Abstract
An ultraviolet-B (UV-B)-resistant mutant, uvi1 (UV-B insensitive 1), of Arabidopsis was isolated from 1,280 M1 seeds that had been exposed to ion beam irradiation. The fresh weight of uvi1 under high-UV-B exposure was more than twice that of the wild type. A root-bending assay indicated that root growth was less inhibited by UV-B exposure in uvi1 than in the wild type. When the seedlings were grown under white light, the UV-B dose required for 50% inhibition was about 6 kJ m−2 for the wild type and 9 kJ m−2 for uvi1. When the seedlings were irradiated with UV-B in darkness, the dose required for 50% inhibition was about 1.5 kJ m−2 for the wild type and 4 kJ m−2 for uvi1. An enzyme-linked immunosorbent assay showed that the reduction in levels of cyclobutane pyrimidine dimers (CPDs) under white light and of (6-4) photoproducts in darkness occurred faster in uvi1 than in the wild type. These results indicate that uvi1 had increased photoreactivation of CPDs and dark repair of (6-4) photoproducts, leading to strong UV-B resistance. Furthermore, the transcript levels of PHR1 (CPD photolyase gene) were much higher in uvi1 than in the wild type both under white light and after UV-B exposure. Placing the plants in the dark before UV-B exposure decreases the early reduction of CPDs in the wild type but not in uvi1. Our results suggest that UVI1 is a negative regulator of two independent DNA repair systems.
Reduction in the stratospheric ozone layer increases the amount of UV-B radiation (290–320 nm) that reaches the earth's surface (McKenzie et al., 1999). The effects of UV-B on plants include damage to DNA, proteins, membranes, and other cellular components, eventually leading to decreased productivity (Jansen et al., 1998). DNA is a sensitive target molecule because it absorbs UV-B efficiently and undergoes phototransformations that lead to the formation of toxic cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts (Sancar and Sancar, 1988; Mitchell and Nairn, 1989). Enhanced solar UV-B has been shown to cause significant increases in DNA damage as well as plant growth inhibition in Gunnera magellanica (Rousseaux et al., 1998, 1999). In a UV-sensitive Japanese rice (Oryza sativa) strain, UV sensitivity was found to be the result of a structure/function alteration of photolyase, an enzyme involved in DNA photorepair (Hidema et al., 2000). An understanding of DNA repair systems in plants may help to improve UV-B tolerance in cultivated species.
Many recent studies have been conducted on UV-induced DNA damage and repair in higher plants (Britt, 1999). DNA repair systems in plants are divided broadly into two categories: photoreactivation, catalyzed by two distinct photolyase activities, and light-independent (dark) repair pathways. In addition, there are mechanisms that protect against DNA damage by filtering UV-B by leaf waxes (Caldwell et al., 1983; Li et al., 1993; Strid et al., 1994) and UV-B absorbing by phenylpropanoid compounds (Bharti and Khurana, 1997; Mazza et al., 2000).
The Arabidopsis plant is thought to have both photoreactivation and dark repair systems. To date, many UV-B-sensitive mutants have been isolated and analyzed. These include mutants hypersensitive to UV-B and/or ionizing radiation (Harlow et al., 1994; Jenkins et al., 1995; Vonarx at al., 1998), dark or photorepair mutants (Jiang et al., 1997a, 1997b). As a result, two genes, namely the genes for CPD photolyase (Landry et al., 1997) and (6-4) photolyase (Nakajima et al., 1998), which function in photoreactivation, have been isolated. Rad1and Rad2 homologous genes that encode nucleotide excision repair endonucleases have also been isolated (Gallego et al., 2000; Liu et al., 2000, 2001). Recently, a dominant mutant with increased UV-absorbing compounds was described and shown to tolerate normally lethal levels of UV-B (Bieza and Lois, 2001). Thus, identifying Arabidopsis mutants with increased or decreased UV-B resistance can help to analyze both repair and protective mechanisms in higher plants.
In this study, we attempted to produce UV-B-resistant mutants of Arabidopsis. We used an ion beam as the mutagen because its high-linear energy transfer (compared with other forms of radiation) gives it a superior ability to create mutations (Tanaka et al., 1997b; Hase et al., 2000; Shikazono et al., 2001). We describe the isolation and characterization of a new Arabidopsis mutant (uvi1) that is more resistant to UV-B than the wild type.
RESULTS
Isolation of UV-Resistant Mutant
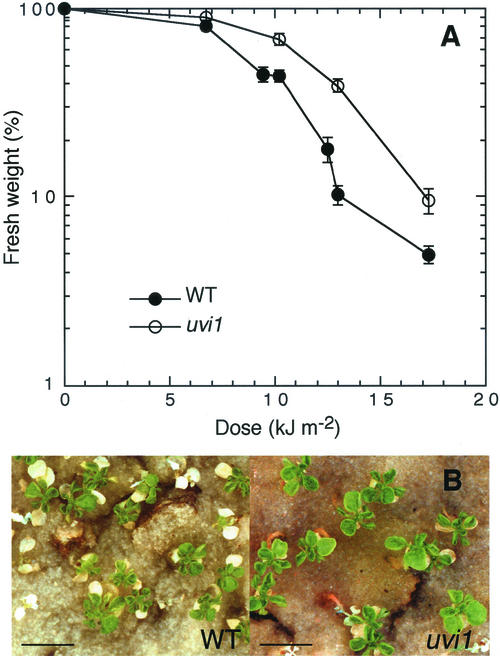
A total of 1,280 dry Arabidopsis seeds were irradiated with an ion beam and then germinated. After flowering, the plants were self-pollinated and the M2 seeds were harvested. Ten-day-old seedlings were exposed to UV-B and the fastest growing plants were selected. To confirm genetic stability of UV-B resistance, the candidates of the M2 through M4 generations were tested for UV-B resistance by both a long-term exposure assay and a root-bending assay. In the M5 generation, four mutant lines, named UV insensitive (uvi), were established to be UV-B-resistant mutants. Among them, uvi1 was used for further study. Under normal growth conditions (16-h photoperiod at 23°C, no UV-B), the mutation in uvi1 affected the plant weight and size. The mean fresh weight, height, length of rosette leaves, and root length (7 d after imbibition on vertical agar plate) were 39.9 ± 3.7 mg (n = 37), 24.1 ± 1.1 cm, 15.9 ± 1.4 mm, and 16.3 ± 1.5 mm (n = 10) in the wild type and 18.9 ± 2.6 mg (n = 25), 18.9 ± 0.4 cm, 10.0 ± 1.3 mm, and 9.2 ± 0.2 mm (n = 10) in uvi1. Other phenotypic traits, such as number of rosette leaves, flowering time, and leaf and root morphology, were normal in uvi1. Although the fresh weight of uvi1 under white-light conditions (no UV-B) was less than that of the wild type, the opposite was true after 1 month of irradiation with a high dose of UV-B (Fig. 1).
Figure 1.
Effects of UV-B exposure on the growth of wild type and uvi1 seedlings. A, UV-B dose-response curves for plant growth (fresh weight) in the wild type and uvi1. Plants were grown in 16-h photoperiod. Ten-day-old seedlings were exposed to the indicated daily doses of UV-B for 20 d. Points represent the mean of 21 to 25 plants. Error bars are se. B, Effects of UV-B on the aerial parts of the wild-type (left) and uvi1 (right) seedlings. Ten-day-old seedlings were exposed to UV-B (daily dose = 13 kJ m−2) for 20 d. The scale bar indicates 10 mm.
For a segregation analysis, uvi1 was backcrossed with the wild type. F1 progeny were selfed and the resulting F2 progenies were scored for segregation of uvi1. Of 232 F2 plants whose aerial parts were subjected to long-term exposure to UV-B, 53 showed the uvi1 phenotype and the remaining 179 showed the wild-type phenotype. This gives a segregation ratio of 3:1 (χ2 = 0.47, P > 0.30). Another 111 F2 families (at least 20 F3 plants per family) were also tested for segregation under UV-B applied against a background of white light or darkness and using the root-bending assay. Of these 111 families, 24 showed the uvi1 phenotype both under light and dark conditions, whereas in the other 87 families, all or most of the F3 plants showed a wild-type phenotype under either condition. These results indicate that uvi1 has a single recessive mutation. The linkage between the uvi1 mutation and 22 simple sequence length polymorphism and cleaved-amplified polymorphic sequence markers was analyzed. The uvi1 mutation was found to be linked to each of the markers on chromosome 4 (nga8 and Det1) but not to any of the other markers. The uvi1 mutation was found to be 29.1 ± 9.3 cM from nga8 and 25.9 ± 8.5 cM from Det1.
DNA Repair and Photoprotective Components in uvi1
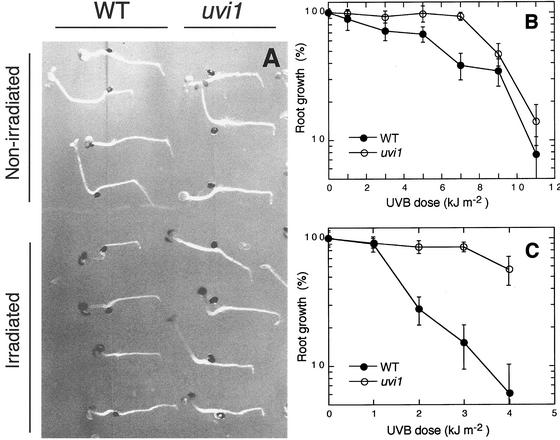
Photoreactivation and dark repair are known to act as repair systems for UV-damaged DNA in plants. To investigate the photoreactivation and dark repair activities of the uvi1 mutant, a root-bending assay was carried out (Fig. 2). Under white light (Fig. 2B), the root growth of uvi1 was more resistant to UV-B irradiation than that of the wild type. The dose required for 50% inhibition of growth was about 6 kJ m−2 for the wild type and about 9 kJ m−2 for uvi1. When the irradiations with UV-B were performed in darkness, the differences between uvi1 and wild-type seedlings in resistance to UV-B were even more marked (Fig. 2, A and C). The dose required for 50% inhibition of growth was about 1.5 kJ m−2 for the wild type and about 4 kJ m−2 for uvi1.
Figure 2.
Inhibition of root growth by UV-B. A, Root-bending assay of wild type and uvi1 seedlings exposed to a single, approximately 11-min pulse of UV-B (3 kJ m−2) against a background of total darkness. For a mock control (nonirradiated), seedlings on the same plate were covered with aluminum foil opaque to UV-B. Although root growth in uvi1 was slower than in the wild type under control (i.e. nonirradiated) conditions, it was hardly affected by exposure to UV-B at 3 kJ m−2. B and C, UV-B dose response curves for root growth in the wild type (●) and uvi1 (○) under light and dark conditions. Three-day-old seedlings grown under continuous white light were exposed to a single pulse of the indicated doses of UV-B. After the UV-B exposure, plants were incubated for 3 d in the light (B) or in the dark (C), and new root growth was measured. The results represent an average of measurements made on 10 to 15 seedlings. Error bars are se.
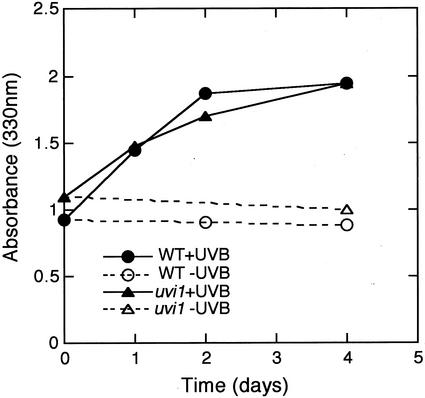
To determine whether the difference in UV-B resistance was because of the accumulation of UV-absorbing compounds, leaves of uvi1 and wild-type plants were exposed to UV-B and then the absorbance of the leaf extracts was measured at 330 nm (Fig. 3). In both the wild type and uvi1, the UV-B-absorbing compounds increased after 1 d of UV-B exposure, and were about 2-fold greater after 4 d of irradiation. No significant differences between uvi1 and wild-type plants were found in sunscreen accumulation.
Figure 3.
Accumulation of UV-absorbing compounds in wild-type and uvi1 seedlings during 4 d of exposure to UV-B. One-month-old plants were incubated in a 16-h photoperiod for 4 d in the absence of UV-B or with 13 kJ m−2 d−1 UV-B supplementation. Leaf extracts from at least three plants were measured.
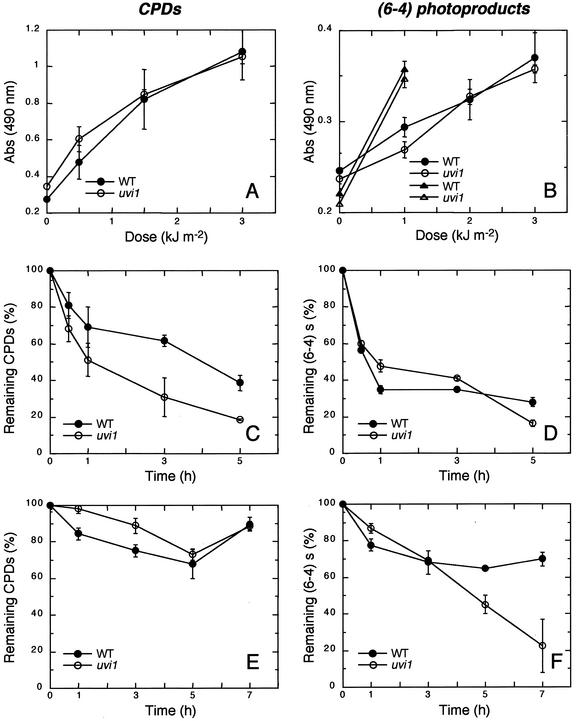
To examine the DNA repair ability of uvi1, an ELISA was carried out to measure the induction and reduction of CPD and (6-4) photoproducts. The induction of CPD and (6-4) photoproducts increased linearly with UV-B exposure up to 3 kJ m−2, and there were no differences between wild type and uvi1 seedlings (Fig. 4, A and B). The reduction of CPDs under white light in uvi1 was faster than that in the wild type (Fig. 4C). After 5 h of incubation, only 20% of the CPDs remained in uvi1, whereas 40% of the initial CPD load remained in the wild type. There was usually no repair of CPDs in darkness either in wild-type or uvi1 seedlings (Fig. 4E). The reduction of (6-4) photoproducts under white light was rapid in uvi1 as well as in the wild type (Fig. 4D). In contrast, in the dark, the amount of (6-4) photoproducts remained fairly constant in the wild type, but it was reduced at a relatively rapid pace in uvi1 (Fig. 4F).
Figure 4.
Induction and reduction of CPDs and (6-4) photoproducts in wild-type and uvi1 seedlings after UV-B exposure. A, Induction of CPDs. Five-day-old seedlings grown in the light were exposed to a single pulse of UV-B (0–3 kJ m−2) and the relative amounts of CPDs were measured by ELISA. C, Reduction of CPDs formed by a single pulse of UV-B (3 kJ m−2) when seedlings were irradiated under white light. E, Reduction of CPDs formed by a single pulse of UV-B (1 kJ m−2) when seedlings were incubated in the dark. B, D, and F, Corresponding results for (6-4) photoproducts. The results represent an average of at least two independent experimental values. Error bars are se.
uvi1 Enhances the Expression of PHR1
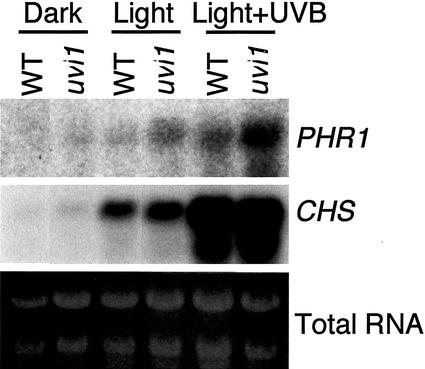
CPD photoreactivation is catalyzed by CPD photolyase in Arabidopsis. Figure 5 compares PHR1 (CPD photolyase) gene expression in the wild type and uvi1. In both genotypes, the expression of PHR1 was hardly detectable in the dark, and was strongly promoted by a white-light treatment, particularly in uvl1. V-B exposure further stimulated the expression of PHR1, and again the expression level was much higher in uvi1 than in the wild type. In contrast, CHS expression, which was also promoted by white light and UV-B exposure, was similar in the wild-type and uvi1 seedlings. Thus, PHR1 expression was specifically up-regulated in uvi1, although no mutation was found in the PHR1 gene of uvi1.
Figure 5.
Effects of white light and UV-B exposure on the expression of PHR1 in wild-type and uvi1 seedlings. Six-day-old etiolated seedlings were kept in the dark, or exposed to white light or white light plus UV-B with a dose of 3.6 kJ m−2 for 6 h. Each lane of the RNA blot was loaded with 15 μg of total RNA.
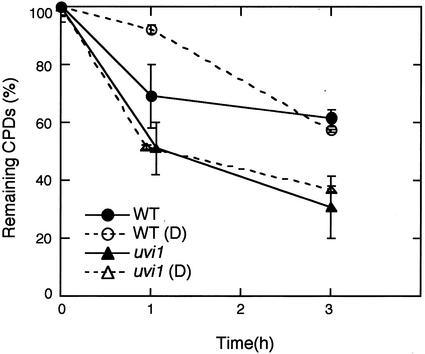
Because light stimulates PHR1 expression in Arabidopsis, we examined the effect of a pretreatment of darkness before the UV-B pulse on CPD repair in wild-type and uvi1 seedlings. Incubation for 5 h in darkness caused a reduction of CPD photorepair in the wild type at 1 h after the exposure, compared with the control without pretreatment (Fig. 6). In contrast, the rapid photorepair of uvi1 was not affected by the pretreatment.
Figure 6.
Effect of a pretreatment in darkness on CPD photoreactivation. Five-day-old seedlings grown in continuous white light were either kept in white light or transferred to darkness (D) for 5 h. Both groups of seedlings were exposed to UV-B (3 kJ m−2) and allowed to photorepair under white light. The relative amounts of CPDs were measured by ELISA. The results represent an average of at least two independent experimental values. Error bars are se.
DISCUSSION
Our study indicates that uvi1 has an enhanced capacity for dark repair of (6-4) photoproducts and photoreactivation of CPDs. Genetic analysis indicated that this mutant has a single recessive mutation. However, we were unable to find a positional marker close to UVI1. The results of recombination frequency using DNA markers of nga8 (26.6 cM on RI map) and Det1 (31.4 cM) indicate that UVI1 is at around 56 to 57 cM or at about 0 to 5.5 cM on chromosome 4, but neither the GA1 (17.7 cM) nor the g4539 (57.6 cM) marker showed a linkage with the uvi1 phenotype. This result may be related to the fact that mutations induced by ion beams frequently involve large DNA alterations, such as inversions, translocations, or deletions (Shikazono et al., 2001). Thus, there may have been a translocation of a chromosomal segment containing the GA1 or g4539 markers, so that they would not show a linkage to the uvi1 phenotype.
On the basis of the root-bending assay, uvi1 appeared to have a high capacity for DNA repair in the dark (Fig. 2, A and C). CPDs were hardly reduced in the dark in either the wild type or uvi1 (Fig. 4E). Other studies were also unable to detect the dark repair of CPDs (Britt et al., 1993; Landry et al., 1997). Our study showed that the amount of (6-4) photoproducts was reduced in the dark (Fig. 4F). Although Britt et al. (1993) clearly observed dark repair of (6-4) photoproducts, Landry et al. (1997) did not. Landry et al. (1997) attributed the discrepancy to either technical differences or to the possibility that the dark repair of (6-4) photoproducts is developmentally regulated. Our results indicate that the strong UV-B resistance of uvi1 in the dark is not because of the dark repair of CPDs, but predominantly because of the gained function of dark repair of (6-4) photoproducts.
uvi1 was also more resistant to UV-B than the wild type when seedlings were irradiated under white-light conditions (Figs. 1 and 2B). In the light condition, the reduction of CPDs was faster in uvi1 than in the wild type (Fig. 4C), but the reduction of (6-4) photoproducts was not affected in uvi1 (Fig. 4D). Thus, uvi1 seemed to gain the function of CPD photoreactivation. In the presence of both light and UV-B, the expression of PHR1 was higher in uvi1 than in the wild type, even though the white light- and UV-B-inducible CHS expression was not different between uvi1 and the wild type (Fig. 5). The promoter region of PHR1 contains two G box-like motifs and five GT-1 box-like motifs that may act positively or negatively in the transcriptional regulation under light conditions (Sakamoto et al., 1998). Because no mutation was found in the PHR1 gene of uvi1, we hypothesize that PHR1 was both positively and negatively regulated in the wild type and that the negative regulation was deficient in uvi1, leading to the higher photoreactivation of CPDs. On the other hand, as shown by uvi1 plants pretreated in the dark, exposure to light before UV-B exposure is not required for the early reduction of CPDs in uvi1. Thus, these results suggest that the photoregulation of CPD photoreactivation may be altered in uvi1.
The existence of the UVI1 gene is interesting from an evolutionary standpoint. UVI1 might be a negative regulator of two different repair systems of photoreactivation and dark repair in Arabidopsis, whereas PHR1 was positively regulated not only by UV-B but also by visible light (Fig. 5). It is likely that higher plants have developed regulatory system of DNA repair both positively and negatively, although the role of a negative regulator is unclear.
MATERIALS AND METHODS
Plant Material and Growth Conditions
A wild type of Arabidopsis, Columbia, was used in this study. The plants were grown in a growth room under continuous white light (approximately 40 μE m−2 s−1) or 16-h photoperiod at 23°C. In general, plants were grown in pots or planters containing a verm-piece:perlite (1:1 [w/v]) commercial substrate and fed with 0.1% (v/v) commercial nutrient (Hyponex Corp., Osaka) once a week.
UV-B Source
UV-B was supplied by a UV lamp cassette (Type CSL-15B, COSMO BIO, Tokyo) that radiates at wavelengths above 280 nm with a high peak at 312 nm (manufactured and measured by Vilber Lourmat, Cedex, France). UV-B doses were measured with a UV-B radiometer (CSV-312, COSMO BIO) whose filter transmits UV-B radiation with a peak at 313 nm and has a half bandwidth of 12 nm.
Mutant Isolation
Seeds were irradiated with carbon ions from an azimuthally varying field cyclotron with a dose of 150 Gray, as previously described (Tanaka et al., 1997a). About 50 to 200 M1 seeds were grown to maturity and harvested together as a M1 group. For each M1 group, 4 times as many M2 seeds (i.e. 200–800 M2 seeds per each M1 group) were used for the first screening of the long-term exposure assay as follows. Ten-day-old seedlings grown under a 16-h photoperiod in a photochamber (BIOTRON, type LDH200-RDS, NK-System, Osaka), were irradiated with UV-B light at 10 to 13 kJ m−2 for 10 h (starting 2 h after the start of the photoperiod) per day for 10 to 20 d. The UV-B dose was controlled by the distance from the UV-B lamp to the plant. The intensity of UV-B light was frequently inspected. Twenty-seven candidates that showed good growth under UV-B exposure were isolated from about 5,100 M2 plants that were derived from 1,280 M1 seeds. These lines were further grown under normal (non-UV-B irradiated) conditions and harvested. Each seed derived from M2 candidates was individually grown and self-pollinated under normal conditions, and harvested to yield M3 families. The long-term exposure assay and the root-bending assay were carried out using 15 to 20 seeds of the M3 and M4 progenies. Six candidates that showed reproducible UV-B resistance in either the long-term exposure assay or the root-bending assay were selected as putative UV-B resistant mutants. Finally, six candidate lines (49 seeds per line) were subjected to long-term exposure, and four lines were selected as homozygous lines. The homogeneity and reproducibility of UV-B resistance of these four lines, which were derived from independent M1 groups, were confirmed in the M5 through M6 generations.
Mapping Analysis
M5 plants were crossed with Landsberg erecta. Two hundred-thirty F3 lines were screened by means of the root-bending assay under dark conditions. Thirty F3 lines selected from the first and second screening were regarded as uvi1 homozygous mutants and used for the mapping analysis. The mapping was done using the methods described by Konieczny and Ausubel (1993) for cleaved-amplified polymorphic sequence markers (THY1, GBF3, GA1, Det1, g4539, and PLC1) and Bell and Ecker (1994) for simple sequence length polymorphism markers (ATEAT1, nga63, Athso392, nga280, AthATPASE, nga1145, nga1126, nga168, mga172, AthGAPAb, nga6, nga8, nga1107, nga249, nga139, and AthPHYC). The genetic distances from uvi1 to nga8, Det1, and g4539 were calculated from 21 homozygous plants. Recombination values were converted to map distances using the Kosambi mapping function.
Root-Bending Assay
The root-bending assay followed the method described by Britt et al. (1993). Three-day-old seedlings vertically grown on a nutritive agar plate (0.1% [v/v] commercial nutrient, Hyponex) were exposed to UV-B light at a rate of about 0.25 kJ m−2 min−1. For a mock control, seedlings on the same plate were covered with aluminum foil that did not transmit UV-B. After the exposure, the plates were rotated by 90° and incubated for another 3 d under the light or dark condition. The length of new root growth after UV-B exposure was measured.
Measurement of UV-B-Absorbing Compounds
To measure the amount of UV-B absorbing compounds, leaves were extracted with 4 volumes of 70% (w/v) methanol/1% (w/v) HCl, cleared by centrifugation, and filtered with glass filter-B (Whatman, Clifton, NJ). The absorbance of the extracts was measured at 330 nm with a model DU60 spectrophotometer (Beckman Instruments, Fullerton, CA) essentially as described by Li et al. (1993).
ELISA
The induction and reduction of CPDs and (6-4) photoproducts were analyzed by ELISA. CPDs and (6-4) photoproducts were detected by the specific antibodies TDM-2 and 64 m-2, respectively, as described previously (Mizuno et al., 1991; Mori et al., 1991; Matsunaga et al., 1993). Five-day-old seedlings (100–150 seedlings per each point) were exposed to UV-B with a dose of 3 kJ m−2 for the light condition and with 1 kJ m−2 for the dark condition. The samples were incubated or collected, followed by immediate freezing in the dark. Fifty microliters of the extracted DNA was placed in each well. The DNA concentration was adjusted to 0.8 μg mL−1 for TDM-1 and 6 μg mL−1 for 64 M-2. In the case of (6-4) photoproducts in the dark condition, 12 μg mL−1 of DNA was used to obtain higher optical density value. The ELISA procedure was chiefly that of Takeuchi et al. (1996). For each sample, the mean value of three wells was calculated and the background was subtracted.
Northern Blots
Six-day-old etiolated seedlings were kept in the dark, or exposed to continuous white light (about 35 μE m−2 s−1) or white light plus UV-B light with a dose of 3.6 kJ m−2 for 6 h. Total RNA was isolated, and 15 μg of each sample was subjected to an RNA blot and hybridized using genomic PHR1 as a probe (Sakamoto et al., 1998).
Sequencing of the PHR1 Gene
Genomic DNA was extracted from vegetative tissues of uvi1. The DNA was partially digested with MboI, then cloned into the BamHI site of λDASH II (Stratagene, La Jolla, CA) to construct a genomic library. The library was screened with a cDNA fragment of PHR1 and one positive clone was isolated. The insert of this clone was extracted by digestion with XbaI, and an approximately 8-kb fragment containing PHR1 gene was subcloned into a pUC vector. Sequencing was done with both strands covering −1,277 to +2,703 of the PHR1 gene.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the materials. Obtaining any permissions will be the responsibility of the requester.
ACKNOWLEDGMENTS
We are grateful to members of Takasaki Ion Accelerators for Advanced Radiation Application facilities (Takasaki, Japan) for the ion beam irradiation in Japan Atomic Energy Research Institute. We thank Dr. James Raymond for his careful review of the manuscript.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010894.
LITERATURE CITED
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bharti AK, Khurana JP. Mutants of Arabidopsis as tools to understand the regulation of phenylpropanoid pathway and UV-B protection mechanisms. Photochem Photobiol. 1997;65:765–776. doi: 10.1111/j.1751-1097.1997.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Bieza K, Lois R. An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiol. 2001;126:1105–1115. doi: 10.1104/pp.126.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt AB. Molecular genetics of DNA repair in higher plants. Trends Plant Sci. 1999;4:20–25. doi: 10.1016/s1360-1385(98)01355-7. [DOI] [PubMed] [Google Scholar]
- Britt AB, Chen JJ, Wykoff D, Mitchell D. A UV-sensitive mutant of Arabidopsis defective in the repair of pyrimidine-pyrimidinone (6-4) dimers. Science. 1993;261:1571–1574. doi: 10.1126/science.8372351. [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Robberecht RR, Flint SD. Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant. 1983;58:445–450. [Google Scholar]
- Gallego F, Fleck O, Li A, Wyrzykowska, Tinland B. AtRAD1, a plant homologue of human and yeast nucleotide excision repair endonucleases, is involved in dark repair of UV damages and recombination. Plant J. 2000;21:507–518. doi: 10.1046/j.1365-313x.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- Harlow GR, Jenkins ME, Pittalwala TS, Mount DW. Isolation of uvh1, an Arabidopsis mutant hypersensitive to ultraviolet light and ionizing radiation. Plant Cell. 1994;6:227–235. doi: 10.1105/tpc.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase Y, Tanaka A, Baba T, Watanabe H. FRL1 is required for petal and sepal development in Arabidopsis. Plant J. 2000;24:21–32. doi: 10.1046/j.1365-313x.2000.00851.x. [DOI] [PubMed] [Google Scholar]
- Hidema J, Kumagai T, Sutherland BM. UV radiation-sensitive Norin 1 rice contains defective cyclobutane pyrimidine dimer photolyase. Plant Cell. 2000;12:1569–1578. doi: 10.1105/tpc.12.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Gaba V, Greenberg B. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Jiang CZ, Yee J, Mitchell DL, Britt AB. Photorepair mutants of Arabidopsis. Proc Natl Acad Sci USA. 1997a;94:7441–7445. doi: 10.1073/pnas.94.14.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CZ, Yen CN, Cronin K, Mitchell D, Britt AB. UV- and gamma-radiation sensitive mutants of Arabidopsis thaliana. Genetics. 1997b;147:1401–1409. doi: 10.1093/genetics/147.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins ME, Harlow GR, Liu Z, Shotwell MA, Ma J, Mount DW. Radiation-sensitive mutants of Arabidopsis thaliana. Genetics. 1995;140:725–732. doi: 10.1093/genetics/140.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Landry LG, Stapleton AE, Lim J, Hoffman P, Hays JB, Walbot V, Last RL. An Arabidopsis photolyase mutant is hypersensitive to ultraviolet-B radiation. Proc Natl Acad Sci USA. 1997;94:328–332. doi: 10.1073/pnas.94.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qu-Lee TM, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UVB irradiation. Plant Cell. 1993;5:171–179. doi: 10.1105/tpc.5.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hall JD, Mount DW. Arabidopsis UVH3 geneis a homolog of Saccharomyces cerevisiae RAD2 and human XPG DNA repair genes. Plant J. 2001;26:329–338. doi: 10.1046/j.1365-313x.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hossain GS, Islas-Osuna MA, Mitchell DL, Mount DW. Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1. Plant J. 2000;21:519–528. doi: 10.1046/j.1365-313x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Hatakeyama Y, Ohta M, Mori T, Nikaido O. Establishment and characterization of a monoclonal antibody recognizing the Dewar isomer of (6-4) photoproducts. Photochem Photobiol. 1993;57:934–940. doi: 10.1111/j.1751-1097.1993.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL. Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol. 2000;122:117–125. doi: 10.1104/pp.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R, Connor B, Bodeker G. Increased summertime UV radiation in New Zealand in response to ozone loss. Science. 1999;285:1709–1711. doi: 10.1126/science.285.5434.1709. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Nairn RS. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49:805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Matsunaga T, Ihara M, Nikaido O. Establishment of a monoclonal antibody recognising cyclobutane-type thymine dimers in DNA: a comparative study with 64M-1 antibody specific for (6-4) photoproducts. Mutation Res. 1991;254:175–184. doi: 10.1016/0921-8777(91)90009-e. [DOI] [PubMed] [Google Scholar]
- Mori T, Nakane M, Hattori T, Matsunaga T, Ihara, Nikaido O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6,4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol. 1991;54:225–232. doi: 10.1111/j.1751-1097.1991.tb02010.x. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Sugiyama M, Iwai S, Hitomi K, Otoshi E, Kim ST, Jiang CZ, Todo T, Britt AB, Yamomoto K. Cloning and characterization of a gene (UVR3) required for photorepair of 6-4 photoproducts in Arabidopsis thaliana. Nucleic Acids Res. 1998;26:638–644. doi: 10.1093/nar/26.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux MC, Ballaré CL, Scopel AL, Searles PS, Caldwell MM. Solar ultraviolet-B radiation affects plant-insect interactions in a natural ecosystem of Tierra del Fuego (southern Argentina) Oecologia. 1998;116:528–535. doi: 10.1007/s004420050618. [DOI] [PubMed] [Google Scholar]
- Rousseaux MC, Ballaré CL, Giordano CV, Scopel AL, Zima AM, Szwarcberg-Bracchitta M, Searles PS, Caldwell MM, Diaz SB. Ozone depletion and UVB radiation: impact on plant DNA damage in southern South America. Proc Natl Acad Sci USA. 1999;96:15310–15315. doi: 10.1073/pnas.96.26.15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Tanaka A, Watanabe H, Tano S. Molecular cloning of Arabidopsis photolyase gene (PHR1) and characterization of its promoter region. DNA Sequence. 1998;9:355–360. doi: 10.3109/10425179809008473. [DOI] [PubMed] [Google Scholar]
- Sancar A, Sancar GB. DNA repair enzymes. Ann Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Shikazono N, Tanaka A, Watanabe H, Tano S. Rearrangements of the DNA in carbon ion-induced mutants of Arabidopsis thaliana. Genetics. 2001;157:379–387. doi: 10.1093/genetics/157.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid A, Chow WS, Anderson JM. UVB damage and protection at the molecular level in plants. Photosynth Res. 1994;39:475–489. doi: 10.1007/BF00014600. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Murakami M, Nakajima N, Kondo N, Nikaido O. Induction and repair of damage to DNA in cucumber cotyledons irradiated with UV-B. Plant Cell Physiol. 1996;37:181–187. [Google Scholar]
- Tanaka A, Shikazono N, Yokota Y, Watanabe, Tano S. Effects of heavy ions on the germination and survival of Arabidopsis thaliana. Int J Radiat Biol. 1997a;72:121–127. doi: 10.1080/095530097143608. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Tano S, Chantes T, Yokota Y, Shikazono N, Watanabe H. A new Arabidopsis mutant induced by ion beams affects flavonoid synthesis with spotted pigmentation in testa. Genes Genet Syst. 1997b;72:141–148. doi: 10.1266/ggs.72.141. [DOI] [PubMed] [Google Scholar]
- Vonarx EJ, Mitchell HL, Karthikeyan R, Chatterjee I, Kunz BA. DNA repair in higher plants. Mutation Res. 1998;400:187–200. doi: 10.1016/s0027-5107(98)00043-8. [DOI] [PubMed] [Google Scholar]