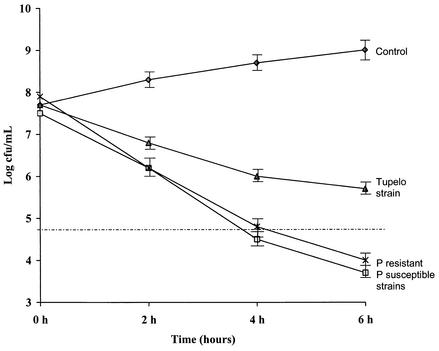
In this study we did not detect any pneumococcal strain that survived in the presence of vancomycin at a concentration of 2.5 mg/liter. In Fig. 1 we show the vancomycin time-kill curve means for the penicillin-susceptible (MICs, <0.12 mg/liter) and penicillin-resistant (MICs, ≥0.12 mg/liter) strains and the time-kill curve for strain Tupelo, which was used as an example of a tolerant strain. Table 2 shows the killing activities of vancomycin at 6 h for control strain Tupelo and penicillin-susceptible and -resistant strains.
FIG. 1.
Complete killing curves for the penicillin-susceptible and resistant (including intermediate-susceptible) strains (mean log CFU per milliliter by time) compared with the control (without antibiotic) and the Tupelo strain. The dotted line indicates a 3 log10 reduction in the initial colony count. P, penicillin.
TABLE 2.
Killing of strain Tupelo and penicillin-susceptible, -intermediate, and -resistant strains by vancomycin at 6 h
| Strain | Mean (SD) log CFU/ml at:
|
Mean (SD) difference in log CFU/ml between 0 and 6 h | |
|---|---|---|---|
| 0 h | 6 h | ||
| Tupeloa | 7.7 (0.1) | 5.7 (0.1) | 2 (0.1) |
| Penicillin resistant (n = 211) | 8.0 (0.3) | 4 (0.1) | 4 (0.2) |
| Penicillin intermediate susceptible (n = 270) | 7.8 (0.3) | 4 (0.2) | 3.8 (0.2) |
| Penicillin susceptible (n = 152) | 7.5 (0.1) | 3.7 (0.1) | 3.8 (0.1) |
The study with strain Tupelo was repeated 10 times.
The tolerance of strains to lytic antibiotics is caused by a defective death response that is due either to a defective autolysin or to a defect in the signal transduction cascade that activates the autolysin. Vancomycin is an important drug in the management of multidrug-resistant pneumococci. No resistance of pneumococci to this drug has yet been reported. However, other groups have reported that the incidence of vancomycin tolerance among laboratory isolates is about 3 to 5% (C. A. Rodriguez, C. G. Whitney, and E. Tuomanen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1777, 2000), and recently a case of clinical failure following vancomycin treatment of meningitis caused by a vancomycin-tolerant isolate (the Tupelo strain) was reported (6).
Henriques et al. (4) determined the prevalence of vancomycin and penicillin tolerance among 116 clinical isolates of pneumococci by monitoring lysis and viability after exposure to each antibiotic for 4 h. Eight percent of the strains were tolerant to penicillin, and 3% were tolerant to vancomycin. The three vancomycin-tolerant strains had reduced susceptibilities to penicillin, and only one was also tolerant to penicillin.
A group from Madrid studied 120 strains of S. pneumoniae isolated in 1999 (1). Although they did not indicate the susceptibilities of the pneumococci in the paper, the final result was that any strain studied was vancomycin tolerant. In our study we included a larger number of strains (n = 633) and strains with a high level of resistance (penicillin MIC, ≥0.12 mg/liter for 481 strains [76%]) because vancomycin tolerance had been detected among penicillin-resistant strains and empirical treatment with vancomycin is needed for meningitis. However, vancomycin tolerance was not detected in any of the strains studied.
Although large numbers of pneumococcal isolates in Spain have reduced susceptibilities to penicillin (intermediate or resistant), strains tolerant to vancomycin, which may compromise the ability to use this antibiotic in the treatment of invasive pneumococcal infections, have not yet been found. Nevertheless, it would be advisable to carry out this type of epidemiological study in order to detect the possible appearance of this sort of strain.
Acknowledgments
M.O. and F.M. were responsible for the microbiological study. A.S. and E.G. aided with the performance of the statistical analysis. M.O. and F.M. wrote the manuscript. J.A.M. and J.M. carried out the critical review of the paper.
REFERENCES
- 1.Antón, N., R. Blazquez, J. L. Gómez- Garcés, and J. I. Alós. 2001. Study of vancomycin tolerance in 120 strains of Streptococcus pneumoniae isolated in 1999 in Madrid, Spain. J. Antimicrob. Chemother. 47:902-903. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F. 1995. Pneumococcal resistance to β-lactam antibiotics: a global geographic overview. Microb. Drug Resist. 1:115-120. [DOI] [PubMed] [Google Scholar]
- 3.Friedland, I., and G. Istre. 1992. Management of penicillin-resistant pneumococcal infections. Pediatr. Infect. Dis. J. 11:433-435. [DOI] [PubMed] [Google Scholar]
- 4.Henriques, B., R. Novak, A. Örtqvist, G. Källenius, E. Tuomanen, and S. Normark. 2001. Clinical isolates of Streptococcus pneumoniae that exhibit tolerance of vancomycin. Clin. Infect. Dis. 32:552-558. [DOI] [PubMed] [Google Scholar]
- 5.Liu, H., and A. Tomasz. 1985. Penicillin tolerance in multiply drug-resistant natural isolates of Streptococcus pneumoniae. J. Infect. Dis. 152:365-372. [DOI] [PubMed] [Google Scholar]
- 6.McCullers, J. A., B. K. English, and R. Novak. 2000. Isolation and characterization of vancomycin-tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J. Infect. Dis. 181:369-373. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. Document M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Novak, R., B. Henriques, E. Charpentier, S. Normak, and E. Tuomanen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399:590-593. [DOI] [PubMed] [Google Scholar]
- 9.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, and B. A. Perkins. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 10.Tuomanen, E. I., R. Austrian, and H. R. Masure. 1995. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 332:1280-1284. [DOI] [PubMed] [Google Scholar]