Abstract
Antioxidant therapy protects against aminoglycoside-induced ototoxicity in animal models. A clinically suitable antioxidant must not affect the therapeutic efficacy of aminoglycosides or exhibit any side effects of its own. In addition, the treatment should be inexpensive and convenient in order to be implemented in developing countries where the use of aminoglycosides is most common. Standardized Salviae miltiorrhizae extracts (Danshen) are used clinically in China and contain diterpene quinones and phenolic acids with antioxidant properties. We combined in vitro and in vivo approaches to investigate the effect of a clinically approved injectable Danshen solution on aminoglycoside-induced free radical generation and ototoxicity. In vitro, Danshen inhibited gentamicin-dependent lipid peroxidation (formation of conjugated dienes from arachidonic acid), as well as the gentamicin-catalyzed formation of superoxide (in a lucigenin-based chemiluminescence assay) and hydroxyl radicals (oxidation of N,N-dimethyl-p-nitrosoaniline). Danshen extracts were then administered to adult CBA mice receiving concurrent treatment with kanamycin (700 mg/kg of body weight twice daily for 15 days). Auditory threshold shifts induced by kanamycin (approximately 50 dB) were significantly attenuated. Danshen did not reduce the levels in serum or antibacterial efficacy of kanamycin. These results suggest that herbal medications may be a significantly underexplored source of antidotes for aminoglycoside ototoxicity. Such traditional medicines are widely used in many developing countries and could become an easily accepted and inexpensive protective therapy.
The side effects of aminoglycoside therapy on the kidneys (nephrotoxicity) and the inner ear (ototoxicity) have largely limited the use of these drugs in a trend promoted in industrialized countries by the development of new, less toxic, alternative antibiotics. In developing countries, however, aminoglycosides remain the antibiotics of choice because of their efficacy, low cost, and easy availability as over-the-counter drugs. Furthermore, the World Health Organization recommends treatment of the rapidly increasing cases of multidrug-resistant tuberculosis with a regimen that includes aminoglycosides, primarily amikacin or streptomycin. This extensive use makes aminoglycosides probably the most commonly used antibiotics worldwide. Ototoxicity associated with aminoglycoside therapy is the major cause of preventable drug-induced hearing loss today (5).
Aminoglycosides have the ability to catalyze the formation of free radicals in vitro and in intact cells (13, 15, 16) as well as in explants of the inner ear (3) by a mechanism that may involve metal chelation (10, 13). Free-radical formation as an underlying cause of ototoxicity has received strong support from the fact that antioxidants attenuate aminoglycoside-induced loss of hearing and balance in guinea pigs and mice in vivo. Effective protective agents include iron chelators and antioxidants, such as 2,3-dihydroxybenzoate, deferoxamine, d-methionine, and salicylate (17, 18, 19).
In recent years, drug development has rediscovered the potential value of phytopharmaceuticals (4), and their incorporation into medical care has been encouraged by the World Health Organization's Traditional Medicines Strategy (21). At the same time, folk remedies in many developing countries have undergone a change from unspecified herbal extracts (or the actual herbs) to well controlled and chemically analyzed preparations. The traditional Chinese medicine Danshen, derived from the dried root or rhizome of Salviae miltiorrhizae Bge, is an example of such a standardized medication. Approved for clinical use in China, indications for Danshen include treatment of angina pectoris and cerebrovascular disorders (25). Danshen extracts contain diterpene quinone and phenolic acid derivatives, including tanshinone (I, IIA, and IIB), cryptotanshinone, isocryptotanshinone, miltirone, tanshinol (I and II), and salviol (7, 25). These compounds have antioxidant properties and protect against lipid peroxidation in vitro and in vivo (12, 24), making them potential antidotes for free-radical-based disorders.
In an effort to improve on prophylaxis of aminoglycoside-induced hearing loss without jeopardizing acceptability and affordability in developing countries, we investigated the effect of Danshen on aminoglycoside-induced free radical generation in vitro and ototoxicity in vivo. The CBA mouse was used because this strain is well established in auditory research and does not carry genes (e.g., ahl) that may predispose to premature hearing loss or sensitivity to stress. Furthermore, the response of CBA mice to ototoxic aminoglycoside antibiotics has been well characterized (22).
MATERIALS AND METHODS
Materials.
Injectio Salvia miltiorrhiza was obtained from Shanghai 1st Pharmaceutical Factory (Shanghai, China). This is a standardized injectable preparation approved for clinical use (approval number 001065; 1995; Department of Drug Administration, Shanghai, China) and widely available over the counter. Lot number 011101 from 20 November 2001 was used in our experiments.
Kanamycin sulfate was purchased from USB Corporation (Cleveland, Ohio) (catalog no. 17924, lot no. 110755), gentamicin sulfate was purchased from Spectrum Chemical Mfg. Corp. (Gardena, Calif.), ketamine (Ketaset) was purchased from Fort Dodge Animal Health (Fort Dodge, Iowa), xylazine (TranquiVed) was purchased from Vedco Inc. (St. Joseph, Mo.), and rhodamine phalloidin was purchased from Molecular Probes Inc. (Eugene, Ore.). All other reagents came from Sigma Chemical Co. (St. Louis, Mo.).
In vitro experiments. (i) Superoxide formation.
The antioxidant properties of Danshen against gentamicin-induced superoxide formation were tested in a chemiluminescence assay according to the method of Gyllenhammar (6) with modifications previously described in detail (16). Superoxide was generated at room temperature from 0.2 mM hypoxanthine and 0.0078 U of xanthine oxidase in an assay with 0.1% arachidonic acid and 0.2 mM lucigenin in the absence or presence of 1 mM gentamicin and various concentrations of Danshen. Luminosity was monitored in a Turner Model 20 luminometer (Cardinal Associates, Santa Fe, N.M.). The amount of luminosity was expressed as relative luminosity units/s, i.e., as the rate of luminosity formation.
(ii) Hydroxyl radical formation.
A copper(II)-gentamicin-H2O2 system served as a source of hydroxyl radicals, and N,N-dimethyl-p-nitrosoaniline (NDMA) served as a reporter molecule. The reaction mixture contained 50 mM sodium phosphate buffer (pH 7.4), 0.05 mM CuCl2, 0.1 mM gentamicin, 0.5 mM H2O2, 0.017 mM NDMA, and various concentrations of Danshen. The absorption measurements were recorded at 25°C on a Perkin-Elmer Lambda 9 spectrophotometer at the characteristic wavelength of NDMA, 440 nm. Hydroxyl radical formation was calculated from the change in NDMA absorbance using an extinction coefficient of 34 × 103 M−1 cm−1.
(iii) Lipid peroxidation.
Arachidonic acid peroxidation was monitored by measuring conjugated diene levels spectrophotometrically according to the method of Buege and Aust (1). The reaction mixtures (200 μl) contained 50 mM sodium phosphate (pH 7.4), 0.25% arachidonic acid (vol/vol), 0.5 mM H2O2, and 0.05 mM CuCl2 with or without 0.1 mM gentamicin sulfate and various concentrations of Danshen. After incubation for 60 min at 37°C, the reaction was stopped by the addition of 1 ml of chloroform-methanol (2:1 [vol/vol]). Following mixing and centrifugation, the lower organic phase material was removed and dried at 45°C under a stream of N2. Cyclohexane (200 μl) was added to the lipid residue, and absorbance was read at 235 nm. Concentration of conjugated dienes was calculated from an extinction coefficient of 2.52 × 104 M−1 cm−1.
In vivo experiments. (i) Experimental animals and drug administration.
Male CBA/J (CBA) mice, at an initial age of 4 weeks, were purchased from Harlan Sprague-Dawley Co. (Indianapolis, Ind.). The animals were given free access to water and a regular mouse diet (Purina, St. Louis, Mo.), and were allowed 1 week to acclimate before treatment. All experimental protocols involving animal use were approved by the University of Michigan Committee on Use and Care of Animals. Animal care was supervised by the University of Michigan's Unit for Laboratory Animal Medicine.
Kanamycin (700 mg of kanamycin base/kg of body weight twice daily) was tested alone and in combination with various concentrations of Danshen. It should be noted that the concentration of Danshen is given by the manufacturer as the equivalent to the original herb: “1 g of Danshen” is the amount of extract derived from 1 g of herb. The study to evaluate auditory effects was comprised of one group serving as the saline control group (n = 11), one group receiving kanamycin injections only (n = 14), five groups (n = 5 each) receiving kanamycin plus Danshen at 1, 4, 6, 10, or 20 g/kg twice daily, and three groups (n = 3 each) receiving Danshen only at 6, 10, or 20 g/kg twice daily. In a separate study, levels of kanamycin in serum were determined for animals receiving either kanamycin alone (700 mg of kanamycin base/kg twice daily) or kanamycin plus Danshen (6 or 10 g/kg twice daily). Kanamycin and Danshen were each dissolved in 0.9% sterile saline, and subcutaneous injections were given separately but simultaneously twice daily for 15 days. Control mice received an equivalent volume of saline.
(ii) Evaluation of auditory function.
Auditory thresholds were measured by evoked auditory brainstem responses (ABR). Thresholds were taken for each animal at the beginning of the study, 2 weeks after the start of drug treatment, and then at 3 weeks and 5 weeks. ABR measurements were performed as described previously (22). In brief, the mice were anesthetized with an intramuscular injection of 100 mg of ketamine and 5 mg of xylazine/kg and kept warm with a heating pad. The positive needle electrode was subdermally inserted at the vertex, the midline of the scalp between the external auditory canals. The negative electrode was placed below the pinna of the left ear, and the ground electrode was inserted contralaterally. Tone bursts of 12 and 24 kHz (10 ms duration, 1 ms rise and fall time) were generated using a SigGen software package (Tucker-Davis Technologies, Gainsville, Fla.) and delivered to the left external auditory meatus in a closed acoustic system through an ear bar connected to a DT-48 transducer (Beyer Dynamic, Farmingdale, N.Y.). Responses from 1020 stimuli were averaged for each frequency, fed to an amplifier, viewed with an oscilloscope, and recorded. Threshold was determined by reducing the sound intensity in 5 dB steps until threshold. Thresholds were defined as the lowest intensity that yielded a reproducible deflection in the evoked response trace and were verified at least twice. Threshold shifts were calculated for individual animals by comparison to their prestudy thresholds. The ABR of each animal was interpreted by an observer without knowledge of the treatment.
(iii) Histopathology.
After the last ABR measurement at 5 weeks, animals were sacrificed, and the temporal bones were removed. The round and oval windows and the apex of the cochlea were opened, perfused with 4% paraformaldehyde in 10 mM phosphate-buffered saline (pH 7.4), and fixed overnight at 4°C. The fixed cochleae were decalcified with 4% EDTA in 10 mM phosphate-buffered saline for 2 to 3 days. After removal of the bony capsule, lateral wall, and tectorial membrane, cochleae were stained with rhodamine phalloidin (14) for 50 min to outline the hair cells. Thereafter, the organ of Corti was separated from the modiolus, microdissected into individual turns, mounted on glass slides in antifade fluorescent mounting media, and coverslipped.
Hair cells in the organ of Corti were counted on a Leitz Orthoplan upright fluorescence microscope, using a ×50 oil immersion objective lens. Evaluation began at the apex and moved down the cochlear spiral to the base, assessing successive 0.19-mm fields. For each field, the area of observation was oriented to include the row of inner hair cells and all three rows of outer hair cells longitudinally. After counting of the entire cochlea, each row was evaluated for the presence or absence of hair cells. Manual count data were entered into a computer program. The percentage of missing hair cells for each row was calculated, utilizing normative counts from control animals as 100%, and cytocochleograms were plotted for the percentage of cell loss.
(iv) Serum levels of kanamycin.
Levels of kanamycin in serum were assayed by high-performance liquid chromatography (HPLC), using a precolumn derivatization procedure with 9-fluorenylmethyl chloroformate (catalog no. F-0378; Sigma Chemical Co.), and modifying the method of Stead and Richards (20). Serum samples and kanamycin standards were prepared by mixing 8 μl of water or kanamycin solution with 2 μl of serum. Proteins were removed by the addition of 60 μl of methanol, vortexing for 10 s, and centrifugation for 5 min at 12,000 × g. The supernatant from each sample was transferred to an autosampler vial, and 30 μl of 0.1 M sodium bicarbonate-carbonate (pH 9.0) was added to the vial, capped, and vortexed. The derivatizing reagent was 2 mg of fluorenylmethyl chloroformate/ml of HPLC-grade acetone. The precolumn derivatization of each sample was performed using a Gibson autosampler. One hundred microliters of the derivatizing reagent was injected into a vial, mixed, and allowed to react for at least 5 min with the sample before 100 μl of the contents was withdrawn and injected into the HPLC column (Bischoff Prontosil C18-AQ, 5 micron, 150- by 4.6-mm analytical column [Bischoff Chromatography, Atlanta, Ga.] and pellicular C18 guard column [Alltech Assoc., Deerfield, Ill.]). Gradient elution started with a mixture of 80% methanol plus 20% water (vol/vol) and ended with 100% methanol. The flow rate was 1 ml/min (Beckman pumps and controller). Fluorescence was detected with excitation at 229 nm and emission at >300 nm (Spectrovision FD 100; Groton Technology Inc., Acton, Mass.). Data were collected using a Shimadzu CR4A integrator (Shimadzu Corp., Columbia, Md.).
The detection limit of the assay was in the low picomolar range, and the lowest-level samples (100 pmol/2 μl of serum) were well above this limit. In fluorescence assays, variation of the intensity of the excitation light source over time will cause variations in the intensity of the emitted light. In order to eliminate such potentially confounding variability, every three serum samples were bracketed by kanamycin standards which served to normalize the data.
Antimicrobial activity.
Efficacy of kanamycin alone and in the presence of Danshen was tested against Escherichia coli (ATCC no. 25922) in a standardized microbiological assay (2). Ten microliters of kanamycin (100 μg/ml) was dispensed onto 1-cm filter paper disks that had been placed on circular 100-mm agar plates previously inoculated with E. coli. Subsequently, 10-μl aliquots of different concentrations of Danshen (0.15 mg/ml, 1.5 mg/ml, and 15 mg/ml) were added to the disks. The concentration of kanamycin was derived from a dose-response curve relating concentration to inhibition zone size; the concentrations of Danshen were chosen to yield the same ratio to kanamycin as administered in the in vivo study, flanked by a concentration 10-fold higher and another 10-fold lower. Kanamycin only was assayed directly or with 10 μl of saline instead of Danshen; since the resulting inhibition zones were not significantly different (P > 0.05), these two groups were collapsed for later statistical comparison to kanamycin plus Danshen. Additionally, kanamycin (100 μg/ml) and Danshen (15 mg/ml) were incubated together for 30 min and then added to the disks in 10-μl aliquots. Control disks with Danshen only were also plated. The inoculated plates were incubated overnight at 37°C in an incubator. The diameter of the inhibition zones was measured with a caliper to the nearest 0.01 mm across each disk.
Statistical analysis.
Data were statistically evaluated by Student's t test and by analyses of variance with a Student-Newman-Keuls posthoc test for significance (P < 0.05) using Primer of Biostatistics software (McGraw-Hill Software, New York, N.Y.).
RESULTS
Danshen inhibits gentamicin-catalyzed free radical formation and lipid peroxidation in vitro.
For the in vitro experiments, gentamicin concentrations were selected based on previous studies of gentamicin-stimulated metal-catalyzed free radical formation (9, 10, 13). Gentamicin binds Cu(II), forming 1:1 complexes which catalyze hydrogen peroxide disproportionation at pH 7.4 in a reaction involving hydroxyl radical formation. We used a 1:2 molar ratio of Cu(II)-gentamicin to maintain Cu(II) ions in the form of the Cu(II)-gentamicin complex and an excess of H2O2 to promote efficient hydroxyl radical formation.
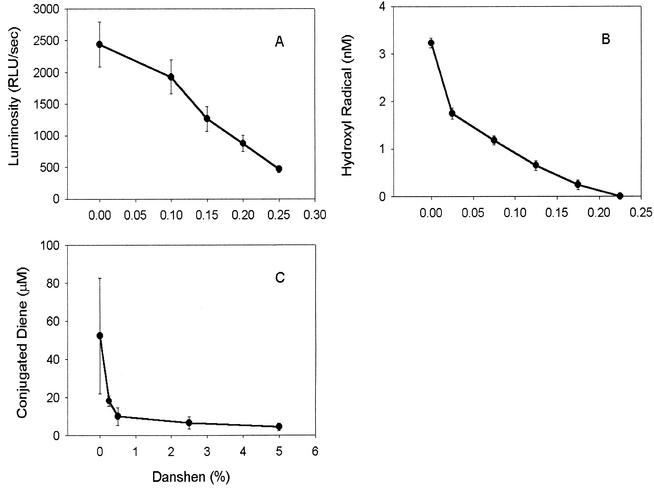
Gentamicin (1 mM) stimulated the formation of lucigenin luminosity, presumably an indicator of superoxide, consistent with our earlier observations (16). Danshen at concentrations ranging from 0.1 to 0.25% significantly suppressed gentamicin-dependent luminosity (P < 0.05). A 50% inhibition of luminosity was observed with 0.15% Dashen (Fig. 1A).
FIG. 1.
Effects of Danshen on gentamicin-mediated free radical reactions. (A) Danshen suppresses gentamicin-catalyzed formation of superoxide. Luminosity was assayed in the hypoxanthine-xanthine oxidase system as described in Materials and Methods. Data are means ± standard deviations for four to six experiments per condition. The concentration of Danshen is given as the final concentration of the commercial extract in the assay. Danshen significantly inhibited gentamicin-stimulated lucigenin luminosity (P < 0.05). Differences among individual concentrations of Danshen were also significant (P < 0.05). RLU, relative luminosity units. (B) Danshen inhibits gentamicin-catalyzed formation of hydroxyl radicals. The formation of hydroxyl radicals was catalyzed by gentamicin-Cu(II) complexes as described in Materials and Methods. The concentration of Danshen is given as the final concentration of the commercial extract in the assay. Data are means ± standard deviations for five experiments. Danshen is effective in suppressing gentamicin-catalyzed hydroxyl radical formation at each concentration (P < 0.05). (C) Danshen decreases gentamicin-induced lipid peroxidation. Lipid peroxidation was measured as described in Materials and Methods. The concentration of Danshen is given as the final concentration of the commercial extract in the assay. Data are means ± standard deviations for four to eight experiments per condition. All values in the presence of Danshen are significantly different from gentamicin alone (P < 0.05).
Hydroxyl radical formation was monitored by the oxidation of the indicator NDMA, which depended on the presence of gentamicin: 0.1 mM gentamicin increased hydroxyl radical formation from 0 to 3.23 nM/min. Danshen inhibited this gentamicin-catalyzed formation of hydroxyl radicals in a dose-dependent fashion (P < 0.05) (Fig. 1B). At a concentration of 0.225%, Danshen completely suppressed the oxidation of NDMA.
Conjugated diene formation from arachidonic acid was significantly increased by the addition of 0.1 mM gentamicin to the H2O2-Cu(II) system. At concentrations ranging from 0.25 to 5%, Danshen effectively suppressed gentamicin-induced lipid peroxidation (P < 0.05), achieving a 50% inhibition around 0.2% (Fig. 1C).
Danshen attenuates kanamycin-induced threshold shifts.
Auditory thresholds (hearing sensitivity) as assessed by ABR were comparable for all animals at the beginning of the study. Saline-injected animals maintained stable thresholds throughout the course of treatment. In contrast, animals receiving kanamycin (700 mg/kg twice daily) developed a progressive hearing loss (Fig. 2). This dosage had previously been established as well tolerated in CBA mice while producing a consistent threshold shift and hair cell loss (22). Auditory thresholds were significantly elevated after 15 days of treatment and remained elevated after cessation of treatment. Following the common pattern of aminoglycoside ototoxicity, the functional deficit was always greater at the higher frequency, and by the end of the fifth week, threshold shifts averaged about 45 dB at 24 kHz and 36 dB at 12 kHz.
FIG. 2.
Danshen reduces kanamycin-induced threshold shifts. Kanamycin (700 mg/kg twice daily) and Danshen (10 g/kg twice daily) were administered, and ABR thresholds were measured at 24 kHz (A) and 12 kHz (B) as described in Materials and Methods. Data are means ± standard deviations. Filled circles (•), saline controls, n = 11; filled squares (▪), kanamycin alone, n = 14; filled triangles (▴), kanamycin plus Danshen, n = 5; cross (x), Danshen alone, n = 3. Cotreatment with Danshen significantly attenuated kanamycin-induced threshold shifts at all times (P < 0.05).
The effect of Danshen on the kanamycin-induced threshold shifts was dose dependent. The dosing range had been selected based on experiments with several animal species showing protective effects of 1 to 4 g of extract/kg on myocardial infarct size or aflatoxin-induced hepatocarcinogenesis (11, 25). No effect on kanamycin-induced threshold shifts was observed at 1 g/kg twice daily, while 4 and 6 g/kg twice daily provided a small but significant (P < 0.05) reduction of the threshold shift at 24 kHz only. A consistent attenuation at both 12 and 24 kHz was seen with 10 g or 20 g of Danshen/kg twice daily. Concurrent treatment with 2 × 10 g of Danshen lowered the final kanamycin-induced threshold shifts to 25 ± 11 dB at 24 kHz and to 17 ± 10 dB at 12 kHz (P < 0.05; Fig. 2). A concentration of 2 × 20 g of Danshen/kg likewise yielded a significant protection at both frequencies (27 ± 13 dB at 24 kHz, 13 ± 7 dB at 12 kHz; P < 0.05) but no improvement over the treatment with 2 × 10 g of Danshen (10 versus 20 g; P > 0.05). Danshen alone up to 20 g/kg twice daily had no effect on auditory thresholds.
Histopathology.
Cochlear pathology (Fig. 3) reflected the functional results obtained from the ABR measurements. Control animals receiving saline injections had a normal complement of outer hair cells with a loss of less than 10% scattered throughout the length of the cochlea (Fig. 3A). Kanamycin-treated animals (700 mg of kanamycin/kg twice daily) exhibited severe to complete hair cell loss in all three rows of outer hair cells in the basal turns of the cochlea, while cells in the apex remained intact. Inner hair cells appeared to be preserved (Fig. 3B). Coadministration of Danshen (10 g/kg twice daily) significantly reduced damage to hair cells, although a 30 to 50% loss was still apparent at the very base of the cochlea (Fig. 3C).
FIG. 3.
Danshen reduces kanamycin-induced hair cell loss. Cytocochleograms were obtained from the surface preparations of the organ of Corti as described in Materials and Methods. The percentage of missing hair cells is plotted for the entire length of the cochlea. In the saline-treated CBA mice, outer hair cells in the basal turn were almost completely present (A). After treatment with kanamycin (700 mg/kg twice daily), most of the outer hair cells in the basal turn of the cochlea disappeared (B). Animals receiving kanamycin plus Danshen (10 g/kg twice daily) demonstrated less outer hair cell loss (C) than animals receiving kanamycin alone. Heavy solid line, inner hair cells; light solid line, outer hair cells of row 1; dashed line, outer hair cells of row 2; solid line with dots, outer hair cells of row 3.
Serum kanamycin levels.
Levels of kanamycin in serum were measured at 20 min and at 1 h in animals receiving kanamycin (700 mg/kg twice daily) or kanamycin plus Danshen (6 or 10 g/kg twice daily). The selected times correspond to peak levels in serum (20 min) and the approximate half-life (1 h) of kanamycin in CBA mice (22). The level of kanamycin in serum at 20 min was 271 ± 61 μg/ml (mean ± standard deviation; n = 5) in the absence of Danshen and remained unchanged in its presence (280 ± 6 μg/ml; n = 3). At one h, the values were 108 ± 86 μg/ml in the kanamycin group (n = 10) and 175 ± 27 μg/ml in the group receiving kanamycin plus Danshen (10 g/kg twice daily; n = 5). Although the values at 1 h were not statistically different, a trend towards higher serum levels in the presence of Danshen seemed supported by somewhat elevated levels in a small group of mice cotreated with 6 g of Danshen/kg twice daily, 137 ± 9 μg/ml (n = 3).
Antimicrobial efficacy.
Growth inhibition zones created by kanamycin on a lawn of E. coli were determined as a measure of antimicrobial efficacy. The concentration of kanamycin for this assay had been derived from a dose-response curve indicating that a 20% reduction of the effective kanamycin concentration would lead to a reduction in zone size of 1 mm. Ten microliters of kanamycin (100 μg/ml) created an inhibition zone of 14.3 ± 0.9 mm. The additional presence of 10 μl of different concentrations of Danshen (0.15, 1.5, and 15 mg/ml) gave inhibitions zones of 14.6 ± 0.14 mm, 15.0 ± 0.8 mm, and 14.6 ± 1.0 mm, respectively (all values are mean ± standard deviation; n = 18 each). These zone sizes were not different from those with kanamycin alone (P > 0.05), indicating that there was no effect of Danshen on the antimicrobial efficacy of kanamycin. Incubation of kanamycin with Danshen for 30 min prior to plating likewise did not affect the inhibition zone size. Danshen plated in the absence of kanamycin had no effect on the growth of the bacterial culture.
DISCUSSION
The results of this study suggest that traditional medicines hold promise as pharmacological protectants against aminoglycoside-induced hearing loss. Both the physiological measurement (ABR) and the morphological assessment of hair cell loss showed significant protection by Danshen against the ototoxicity of kanamycin at both the structural and functional levels. Kanamycin induced a massive destruction of outer hair cells at the base of the cochlea where high frequencies are being processed. The apical region was less affected, consistent with a lesser threshold shift at 12 kHz than at 24 kHz. The reduction of hair cell loss in the middle and the apex of the cochlea by cotreatment with Danshen was consistent with the observed protection against functional loss at both 12 and 24 kHz. The preferential loss of outer hair cells at the base and the apparent sparing of inner hair cells is a general pattern of aminoglycoside-induced ototoxicity. While a correlation between preservation of hair cells and function along the length of the cochlea is obvious in our experiments, a quantitative relationship cannot be expected. The histology (cytocochleogram) assesses the presence of sensory cells without insight into possible structural changes that might affect function. Conversely, a small and scattered loss of hair cells as seen in control animals or in the apex of treated animals may not lead to a noticeable threshold shift. Hence, both morphological and functional measures have to be combined to determine ototoxic actions and protection.
Danshen has been widely used clinically in China since its introduction in the 1960s as an effective remedy for cerebrovascular disorders, angina pectoris, and hypertension with minimal side effects (25). Its components provide a wide spectrum of antioxidant activity. Tanshinone and tanshinol derivatives protect against lipid peroxidation in vitro and in vivo (12, 24). Three of the water-soluble components, salvianolic acid A, salvianolic acid B, and rosmarinic acid, inhibited NADPH-vitamin C- and Fe(II)-cysteine-induced lipid peroxidation in microsomes and the production of superoxide in a xanthine-xanthine oxidase system (8). Another ingredient, magnesium lithospermate B, is a hydroxyl radical scavenger (23). In addition to a direct antioxidant action, Danshen may protect against oxidant stress in vivo by regulating the activities of antioxidant enzymes. Sodium tanshinone IIA sulfonate protected against doxorubicin hydrochloride (Adriamycin)-induced lipid peroxidation in mouse hearts by increasing the activities of endogenous antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and catalase (24). As we show here, Danshen acts as an antioxidant against aminoglycoside-induced free-radical formation and lipid peroxidation, showing similar efficacy in three different in vitro assay systems. Whether Danshen also influences gene activation in the inner ear remains to be established. In any case, the correlation between antioxidant actions in vitro and the attenuation of hearing loss in vivo reinforces the connection between free radical formation and ototoxicity (5).
The dosage of kanamycin used here by far exceeds dosages that produce ototoxicity in other animals, such as guinea pigs, or that are commonly administered to patients in antimicrobial therapy. In fact, on a body weight basis, the kanamycin dose for CBA mice is 100 times the human dose. These high doses, however, are well tolerated by the mice without signs of nephrotoxicity, and the necessity for such high concentrations to achieve auditory damage in mice has been discussed at length elsewhere (22). The reasons may include different pharmacokinetics in small animals and species differences in susceptibility. The dose of Danshen (per kilogram of body weight) used in our experimental animals is also higher than in humans but to a lesser degree than in the case of kanamycin. In clinical applications, Danshen is administered intramuscularly at 9 to 15 g per day (25) or intravenously at up to 24 g/day, corresponding to 0.2 to 0.3 g/kg. A salient point to consider here is the fact that the efficacy of protection depends on the relative concentrations of the aminoglycoside and the protectant (22). Since Danshen is effective in the presence of high levels of kanamycin in animals, it should be equally or more effective at clinical concentrations of aminoglycosides.
The clinical equivalent of the model studied here (kanamycin injections for 2 weeks) would be a treatment for acute infections. Tuberculosis patients, however, may receive aminoglycosides for up to 6 months, and it is an important question whether Danshen could be useful in such a protracted regimen. An extension of our experiments would be interesting, but at the moment, no animal model exists that mimics extremely long-term, low-dose application of aminoglycosides.
A crucial question in view of a possible clinical application of Danshen is potential interference with the pharmacological activity of kanamycin. No such interference seems to exist. First, Danshen does not lower levels of kanamycin in serum, which would be a confounding factor in establishing its protective potential. In fact, the trend towards higher serum levels in its presence rules out kanamycin pharmacokinetics as an explanation of the attenuation of auditory damage. Second, Danshen does not adversely affect the antibacterial efficacy of kanamycin even when present at a 10-fold-higher ratio than is administered in vivo. Since traditional medicines are frequently used in many developing countries, they could easily become an accepted and inexpensive therapeutic prophylaxis that could curtail the adverse side effects of commonly used aminoglycoside antibiotics.
Acknowledgments
This research was supported by research grant DC-03685 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
We thank John McLaren for analyzing the serum kanamycin levels and Andra E. Talaska for the microbiological assays.
REFERENCES
- 1.Buege, J. A., and S. D. Aust. 1978. Microsomal lipid peroxidation. Methods Enzymol. 52:302-310. [DOI] [PubMed] [Google Scholar]
- 2.Chapin-Robertson, K., and S. C. Edberg. 1991. The measurement of antibiotics in human body fluids: techniques and significance, p 295-366. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, Md.
- 3.Clerici, W. J., K. Hensley, D. L. Dimartino, and D. A. Butterfield. 1996. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear. Res. 98:116-124. [DOI] [PubMed] [Google Scholar]
- 4.Cyranoski, D. 2001. Hong Kong seeks secrets of Chinese medicine. Nature 412:7.. [DOI] [PubMed] [Google Scholar]
- 5.Forge, A., and J. Schacht. 2000. Aminoglycoside antibiotics. Audiol. Neuro-Otol. 5:3-22. [DOI] [PubMed] [Google Scholar]
- 6.Gyllenhammar, H. 1987. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J. Immunol. Methods 97:209-213. [DOI] [PubMed] [Google Scholar]
- 7.Huang, K. C. 1999. The pharmacology of Chinese herbs, p. 91-94. CRC Press, Boca Raton, Fla.
- 8.Huang, Y. S., and J. T. Zhang. 1992. Antioxidative effect of three water-soluble components isolated from Salvia Miltiorrhiza in vitro (Chinese). Yao Xue Xue Bao. 27:96-100. [PubMed] [Google Scholar]
- 9.Jezowska-Bojczuk, M., W. Lesniak, W. Bal, H. Kozlowski, K. Gatner, A. Jezierski, J. Sobczak, S. Mangani, and W. Meyer-Klaucke. 2001. Molecular mechanism of hydrogen peroxide conversion and activation by Cu(II)-amikacin complexes. Chem. Res. Toxicol. 14:1353-1362. [DOI] [PubMed] [Google Scholar]
- 10.Lesniak, W., W. R. Harris, J. Y. Kravitz, J. Schacht, and V. L. Pecoraro. 2003. Solution chemistry of copper(II)-gentamicin complexes: relevance to metal-related aminoglycoside toxicity. Inorg. Chem. 42:1420-1429. [DOI] [PubMed] [Google Scholar]
- 11.Liu, J., C. F. Yang, S. Wasser, H. M. Shen, C. E. L. Tan, and C. N. Ong. 2001. Protection of Salvia Miltiorrhiza against aflatoxin-B1-induced hepatocarcinogenesis in Fischer 344 rats. Life Sci. 69:309-326. [DOI] [PubMed] [Google Scholar]
- 12.Niu, X.-L., K. Ichimori, X. Yang, Y. Hirota, K. Hoshiai, M. Li, and H. Nakazawa. 2000. Tanshinone II-A inhibits low density lipoprotein oxidation in vitro. Free Radic. Res. 33:305-312. [DOI] [PubMed] [Google Scholar]
- 13.Priuska, E. M., and J. Schacht. 1995. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem. Pharmacol. 50:1749-1752. [DOI] [PubMed] [Google Scholar]
- 14.Raphael, Y., and R. A. Altschuler. 1991. Scar formation after drug-induced cochlear insult. Hear. Res. 51:173-183. [DOI] [PubMed] [Google Scholar]
- 15.Sha, S.-H., and J. Schacht. 1999. Formation of reactive oxygen species following bioactivation of gentamicin. Free Radic. Biol. Med. 26:341-347. [DOI] [PubMed] [Google Scholar]
- 16.Sha, S.-H., and J. Schacht. 1999. Stimulation of free radical formation by aminoglycoside antibiotics. Hear. Res. 128:112-118. [DOI] [PubMed] [Google Scholar]
- 17.Sha, S.-H. and J. Schacht. 1999. Salicylate attenuates gentamicin-induced ototoxicity. Lab. Investig. 79:807-813. [PubMed] [Google Scholar]
- 18.Song, B.-B., D. J. Anderson, and J. Schacht. 1997. Protection from gentamicin ototoxicity by iron chelators in guinea pig in vivo. J. Pharmacol. Exp. Ther. 282:369-377. [PubMed] [Google Scholar]
- 19.Song, B.-B., S.-H. Sha, and J. Schacht. 1998. Iron chelators protect from aminoglycoside-induced cochleo- and vestibulotoxicity in guinea pig. Free Radic. Biol. Med. 25:189-195. [DOI] [PubMed] [Google Scholar]
- 20.Stead, D. A., and R. M. Richards. 1996. Sensitive fluorimetric determination of gentamicin sulfate in biological matrices using solid-phase extraction, pre-column derivatization with 9-fluorenylmethyl chloroformate and liquid chromatography. J. Chromatogr. Biomed. Appl. 679:69-78. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2001. Traditional medicine. [Online.] http://www.who.int/m/topics/traditional_medicine/en/index.html.
- 22.Wu, W.-J., S.-H. Sha, J. D. McLaren, K. Kawamoto, Y. Raphael, and J. Schacht. 2001. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear. Res. 158:165-178. [DOI] [PubMed] [Google Scholar]
- 23.Yokozawa, T., H. Y. Chung, E. Dong, and H. Oura. 1995. Confirmation that magnesium lithospermate B has a hydroxyl radical-scavenging action. Exp. Toxicol. Pathol. 47:341-344. [DOI] [PubMed] [Google Scholar]
- 24.Zhou, G.-Y., B.-L. Zhao, J.-W. Hou, G.-E. Ma, and W.-J. Xin. 1999. Protective effects of sodium tanshinone IIA sulphonate against Adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacol. Res. 40:487-491. [DOI] [PubMed] [Google Scholar]
- 25.Zhu, Y.-P. 1998. Chinese materia medica: chemistry, pharmacology and applications, p. 459-463. Harwood Academic Publishers, Amsterdam, The Netherlands.