Abstract
Using reversible electropermeabilization of cells and spheroplasts, we show that the cell wall and plasma membrane partly account for bleomycin resistance by acting as two independent barriers. We also report on the presence of a membrane protein that may be responsible for bleomycin internalization and toxicity in Saccharomyces cerevisiae.
The antitumor drug bleomycin (BLM) was used to analyze the contributions of the cell wall and the cell membrane in limiting the uptake of hydrophilic cytotoxic molecules during the stationary phase of the yeast Saccharomyces cerevisiae. Two yeast strains with different sensitivities to BLM were tested: YPH-1 (MATa ura3-52 lys2-801am ade2-101oc) (14) and FY67 (MATa trp1-Δ36) (15).
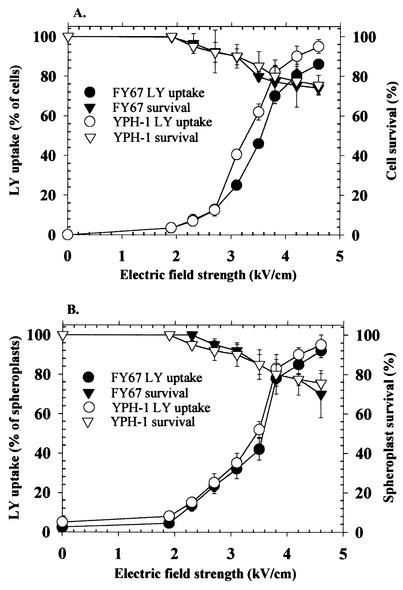
Yeast cells were grown to a density of approximately 108 cells/ml through shaking and aeration in complete YPD (yeast extract-peptane-dextrose) medium (13) at 30°C. We determined the percentage of reversibly permeabilized cells by the lucifer yellow test (6) and prepared spheroplasts as described previously (2) with lyticase from Sigma. A total of 5 × 107 cells were electropermeabilized as described previously (1) in the absence of dithiothreitol by using eight 100-μs square-wave pulses at a frequency of 1 Hz. Spheroplasts were electroporated in 1.2 M sorbitol-1 mM MgCl2-10 mM Tris-HCl (pH 7.5). No effect on survival was detected when electric fields from 0 to 2,000 V/cm were applied to either the cells or the spheroplasts. Optimal conditions were chosen such that the electric field resulted in 70 to 75% survival and electropermeabilization of approximately 85% of those surviving cells (i.e., 4,640 and 3,920 V/cm for FY67 and YPH-1 cells, respectively, and 4,300 and 3,900 V/cm for FY67 and YPH-1 spheroplasts, respectively) (Fig. 1). Our results show that the optimal permeabilization value is a function of the difference between the mean cell diameter for the two strains, as determined by flow cytometry (50.5 ± 2.12 and 58 ± 2.83, in arbitrary units, for FY67 and YPH-1 cells, respectively, and 49 ± 2.15 and 56.5 ± 1.41 for FY67 and YPH-1 spheroplasts, respectively). Our data also show that the removal of the cell wall does not significantly alter the cell diameter and has almost no influence on the optimal electrical field strength applied to the spheroplasts. Thus, the presence of the cell wall does not affect the electropermeabilization of the plasma membrane.
FIG. 1.
Influence of electric field strength on yeast cell (A) or spheroplast (B) permeabilization and survival. Each point represents the mean of three independent experiments performed in triplicate. Bars represent standard errors of the means. LY, lucifer yellow.
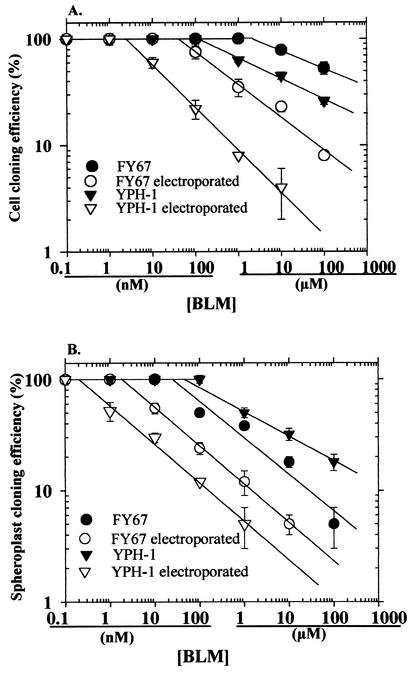
Both yeast strains were exposed to BLM for 5 min at concentrations ranging from 0.3 nM to 100 μM, with and without application of the electric pulses. In this study, a BLM-Fe complex in a 1:3 molar ratio was used to induce cytotoxicity in the yeasts (7). The cytotoxicity of BLM was determined by determination of the relative cloning efficiencies of the treated cells. The BLM concentration that induced 50% lethality (the minimal bactericidal concentration for 50% of the cells [MBC50]) for the nonelectropermeabilized YPH-1 yeast cells was 4 μM. The FY67 yeast cells were approximately 30 times more resistant to BLM, with an MBC50 of 120 μM (Fig. 2A and Table 1). When the cells were electropermeabilized in the presence of BLM, FY67 cells were 27 times more resistant to BLM than the YPH-1 cells (Fig. 2A and Table 1). Electropermeabilization had no effect on the susceptibilities of the strains to BLM; however, it increased the BLM-induced cytotoxicities 300-fold for FY67 and 267-fold for YPH-1 yeast cells (Table 1). The increase in the cytotoxicity of BLM for yeast cells could be the result of a higher level of internalization of BLM. Indeed, by using the radiolabeled 57Co-BLM complex (9), we found that more57Co-BLM molecules were associated with electropermeabilized yeast cells than with nonelectropermeabilized yeast cells (data not shown). Similar increases in levels of cytotoxicity and BLM internalization have previously been demonstrated in animal cells (4, 5, 8, 10).
FIG. 2.
BLM survival response curves for FY67 and YPH-1 cells (A) or spheroplasts (B) exposed or not exposed to permeabilizing electric pulses. Each point represents the mean of three independent experiments performed in triplicate. Bars represent standard errors of the means.
TABLE 1.
Contributions of the cell wall and the plasma membrane in limiting the cytotoxicity of BLM for yeast cells and spheroplastsa
| Strain | MBC50 (nM) in the absence of electropermeabilization | f′CW | MBC50 (nM) in the presence of electropermeabilization | fCW | fEPN |
|---|---|---|---|---|---|
| FY67 cells | 120,000 | 600 | 400 | 34 | 300 |
| FY67 spheroplasts | 200 | 12 | 17 | ||
| YPH-1 cells | 4,000 | 4 | 15 | 10 | 267 |
| YPH-1 spheroplasts | 1,000 | 1.5 | 667 |
fCW, cell wall filtering factor, determined as the ratio of the MBC50 for electropermeabilized cells and the MBC50 for electropermeabilized spheroplasts (when the plasma membrane restrictions have been removed by electroporation); f′CW, cell wall pseudo-filtering factor, determined as the ratio of the MBC50 for nonelectropermeabilized yeast cells and the MBC50 for nonelectropermeabilized spheroplasts (when the plasma membrane restrictions have not been removed); fEPN, electropermeabilization factor, determined as the ratio of the MBC50 for nonelectropermeabilized cells or spheroplasts and the MBC50 for electropermeabilized cells or spheroplasts.
The MBC50s for intact spheroplasts were 200 nM for FY67 and 1 μM for YPH-1 (Fig. 2B and Table 1). Thus, intact YPH-1 spheroplasts were five times more resistant to BLM than intact FY67 spheroplasts. As such, it was shown that the presence of the cell wall had a significant, strain-dependent influence on the cytotoxicity of BLM. The conferral of such a decrease in toxicity by the cell wall has previously been described for another small hydrophilic polypeptide molecule, nisin (3).
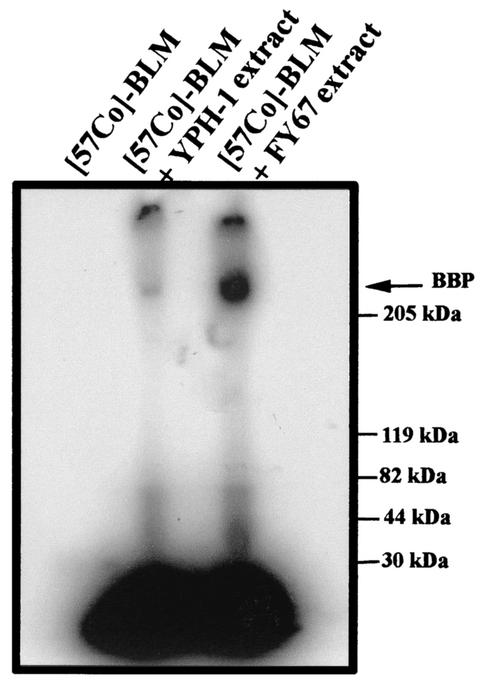
Electroporation of spheroplasts increased the cytotoxicity of BLM 17-fold for FY67 spheroplasts and 667-fold for YPH-1 spheroplasts (Table 1). Unexpectedly, when electropermeabilization was used, electroporated YPH-1 spheroplasts were eight times less resistant to BLM than FY67 spheroplasts (Fig. 2B and Table 1). Thus, in the absence of the cell wall, the actual levels of BLM uptake and cytotoxicity were 40 times (8 × 5) higher for the YPH-1 strain than for the FY67 strain. We have previously demonstrated the presence of a BLM-binding protein (BBP) that plays a role in the internalization and cytotoxicity of BLM in mammalian cells (11, 12). The procedure previously implemented to prepare this mammalian plasma membrane protein was repeated with yeast spheroplasts (11). As a result of the studies with the 57Co-BLM complex (9), we report here for the first time the detection of a BBP of approximately 250 kDa in the membrane extracts of yeast cells (Fig. 3). Quantification of the 57Co-BLM-BBP complex indicates that the intensity of the radioactive band was approximately seven times lower for the YPH-1 strain extract than for the FY67 strain extract (results not shown). Assuming that this protein is involved in the uptake and cytotoxicity of BLM in yeast cells like it is in animal cells (12), the 7-fold increase in BBP could explain the 40-fold difference in resistance between YPH-1 and FY67 spheroplasts. Indeed, in animal cells a 3-fold increase in the level of BBP was found to cause a 22-fold increase in cell sensitivity to BLM (12).
FIG. 3.
Visualization of the BBPs in crude yeast membrane extracts. Reported molecular weights correspond to the high-molecular-weight-range color markers (molecular weights, 29,000 to 205,000; Sigma) that migrated in parallel. The arrow indicates the position of the radioactive BBP band detected at about 250 kDa.
Furthermore, the filtering factor of the cell wall between both strains have ratios of 150 in the presence of the cell membrane (f′CW) and 3.4 in the absence of the cell membrane (fCW) (Table 1). This means that the cell membrane imposes an ≈40-fold (150/3.4 = 44.1) decrease in BLM uptake in the presence of the cell wall, similar to the decrease calculated for the spheroplasts. These results suggest that both barriers act independently.
In conclusion, this work demonstrates that both the cell wall and the cell membrane limit the uptake of small hydrophilic molecules (e.g., BLM) by yeast cells. The restrictions imposed by the cell wall and the plasma membrane are independent of each other but are cell type dependent. We also demonstrate that cell electropermeabilization is an interesting method that can be used to increase the levels of uptake of small hydrophilic molecules in yeast.
Acknowledgments
We thank P. Thuriaux for providing the yeast strains and Yann Lecluse (IFR 54) for evaluation of cell diameters by flow cytometry. We also thank Tracy Petzke and Rafael Davalos for critical linguistic reading of the paper.
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Institut Gustave-Roussy, the Association pour la Recherche contre le Cancer, and the Ministère de l'Enseignement Supérieur de Tunisie. We thank the CNRS (France) and DGRST (Tunisia) for travel support to M.A., O.T., and L.M.M. for scientific exchanges. L.M.M. also acknowledges the support of the EU commission (grant QLK3-1999-00484).
REFERENCES
- 1.Barre, F. X., L. M. Mir, Y. Lecluse, and A. Harel-Bellan. 1998. Highly efficient oligonucleotide transfer into intact yeast cells using square-wave pulse electroporation. BioTechniques 25:294-296. [DOI] [PubMed] [Google Scholar]
- 2.Birren, B., E. D. Green, S. Klapholzy, R. M. Myers, and J. Roskams. 1997. Genome analysis, a laboratory manual, vol. 1, p. 26-29. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Dielbandhoesing, S. K., H. Zhang, L. H. Caro, J. M. van der Vaart, F. M. Klis, C. T. Verrips, and S. Brul.1998. Specific cell wall proteins confer resistance to nisin upon yeast cells. Appl. Environ. Microbiol. 64:4047-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehl, J., T. Skovsgaard, and L. M. Mir. 1998. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs 9:319-325. [DOI] [PubMed] [Google Scholar]
- 5.Jaroszeski, M. J., V. Dang, C. Pottinger, J. Hickey, R. Gilbert, and R. Heller. 2000. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs 11:201-208. [DOI] [PubMed] [Google Scholar]
- 6.Mir, L. M., H. Banoun, and C. Paoletti. 1988. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: direct access to the cytosol. Exp. Cell Res. 175:15-25. [DOI] [PubMed] [Google Scholar]
- 7.Moore, C. W. 1994. Potentiation of bleomycin cytotoxicity in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 38:1615-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlowski, S., J. Belehradek, Jr., C. Paoletti, and L. M. Mir. 1988. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 37:4727-4733. [DOI] [PubMed] [Google Scholar]
- 9.Poddevin, B., J. Belehradek, Jr., and L. M. Mir. 1990. Stable [57Co]-bleomycin complex with a very high specific radioactivity for use at very low concentrations. Biochem. Biophys. Res. Commun. 173:259-264. [DOI] [PubMed] [Google Scholar]
- 10.Poddevin, B., S. Orlowski, J. Belehradek, Jr., and L. M. Mir. 1991. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochem. Pharmacol. 42(Suppl.):S67-S75. [DOI] [PubMed] [Google Scholar]
- 11.Pron, G., J. Belehradek, Jr., and L. M. Mir. 1993. Identification of a plasma membrane protein that specifically binds bleomycin. Biochem. Biophys. Res. Commun. 194:333-337. [DOI] [PubMed] [Google Scholar]
- 12.Pron, G., J. Belehradek, Jr., S. Orlowski, and L. M. Mir. 1994. Involvement of membrane bleomycin-binding sites in bleomycin cytotoxicity. Biochem. Pharmacol. 48:301-310. [DOI] [PubMed] [Google Scholar]
- 13.Sherman, F., G. R. Fink, and J. B. Hicks. 1986. Methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 14.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]