Abstract
The influence of reduced susceptibilities to glycopeptides on the activities of vancomycin and teicoplanin against an isogenic pair of clinical Staphylococcus aureus strains in experimental endocarditis was investigated. While vancomycin was similarly active against both strains, teicoplanin was approximately 100-fold less active against the resistant strain and selected for the emergence of more resistant subpopulations.
The emergence of Staphylococcus aureus strains with reduced susceptibilities to glycopeptides (glycopeptide-intermediate-resistant S. aureus [GISA]) in Japan (13), the United States (5; S. Fridkin, J. Hageman, M. Kellum, S. McAllister, J. Mohammed, and F. Tenover, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-1230, 2001), and Europe (17) has raised the question of the clinical relevance of this low level of resistance and of the efficacies of glycopeptides for the treatment of infections due to GISA (1-3, 6; M. Dudley, D. Griffith, E. Corcoran, C. Liu, K. Sorensen, V. Tembe, D. Cotter, S. Chamberland, and S. Chen, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2031, 1999). In order to investigate the in vivo therapeutic relevance of reduced susceptibilities to glycopeptides on the activities of vancomycin and teicoplanin, we used an isogenic pair of clinical methicillin-resistant S. aureus strains in a rabbit endocarditis model to yield a high inoculum at the start of therapy.
A pair of isogenic clinical strains isolated from a patient before and after treatment failure with vancomycin and teicoplanin was used: S. aureus Lim-1 was susceptible to vancomycin and teicoplanin, whereas Lim-2 had reduced susceptibilities to both glycopeptides (17). Cation-adjusted Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) was used for MIC determinations, and brain heart infusion (BHI) agar and broth were used for all other experiments (Difco, Detroit, Mich.). The antibiotics were provided by their respective manufacturers: teicoplanin by Aventis (Vitry sur Seine, France) and vancomycin by Lilly (Saint-Cloud, France). MICs were determined by the broth microdilution method in cation-adjusted Mueller-Hinton broth, according to the standards of the National Committee for Clinical Laboratory Standards (15). Population analysis was performed with overnight cultures of the isolates (∼108 to 109 CFU/ml), which were serially diluted and plated onto BHI agar containing increasing concentrations of vancomycin or teicoplanin and incubated for 48 h at 37°C before enumeration of the CFU. Time-kill curve studies were done in fresh BHI agar in 10-ml glass tubes to yield an inoculum of ∼106 CFU/ml. Vancomycin and teicoplanin were used at concentrations of 8, 16, and 32 μg/ml and 4, 20, and 50 μg/ml, respectively, in order to study the peak and trough concentrations achievable in serum during therapy. After 0, 3, 6, and 24 h of incubation at 37°C, serial dilutions of 100-μl samples were subcultured and incubated for 48 h at 37°C before enumeration of the CFU (16).
Aortic endocarditis was induced in New Zealand White female rabbits (weight, 2.2 to 2.5 kg) as described previously (10). Twenty-four hours after catheter insertion, each rabbit was inoculated via the ear vein with 106 CFU of S. aureus in 1 ml of sterile saline. Forty-eight hours after the inoculation, the rabbits were killed and served as control animals or were treated intramuscularly for 4 days with one of the following regimens: vancomycin at a standard dose of 40 mg/kg of body weight every 12 h, vancomycin at a high dose of 50 mg/kg every 8 h, teicoplanin at a standard dose of 20 mg/kg every 12 h after administration of a loading dose of 40 mg/kg, and teicoplanin at a high dose of 40 mg/kg every 12 h after administration of a loading dose of 80 mg/kg. The animals were killed 8 to 12 h after the last antibiotic injection. All the vegetations from each rabbit were excised, immediately put on ice after excision, rinsed in sterile saline, pooled, weighed, and homogenized in 1 ml of sterile water. Vegetation homogenates were diluted 10-fold and plated on agar to count the surviving bacteria after 24 h of incubation. These dilutions avoided any significant in vivo carryover (10). Since the stability of the glycopeptide intermediate resistance of S. aureus has been questioned (4), population analyses were performed ex vivo, as described above, with vegetation homogenates sampled 48 h after bacterial challenge to confirm the persistence of the in vivo expression of resistance by strain Lim-2 before the start of therapy. In addition, at the end of therapy, 0.1-ml vegetation homogenates were plated onto BHI agar containing 4 or 8 μg of vancomycin per ml and 8 or 32 μg of teicoplanin per ml for strains Lim-1 and Lim-2, respectively, to detect the emergence of subpopulations more resistant to glycopeptides. The agar plates were then incubated for 48 h at 37°C before enumeration of the CFU. MICs were determined for colonies growing on antibiotic-containing agar.
The concentrations in the sera of three uninfected rabbits were determined at 0, 0.25, 0.5, 1, 2, 4, 8, 12, and 24 h after a single injection of 40 or 50 mg of vancomycin per kg or 20 or 40 mg of teicoplanin per kg. In addition, concentrations in serum were determined at the time of killing of the animals with experimental endocarditis treated with teicoplanin, since a loading dose was given to those animals, and after the administration of a single dose in uninfected animals. Vancomycin and teicoplanin concentrations were measured by fluorescence polarization immunoassay. The sensitivities of the procedure were 2 and 1.7 μg/ml for vancomycin (Ax-SYM; Abbott) and teicoplanin (TDx; Abbott), respectively. The area under the concentration-time curve (AUC) was calculated by noncompartmental analysis. The WinNonLin program (Scientific Consulting, Inc., Apex, N.C.) was used to fit the data.
Variance analysis followed by the Fisher test for multiple comparisons was used to compare the bacterial counts in vegetations from groups of animals infected with the same strain and treated with various regimens.
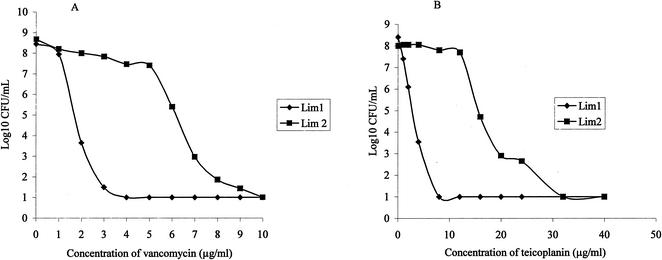
The MICs of vancomycin and teicoplanin were 2 and 4 μg/ml, respectively, for strain Lim-1, and 8 and 16 μg/ml, respectively, for strain Lim-2. Vancomycin and teicoplanin resistance was homogeneously expressed by Lim-2, as shown by population analysis (Fig. 1), while Lim-1 was fully susceptible to both glycopeptides.
FIG. 1.
In vitro population analysis of S. aureus Lim-1 and Lim-2 on vancomycin (A) and teicoplanin (B) agar. Population analyses were performed with overnight cultures (∼108 to 109 CFU/ml) that were serially diluted and plated onto BHI agar containing increasing concentrations of vancomycin (0 to 10 μg/ml) or teicoplanin (0 to 40 μg/ml) and incubated for 48 h at 37°C before enumeration of the CFU.
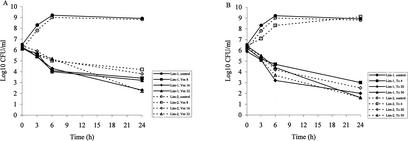
Concentrations of vancomycin ranging from 8 to 32 μg/ml produced comparable effects against strain Lim-1, with bacterial killing of approximately 2 to 2.5 log10 CFU/ml after 6 h of incubation and 3 to 4 log10 CFU/ml after 24 h of incubation (Fig. 2A). The time of killing of strain Lim-2 by vancomycin at the same concentrations was delayed compared to the time of killing of strain Lim-1, with a reduction of 1.0 to 1.3 log10 CFU/ml after 6 h of exposure for strain Lim-2. After 24 h of incubation, the intensity of the bacterial killing by vancomycin ranged between 2.1 and 4.0 log10 CFU/ml.
FIG. 2.
Time-kill curve studies with vancomycin (Vm) (A) and teicoplanin (Tc) (B) at various concentrations against S. aureus Lim-1 and Lim-2.
For teicoplanin, concentrations of 4, 20, and 50 μg/ml were bactericidal for strain Lim-1 after 24 h of incubation (Fig. 2B), while the last two concentrations were bactericidal against Lim-2. The bactericidal activities of teicoplanin at concentrations of 20 and 50 μg/ml were similar against both strains, with reductions of 3.9 and 4.8 log10 CFU/ml, respectively, after 24 h of incubation.
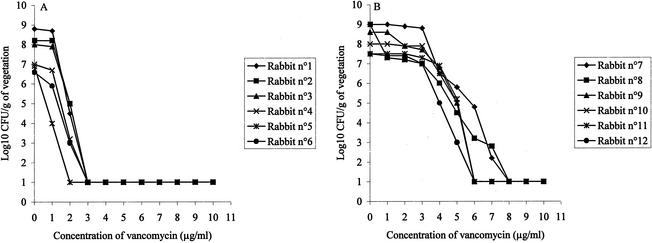
As in the in vitro experiments (Fig. 2), the growth rates of both strains were similar in vivo, and the aortic valve vegetations of the control animals yielded comparable concentrations of both strains 48 h after inoculation (Table 1). Population analysis performed ex vivo with cardiac vegetations infected with Lim-1 or Lim-2 before the start of therapy showed that the strains exhibited a pattern of resistance to vancomycin comparable to that observed in vitro (Fig. 1A), as shown in Fig. 3 for six individuals animals. While the entire Lim-1 population from the six different animals was inhibited by less than 4 μg of vancomycin per ml (Fig. 3A), 8 μg of vancomycin per ml was required to inhibit the growth of the entire population of Lim-2 from six different animals (Fig. 3B).
TABLE 1.
Efficacies of 4-day antibiotic regimens for treatment of experimental endocarditis due to S. aureus
| Treatment | Regimen | Mean ± log10 CFU/g of vegetation (no. of sterile vegetations/total no. of vegetations) for strain:
|
|
|---|---|---|---|
| Lim-1 | Lim-2 | ||
| None (start-of- therapy controls) | 9.4 ± 0.9 (0/14) | 9.2 ± 1.3 (0/11) | |
| Vancomycin | |||
| Standard dose | 40 mg/kg every 12 h | 7.0 ± 2.3a (1/8) | 6.5 ± 2.4a (1/8) |
| High dose | 50 mg/kg every 8 h | 6.2 ± 2.0a (2/10) | 5.8 ± 2.8a (2/8) |
| Teicoplanin | |||
| Standard dose | 20 mg/kg every 12 hb | 6.1 ± 2.4a (1/8) | 8.3 ± 1.9 (0/14) |
| High dose | 40 mg/kg q 12 hc | Not done | 7.0 ± 1.6a (0/9) |
P < 0.05 versus controls.
After a loading dose of 40 mg/kg.
After a loading dose of 80 mg/kg.
FIG. 3.
Ex vivo population analysis of S. aureus Lim-1 (A) and Lim-2 (B) on vancomycin agar. The two strains were from the vegetations of six different infected rabbits sampled at the start of therapy.
As shown in Table 2, the vancomycin and teicoplanin levels in the sera of uninfected animals were comparable to those achieved in humans with standard or high-dose regimens. The peak and trough levels of teicoplanin in the sera of infected animals were determined on the last day of therapy and were 44 ± 7 and 21 ± 5 μg/ml (n = 3), respectively, for the standard regimen and 85 ± 6 and 45 ± 7 μg/ml (n = 5), respectively, for the high-dose regimen. The 24-h AUC/MIC ratios for the different glycopeptide regimens ranged from 84 (standard dose against Lim-2) to 372 (high dose against Lim-1) for vancomycin and from 47 (standard dose against Lim-2) to 187 (standard dose against Lim-1) for teicoplanin. As shown in Table 1, the activities of vancomycin in experimental endocarditis were similar for rabbits infected with Lim-1 or Lim-2, regardless of the dosing regimen used. The standard dosage of vancomycin produced a reduction of 2.5 to 2.7 log10 CFU/g of vegetation compared to the initial bacterial titer, while the high dose produced a reduction of 3.2 to 3.4 log10 CFU/g of vegetation compared to the initial bacterial titer (P < 0.05). In contrast, the activities of teicoplanin against both strains differed significantly. While the standard dose of teicoplanin was as active as the standard dose of vancomycin against Lim-1 (P < 0.05 versus controls), the same dosage regimen was approximately 100-fold less active against Lim-2 and did not produce any significant reduction in bacterial counts in the vegetations compared to those in the vegetations from the controls. Use of a high-dose teicoplanin regimen was necessary to achieve a significant reduction in bacterial counts compared to the reduction in bacterial counts in the controls infected with Lim-2 (Table 1). No resistant subpopulation was detected during therapy with vancomycin, regardless of the strain or the dosing regimen tested. In contrast, when isolates from nine animals infected with Lim-2 and treated with a standard dose of teicoplanin were evaluated for the emergence of resistance, it was found that bacterial subpopulations of the isolates from two of the animals grew on agar containing 8 μg of vancomycin per ml and 32 μg of teicoplanin per ml. The MICs for the two resistant clones were 8 μg/ml for vancomycin and 32 μg/ml for teicoplanin.
TABLE 2.
Concentrations of vancomycin and teicoplanin in the sera of three uninfected rabbits after the intramuscular administration of a single antibiotic dose
| Regimen (dose [mg/kg]) | Concn (μg/ml) in serum (time [h])
|
|
|---|---|---|
| Peak | Trough | |
| Vancomycin (40) | 39 ± 12 (2) | 12 ± 3 (12) |
| Vancomycin (50) | 45 ± 8 (2) | 20 ± 5 (8) |
| Teicoplanin (20) | 44 ± 5 (4) | 24 ± 1 (12) |
| Teicoplanin (40) | 95 ± 17 (4) | 59 ± 21 (12) |
An important result from our study was that vancomycin was as effective against Lim-2 as it was against Lim-1, regardless of the dosing regimen used. However, it must be acknowledged that, for technical reasons, therapy lasted only 4 days. Because the emergence of GISA strains has often been detected in clinical practice after several weeks of antibiotic pressure (11), one cannot exclude the possibility that differences between Lim-1 and Lim-2 would be evidenced with a more prolonged duration of vancomycin therapy.
In contrast to the results obtained with vancomycin, a clear impact of the GISA phenotype was observed with teicoplanin both in terms of bactericidal activity (∼100-fold decrease activity) and in terms of the selection of subpopulations that became more resistant to glycopeptides. It is important that this result was achieved within a relatively short period of time and with concentrations in serum that were in accordance with those obtained in humans. High levels of protein binding and the poor diffusion of teicoplanin in cardiac vegetations have already been reported as possible pharmacokinetic causes of teicoplanin failure in experimental and clinical endocarditis (8, 10, 12, 14). In our study, teicoplanin treatment of an infection caused by a GISA strain for which the initial MIC was 16 μg/ml led in two animals to the selection of strains fully resistant to teicoplanin (32 μg/ml), according to the breakpoints (7, 15). Therefore, it can be anticipated that the widespread use of teicoplanin may favor the selection of strains with decreased susceptibilities to other glycopeptides.
From a pharmacodynamic-pharmacokinetic point of view, it must be indicated that all the dosing regimens were considered clinically relevant and associated with levels in serum exceeding the MIC during the entire course of therapy (Table 2). In terms of the 24-h AUC/MIC ratios, our results suggest that values of ∼80 were associated with effective regimens for vancomycin and might also be adequate for teicoplanin. Finally, our observations demonstrate that the higher MICs observed for teicoplanin compared with those observed for vancomycin for GISA strains (9; Fridkin et al. 41st ICAAC), which may be used in the laboratory for the detection of such strains (7), actually correspond to decreased in vivo activity.
REFERENCES
- 1.Aeschlimann, J. R., G. P. Allen, E. Hershberger, and M. J. Ryback. 2000. Activities of LY333328 and vancomycin administered alone or in combination with gentamicin against three strains of vancomycin-intermediate Staphylococcus aureus in an in vitro pharmacodynamic infection model. Antimicrob. Agents Chemother. 44:2991-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aeschlimann, J. R., E. Hershberger, and M. J. Ryback. 1999. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backo, M., E. Gaenger, A. Burkart, Y. L. Chai, and A. S. Bayer. 1999. Treatment of experimental staphylococcal endocarditis due to a strain with reduced susceptibility to vancomycin: efficacy of ampicillin-sulbactam. Antimicrob. Agents Chemother. 43:2565-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle-Vavra, S., S. Berke, J. C. Lee, and R. S. Daum. 2000. Reversion of the glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 44:272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 6.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comité de l'Antibiogramme de la Société Française de Microbiologie. 1998. Communiqué. Pathol. Biol. 46:1-16. [Google Scholar]
- 8.Crémieux, A. C., B. Mazière, J.M.Vallois, M. Ottaviani, A. Azancot, H. Raffoul, A. Bouvet, J. J. Pocidalo, and C. Carbon. 1988. Evaluation of antibiotic duffusion into cardiac vegetations by quantitative autoradiography. J. Infect. Dis. 159:938-944. [DOI] [PubMed] [Google Scholar]
- 9.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in United States, Canada, Latin America, Europe, and the Western Pacific region for the Sentry antimicrobial surveillance programme, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed]
- 10.Fantin, B., R. Leclercq, M. Arthur, J. Duval, and C. Carbon. 1991. Influence of low-level resistance to vancomycin on efficacy of teicoplanin and vancomycin for treatment of experimental endocarditis due to Enterococcus faecium. Antimicrob. Agents Chemother. 35:1570-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fridkin, S. K. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus. What the infectious disease specialist needs to know. Clin. Infect. Dis. 32:108-115. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, D. N., C. A. Wood, R.C. Kimbrough, and the Infectious Diseases Consortium of Oregon. 1991. Failure of treatment with teicoplanin at 6 milligrams/kilogram/day in patients with Staphylococcus aureus intravascular infection. Antimicrob. Agents Chemother. 35:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 14.Kaatz, G. W., S. M. Seo, N. J. Dorman, and S. A. Lerner. 1990. Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J. Infect. Dis. 162:103-108. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2001. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Pearson, R., R. T. Steigbigel, H. T. Davis, and S. W. Chapman. 1980. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis. 1998. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351:1212.. [DOI] [PubMed] [Google Scholar]