Abstract
At least two 1-aminocyclopropane-1-carboxylic acid synthase genes (ACS) are implicated in the submergence response of rice (Oryza sativa). Previously, the OS-ACS5 gene has been shown to be induced during short- as well as long-term complete submergence of seedlings and to be controlled by a balance of gibberellin and abscisic acid in both lowland and deepwater rice. This study demonstrates that OS-ACS5 mRNA is localized in specific tissues and cells both during normal development and in response to complete submergence. The temporal and spatial regulation of OS-ACS5 expression is presented by in situ hybridization and histochemical analysis of β-glucuronidase (GUS) activity in transgenic rice carrying an OS-ACS5-gus fusion. Whole-mount in situ hybridization revealed that in air-grown rice seedlings, OS-ACS5 was expressed at a low level in the shoot apex, meristems, leaf, and adventitious root primordia, and in vascular tissues of nonelongated stems and leaf sheaths. In response to complete submergence, the expression in vascular bundles of young stems and leaf sheaths was strongly induced. The results of histochemical GUS assays were consistent with those found by whole-mount in situ hybridization. Our findings suggest that OS-ACS5 plays a role in vegetative growth of rice under normal conditions and is also recruited for enhanced growth upon complete submergence. The possible implication of OS-ACS5 in root-shoot communication during submergence stress and its putative role in aerenchyma formation upon low-oxygen stress are discussed.
Although being relatively well adapted to submergence stress, rice (Oryza sativa) plants sometimes cope with severe stress conditions due to excessively high and long-lasting floods (Kende et al., 1998). Most rice cultivars tolerate partial submergence for a few days; however, some deepwater rice varieties can survive in several meters of water for up to several months. Problems of submergence are most often associated with heavy monsoonal rains and, therefore, linked to the Asian continent (Herdt, 1991). Submergence, however, is also a very important problem in rice culture in Latin America, where rice is sown on irrigated land rather than transplanted as done traditionally in Asia. In this case, seedlings grow under fully submerged conditions for a few days and losses caused by limited flooding tolerance are considerable.
The adaptation of rice plants to conditions of flooding is mainly based on growth (typical for deepwater rice) or submergence tolerance (as often observed in fully submerged lowland rice and in fully submerged seedlings; Setter et al., 1997; Vartapetian and Jackson, 1997). Submergence causes a decrease in the endogenous oxygen concentration and an increase in the production and accumulation of ethylene (Kende et al., 1998). These changes induce the growth of coleoptile and mesocotyl of seedlings (Ohwaki and Nagao, 1967) and enhance internodal cell division and elongation in adult plants (Métraux and Kende, 1983; Lorbiecke and Sauter, 1998). In seedlings, the response differs somewhat from that of adult plants. When full submergence causes anaerobiosis (0% [v/v] oxygen, in stagnant water in the dark), seedlings do not produce ethylene. The mechanism by which coleoptile growth is induced under these conditions is not fully understood (Pearce and Jackson, 1991; Pearce et al., 1992). In addition to its involvement in elongation growth upon flooding, ethylene also accelerates the formation of lysigenous aerenchyma (at least in several rice cultivars), which helps plants to cope with flooding (Justin and Armstrong, 1991; He et al., 1996; Jackson and Armstrong, 1999; Drew et al., 2000; Colmer et al., 2001). Likewise, epidermal cell death at the site of emergence of adventitious roots in deepwater rice is also controlled by ethylene (Mergemann and Sauter, 2000). Recent findings support a role for ethylene in delaying rice seedling senescence upon complete submergence. When seedlings of lowland rice are treated with 20 μm 1-aminocyclopropane-1-carboxylic acid (ACC) during long-term full submergence, an increase in leaf elongation growth and a significant delay in chlorophyll breakdown are observed (Van Der Straeten et al., 2001). A delayed senescence is also characteristic for more flooding-tolerant rice cultivars (Krishnan et al., 1999).
One of the genes induced by the hypoxic conditions caused by submergence encodes ACC synthase (ACS; Dennis et al., 2000). The formation of the ethylene precursor ACC by ACS is considered a regulatory step in ethylene biosynthesis. An increase in ACS activity was found in partially submerged deepwater rice (Cohen and Kende, 1987). ACS is encoded by a multigene family, consisting of at least five members in rice (Zarembinski and Theologis, 1993, 1997; Van Der Straeten et al., 1997, 2001). Three rice ACS genes are suppressed upon submergence, but play a role in response to other environmental and developmental cues (Van Der Straeten et al., 1997). Another ACS gene, OS-ACS1, has been implicated in the partial submergence response (Zarembinski and Theologis, 1997). Its expression was suggested to contribute to the longer term ethylene production, but not to the initial increase in ethylene synthesis. Recently, we have characterized a second submergence-induced ACS gene from rice (OS-ACS5; Van Der Straeten et al., 2001; Zhou et al., 2001). Both short- and long-term complete submergence treatments induced OS-ACS5. OS-ACS5 expression was positively controlled by low oxygen, ethylene, and GA, and negatively regulated by abscisic acid. In addition, the stimulated elongation growth of seedling leaves upon full submergence was correlated with an induction of OS-ACS5 and with higher levels of ACC oxidase (ACO) activity and ethylene emanation after desubmergence.
To determine whether OS-ACS5 induction is confined to specific cell and tissue types, we have analyzed the temporal and spatial distribution of OS-ACS5 mRNA. The expression patterns were studied in air-grown and in fully submerged seedlings. Both promoter-β-glucuronidase (GUS) fusion analysis and in situ hybridization approaches were followed. The results show that OS-ACS5 is predominantly expressed in young, growing, and vascular tissues. Its importance in development and possible involvement in root-to-shoot signaling and aerenchyma formation upon submergence stress are discussed.
RESULTS
Regeneration and Evaluation of Transgenic Rice Carrying OS-ACS5-gus Constructs
Four independent Agrobacterium tumefaciens transformation experiments were carried out. Two rounds of transformation with pRG11, containing the full-length pOS-ACS5-gus construct, yielded 37 independent T0 transgenic lines, 28 of which were fertile. The pRG13 construct represents a partially deleted OS-ACS5 promoter-GUS construct carrying a 357-bp fragment of the OS-ACS5 5′-untranslated region (UTR; Fig. 1). Twenty-five independent lines were obtained, nine of which turned out to be fertile. The third construct, pRG16, contains a pCaMV35S-GUS combination and was used as a positive control for transformation and also allowed the estimation of the OS-ACS5 promoter strength relative to the 35S promoter. Eight lines of 18 were further analyzed, all of which were fertile.
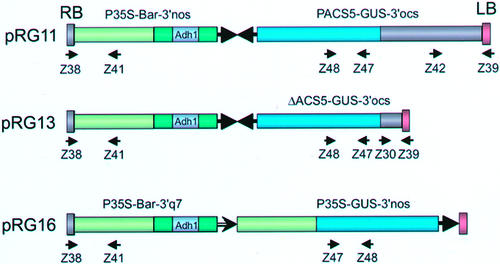
Figure 1.
Scheme of T-DNA constructs used in transformation experiments. The direction of transcription is marked with an arrow. The small arrows and numbers under the constructs mark the positions and the orientation of primers used in PCR amplifications. PACS5, 1,783-bp promoter fragment of the OS-ACS5 gene (gray bar); ΔPACS5, partial deletion of the promoter fragment covering 357 bp of the 5′-UTR (short gray bar); P35S, promoter of the cauliflower mosaic virus (CaMV) 35S gene (green bar); GUS (blue bar).
T-DNA integration into the genome was examined in primary transgenic plants by PCR. Figure 1 gives an overview of the primer combinations used for the three constructs. In each case, the presence of an intact left and right T-DNA border, as well as the presence of the gus gene, were tested. PCR analysis on plants transformed with the pRG11 construct showed that all transgenic lines regenerated from a culture medium containing phosphinothricin (PPT) carried the fragment representing the GUS-coding region (primers Z47/Z48; Fig. 1). However, only 31 of the 37 pRG11 lines had an intact fragment covering the right border and the neighboring p35S promoter region (primers Z38/Z41). Nevertheless, a Liberty resistance test showed that all lines were PPT resistant. Therefore, the failure in generating the expected PCR fragment was probably due to rearrangements within a short region of the right border. Rearrangements were also noticed in the left border region: In approximately 40% of all pRG11 lines, PCR amplification of the left border region (primers Z39/Z42) failed to produce the desired fragment. Only those lines harboring the entire left border region were selected for subsequent GUS histochemical assays. No further rearrangements were observed in T1 and T2 progenies as assessed by PCR analysis.
T1 seedlings from selected PPT-resistant lines were examined both by histochemical and quantitative GUS assays. Histochemical staining failed to produce any detectable blue color in most of the air-grown pRG11 lines. In four lines (15%), air-grown seedlings displayed faint GUS activity in the immature, nonelongated stem. However, when seedlings were submerged for 24 h, all pRG11 lines showed detectable, albeit variable GUS staining. Figure 2, A and B, show the effect of submergence in pRG11-2 seedlings, at 3 and 9 d of age, respectively. Quantitative GUS activity measurements in nonelongated stems (averaged over four randomly selected lines, pRG11-1, pRG11-2, pRG11-38, and pRG11-174) revealed an 8-fold enhancement of GUS activity after 24 h of submergence (Fig. 2C). In contrast, most pRG13 lines had no detectable GUS staining even after a submergence treatment, with exception of pRG13-72 and pRG13-164, in which weak expression was observed after submergence. Quantitative GUS measurements in the youngest node and internode of 2-month-old T1 plants of eight independent lines generated with pRG11 and pRG13 constructs, showed the effect of the deletion on the intensity of the GUS activity (on average 99.4 units mg−1 protein for pRG11 versus 14.4 units mg−1 protein for pRG13). Nevertheless, the first 357 bp of the 5′-UTR of OS-ACS5 in pRG13 supported an expression pattern similar to that of the full-length promoter, even after submergence treatment, albeit at a much reduced level (data not shown). An average of 1,239.2 units mg−1 protein was found in p35S-GUS transgenic plants.
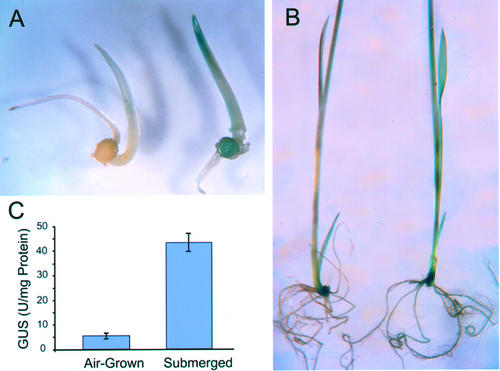
Figure 2.
Expression of the OS-ACS5 gene in transgenic rice seedlings upon submergence as assessed by a promoter-GUS fusion analysis. Histochemical assays were performed in 3- (A) and 9- (B) d-old T2 seedlings of line pRG11-2, carrying the full-length promoter fragment. Air-grown pRG11-2 seedlings are shown on the left, seedlings after 24 h of submergence are on the right. Quantification of GUS activities in immature stems of 9-d-old air-grown seedlings or after 24 h of submergence (C). Data represent the mean of four different, randomly chosen, transgenic lines (±sd).
Developmental Control of OS-ACS5 Expression
Based on the level of GUS activity and the results of PCR amplifications, transgenic lines pRG11-2, pRG11-85, and pRG13-164 were chosen for a detailed histochemical analysis. In each of these lines, segregation of PPT resistance was consistent with the integration of a single T-DNA copy. All patterns described below were similar in the three lines.
To describe the GUS activity patterns in function of development, we adopted the classification system for different growth stages of rice proposed by Meier (1997). According to this system, the codes for germination, leaf development, tillering, and stem elongation are stages 0, 1, 2, and 3, respectively. Each stage is divided into 10 substages corresponding to specific developmental processes. For instance, stage 07 indicates coleoptile emergence from caryopsis and stage 12 refers to unfolding of the second leaf. The organ appearing immediately after the coleoptile is called “imperfect leaf” because this leaf-like organ does not bear a lamina. Subsequently emerging leaves are designated first leaf, second leaf, and so on. The nodes where leaves emerge are named likewise.
As mentioned above, the intensity of GUS staining was strongly enhanced upon submergence. However, the patterns of expression were not notably affected. The results of histochemical analyses presented below are all from samples submerged for 24 h, unless stated otherwise. Figure 3 presents the progression of OS-ACS5 gene expression during vegetative growth. GUS activity was first detected 2 d after germination (stage 07). The staining was restricted to the basal area of the shoot (mesocotyl and coleoptile node; Fig. 3A) and to the scutellum. Intense staining was also observed in the coleoptile tip and the emerging imperfect leaf of 3- to 4-d-old seedlings (stage 09; Fig. 3B).
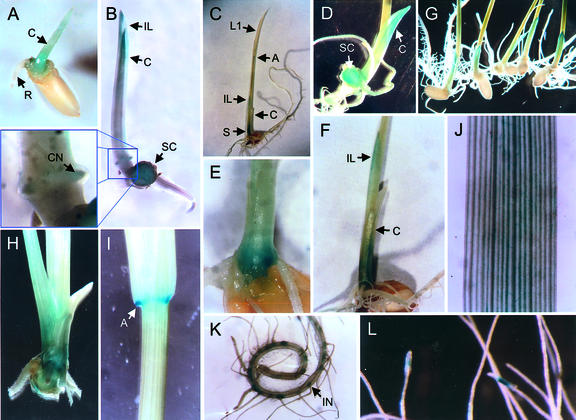
Figure 3.
Expression of the OS-ACS5 gene during development. Macroscopic histochemical analysis of transgenic line pRG11-2. A through D and G, Seedlings (in B, the endosperm was removed to reveal the scutellum); E, F, and H, part of the immature seedling stem; I, junction of lamina and leaf sheath of the first leaf, staining in auricle area; J, part of the lamina of the second leaf; K, part of a primary root and lateral roots; L, lateral roots. Plant age: 2 d (A), 3 d (B), 5 d (C–G), 9 d (H-K), 8 weeks (L). A, Auricle; C, coleoptile; CN, coleoptile node; IL, imperfect leaf; IN, initiation site of the lateral root; L1, first true leaf; R, radicle; S, stem; SC, scutellum.
A more detailed analysis of a longitudinal section of the scutellum of 4-d-old seedlings revealed that the OS-ACS5 promoter activity was mainly detected in the epithelium layer (Fig. 4A). In contrast, in the mesocotyl and coleoptile node, gene expression was restricted to vascular bundles (Fig. 4, B and C). A less intense staining was observed in the two vascular bundles of the coleoptile node and in adventitious root primordia (Fig. 3B and magnification). Leaf development (stage 10) started 5 to 6 d after germination (Fig. 3C). At this stage, the promoter was most active in the scutellum and vascular bundles of the fully developed coleoptile (Fig. 3D) and in the nonelongated stem and tip of the imperfect leaf (Fig. 3, E–G). Transverse sectioning of coleoptiles of 5-d-old seedlings confirmed these data and showed a reduced promoter activity in the epidermal layer when compared with 4-d-old seedlings (Fig. 4D). At 9 d of age (stage 12), the second leaf had emerged and elongated very fast, whereas the coleoptile and the scutellum were senescing. GUS activity at this stage was mainly observed in the nonelongated stem, the basal part of leaf sheaths, and the auricles of the first and second leaf (Fig. 3, H and I). In the lamina of the second leaf, faint, blue staining was observed along the vascular tissue (Fig. 3J). However, laminae of leaves emerging thereafter hardly showed any GUS staining. In the primary root, OS-ACS5 expression was found at the basis of lateral roots, resulting in a spotted pattern (Fig. 3K).
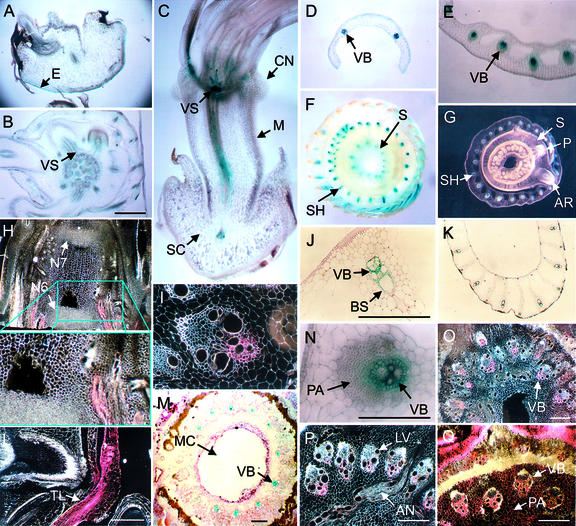
Figure 4.
Expression of the OS-ACS5 gene during development. Representation of longitudinal (A, C, H, and L) and transverse sections of different tissues of transgenic line pRG11-2, as well as a transverse stem section of the control line pRG16-73 (Q). A through G, J, K, M, and N are bright-field images, in which the blue color represents GUS activity, whereas H, I, L, and O–Q are dark-field images, in which GUS appears purple. A, Scutellum; B, coleoptile node; C, scutellum, mesocotyl, and coleoptile node; D, coleoptile and its two vascular bundles; E, leaf sheath; F, stem above node 6 (the highest internode); G, stem above node 4; H, young stem; I, detailed view of stem vascular bundle; J, vascular bundles in mature leaf sheath; K, sheath of leaf 4; L, initiation of tiller; M, stem; N, developing vascular bundles in a young leaf sheath; O, stem vascular bundles; P, node 6. Plant age: 4 d (A–D), 9 d (E), and 8 weeks (F–Q). AN, Anastomosis; AR, adventitious root; BS, bundle sheath cell layer; CN, coleoptile node; E, epithelium; IN, imperfect leaf node; LV, longitudinally orientated vascular bundle in node; M, mesocotyl; N6 and N7, sixth and seventh node, respectively; PA, parenchyma; S, stem; SC, scutellum; SH, leaf sheath; TL, tiller; VB, vascular bundles; VS, vascular system.
In summary, during germination and leaf development stages (stages 0 and 1, respectively), OS-ACS5 expression was closely associated with the development of new organs. A decrease in the overall GUS activity was observed with aging. However, transient activity of the OS-ACS5 promoter was detected in every leaf sheath during emergence and elongation phases. After full elongation and expansion, GUS staining became undetectable. In addition, leaves and stems showed a differential timing in OS-ACS5 regulation. Under the growth conditions described, 8-week-old plants (stage 17) bear six to seven true leaves. Longitudinal sectioning revealed that plants at this stage have a nonelongated highest internode (between nodes 7 and 6) and an elongating second highest internode (beneath node 6). GUS staining was detected in the area covering both internodes and node 6, as illustrated in Figure 4H (dark-field image; note the purple color indicating GUS activity close to the cell wall of the highly vacuolated cells of the internode [weak signal] and in the vascular strands on the left and right sides in node 6 [strong signal; see magnification of the inset of Fig. 4H]). No GUS staining could be observed in older parts of the stem (below node 5). In contrast, the corresponding leaf sheaths showed prolonged gene activation because in 8-week-old plants, GUS staining could still be observed in the fourth leaf sheath. Furthermore, OS-ACS5 activity was observed in some lateral roots, mainly in the elongation zone, approximately 1 mm behind the root tip (Fig. 3L). Finally, GUS activity was also correlated with tiller development (Fig. 4L, dark-field image; purple color corresponds to GUS activity).
Association of OS-ACS5 Expression to the Vascular System
Submergence-induced OS-ACS5 gene activity proved to be associated with the vascular system in all examined transgenic plants of 2 d to 8 weeks of age. During early developmental stages, seedlings also exhibited some promoter activity in the epidermal layer of the scutellum and coleoptile, whereas mature plants showed only vascular-specific GUS staining in stems and leaf sheaths (Fig. 4, F, G, M, O, and P for stems; J, K, and N for leaf sheaths). Upon close examination of stem sections, promoter activity appeared confined to xylem tissue (Fig. 4I). In leaf sheaths, the signal was present all over the vascular bundle (Fig. 4J). It is worthwhile to mention that during the early development of sheaths, faint GUS staining was observed in parenchyma cells surrounding the intensely stained developing vascular bundle (Fig. 4N). Because samples were incubated with ice-cold 90% (v/v) acetone before GUS staining and because the staining buffer was supplemented with 0.5 mm of both potassium ferro- and ferricyanide (generally regarded as the most reliable method for histochemical GUS assays), it can be assumed safely that the signal in the parenchyma cells did not result from diffusion (Rueb and Hensgens, 1997; de Almeida Engler et al., 1999). Moreover, the weak signal in parenchyma cells around the vascular bundle was consistently present. In mature vascular bundles of leaf sheaths, staining was restricted within the bundle (Fig. 4, J and K). Interestingly, OS-ACS5 was only active in longitudinally oriented vascular bundles of stem nodes. The transversally oriented vascular tissue (anastomosis) never showed detectable GUS activity (Fig. 4L).
Tissue Specificity of OS-ACS5 Expression as Assessed by in Situ Hybridization
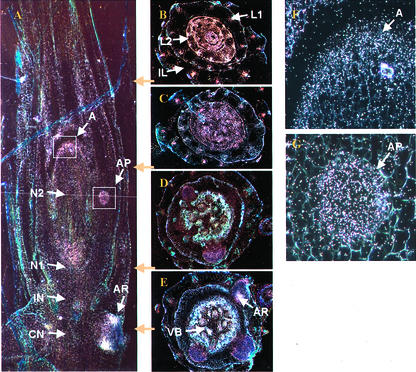
In situ hybridization with an OS-ACS5 antisense probe was performed on nonelongated stems and leaf sheaths of 9-d-old seedlings of both lowland rice (cv IR36) and deepwater rice (cv Plai Ngam) to confirm whether OS-ACS5 activity assessed by promoter-GUS analysis reflected the actual OS-ACS5 transcript accumulation in the same tissue and cell types. At 9 d of age, seedlings had unfolded the imperfect leaf and the first and second leaves. In air-grown rice cv Plai Ngam, a low OS-ACS5 promoter activity was detected in the shoot apex, in the nonelongated stem portion around the first node, and in leaf and adventitious root primordia (Fig. 5A; magnifications of apex and adventitious root primordium are shown in Fig. 5, F and G, respectively, with hybridization signals seen as white spots). Transversal sections revealed that promoter activity was mainly localized in vascular bundles of sheaths and nonelongated stems (Fig. 5, B–E). Although mRNA accumulated in the main vascular bundles of all emerged leaf sheaths, the transcription level was significantly higher in younger tissues (Fig. 5, B and C). In addition, air-grown rice cv IR36 plants showed an expression pattern comparable with that of air-grown deepwater rice. Sections hybridized with a sense probe showed no detectable signal (data not shown).
Figure 5.
In situ hybridization of OS-ACS5 mRNA in air-grown deepwater rice seedlings (9 d). The hybridization signal is seen as bright-white spots. A, Longitudinal stem section; B through E, transverse sections of nonelongating stem and leaf sheaths at indicated positions; F, magnification of apex seen in A; G, magnification of adventitious root primordium seen in A. A, Shoot apex; AP, adventitious root primordium; AR, adventitious root; CN, coleoptile node; IL, imperfect leaf; IN, imperfect leaf node; L1 and L2, leaves; N1 and N2, corresponding nodes; VB, vascular bundle.
Submergence Induction of ACS
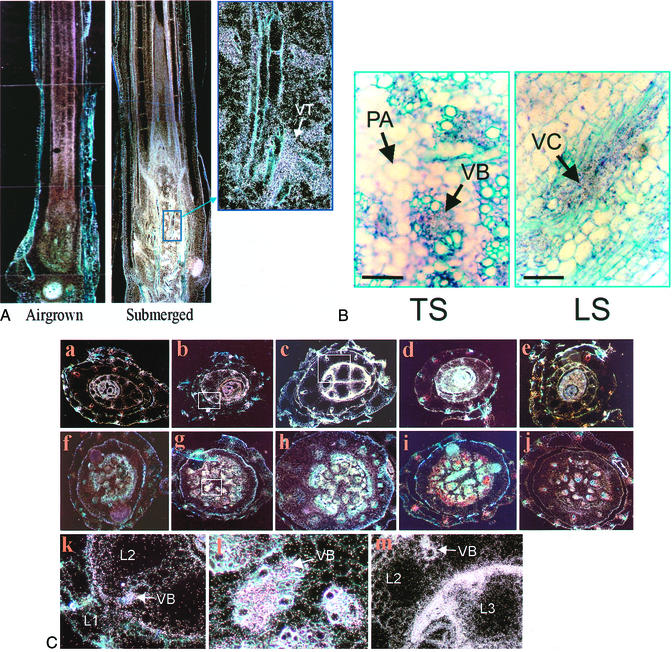
The accumulation of OS-ACS5 mRNA in submerged deepwater rice has recently been demonstrated by RNA gel-blot analysis and reverse transcriptase-PCR (Van Der Straeten et al., 2001; Zhou et al., 2001). Histochemical studies of rice plants transformed with OS-ACS5 promoter-GUS constructs refined these observations enabling temporal and spatial analysis of gene expression (Figs. 2–4). Moreover, in situ hybridization confirmed these results (Figs. 6 and 7). Submergence-induced gene expression was essentially localized to the vascular system (Fig. 6A; dark-field image, hybridization signal is seen as white spots). Higher magnification showed deposition of silver grains in both xylem and phloem elements in nonelongated stems (Fig. 6B; bright-field image, hybridization signal is seen as black spots).
Figure 6.
In situ hybridization of OS-ACS5 mRNA in 9-d-old submerged deepwater rice seedlings. Dark-field (A and C) and bright-field (B) images in which the hybridization signal appears as white and black spots, respectively. Toluidine blue stain was used to reveal cell walls. A, Longitudinal stem sections of air-grown (left) and seedlings submerged for 2 d (middle), with a close-up of vascular tissue (right); B, vascular system in stem at high magnification (submerged for 2 d); C, transverse sections of leaves (a–e) and immature stems (f–j). Time of submergence: 0 h (a and f), 4 h (b and g), 2 d (c and h), 7 d (d and i), and 14 d (e and j). k–m, Close-ups of delineated zones in b, g, and c, respectively. L1, L2, L3, First, second and third true leaf; LS, longitudinal section; PA, parenchyma cells; TS, transverse section; VB, vascular bundle; VC, vascular cylinder.
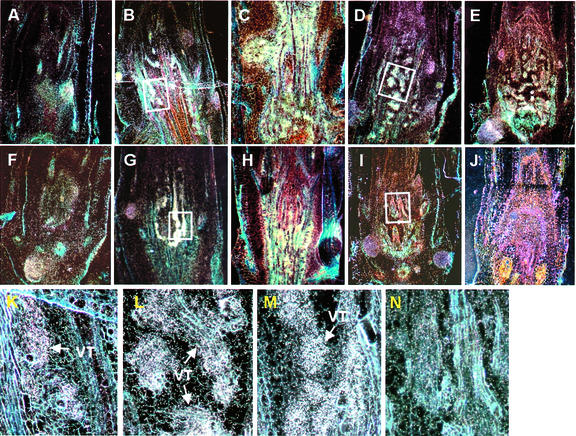
Figure 7.
Comparison of the submergence response of deepwater and lowland rice seedlings. Dark-field images with hybridization signals as white spots. Longitudinal sections through immature stems of rice cv Plai Ngam (upper row, A–E) and rice cv IR36 (middle row, F–J) were hybridized with an antisense OS-ACS5 probe. Time of submergence: 0 h (A and F), 4 h (B and G), 2 d (C and H), 7 d (D and I), and 14 d (E and J). K–N represent close-ups of marked zones corresponding to B, G, D, and I, respectively.
In rice cv Plai Ngam, the signal in nonelongated stems and leaf sheaths of air-grown seedlings was weak, but detectable. However, after 4 h of submergence, the transcription in both nonelongated stem and leaf sheath was significantly increased (Fig. 6C). Further magnification revealed that the increase of signal intensity was most pronounced in the vascular system of nonelongated stems (Fig. 6C, l) and that the expression was stronger in younger leaves (compare leaves 1 and 2 in Fig. 6C, k). The induction reached a maximum in samples submerged for 2 d, when seedlings displayed a significant stimulation of elongation as well as a strong development of the vascular system (Fig. 6, A and C, c, h, and m). After 7 d of submergence, seedlings of rice cv Plai Ngam still showed a very high expression level in their second leaf and corresponding stem region (Fig. 6C, d and i). However, in the first and the imperfect leaves, mRNA levels gradually decreased, although remaining higher than in air-grown seedlings. The expression in nonelongated stems and young leaves was observed throughout the entire period of treatment (up to 14 d; Fig. 6C, e and j).
Lowland Rice Lacks a Sustainable Submergence Response
Figure 7 presents a comparison of in situ hybridization data for OS-ACS5 from fully submerged deepwater and lowland rice seedlings. In the first 2 d of treatment, lowland rice responded in a way similar to deepwater rice (compare Fig. 7, A–C and F–H, close-ups in K and L, for deepwater and lowland rice, respectively). The development of the vascular system in elongating stems and leaf sheaths was accelerated, which coincided with OS-ACS5 induction in these tissues. However, differences between the two varieties appeared when submergence was sustained (Fig. 7). In rice cv IR36 plants submerged for 7 d, the OS-ACS5 mRNA level was quickly reduced to that in air-grown plants (Fig. 7N; compare with M for deepwater rice). In addition, it is worth mentioning that the signal in adventitious root primordia after 7 d of submergence did not differ from that of air-grown controls. After 2 weeks of submergence, gene expression could still be observed in rice cv Plai Ngam, reflected by the bright-white stain (Fig. 7E). In contrast, no signal was observed in rice cv IR36 (Fig. 7J; the dispersed white color corresponds to autofluorescence of dead cells and is clearly different from the silver grain spots under higher magnification).
DISCUSSION
Growth of rice under flooded conditions has been associated with the induction of the ethylene biosynthesis genes ACS and ACO (Mekhedov and Kende, 1996; Zarembinski and Theologis, 1997; Van Der Straeten et al., 2001; Zhou et al., 2001). At least two ACS genes are implicated in the growth response of rice seedlings upon submergence (partial or complete), from which the OS-ACS5 gene is highly expressed and responsive upon short- and long-term full submergence (Van Der Straeten et al., 2001). Moreover, enhanced ACS expression was correlated with an increase in the capacity to convert ACC to ethylene (Van Der Straeten et al., 2001). In addition, the duration of submergence was shown to enhance the subsequent ethylene release, suggesting a higher production during the submergence phase. Although measurement of ethylene from submerged plants would be a more definite experiment, it is practically complicated. However, our findings confirm previous studies in both rice and Rumex sp. that indicated that when submergence terminated, ethylene release was strongly correlated with submergence tolerance (Khan et al., 1987; Vriezen, 2000). The present study illustrates the detailed pattern of OS-ACS5 expression in function of development during the vegetative stage and upon submergence, as assessed by both promoter-GUS fusions and by in situ hybridization. The fact that the latter technique confirmed the data obtained by promoter-GUS fusion analysis indicates that probably no essential control elements reside out of the 1,783-bp OS-ACS5 promoter fragment used here. Furthermore, transgenic plants carrying the OS-ACS5 promoter-GUS construct with the truncated promoter of 357 bp showed a comparable GUS activity pattern, though with a much reduced intensity. These observations suggest that the cis elements that control submergence induction are probably located in the proximal promoter, whereas an enhancer element farther upstream might regulate the intensity of response.
A stimulation of ACS expression has been correlated mainly with developmental and stress responses (Fluhr and Mattoo, 1996). A close association of OS-ACS5 expression with young developing tissues and decrease with aging is reminiscent of the patterns seen in Arabidopsis for AT-ACS1 (Rodrigues-Pousada et al., 1993; Smalle et al., 1999). As was proposed for AT-ACS1, such pattern implicates involvement of OS-ACS5 expression in growth and development. This was further supported by the fact that the level of OS-ACS5 was enhanced upon complete submergence, as assessed by quantitative GUS assays and by in situ hybridization in this study as well as by previous RNA gel-blot experiments. This enhancement has been correlated with leaf growth patterns both in lowland and deepwater rice (Van Der Straeten et al., 2001; Zhou et al., 2001). The stimulation of expression upon submergence also confirmed the specificity of the GUS staining and in situ hybridizations as not merely being associated to cells with dense cytoplasm and high RNA content. This observation was supported by the absence of OS-ACS5 promoter activity in meristematic tissue, which is known to contain very dense, RNA-rich cells (see root primordia and emerging lateral root tips in Fig. 4G). In addition, in the control line pRG16 that carries the gus gene under control of the CaMV 35S promoter, very little difference in signal intensity was seen between cells in the vascular bundles of the stem and surrounding parenchymatous tissue (Fig. 4Q), whereas the latter was devoid of any signal in pRG11 stems of the same age (Fig. 4O). The above-mentioned facts clearly indicate that the expression pattern of OS-ACS5 is not simply confined to cells with dense cytoplasm.
Besides being highly expressed in emerging and immature leaf tissues, GUS activity and mRNA accumulation were also demonstrated in adventitious and lateral root primordia and differentiating vascular bundles. Adventitious root formation in rice was shown to be induced by ethylene rather than by auxin (Bleecker et al., 1987; Lorbiecke and Sauter, 1999; Mergemann and Sauter, 2000). Although it cannot be excluded that other OS-ACS genes might also play a role in the initiation of adventitious roots, our data present evidence for the involvement of OS-ACS5 in this process, which is a typical morphological adaptation to low-oxygen stress.
The overall tissue localization pattern has certain similarities with that found for expansin in rice (Cho and Kende, 1998). There is evidence for the existence of a multigene family for expansins in rice with at least four members (Cho and Kende, 1997). OS-EXP4 accumulation is induced upon submergence and GA treatment before the growth rate starts to increase. Although it remains to be proven, OS-EXP4 may also be responsive to ethylene. An ethylene-induced α-expansin has already been isolated from Rumex palustris (Vriezen et al., 2000). Close association of OS-ACS5 and OS-EXP4 expression might indicate a common role in the cascade of submergence-induced organ elongation.
With respect to cell-type specificity, OS-ACS5 expression was mainly associated with vascular tissues. Parenchymatous cells surrounding the differentiating vascular bundle showed GUS activity in young leaf tissues, which disappeared in the bundle sheath of mature vascular bundles. It is important to mention that OS-ACS5 expression was confined to vertical (longitudinal) vascular bundles. Formation of constitutive aerenchyma, typical for rice, leaves large gas-filled pores, which allow gas exchange with the environment. Although it remains largely unknown how the actual process of aerenchyma formation is initiated, it is clear that ethylene plays a role in this adaptive response, at least in some rice cultivars (Justin and Armstrong, 1991; Jackson and Armstrong, 1999; Drew et al., 2000; Colmer et al., 2001). Large gas spaces can be seen on transverse sections (Fig. 4, E and K) as well as a limited number of cell layers that surround the vascular bundle. In a recent report, Matsukura et al. (2000) showed that transverse vein differentiation is associated with gas space formation. Both events are initiated at a cell-specific position. A detailed analysis of different stages of leaf development should be done to demonstrate whether OS-ACS5 expression is confined to particular cell(s) and occurs at a particular time in development. Alternatively, it remains possible that the expression in the vertical vascular bundles is correlated with a local, enhanced ethylene production, targeting neighboring cells for programmed cell death. With respect to this hypothesis, it would be interesting to compare the tissue-specific pattern of OS-ACO with that found for OS-ACS5. The mechanisms that target certain cells to undergo ethylene action remain to be unraveled. Tissue localization of ethylene receptor genes as markers for ethylene responsiveness could provide a clue to understand at what level of the ethylene cascade major regulatory elements reside.
Finally, it is worth considering that the correlation of OS-ACS5 expression with conducting tissues may also be functionally related to ACC transport. Despite full submergence conditions and therefore absence of transpiration, long-distance mass flow of water occurs in aquatic plants and is controlled by root pressure (Pedersen, 1993; Pedersen et al., 1997). Based on what is known in several truly submerged aquatic species, it can be assumed that root pressure control of water flux also occurs in fully submerged rice plants. Although there is ample evidence for the importance of ACC transport in root-shoot signaling of flooded tomato (Lycopersicon esculentum) plants (Jackson, 1997), it remains to be proven how much this process would be involved in the adaptive response of rice to submergence. A careful analysis of ACC flux rates in conducting tissues could provide a clue to whether changes in ACC transport occur and how fast these changes lead to downstream processes. Because OS-ACS5 expression was subjected to a positive feedback control by ACC/ethylene (Van Der Straeten et al., 2001), ACC transport might further enhance the signal over the vascular system, thereby acting as a catalyst to maximize the response. This could be of great importance during short-term submergence. Future research will be needed to gain further insight into these processes.
MATERIALS AND METHODS
Constructs
The 1,783-bp of the 5′ region upstream of the start codon of the OS-ACS5 gene (GenBank accession no. X97066) were modified by PCR to allow subcloning in the NcoI site of the gus gene in pGUS1 (Aventis CropScience N.V., Gent, Belgium), resulting in an ATG fusion. After sequence confirmation, the 4.3-kb XbaI fragment covering the 5′ region of OS-ACS5, gus and the 3′-UTR of the octopine synthase gene was subcloned in pTCO114, a T-DNA vector containing a PPT resistance gene as a selectable marker (CaMV35S/bar/3′nos; D'Halluin, 1998). The resulting plasmid was named pRG11. Likewise, a construct was made containing a partially deleted 5′-UTR (1,424–1,783 bp) of the OS-ACS5 gene and designated pRG13. Finally, a CaMV35S/gus/3′nos cassette (Rodrigues-Pousada et al., 1993) was inserted into pTCO114 as an XbaI fragment, yielding pRG16, used as a positive control in the transformation procedure. Introduction into Agrobacterium tumefaciens LBA4404 (Hoekema et al., 1983) was by triparental mating (Van Haute et al., 1983).
Plant Material and Growth Conditions
All transformations were carried out on the japonica rice (Oryza sativa var Nippon Bare). In situ hybridization experiments were on rice cv IR36 (lowland rice) and rice cv Plai Ngam (deepwater rice). Seeds of rice var Nippon Bare and rice cv IR36 were provided by Dr. Gurdev Khush (International Rice Research Institute, Los Banos, Philippines), and rice cv Plai Ngam seeds were a kind gift from Dr. Somboon Anuntalabhochai (Chiang Mai University, Thailand).
For in situ hybridization, rice seeds (cv IR36 and cv Plai Ngam) were soaked in distilled water and kept at 24°C in the dark for 2 d. Imbibed seeds were germinated in the dark on vermiculite impregnated with one-half-strength Hoagland solution (Hoagland and Arnon, 1938). Seedlings were kept in a growth chamber at 28°C and 70% relative humidity, under a photoperiod of 16 h of light/8 h of darkness, with a light intensity of 100 μmol m−2 s−1. When 9 d old, plantlets were subjected to a submergence treatment lasting from 4 h to 14 d, as described previously (Van Der Straeten et al., 2001; Zhou et al., 2001).
Production of Transgenic Rice
Rice was transformed by coincubation of A. tumefaciens with wounded, compact embryogenic callus pieces derived from scutellum tissue of mature rice seeds (D'Halluin and Göbel, 1992; D'Halluin, 1998). Infected callus was subcultured under the conditions mentioned above on 2N6 medium. N6 medium (Chu et al., 1975) was supplemented with 30 g L−1 Suc, 2 mg L−1 2,4-dichlorophenoxyacetic acid, and 1.6 g L−1 Gelrite (Sigma-Aldrich, St. Louis) at pH 5.8. Selection of transformed calli and subsequent regeneration was on medium containing 5 μg mL−1 glufosinate-ammonium (ammonium-dl-homo-Ala-4-ylmethylphosphinate), an ammonium form of l-PPT (Riedel-de Haën AG, Seeize, Germany). When the primary transgenic plants were approximately 20 cm high (bearing four–five true leaves), they were transferred to a 2:1:1 (v/v) mixture of potting soil, clay, and sand and placed in the growth chamber. Flowering was induced by exposing plants to short-day conditions (10 h of light/14 h of darkness). T1 seeds were harvested 5 months after transfer to soil.
Selection of Transformants by PCR
Genomic DNA was isolated from pieces of callus or leaf blades using the method of Edwards et al. (1991), with minor modifications. In primary transgenic plants, every tiller was sampled. The tissue was ground in an Eppendorf tube on ice with a plastic micropestle (Eppendorf, Hamburg, Germany) and extracted with extraction buffer (200 mm Tris/HCl, pH 7.5; 250 mm NaCl; 25 mm EDTA; and 0.5% [w/v] SDS). Crude DNA was precipitated with isopropanol. The DNA extract was resuspended in 200 μL of Tris-EDTA buffer (10 mm Tris-HCl and 1 mm EDTA, pH 8). For each PCR reaction, 5 μL of the extract was used. The following primers were added (melting temperatures calculated using primer analysis software OLIGO 4.0 are mentioned in parentheses): Z30, 5′-GGTGTGTGAGATGAGATGATCC-3′ (50.5°C); Z38, 5′-TACCCGGAATTCCAATC-3′ (44.8°C); Z39, 5′-TGGCTCCATGGATCTAAG-3′ (45.1°C); Z41, 5′-TTGAATCGTCCATACTGG-3′ (43.0°C); Z42, 5′-CATGGACGACTTGTCTAGC-3′ (44.9°C); Z47, 5′-GCCAGGCAGTTTTAACGATC-3′ (51.6°C); and Z48, 5′-TGTCTGGCTTTTGGCTGTGA-3′ (54.1°C). An overview of the annealing positions of the primers is given in Figure 1. PCR reactions were performed on a thermal cycler (OmniGene, Middlesex, UK) following a standard protocol (Zhou et al., 2001).
Selection of Transformants by Herbicide Resistance
Rice seeds were placed on water-soaked 3MM paper (Whatman, Clifton, NJ) in a petri dish and kept in the dark at 27°C for 2 d. Germinated seeds were transferred to a petri dish containing 5 μg mL−1 PPT in water and kept in the growth chamber (16 h of light/8 h of darkness) for 4 d before scoring for resistance. Resistant seedlings grew normally under the selective pressure. In contrast, the development of sensitive seedlings was completely blocked: The plantlets had pale, barely developed coleoptiles (less than 5 mm high) and remained at that stage for a few days before cell death.
In adult plants, herbicide resistance was assayed on mature leaves by the leaf-painting procedure (Kumpatla et al., 1997). Leaves from sensitive plants turned yellow and dry, whereas those from resistant plants remained green.
Protein Extraction and Histochemical and Quantitative GUS Assays
Protein extraction was performed as described previously (Jefferson, 1987). Measurements of protein concentration were according to Bradford (1976) and spectrophotometric measurements were done in a computer-directed microtiter plate reader (340-ATTC colorimetric reader equipped with SOFT 2000 software; SLT Lab Instruments, Salzburg, Austria).
An improved histochemical staining method for GUS activity was used to monitor the expression of the OS-ACS5 promoter in transgenic rice (Rueb and Hensgens, 1997). Photographs of whole mounts were taken at low magnification using bright-field optics. To gain details of staining, GUS-positive tissues were further sectioned either by using a vibroslicer (Campden Instruments Ltd., London) or a microtome 2050 (Reichert-Jung, Nußloch, Germany). Thick Vibroslicer sections (70–80 μm) were examined with a binocular (model Stemi NV 11; Zeiss, Jena, Germany) to observe blue staining. Paraffin-embedded thin sections (10 μm) were examined under a microscope (model Stemi SV11; Zeiss) in bright field (GUS staining was blue) or in dark field (GUS staining was red).
Quantitative GUS activity measurements were performed as reported previously (Jefferson, 1987), with 4-methyl-umbelliferyl glucuronide as substrate in a buffer containing 50 mm Na2EDTA and 0.1% (v/v) Triton X-100. The fluorometric method (Breyne et al., 1993) was adapted for determination of GUS activity by kinetic analysis at 37°C in a computer-directed microtiter plate reader (Fluoroscan II; Labsystems, Helsinki). The results are expressed in units of GUS per mg of protein. In each line, four PPT-resistant plants (2 months of age) were randomly sampled and the stems were used for protein extraction and GUS measurements. Data were averaged from four independent extractions; each sample was measured twice.
In Situ Hybridization
The procedure was essentially according to de Almeida Engler et al. (1998). At harvest, seedlings (approximately 50 for each time point) were surface dried and quickly submerged in the fixative. On average, 15 longitudinal and 20 transverse sections per seedling were selected for hybridization. To detect the abundance of OS-ACS5 mRNA, the antisense transcript of a 570-bp carboxyl-terminal fragment (2,795–3,364 in X97066) was used as a probe. This fragment was chosen because of its significant divergence to all other ACS family members. The sense transcript of the same region was used as a negative control. The probes were prepared with the Riboprobe Gemini II kit (Promega, Madison, WI) by in vitro transcription in the presence of 35S-UTP. For signal detection, the slides were exposed to the NTB-2 emulsion (Eastman-Kodak, Rochester, NY) for 8 weeks at 4°C. Three independent hybridization experiments were performed. A dark-field microscope was used to visualize the hybridization signal, reflected as bright silver grain spots. To reveal cell walls, a toluidine blue staining was performed as described previously (de Almeida Engler et al., 1998).
ACKNOWLEDGMENTS
The authors gratefully acknowledge Gurdev Khush (International Rice Research Institute, Manila, Philippines) and Somboon Anuntalabhochai (Chiang Mai University, Thailand) for providing rice seeds (cv IR36 and cv Plai Ngam, respectively), and thank Margret Sauter (Institut für Allgemeine Botanik, Hamburg University, Germany) for helpful discussions, Wim Vriezen (Department of Molecular Genetics, Ghent University, Belgium) for critical reading of the manuscript, Ruth De Groot (Department of Molecular Genetics, Ghent University) for help with digital imaging of in situ hybridizations, Martine De Cock (Department of Molecular Genetics, Ghent University) for layout, and Rebecca Verbanck (Department of Molecular Genetics, Ghent University) for art work.
Footnotes
This work was supported by the European Union (grant no. ISC China CI1*–CT93–0082) and by the Geconcerteerde Overlegde Acties (grant no. GOA 96016).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001206.
LITERATURE CITED
- Bleecker AB, Rose-John S, Kende H. An evaluation of 2,5-norbornadiene as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiol. 1987;84:395–398. doi: 10.1104/pp.84.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breyne P, De Loose M, Dedonder A, Van Montagu M, Depicker A. Quantitative kinetic analysis of β-glucuronidase activities using a computer-directed microtiter plate reader. Plant Mol Biol Rep. 1993;11:21–31. [Google Scholar]
- Cho H-T, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-T, Kende H. Tissue localization of expansins in deepwater rice. Plant J. 1998;15:805–812. doi: 10.1046/j.1365-313x.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- Chu CC, Wang CC, Sun SC, Hsu SC, Yin KC, Chu CY, Bi FY. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci Sin. 1975;18:659–668. [Google Scholar]
- Cohen E, Kende H. In vivo 1-aminocyclopropane-1-carboxylate synthase activity in internodes of deepwater rice. Enhancement by submergence and low oxygen levels. Plant Physiol. 1987;84:282–286. doi: 10.1104/pp.84.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Cox MCH, Voesenek LACJ. 7th Conference of the International Society for Plant Anaerobiosis (ISPA), June 12–16, 2001. Nigmegen, The Netherlands: University of Nijmegen; 2001. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in rice (abstract no. 30) p. 27. [Google Scholar]
- D'Halluin K, inventor. August 27, 1998. Improved transformation method for plants. Patent Application No. PCT/IB98/00220, WO98/37212.
- D'Halluin K, Göbel E, inventors. June 24, 1992. Process for transforming monocotyledonous plants. Patent Application No. PCT/EP91/02198, WO92/09696.
- de Almeida Engler J, Van Montagu M, Engler G. Whole-mount in situ hybridization in plants. In: Martínez-Zapater JM, Salinas J, editors. Arabidopsis Protocols, Methods in Molecular Biology. Vol. 82. Totowa, NJ: Humana Press; 1998. pp. 373–384. [DOI] [PubMed] [Google Scholar]
- de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL, Jr, Inzé D, Van Montagu M, Engler G, Gheysen G. Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell. 1999;11:793–807. doi: 10.1105/tpc.11.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ES, Dolferus R, Ellis M, Rahman M, Wu Y, Hoeren FU, Grover A, Ismond KP, Good AG, Peacock WJ. Molecular strategies for improving waterlogging tolerance in plants. J Exp Bot. 2000;51:89–97. [PubMed] [Google Scholar]
- Drew MC, He C-J, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991;19:1349–1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R, Mattoo AK. Ethylene: biosynthesis and perception. Crit Rev Plant Sci. 1996;15:479–523. [Google Scholar]
- He C-J, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdt RW. Research priorities for rice biotechnology. In: Khush GS, Toenniessen GH, editors. Rice Biotechnology. Wallingford, UK: CAB International; 1991. pp. 19–54. [Google Scholar]
- Hoagland DT, Arnon DI. Water culture method for growing plants without soil. Univ Calif Agric Exp Stu Circ. 1938;347:1–39. [Google Scholar]
- Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA. A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature. 1983;303:179–180. [Google Scholar]
- Jackson M. Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci. 1997;2:22–28. [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999;1:274–287. [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Justin SHFW, Armstrong W. Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.) New Phytol. 1991;118:49–62. [Google Scholar]
- Kende H, van der Knaap E, Cho H-T. Deepwater rice: a model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Thakur R, Akbar M, HilleRisLambers D, Seshu DV. Relationship of ethylene production to elongation in deepwater rice. Crop Sci. 1987;27:1188–1196. [Google Scholar]
- Krishnan P, Ravi I, Krishnayya GR. Leaf senescence in submerged rice plants. Exp Agric. 1999;35:345–355. [Google Scholar]
- Kumpatla SP, Teng W, Buchholz WG, Hall TC. Epigenetic transcriptional silencing and 5-azacytidine-mediated reactivation of a complex transgene in rice. Plant Physiol. 1997;115:361–373. doi: 10.1104/pp.115.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M. Induction of cell growth and cell division in the intercalary meristem of submerged deepwater rice (Oryza sativa L.) Planta. 1998;204:140–145. [Google Scholar]
- Lorbiecke R, Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–29. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura C, Kawai M, Toyofuku K, Barrero RA, Uchimiya H, Yamaguchi J. Transverse vein differentiation associated with gas space formation: fate of the middle cell layer in leaf sheath development of rice. Ann Bot. 2000;85:19–27. [Google Scholar]
- Meier U. Growth Stages of Mono- and Dicotyledonous Plants (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie [BBCH]-Monograph). Berlin: Blackwell Wissenschafts-Verlag; 1997. [Google Scholar]
- Mekhedov SI, Kende H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 1996;37:531–537. doi: 10.1093/oxfordjournals.pcp.a028976. [DOI] [PubMed] [Google Scholar]
- Mergemann H, Sauter M. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 2000;124:609–614. doi: 10.1104/pp.124.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J-P, Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwaki Y, Nagao M. Growth of rice coleoptiles in relation to oxygen concentration. Sci Rep Tohoku Univ Series V Biol. 1967;33:1–5. [Google Scholar]
- Pearce DME, Hall KC, Jackson MB. The effects of oxygen, carbon dioxide and ethylene on ethylene biosynthesis in relation to shoot extension in seedlings of rice (Oryza sativa) and barnyard grass (Echinochloa oryzoides) Ann Bot. 1992;69:441–447. [Google Scholar]
- Pearce DME, Jackson MB. Comparison of growth responses of barnyard grass (Echinochloa oryzoides) and rice (Oryza sativa) to submergence, ethylene, carbon dioxide and oxygen shortage. Ann Bot. 1991;68:201–209. [Google Scholar]
- Pedersen O. Long-distance water transport in aquatic plants. Plant Physiol. 1993;103:1369–1375. doi: 10.1104/pp.103.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Jørgensen LB, Sand-Jensen K. Through-flow of water in leaves of a submerged plant is influenced by the apical opening. Planta. 1997;202:43–50. [Google Scholar]
- Rodrigues-Pousada RA, De Rycke R, Dedonder A, Van Caeneghem W, Engler G, Van Montagu M, Van Der Straeten D. The Arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell. 1993;5:897–911. doi: 10.1105/tpc.5.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueb S, Hensgens LAM. Improved histochemical staining for β-d-glucuronidase activity in monocotyledonous plants. Rice Genet Newslett. 1997;6:168. [Google Scholar]
- Setter TL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta D. Physiology and genetics of submergence tolerance in rice. Ann Bot Suppl. 1997;69:67–77. [Google Scholar]
- Smalle J, Haegman M, Mertens J, Vangronsveld J, Van Montagu M, Van Der Straeten D. The expression pattern of the Arabidopsis ACC synthase gene 1 during rosette leaf development. J Exp Bot. 1999;50:1561–1566. [Google Scholar]
- Van Der Straeten D, Anuntalabhochai S, Van Caeneghem W, Zhou Z, Gielen J, Van Montagu M. Expression of three members of the ACC synthase gene family in deepwater rice by submergence, wounding and hormonal treatments. Plant Sci. 1997;124:79–87. [Google Scholar]
- Van Der Straeten D, Zhou Z, Prinsen E, Van Onckelen H, Van Montagu M. A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol. 2001;125:955–968. doi: 10.1104/pp.125.2.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E, Joos H, Maes M, Warren G, Van Montagu M, Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2:411–418. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot Suppl. 1997;79:3–20. [Google Scholar]
- Vriezen W. Molecular regulation of submergence induced cell elongation. PhD thesis. Nijmegen, The Netherlands: The Katholieke Universiteit Nijmegen; 2000. [Google Scholar]
- Vriezen WH, De Graaf B, Mariani C, Voesenek LACJ. Submergence induces expansin gene expression in flooding-tolerant Rumex palustris and not in flooding-intolerant R. acetosa. Planta. 2000;210:956–963. doi: 10.1007/s004250050703. [DOI] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Anaerobiosis and plant growth hormones induce two genes encoding 1- aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.) Mol Biol Cell. 1993;4:363–373. doi: 10.1091/mbc.4.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Expression characteristics of OS-ACS1 and OS-ACS2, two members of the 1-aminocyclopropane-1-carboxylate synthase gene family in rice (Oryza sativa L. cv. Habiganj Aman II) during partial submergence. Plant Mol Biol. 1997;33:71–77. doi: 10.1023/b:plan.0000009693.26740.c3. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Vriezen W, Van Caeneghem W, Van Montagu M, Van Der Straeten D. Rapid induction of a novel ACC synthase gene in deepwater rice seedlings upon complete submergence. Euphytica. 2001;121:137–143. [Google Scholar]