Abstract
Receipt of a broad-spectrum cephalosporin is a strong risk factor for isolation of broad-spectrum cephalosporin-resistant Enterobacter species, and yet the risk from other broad-spectrum β-lactams hydrolyzed by group 1 β-lactamases has not been well characterized. We compared the risk conferred by broad-spectrum cephalosporins to that conferred by piperacillin-tazobactam, alone or in combination with an aminoglycoside or a fluoroquinolone. A retrospective cohort was monitored from treatment onset until a broad-spectrum cephalosporin-resistant Enterobacter strain was isolated or the patient was discharged. There were 447 patients in the piperacillin-tazobactam group and 2,341 patients in the broad-spectrum cephalosporin group. Groups were similar in age (mean, 62.5 years). The piperacillin-tazobactam group had a smaller percentage of men (32% versus 44%, P < 0.001) and a lower rate of intensive care unit stay (25% versus 38%, P < 0.001) but a higher rate of surgery (41% versus 26%, P < 0.001). Groups differed in the distribution of comorbidities. Resistant Enterobacter strains were isolated from 62 patients, 2% in each group (hazard ratio [RR] = 1.02 [P = 0.95]). In multivariable analysis, risk was similar among treatment groups (RR = 0.71 [P = 0.32]). Intensive care unit stay and surgery were associated with increased risk (RR = 4.53 [P < 0.001] and RR = 1.97 [P = 0.015], respectively), fluoroquinolones were protective (RR = 0.24 [P = 0.003]), and aminoglycosides did not affect risk (RR = 0.98 [P = 0.95]). The protective effect of fluoroquinolones against isolation of broad-spectrum cephalosporin-resistant Enterobacter spp. and the equivalence in risk associated with piperacillin-tazobactam and broad-spectrum cephalosporins may have important clinical and epidemiologic implications.
Enterobacter spp. are among the most common gram-negative healthcare-associated pathogens. They are responsible for 5 to 7% of cases of nosocomial bacteremia and are the second most common gram-negative pathogen causing intensive care unit-associated pneumonia (11, 12). In recent years, Enterobacter strains have been implicated in community-acquired infections as well (13). Enterobacter isolates have a distinctive ability to develop broad-spectrum antimicrobial resistance during therapy, owing largely to their chromosomal group 1 β-lactamase gene, which facilitates the emergence, under antibiotic pressure, of highly resistant, stably derepressed mutants (3, 12, 13). These strains are resistant to broad-spectrum cephalosporins, a feature that complicates treatment of infections caused by them and that is associated with increased mortality, as well as other adverse clinical and economic outcomes (2, 4).
As Cosgrove et al. have noted, individuals harboring broad-spectrum cephalosporin-resistant Enterobacter strains actually comprise two different patient populations: (i) those from whom a resistant isolate is cultured initially and (ii) those with originally susceptible isolates in whom resistance emerges during therapy (4). The importance of distinguishing between these two populations has been illustrated by Carmeli et al., who demonstrated differing outcomes by population in patients infected with Pseudomonas aeruginosa (1). Chow et al. considered both of these populations in their study of Enterobacter bacteremia over a decade ago (2). These authors noted a connection between prior exposure to broad-spectrum cephalosporins and the isolation of multiresistant bloodstream isolates, an association not found with other individual classes of antibiotics. Chow et al. also noted the emergence of broad-spectrum cephalosporin resistance in 19% of isolates from patients receiving this class of antibiotic.
The prospective nature of Chow et al.'s study, however, resulted in a sample size of only 129 patients, of whom only 37 had a resistant isolate on initial culture, and only 6 of whom had an initially susceptible isolate in which broad-spectrum cephalosporin resistance emerged. Their study, therefore, had limited power to explore the question of risk factors for emergence of resistance and for initial isolation of a resistant isolate. Kaye et al. recently reported results from an analysis of a retrospective cohort of 477 patients with initially susceptible Enterobacter isolates in an attempt to determine risk factors for emergence of broad-spectrum cephalosporin resistance (9). These authors found that broad-spectrum cephalosporins were an independent risk factor for the emergence of resistance, whereas fluoroquinolones were protective.
Following the report of Kaye et al., we conducted a matched case-control study exploring risk factors for isolation of broad-spectrum cephalosporin resistant gram-negative nosocomial pathogens (M. J. Schwaber, S. E. Cosgrove, H. S. Gold, K. S. Kaye, and Y. Carmeli, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-1182, 2001). In contrast to the results of Kaye et al., we found ureidopenicillins and β-lactam-β-lactamase inhibitor combinations to be equal in importance to broad-spectrum cephalosporins as risk factors for isolation of resistant Enterobacter spp. in a subgroup analysis. We hypothesize that the discrepancy between our findings and those of Kaye et al. results from differing risk factors for the emergence of resistance in a previously susceptible isolate and the isolation of an already-resistant isolate. It is possible, for example, that broad-spectrum cephalosporins are more selective than ureidopenicillins and β-lactam-β-lactamase inhibitor combinations for derepressed mutants but that, once these mutants are selected, since they are resistant to all three classes of antimicrobials, equivalent selective pressure is provided by all of them.
We conducted this study in order to compare directly the extent of risk of isolation of an already-resistant isolate conferred by piperacillin-tazobactam with that conferred by a broad-spectrum cephalosporin. We did not distinguish between patients from whom Enterobacter was never before isolated and those who may have harbored a susceptible isolate prior to enrollment in the study, since Enterobacter can be a component of normal endogenous flora. Our focus was on the initial culture of a resistant isolate after enrollment. Piperacillin-tazobactam was chosen as the agent of comparison since it is a newer, broad-spectrum ureidopenicillin-β-lactamase combination that, like broad-spectrum cephalosporins, provides broad coverage of Enterobacteriaceae. We also attempted to determine the effect of combination therapy with either an aminoglycoside or a fluoroquinolone upon initial isolation of broad-spectrum cephalosporin-resistant Enterobacter strains.
MATERIALS AND METHODS
Hospital setting, study design, and microbiology.
This study was performed at the Beth Israel Deaconess Medical Center, West Campus, a 320-bed, urban, tertiary-care teaching hospital serving a nonobstetric adult population in Boston, Mass. It has 24 intensive care unit beds and about 12,000 admissions annually. The study database was assembled from the records of patients hospitalized at this institution from 1 November 1 1994 through 31 December 1997. Data were collected from administrative, laboratory, and pharmacy databases by using relational database software (Access97; Microsoft, Redmond, Wash.) and were linked by a unique patient identifier. The study was designed as a retrospective cohort study.
The pharmacy database was searched to identify all patients treated with either piperacillin-tazobactam (typical dose, 3 g of piperacillin and 0.375 g of tazobactam every 6 h [q6]) or a broad-spectrum cephalosporin (ceftazidime at a typical dose of 1 g q8 or ceftriaxone at a typical dose of 1 g q24). Patients were included in the study from the day of the start of treatment with either agent and were monitored until the isolation of broad-spectrum cephalosporin-resistant Enterobacter from a clinical culture. Resistance was defined as an MIC of ceftazidime or ceftriaxone of ≥16 μg/ml. Observations were censored at hospital discharge, death, or a switch to the alternate study antibiotic. Patients from whom resistant Enterobacter strains were isolated prior to the start of one of the study agents were excluded.
Clinical data collected on study subjects included demographics (age and sex), comorbidities (cardiovascular disease, diabetes mellitus, malignancy, hepatic disease, renal disease, pulmonary disease, AIDS, and a history of transplantation), hospital events (transfer from another facility, duration of stay prior to study entry, number of days in the study, surgery, and intensive care unit stay), and antimicrobial exposures (fluoroquinolones [predominantly ciprofloxacin and ofloxacin, typically dosed twice daily] and aminoglycosides [predominantly gentamicin, typically dosed three times daily]). The route of administration of the antibiotics was not considered, since for all except the fluoroquinolones the route was parenteral, and for fluoroquinolones the bioavailablility was considered equivalent for oral and parenteral administration.
Statistical analysis.
Statistical analyses were performed by using SAS software (version 8e; SAS Institute, Inc., Cary, N.C.). Univariate analysis was performed comparing individual characteristics of the cohort by antibiotic group (broad-spectrum cephalosporins versus piperacillin-tazobactam). Categorical data were compared by using the chi-square test. Continuous data were compared via either the two-sample Student t test for equal variances or the Wilcoxon rank-sum test, depending on the normality of the distribution.
The log-rank test was performed, and a Kaplan-Meier curve was generated, comparing the proportion of the study population free of resistant Enterobacter over time, stratified by antibiotic group. An unadjusted Cox proportional hazards model was run, comparing the time to isolation of resistant Enterobacter strains by antibiotic group. The effect estimate was recorded as the hazard ratio (RR). The β-coefficient from the regression model was recorded.
Purported confounders were evaluated by introducing each studied covariate individually in a bivariable Cox proportional hazards model, including the antibiotic group. In addition, we constructed bivariable models comparing the effect of intensive care unit exposure, receipt of a fluoroquinolone, and receipt of an aminoglycoside as time-dependent variables in each antibiotic group. The variables that caused a change of >10% in the β-coefficient of the “antibiotic group” covariate were considered confounders and included in the multivariable model-building algorithm.
We constructed a multivariable Cox regression model by including each of the identified confounders of the effect of antibiotic group in a forward selection algorithm, the robustness of which was then confirmed via backward elimination and stepwise selection. Once the statistically significant covariates were identified, our final model was assembled by incorporating these covariates into a multiple Cox regression model with the covariate of antibiotic group forced in.
We evaluated our model for effect modification by constructing interaction terms between antibiotic group and each of the other covariates in the multivariable model. We tested the validity of the proportional hazards assumption by constructing interaction terms between the time variable and each of the covariates in the multivariable model, incorporating each interaction term separately into individual Cox regression models that included all of the terms in our final model. For all statistical tests, a P value of ≤0.05 was considered significant.
RESULTS
A total of 2,788 inpatients were eligible for study by virtue of treatment with either piperacillin-tazobactam (n = 447) or a broad-spectrum cephalosporin (n = 2,341) during the period of observation. Baseline characteristics of this cohort are presented in Table 1. Resistant Enterobacter strains were isolated from 11 patients in the piperacillin-tazobactam group and from 51 patients in the broad-spectrum cephalosporin group. Distribution of species and sites of isolation were similar in each antibiotic group: ca. 80% of the isolates were E. cloacae, and the remainder were E. aerogenes, and the predominant site of isolation was the respiratory tract, which accounted for approximately one-half of the isolates. Other sites of isolation included urine, wounds, blood, and peritoneal fluid.
TABLE 1.
Descriptive characteristics of cohort and univariate analysis
| Variablea | Entire cohort (n = 2,788) | Patients on piperacillin- tazobactam (n = 447) | Patients on broad- spectrum cephalosporin (n = 2,341) | P |
|---|---|---|---|---|
| Demographics | ||||
| Mean age (yr) ± SD | 62.5 ± 16.3 | 62.0 ± 15.3 | 62.6 ± 16.5 | 0.52 |
| No. of males (%) | 1,174 (42) | 145 (32) | 1,029 (44) | <0.001 |
| Comorbidities (n [%]) | ||||
| Cardiovascular disease | 1,995 (72) | 344 (77) | 1,651 (71) | 0.006 |
| Diabetes mellitus | 1,305 (47) | 294 (66) | 1,011 (43) | <0.001 |
| Malignancy | 438 (16) | 53 (12) | 385 (16) | 0.015 |
| Liver disease | 320 (11) | 45 (10) | 275 (12) | 0.31 |
| Renal disease | 419 (15) | 72 (16) | 347 (15) | 0.49 |
| Lung disease | 452 (16) | 46 (10) | 406 (17) | <0.001 |
| AIDS | 166 (6) | 14 (3) | 152 (6) | 0.006 |
| Organ transplant | 142 (5) | 34 (8) | 108 (5) | 0.008 |
| Hospital exposures | ||||
| Median length of hospital stay (days) before entry into cohort (interquartile range) | 1 (0-4) | 1 (0-4) | 1 (0-4) | 0.36 |
| Median no. of days in cohort (interquartile range) | 6 (3-11) | 7 (4-13) | 6 (3-11) | 0.007 |
| No. of transfers (%) from other institutions | 806 (29) | 137 (31) | 669 (29) | 0.38 |
| Intensive care unit stay (n [%]) | 995 (36) | 111 (25%) | 884 (38) | <0.001 |
| Surgical procedure (n [%]) | 798 (29) | 183 (41%) | 615 (26) | <0.001 |
| Exposure to other antibiotics (n [%]) | ||||
| Aminoglycosides | 409 (15) | 63 (14) | 346 (15) | 0.71 |
| Fluoroquinolones | 373 (13) | 69 (15) | 304 (13) | 0.16 |
| Resistant Enterobacter (n [%]) | 62 (2) | 11 (2) | 51 (2) | 0.71 |
n = number of isolates.
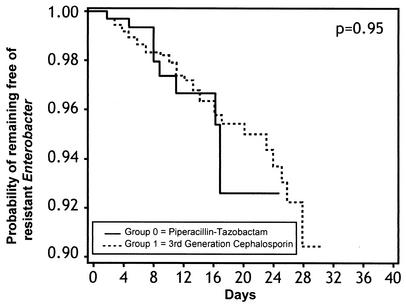
Groups were similar in age (mean, 62.5 years). The proportion of patients from whom resistant Enterobacter strains were isolated did not differ among the treatment groups (P = 0.71 [chi-square analysis]). Of the variables examined, cardiovascular disease, diabetes, organ transplantation, larger number of days in the cohort, and having had a surgical procedure were significantly associated with piperacillin-tazobactam use, whereas male sex, malignancy, lung disease, AIDS, and having had an intensive care unit stay were significantly associated with broad-spectrum cephalosporin use. Estimates of risk for isolation of resistant Enterobacter did not differ between the group treated with a broad-spectrum cephalosporin and the group receiving piperacillin-tazobactam (RR = 1.02, 95% confidence interval [CI] = 0.53 to 1.96 [P = 0.95]; Fig. 1).
FIG. 1.
Kaplan-Meier curve comparing the probability of remaining free of resistant Enterobacter over time in two antibiotic treatment groups.
We constructed a multivariable model to adjust for confounding variables. The following variables were identified as confounders of the effect of antibiotic group on the outcome, and introduced into the model selection algorithm: sex, diabetes mellitus, liver disease, renal disease, organ transplantation, transfer from another institution, surgical procedure, length of stay before enrollment, admission to an intensive care unit, and use of a fluoroquinolone. In the final model, intensive care unit stay was found to be a strong risk factor for isolation of resistant Enterobacter (RR = 4.53, 95% CI = 2.18 to 9.40 [P < 0.001]). Having had a surgical procedure also increased the risk of isolation of resistant Enterobacter (RR = 1.97, 95% CI = 1.14 to 3.40 [P = 0.015]). Fluoroquinolone use, in contrast, was protective (RR = 0.24, 95% CI = 0.09 to 0.62 [P = 0.003]). Study antibiotic group (i.e., broad-spectrum cephalosporin versus piperacillin-tazobactam) neither increased nor reduced risk in multivariable analysis (RR = 0.71, 95% CI = 0.37 to 1.39 [P = 0.32]). Aminoglycoside use, when forced into the multivariable model, likewise neither increased nor decreased risk (RR = 0.98, 95% CI = 0.52 to 1.85 [P = 0.95]).
Introducing individual interaction terms for each variable in the model revealed no significant effect modification between antibiotic group and the covariates found to be predictive of the outcome. The proportional hazards assumption was determined to be valid for each term in the model.
DISCUSSION
Enterobacter species are increasingly common hospital-associated pathogens (13). All Enterobacter isolates have a chromosomal group 1 β-lactamase which, expressed in large quantity, can hydrolyze expanded-spectrum cephalosporins and penicillins, including broad-spectrum cephalosporins and piperacillin. These enzymes are not inhibited by β-lactamase inhibitors available for clinical use. Broad-spectrum cephalosporin resistance is a common finding among Enterobacter isolates, affecting approximately one-third of strains associated with nosocomial infections in intensive care unit patients in a recent survey of U.S. hospitals (10). Moreover, resistance emerges frequently during treatment, and when it emerges in a susceptible isolate it is associated with increased mortality, lengthier hospital stays, and increased hospital charges (3, 4).
The link between broad-spectrum cephalosporin use and isolation of Enterobacter strains resistant to this class of antibiotic was demonstrated over a decade ago by Chow et al. (2). In their prospective study of 129 patients with Enterobacter bacteremia, these authors found 29% of isolates to be multiresistant. Patients who had received any antibiotics in the 2 weeks preceding culture were significantly more likely to have a resistant isolate and, among this subgroup, patients whose regimen included broad-spectrum cephalosporins were more likely to have a multiresistant isolate than were patients whose antimicrobial regimen did not. No similar association was shown for any other inidividual antibiotic class.
In the years since the publication of the report by Chow et al., additional broad-spectrum antimicrobial agents have emerged that are effective against gram-negative nosocomial pathogens. Notable among these is the β-lactam-β-lactamase inhibitor combination piperacillin-tazobactam. This agent might be used interchangeably with a broad-spectrum cephalosporin as part of broad-spectrum empirical treatment of infection, prophylactic coverage, or targeted therapy for an identified pathogen. By comparing the risk of this agent with that of broad-spectrum cephalosporins for isolation of broad-spectrum cephalosporin-resistant Enterobacter, we hoped to determine whether broad-spectrum cephalosporins represent a unique risk for isolation of multiresistant strains and, as such, should be subject to particularly stringent safeguards, or whether this risk applies to other agents as well, that can lead to resistance either by induction or, more likely in this case, selection of derepressed mutants (6).
In addition to the report by Chow et al., a number of more recent studies framed the background for our analysis. Kaye et al. applied analytical methods to a retrospective cohort that enabled a comprehensive investigation of risk factors for the emergence of resistance to broad-spectrum cephalosporins (9). These authors found that therapy with an antibiotic from this class was strongly associated with emergence of resistance, although this association did not hold for patients who received both broad-spectrum cephalosporins and either an aminoglycoside or imipenem, agents that are not hydrolyzed by the chromosomal group 1 β-lactamase of Enterobacter. In addition, Kaye et al. found no association between the emergence of broad-spectrum cephalosporin resistance and exposure to narrow- and extended-spectrum cephalosporins, ureidopenicillins, or β-lactam-β-lactamase inhibitor combinations. Finally, these authors found fluoroquinolone therapy to be protective against emergence of broad-spectrum cephalosporin resistance.
Although the study by Kaye et al. provided statistical power lacking in the initial bacteremia study by Chow et al., it addressed only one of the two patient populations with multiresistant Enterobacter infections cited by Cosgrove et al. (4): those with isolates in which resistance emerges during treatment. The second population, those whose initial isolates are resistant, were examined in an earlier study as part of a cohort of patients with cultures positive for group 1 β-lactamase-producing organisms (8). While that study did note an association between resistance and piperacillin use (as well as broad-spectrum cephalosporin use), it antedated the widespread use of piperacillin-tazobactam, and no data were presented on fluroquinolone use. Moreover, the choice of reference group likely introduced bias into the results (7). The association of antecedent antimicrobial use and initial isolation of broad-spectrum cephalosporin-resistant Enterobacter thus remained to be studied in a comprehensive manner.
We recently reported on the risks and protective factors in isolation of the three most common broad-spectrum cephalosporin-resistant gram-negative nosocomial pathogens: Enterobacter spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae (Schwaber et al., 41st ICAAC). We found that exposure to broad-spectrum cephalosporins was a risk factor, as was exposure to β-lactam-β-lactamase inhibitor combinations and ureidopenicillins. In addition, surgery and intensive care unit exposure during hospitalization were independent risk factors, and fluoroquinolones were protective. Subgroup analysis of the Enterobacter isolates alone yielded similar results.
The present study takes a closer look at isolation of initially resistant Enterobacter spp. Although differences exist in the underlying comorbidities, as well as in the hospital events, between patients who received piperacillin-tazobactam and those who received broad-spectrum cephalosporins, there was no difference between the groups in the proportion from whom resistant Enterobacter strains were isolated (2% in each). After controlling for the background differences between the two groups of patients, we found that the risk conferred by piperacillin-tazobactam was similar to that conferred by broad-spectrum cephalosporins for isolation of resistant Enterobacter.
Although molecular typing data were not available in this large retrospective cohort, an earlier study done at the same institution during our study period demonstrated that gram-negative bacilli in intensive care units during a nonoutbreak period involved diverse strains (5). That intensive care unit stay and surgery conferred added risk for isolation of multiresistant Enterobacter is consistent with findings from other studies (13; Schwaber et al., 41st ICAAC). Again, we found that fluoroquinolones represent an independent protective factor against de novo isolation of broad-spectrum cephalosporin-resistant Enterobacter.
This last finding complements that of Kaye et al. regarding the protective effect of fluoroquinolones on the emergence of resistance. In the present study, we found that flouroquinolones protect also against de novo isolation of resistant Enterobacter. Our results add strength to the findings that we reported in our earlier study (Schwaber et al., 41st ICAAC). In that study, we could not rule out the possibility that fluoroquinolones appeared to be protective against the emergence of resistance simply because they were given in lieu of broad-spectrum cephalosporins. However, in the present study, fluoroquinolones, when given, were administered in addition to either piperacillin-tazobactam, or a broad-spectrum cephalosporin. Use of an aminoglycoside with one of these agents, unlike the use of a fluoroquinolone, did not confer protection against isolation of multiresistant Enterobacter strains. This discrepancy cannot be explained by coselection of resistance, since a survey of Enterobacter isolates at our institution at the end of our study period demonstrated 100% susceptibility to gentamicin. At the same time, 93% of these isolates were susceptible to ciprofloxacin. We hypothesize that fluoroquinolones were protective in circumstances in which aminoglycosides were not due to their differences in penetration of the respiratory tract, where more than half of the Enterobacter species were found.
The selection of appropriate antibiotic therapy in the inpatient setting remains a challenge for the physician, who must balance the need for prevention or eradication of infection against the selection of resistant pathogens with potentially deleterious consequences for the patient. Our study indicates that there is no difference in risk of de novo isolation of broad-spectrum cephalosporin-resistant Enterobacter strains in hospitalized patients receiving either piperacillin-tazobactam or a broad-spectrum cephalosporin. Fluoroquinolones, given in addition to one of these agents, confer protection.
The protective effect of fluoroquinolones may be offset, however, by the potential risk for the selection of fluroquinolone-resistant pathogens as the result of an increase in the use of fluoroquinolones by hospitals attempting to reduce their burden of broad-spectrum β-lactam-resistant Enterobacter species. Should such a scenario occur, neutralizing the utility of fluoroquinolones in the treatment of serious gram-negative infections, the net effect of more widespread use of fluoroquinolones on hospital wards and intensive care units may be a reduced ability to treat the sickest patients. Alternatively, the enhanced use of fluoroquinolones may lead to a lower burden of β-lactam-resistant hospital pathogens, thereby ultimately diminishing the incidence of multidrug-resistant infections and the need for fluoroquinolones to treat them. The role of fluoroquinolones in preventing the spread of broad-spectrum cephalosporin resistance by organisms carrying chromosomal group 1 β-lactamases, the potential for formulary interventions that would replace broad-spectrum cephalosporins with fluoroquinolones in order to reduce resistance, the issue of whether widespread use of fluoroquinolones in the hospital setting will be associated with an increased prevalence of fluoroquinolone-resistant pathogens, and the ultimate effect of such use on our ability to fight healthcare-associated gram-negative infections are issues for further investigation.
Acknowledgments
M.J.S. was supported in part by the Centers for Disease Control and Prevention Postdoctoral Fellowship Training Program in Infectious Diseases (TO1/CCU111438) and is the recipient of a fellowship from the American Physicians Fellowship for Medicine in Israel.
REFERENCES
- 1.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127-1132. [DOI] [PubMed] [Google Scholar]
- 2.Chow, J. W., M. J, Fine, D. M. Shlaes, J. P. Quinn, D. C. Hooper, M. P. Johnson, R. Ramphal, M. M. Wagener, D. K. Miyashiro, and V. L. Yu. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 115:585-590. [DOI] [PubMed] [Google Scholar]
- 3.Chow, J. W., V. L. Yu, and D. M. Shlaes. 1994. Epidemiologic perspectives on Enterobacter for the infection control professional. Am. J. Infect. Control 22:195-201. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulous, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 5.D'Agata, E., L. Venkataraman, P. DeGirolami, and M. Samore. 1997. Molecular epidemiology of acquisition of ceftazidime-resistant gram-negative bacilli in a nonoutbreak setting. J. Clin. Microbiol. 10:2602-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein, F. W. 2002. Cephalosporinase induction and cephalosporin resistance: a longstanding misinterpretation. Clin. Microbiol. Infect. 8:823-825. [DOI] [PubMed] [Google Scholar]
- 7.Harris, A. D., T. B. Karchmer, Y. Carmeli, and M. H. Samore. 2001. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin. Infect. Dis. 32:1055-1061. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson, K. L., S. H. Cohen, J. F. Inciardi, J. H. King, W. E. Lippert, T. Iglesias, and C. J. VanCouwenberghe. 1995. The relationship between antecedent antibiotic use and resistance to extended-spectrum cephalosporins in group I β-lactamase-producing organisms. Clin. Infect. Dis. 21:1107-1113. [DOI] [PubMed] [Google Scholar]
- 9.Kaye, K. S., S. Cosgrove, A. Harris, G. M. Eliopoulos, and Y. Carmeli. 2001. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob. Agents Chemother. 45:2628-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Nosocomial Infections Surveillance Syst. 2000. National Nosocomial Infections Surveillance (NNIS) System report: data summary from January 1992 to April 2000. Am. J. Infect. Control 28:429-448. [DOI] [PubMed] [Google Scholar]
- 11.National Nosocomial Infections Surveillance Syst. 1999. National Nosocomial Infections Surveillance (NNIS) System report: data summary from January 1990 to May 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 12.Pitout, J. D., E. S. Moland, C. C. Sanders, K. S. Thomson, and S. R. Fitzsimmons. 1997. Beta-lactamases and detection of beta-lactam resistance in Enterobacter spp. Antimicrob. Agents Chemother. 41:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders, W. E., Jr., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]