Abstract
Pectin is a class of complex cell wall polysaccharides with multiple roles during cell development. Assigning specific functions to particular polysaccharides is in its infancy, in part, because of the limited number of mutants and transformants available with modified pectic polymers in their walls. Pectins are also important polymers with diverse applications in the food and pharmaceutical industries, which would benefit from technology for producing pectins with specific functional properties. In this report, we describe the generation of potato (Solanum tuberosum L. cv Posmo) tuber transformants producing pectic rhamnogalacturonan I (RGI) with a low level of arabinosylation. This was achieved by the expression of a Golgi membrane-anchored endo-α-1,5-arabinanase. Sugar composition analysis of RGI isolated from transformed and wild-type tubers showed that the arabinose content was decreased by approximately 70% in transformed cell walls compared with wild type. The modification of the RGI was confirmed by immunolabeling with an antibody recognizing α-1,5-arabinan. This is the first time, to our knowledge, that the biosynthesis of a plant cell wall polysaccharide has been manipulated through the action of a glycosyl hydrolase targeted to the Golgi compartment.
Current models of the plant cell wall present pectins as complex matrix polysaccharides embedding the load-bearing structures of the wall (cellulose microfibrils and hemicelluloses) and forming the middle lamella, which cements neighboring cells together (Carpita and Gibeaut, 1993). The pectic matrix has been described as coextensive with the microfibrillar and hemicellulosic polymers of the wall (Roberts, 1994), suggesting that some pectic polymers may be structural components rather than mere fillers of cell wall pores. Pectin constitutes a very complex class of polysaccharides (Ridley et al., 2001) and their large-scale organization in the cell wall is far from resolved. The prevailing view of pectin fine structure (Schols and Voragen, 1996) and conformation and architecture (Pérez et al., 2000) has recently been challenged and a new pectin model is being drafted (J.-P. Vincken, A. Voragen, and H. Schols, personal communication). Neither model directly suggests roles for pectic side-chains, for example, arabinans, the polymer of interest to the present investigation. Arabinans are very flexible molecules in aqueous solution (Cros et al., 1994), whereas 13C-NMR studies by Renard and Jarvis (1999) demonstrate that they are also very mobile molecules in muro. The authors concluded that arabinans are not structural components; rather, they propose a role for them as plasticizers and water binding agents in the wall. Testing this working hypothesis requires plants in which the arabinan structure or content is modified, and a technology for producing such plants is presented in this report.
Because they are the most abundant bio-polymers on Earth (Prade et al., 1999), cell wall polysaccharides are of fundamental interest and are used by industry for both food and non-food applications. Biotechnological approaches for their modification and further exploitation have so far been limited because modification and production of carbohydrates has focused primarily on the generation of novel starches and fructans (Heyer et al., 1999). The primary reason for this slow progress in bioengineering is the fact that the biosynthetic pathways of cell wall polysaccharides have not been fully characterized at the molecular level. Despite significant efforts to elucidate the biogenesis of cell wall carbohydrates through mutant screening programs (Zablackis et al., 1996; Reiter et al., 1997) and through cloning and characterization of enzymes involved in cellulose (Arioli et al., 1998), xyloglucan (Perrin et al., 1999), and galactomannan (Edwards et al., 1999) biosynthesis, the cell wall polysaccharide biosynthetic apparatus will remain elusive for quite a while given the large number of genes predicted to be involved (Mohnen, 1999). Simpler approaches are called for. We have previously demonstrated that β-1,4-galactan side-chains of the pectic polymer rhamnogalacturonan I (RGI) can be enzymatically cleaved post deposition in the cell wall without compromising plant viability (Sørensen et al., 2000). This was achieved through the targeting of a fungal endo-1,4-β-d-galactanase to the apoplast in potato (Solanum tuberosum L. cv Posmo) tubers. In this paper, we present technology for direct interference with pectin biosynthesis in Golgi vesicles. By targeting a rat α-2,6 sialyl transferase-endo-α-1,5-arabinanase fusion protein to the Golgi compartment of potato tuber cells, arabinan side-chains on RGI can be hydrolyzed at the site of pectin biosynthesis. We demonstrate that this approach reduces the biosynthesis of RGI-arabinans in transgenic potato tubers without compromising the viability of plants.
RESULTS
The Endo-Arabinanase Displays Activity toward Potato Rhamnogalacturonan I in Vitro
A purified recombinant endo-arabinanase from Aspergillus aculeatus shows endo-activity in vitro against debranched sugar beet arabinan releasing primarily arabinobiose and arabinotriose (Skjøt et al., 2001). We verified that it is also active toward RGI isolated from wild-type (WT) potato tubers. Monosaccharide analysis of isolated RGI from potato treated with the arabinanase, showed that enzyme treatment resulted in a 75% reduction in the Ara content compared with the untreated sample (not shown).
Tubers Are Not Recovered if Arabinanase Is Targeted to the Apoplast
The cDNA encoding an A. aculeatus endo-α-1,5-arabinanase including the fungal secretion signal (Skjøt et al., 2001) was transcriptionally fused to the tuber-specific granule-bound starch synthase promoter (Visser et al., 1991), giving the vector pGED/ARA (Fig. 1). Transformation with pGED/ARA reduced the transformation frequency: 27% compared with approximately 80% for the empty pGED vector. Regenerated in vitro plantlets were tested for the presence of the endo-arabinanase cDNA by Southern analysis (not shown), and 10 independent transformants were transferred to soil. After 16 weeks of growth, transgenic pGED/ARA potato plants showed a severe phenotype lacking side shoots (Fig. 2), flowers, stolons, and tubers (not shown). Endo-arabinanase activity could be detected in induced leaves from in vitro-grown plantlets using an endo-arabinanase-specific plate-assay. The granule-bound starch synthase promoter was induced using the method described previously (Visser et al., 1991). In addition, polyclonal antibodies raised against the endo-arabinanase recognized a protein in the extracts from induced plantlets. The molecular mass of this protein was in range with the 34 kD (Skjøt et al., 2001) determined for the arabinanase when expressed in Aspergillus oryzae (Fig. 3). The absence of tubers from the pGED/ARA plants made it particularly relevant to develop a Golgi-anchored version of the arabinanase as an alternative approach to modifying pectins in planta.
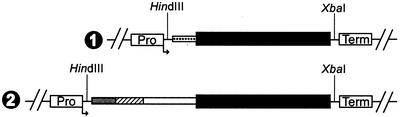
Figure 1.
Expression cassettes. 1, The pGED/ARA expression cassette for apoplastic targeting. 2, The pGED/ST::ARA cassette conferring Golgi targeting. Dotted bar, Region encoding the A. aculeatus α-1,5-endo-arabinanase signal sequence. Black bar, Mature A. aculeatus α-1,5-endo-arabinanase. Gray bar, Region encoding the β-galactoside α-2,6-sialyltransferase cytoplasmic domain. Hatched bar, Region encoding the β-galactoside α-2,6-sialyltransferase membrane spanning/signal sequence domain. White bar, Region encoding a truncated β-galactoside α-2,6-sialyltransferase catalytic domain. Pro, Tuber-specific granule-bound starch synthase promoter. Term, Nopaline synthase terminator and polyadenylation site.
Figure 2.
Phenotypic appearance of WT and endo-arabinanase expressing potato plants. 1, Transformant harboring the pGED/ARA construct for apoplastic targeting. 2, Transformant harboring the pGED/ST::ARA construct for Golgi targeting. 3, WT potato plant.
Figure 3.
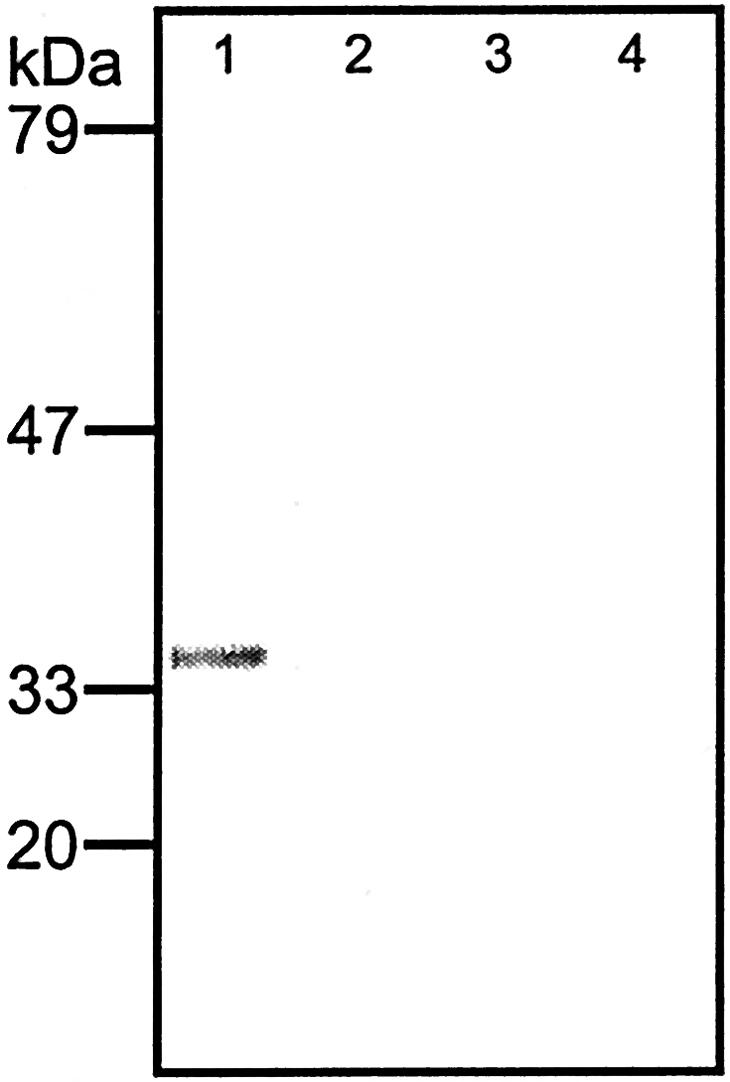
Western analysis of protein extracts from pGED/ARA leaves after 24-h induction of the granule-bound starch synthase promoter with Suc and light. 1, Induced pGED/ARA leaves. 2, Noninduced pGED/ARA leaves. 3, Induced WT leaves. 4, Noninduced WT leaves. Blot was probed with an antiserum raised against the A. aculeatus endo-arabinanase.
Generation of Potato Plants Expressing a Golgi-Localized Arabinanase
To generate a Golgi-localized enzyme, we fused the 52 N-terminal amino acids of a rat α-2,6 sialyl transferase (ST; Munro, 1991) to the mature part of the arabinanase creating pGED/ST::ARA (Fig. 1). The fusion protein has a calculated molecular mass of 39 kD. Previous analyses of the primary structure of ST suggest that the protein comprises a nine-residue N-terminal cytoplasmic domain, a 17-residue hydrophobic sequence that serves as a membrane anchor and signal sequence, and a large Golgi-lumenal, catalytic domain (Weinstein et al., 1987). The region including the N-terminal cytoplasmic domain, the hydrophobic sequence, and 26 residues of the catalytic domain has previously been shown to direct Golgi targeting of green fluorescent protein (Boevink et al., 1998) and lysozyme (Munro, 1991) in planta.
Transformation with pGED/ST::ARA resulted in transformation frequencies comparable with those obtained with the empty pGED vector. All tested plantlets contained the DNA encoding the ST-arabinanase (ST-ARA) fusion. Ten plantlets expressing ST-ARA were transferred to soil, and their growth was monitored for 16 weeks; during this period they produced stolons and tubers in numbers comparable with the WT plants (data not shown). Furthermore, transgenic tubers sprouted as efficiently as WT tubers and gave rise to plants with WT phenotypic traits (Fig. 2).
The Sialyltransferase-Arabinanase Fusion Product Has Endo-Arabinanase Activity
Two transgenic pGED/ST::ARA lines (see “Materials and Methods”) whose tuber protein extracts gave rise to intermediate (T5.2) and high endo-arabinanase activities (T7.2) were selected for further quantitative analysis. A colorimetric assay using sugar-beet arabinan as substrate demonstrated endo-arabinanase activities of 129, 405, and 940 milliunits g−1 fresh tuber weight in extracts from WT, T5.2, and T7.2, respectively.
The Sialyltransferase-Arabinanase Fusion Product Is Directed to the Golgi Compartment
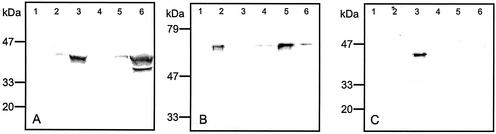
We investigated the cellular localization of the ST-ARA fusion protein in extracts of WT and T7.2 using classical organelle separation by Suc gradient centrifugation. The enrichment of Golgi and endoplasmic reticulum (ER) vesicles in gradient fractions was investigated by immunoblotting using monoclonal antibodies (mAbs) against the Golgi marker RGP1 (reversibly glycosylated polypeptide from pea [Pisum sativum; Dhugga et al., 1997]; Fig. 4A) and the ER marker CRH (Calnexin/Calreticulin from barley [Hordeum vulgare; Møgelsvang and Simpson, 1998]; Fig. 4B). The results confirmed that particular fractions were enriched in the expected organelles. However, we detected minor contamination of the ER preparations with Golgi vesicles, whereas the cytosolic/soluble protein and Golgi preparations contained traces of the ER marker protein.
Figure 4.
Immunoblots of fractions collected during separation of organelles from T7.2 and WT tuber tissue. 1, Cytosolic fraction from T7.2. 2, ER fraction from T7.2. 3, Golgi fraction from T7.2. 4, Putative cytosolic fraction from WT. 5, Putative ER fraction from WT. 6, Golgi fraction from WT. A, Western blot probed with mAbs recognizing the Golgi marker RGP1. B, Western blot probed with mAbs recognizing the ER marker CRH. C, Western blot probed with polyclonal antibodies raised against the A. aculeatus endo-arabinanase. The observed molecular masses are in agreement with the 41.5 kD determined for pea RGP1 (Dhugga et al., 1997), the 60 kD determined for the tobacco CRH homolog (Denecke et al., 1995), and the predicted molecular mass of 39 kD for the ST-ARA fusion protein.
Western blots of the organelle fractions were probed for ST-ARA using an antibody raised against the endo-arabinanase (Fig. 4C). These experiments showed that ST-ARA was localized specifically to the Golgi compartment of the transgenic tubers.
Targeting of the Arabinanase to the Golgi Apparatus Leads to Changes in Cell Wall Pectins
Total cell wall material was isolated from transgenic (T7.2 and T5.2) and WT tubers taking precautions to avoid enzymatic degradation of cell walls by the ST-ARA enzyme and any endogenous arabinanase activities (see “Materials and Methods”). The isolated cell wall material was subjected to Seaman hydrolysis (Selvendran et al., 1979), and the relative abundance of the resulting monosaccharides was determined. This analysis did not detect any significant differences between total cell walls prepared from WT (Sørensen et al., 2000) and ST-ARA transformants (data not shown). To achieve a more detailed insight into the composition of the pectic polymers, walls from WT and transgenic tubers were enzymatically digested with a combination of pectin methyl esterase and endo-polygalacturonase, a procedure that solubilizes almost all of the RGI present in the walls (Sørensen et al., 2000). The solubilized pectic fragments were separated using size exclusion chromatography resulting in the isolation of an RGI-enriched fraction. The total yield of the solubilized pectins (0.94 mg g−1 fresh tuber weight) and their molecular size profiles did not differ between the transgenic and WT pectins (data not shown). However, sugar analyses performed on the samples enriched in RGI (Table I) showed that the pectic arabinan content of both T7.2 and T5.2 was reduced by 69% compared with the WT values.
Table I.
Sugar compositions (mol%) of RGI material obtained from tubers of two pGED/ST∷ARA transformants (T7.2, T5.2) and wild type (WT)
| WT | T7.2 | T5.2 | |
|---|---|---|---|
| Fuc | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| Ara | 15.8 ± 1.2 | 4.9 ± 0.3 | 5.2 ± 0.5 |
| Rha | 5.7 ± 2.0 | 6.1 ± 1.4 | 6.2 ± 0.3 |
| Gal | 64.6 ± 4.1 | 77.0 ± 3.5 | 76.3 ± 4.3 |
| Glc | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 |
| Xyl | 0.4 ± 0.3 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| GalA | 13.0 ± 2.5 | 11.3 ± 1.5 | 11.8 ± 1.1 |
Data (±sd) are the average of three independent experiments.
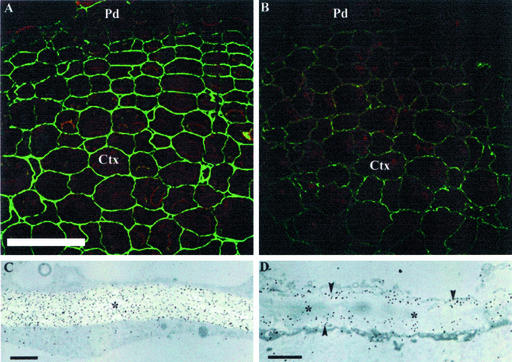
The mAb LM6 (Willats et al., 1998) was used to monitor the abundance and localization of 1,5-α-arabinan in WT and transgenic tuber walls (Fig. 5). Reflection confocal imaging of WT parenchymal tuber cell walls labeled with LM6 showed that the epitope was abundant (Fig. 5A), but significantly reduced in both transgenic lines (Fig. 5B). This reduction was apparent in both cortical (Fig. 5, A and B) and perimedullary (Fig. 5, C and D) cell walls. Transmission electron microscopy confirmed these observations and showed that the reduced labeling in the transgenic tuber walls was because of a reduction of epitopes in the primary wall and a complete loss from the middle lamella (Fig. 5, C and D). These changes were specific to 1,5-α-arabinan; labeling of serial sections with mAb LM5 that recognizes 1,4-β-galactan (Jones et al., 1997), showed no differences between parenchymal cell walls from WT and transgenic tubers (not shown).
Figure 5.
Sections of WT (A and C) and ST-ARA-expressing (T7.2, B and D) potato tubers gold-labeled with mAb LM6, silver-enhanced and viewed by reflection confocal laser scanning microscopy (A and B) and transmission electron microscopy (C and D). WT parenchymal walls are strongly labeled (green in A; black particles in C); whereas, in contrast, the LM6 arabinan epitope abundance in the equivalent T7.2 walls is greatly reduced and localized to the primary wall (arrowheads in D) and absent from the middle lamella (asterisks in C and D). Pd and Ctx indicate periderm and cortical regions, respectively; C and D show perimedullary walls. Scale bars represent 200 μm (in A and B) and 2 μm (in C and D).
DISCUSSION
To our knowledge, this is the first report of the generation of a novel pectin by the expression of a Golgi-targeted polysaccharide-modifying enzyme in planta. This technique offers significant potential for bioengineering plant cell wall polysaccharides and holds promise for the generation of novel polymers for industrial use.
Pectic polysaccharides are multifunctional polymers implicated in the regulation of cell wall mechanical properties (McCann and Roberts, 1994; Chanliaud and Gidley, 1999), cell-cell adhesion (Satoh, 1998), cell wall porosity (Baron-Epel et al., 1988; Fleischer et al., 1998), and embryogenesis (Satoh, 1998) and to be a source of signaling molecules released under herbivore attack (Côté and Hahn, 1994). Deposition of pectic arabinans is known to be under strict developmental control in potato (Bush et al., 2001). In accordance, in planta manipulation of pectic polysaccharides may lead to changes in the physiology of the plant. The severe phenotype of the pGED/ARA plants indicates that there are limits to which plants can tolerate transgenic manipulation. The severe phenotype of the apoplastic arabinanase plants may be a specific stress response to the release into the wall of arabinosyl-containing oligosaccharides or a similar response to the arabinanase enzyme protein; plants expressing an apoplastic endo-galactanase (Sørensen et al., 2000) developed normally. When the arabinanase is targeted to the Golgi, these oligosaccharides may be retained in this organelle and recycled. Furthermore, modification of RGI before its deposition into the wall may allow for the plant to compensate for the imposed changes in polysaccharide structure. The plants expressing Golgi-targeted arabinanase certainly tolerated a major loss of Ara from their wall-bound RGI and yet remained viable and developed normally. A decrease in arabinosyl content not accompanied by any apparent phenotype has previously been reported for the Arabidopsis mur4 mutant (Burget and Reiter, 1999) that shows a reduction to 50% of the WT Ara level in leaf tissue.
The amount of arabinosyl residues removed from RGI in planta was similar to that removed in vitro (69% versus 75%). Analysis of crude cell wall material did not detect any significant differences in monosaccharide abundance between WT and ST-ARA transformants, indicating the necessity to isolate the modified pectin polymer before analysis. The mAb LM6 arabinan epitope in WT tubers is localized throughout all parenchymal walls except at cell corners, where it is absent from the expanded middle lamella (Bush and McCann, 1999). In potato tubers expressing the ST-ARA fusion protein, this epitope was significantly reduced in abundance, which correlated well with the decreased Ara content of RGI from ST-ARA transformants as shown by sugar analysis.
The prospects of controlling pectin structure in higher plants are attractive and far-reaching. Because of significant interspecies differences in pectic quality, pectins are currently only extracted on an industrial scale from a few crop plants (Voragen et al., 1995). Other sources of pectin, such as potato and sugar beet pulp exist, but the abundance of side-chains composed of neutral sugars is believed to impair the gelling ability of the pectin (Hwang and Kokini, 1991; Hwang et al., 1993). In vitro enzymatic removal of Ara side chains combined with enzymatic demethoxylation and deacetylation has been shown to improve gelling of sugar beet pectin significantly (Matthew et al., 1990).
The use of pectin as a fat and sugar replacer in low-calorie foods is expected to increase in the future (Thakur et al., 1997). In addition, the immunostimulatory activity of RGI derived polysaccharides (Guo et al., 2000) indicate that pharmaceutical applications of this cell wall component may emerge. Pharmaceutical activity of RGI polymers has especially been demonstrated in preparations originating from non-crop plants (Paulsen, 2000), for which neither cultivation systems nor processing plants exist. The production of these polysaccharides in crop plants engineered following the principles presented here may prove to be more cost-effective than cultivating WT exotic plants that produce the native polysaccharides. The manipulation of cell wall polymers in planta to generate, for instance, pectins modified to suit a specific commercial use will be a significant advance in biotechnology.
MATERIALS AND METHODS
Vector Construction and Potato (Solanum tuberosum L. cv Posmo) Transformation
The 1.2-kb ara1 cDNA encoding an Aspergillus aculeatus endo-α-1,5-arabinanase (ARA1) was excised from the vector pC1A4 (Skjøt et al., 2001) by digestion with HindIII and XbaI and cloned in the pGED vector (Sørensen et al., 2000) giving pGED/ARA.
A fragment encoding 52 N-terminal amino acid residues of ST was amplified by PCR with primers (ST specific sequences underlined) 5′ GAC GAA GCT TAT GAT TCA TAC CAA CTT G 3′ (primer1, HindIII site included) and 5′ GGA GCC GGG GTT GGC GTA GGC CAC TTT CTC CTG GCT C 3′ (primer 2) from pST-MYC (Munro, 1991). The mature part of ARA1 (302 residues) was amplified from pC1A4 (Skjøt et al., 2001) with primers (ara1 specific sequences in bold) 5′ GAG CCA GGA GAA AGT GGC CTA CGC CAA CCC CGG CTC C 3′ (primer 3) and 5′ CAG TCT AGA CTA CAC AAC AGG CCA GCC 3′ (primer 4, XbaI site included). The two products were combined by sequence overlap extension PCR (Higuchi et al., 1988) with primers 1 and 4. The fusion product was cloned HindIII/XbaI in pGED (Sørensen et al., 2000) giving pGED/ST::ARA. pGED/ARA and pGED/ST::ARA were transferred to Agrobacterium tumefaciens strain LBA 4404 by electroporation. Transformation of potato leaf discs essentially followed a regeneration procedure described previously (Edwards et al., 1991) using kanamycin selection. Regenerated transgenic and WT plants were grown for 16 weeks in the greenhouse before tuber harvest. The transgene state was confirmed by detection of an immunoreactive gene product of the correct size and by arabinanase activity toward azurine blue cross-linked arabinan in a plate assay described previously (Skjøt et al., 2001). There is no background activity in WT tuber cells using this substrate. Southern analysis was performed using the ara1 cDNA (Skjøt et al., 2001) as probe under standard conditions (Sambrook et al., 1989).
Extraction of Potato Tuber Protein, Endo-Arabinanase Assays, and Western Analysis
Potato tuber extracts were prepared as described by Sørensen et al. (2000), except that the extraction buffer was 0.1 m citric/trisodium citrate buffer, pH 5.5, supplemented with Complete Proteinase Inhibitor cocktail tablets according to the manufacturer (Boehringer Mannheim, Kvistgaard, Denmark) and that sea sand was present during grinding. Extracts were screened for the presence of the ST-arabinanase fusion protein using standard western analysis procedures. Enzyme activity plate assays were performed as described previously (Skjøt et al., 2001). The endo-arabinanase assay using debranched arabinan colored with Procion red dye was performed as described by the manufacturer (Megazyme, Bray, Ireland) using purified recombinant A. aculeatus endo-arabinanase as standard. One unit was defined as the amount of enzyme releasing 1 μmol Ara reducing sugar equivalents min−1 from debranched sugar-beet arabinan.
Isolation and Analysis of Cell Wall Material
Potato tuber cell walls and pectic polysaccharides were isolated and analyzed using the methods described previously (Sørensen et al., 2000) but with minor modifications: Frozen comminuted tuber tissue (30 g) was homogenized and treated as described (Sørensen et al., 2000), except that washes of the residue with buffer C were performed directly on the nylon mesh and not overnight. The residue on the filter was immediately extracted with phenol:glacial acetic acid:water (2:1:1, v/v) at room temperature. All other steps were carried out at 4°C.
To investigate whether the cell wall arabinanase activity was fully inactivated during the cell wall isolations, we tested the activity of 0.5 unit of purified endo-arabinanase in our extraction buffer using the plate assay. After 20 h of incubation at 5°C, no arabinanase activity could be detected, demonstrating that under the extraction conditions used, the arabinanase was not able to modify arabinans during cell wall isolation.
Immunogold Labeling
Transgenic and WT tuber samples were processed for low temperature resin-embedding and immunogold-labeling as reported previously (Bush and McCann, 1999) using mAbs LM5 (recognizes a tetrasaccharide composed of 1,4-linked β-d-Galp residues [Jones et al., 1997]) and LM6 (recognizes a pentasaccharide of 1,5-linked α-l-Araf residues [Willats et al., 1998]). Sections were viewed with an TCS NT confocal laser scanning microscope (Leica, Wetzlar, Germany) or a 1200EX transmission electron microscopy (JEOL, Tokyo; Bush and McCann, 1999).
Organelle Separation and Analysis
The procedure (Munoz et al., 1996) used for organelle isolation was slightly modified: Dithiothreitol was replaced with 20 mm ascorbate. Monoclonal Abs raised against RGP1 from pea (Pisum sativum; Dhugga et al., 1997), and CRH from barley (Hordeum vulgare; Møgelsvang and Simpson, 1998) were used to detect Golgi or ER vesicles, respectively, in the preparations.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Dr. G. Libiakova for sharing her expertise on potato transformation; Dr. Sean Munro for pST-MYC; Dr. K.S. Dhugga for the RGP1 Abs; Dr. D.J. Simpson for the CRH Abs; Dr. K. Schnorr for the pectin methyl esterase; and W. Dam, D. Christiansen, and M. Stephensen for skillful technical assistance.
Footnotes
This work was supported by the Danish Research Council's Technology by Highly Oriented Research program, by The Danish National Research Foundation, by the European Commission (grant no. BIOTECH CT97–2224), and by a Royal Society University Research Fellowship (to M.C.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010948.
LITERATURE CITED
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–720. doi: 10.1126/science.279.5351.717. [DOI] [PubMed] [Google Scholar]
- Baron-Epel O, Gharyal PK, Schindler M. Pectins as mediators of wall porosity in soybean cells. Planta. 1988;175:389–395. doi: 10.1007/BF00396345. [DOI] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- Burget E, Reiter W. The mur4 mutant of Arabidopsis is partially defective in the de novo synthesis of uridine diphospho l-arabinose. Plant Physiol. 1999;121:383–389. doi: 10.1104/pp.121.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush MS, Marry M, Huxham M, Jarvis MC, McCann MC. Developmental regulation of pectic epitopes during potato tuberization. Planta. 2001;213:869–880. doi: 10.1007/s004250100570. [DOI] [PubMed] [Google Scholar]
- Bush MS, McCann MC. Pectic epitopes are differentially distributed in the cell walls of potato (Solanum tuberosum) tubers. Physiol Plant. 1999;107:201–213. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Chanliaud E, Gidley MJ. In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J. 1999;20:25–35. doi: 10.1046/j.1365-313x.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Cros S, Imberty A, Bouchemal N, Dupenhoat CH, Perez S. Modeling of arabinofuranose and arabinan: 2. Nmr and conformational-analysis of arabinobiose and arabinan. Biopolymers. 1994;34:1433–1447. [Google Scholar]
- Denecke J, Carlsson LE, Vidal S, Hoglund AS, Ek B, van Zeijl MJ, Sinjorgo KM, Palva ET. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell. 1995;7:391–406. doi: 10.1105/tpc.7.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhugga KS, Tiwari SC, Ray PM. A reversibly glycosylated polypeptide (RGP1) possibly involved in plant cell wall synthesis: purification, gene cloning, and trans-Golgi localization. Proc Natl Acad Sci USA. 1997;94:7679–7684. doi: 10.1073/pnas.94.14.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GA, Hepher A, Clerk SP, Boulter D. Pea lectin is correctly processed, stable and active in leaves of transgenic potato plants. Plant Mol Biol. 1991;17:89–100. doi: 10.1007/BF00036809. [DOI] [PubMed] [Google Scholar]
- Edwards ME, Dickson CA, Chengappa S, Sidebottom C, Gidley MJ, Reid JSG. Molecular characterization of a membrane-bound galactosyltransferase of plant cell wall matrix polysaccharide biosynthesis. Plant J. 1999;19:691–697. doi: 10.1046/j.1365-313x.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Fleischer A, Titel C, Ehwald R. The boron requirement and cell wall properties of growing and stationary suspension-cultured Chenopodium album L. cells. Plant Physiol. 1998;117:1401–1410. doi: 10.1104/pp.117.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Matsumoto T, Kikuchi Y, Ikejima T, Wang B, Yamada H. Effects of a pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. on interleukin 6 production of murine B cells and B cell lines. Immunopharmacology. 2000;49:307–316. doi: 10.1016/s0162-3109(00)00245-9. [DOI] [PubMed] [Google Scholar]
- Heyer GH, Lloyd JR, Kossmann J. Production of modified polymeric carbohydrates. Curr Opin Biotechnol. 1999;10:169–174. doi: 10.1016/s0958-1669(99)80030-5. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Pyun YR, Kokini JL. Sidechains of pectins: some thoughts on their role in plant cell walls and foods. Food Hydrocolloids. 1993;7:39–53. [Google Scholar]
- Hwang JW, Kokini JL. Structure and rheological function of side branches of carbohydrate polymers. J Texture Stud. 1991;22:123–167. [Google Scholar]
- Jones L, Seymour GB, Knox JP. Localization of pectin galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-d-galactan. Plant Physiol. 1997;113:1405–1412. doi: 10.1104/pp.113.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew JA, Howson SJ, Keenan MHJ, Belton PS. Improvement of the gelation properties of sugarbeet pectin following treatment with an enzyme preparation derived from Aspergillus niger: comparison with a chemical modification. Carbohydr Polym. 1990;12:295–306. [Google Scholar]
- McCann MC, Roberts K. Changes in cell wall architecture during cell elongation. J Exp Bot. 1994;45:1683–1691. [Google Scholar]
- Møgelsvang S, Simpson DJ. Changes in the levels of seven proteins involved in polypeptide folding and transport during endosperm development of two barley genotypes differing in storage protein localization. Plant Mol Biol. 1998;36:541–552. doi: 10.1023/a:1005916427024. [DOI] [PubMed] [Google Scholar]
- Mohnen D. Biosynthesis of pectins and galactomannans. In: Pinto BM, editor. Carbohydrates and Their Derivatives Including Tannins, Cellulose, and Related Lignins. Amsterdam: Elsevier/North-Holland Publishing; 1999. pp. 497–527. [Google Scholar]
- Munoz P, Norambuena L, Orellana A. Evidence for a UDP-glucose transporter in Golgi apparatus-derived vesicles from pea and its possible role in polysaccharide biosynthesis. Plant Physiol. 1996;112:1585–1594. doi: 10.1104/pp.112.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991;10:3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen BS. Bioactive Carbohydrate Polymers: Occurrence, Function and Use. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- Pérez S, Mazeau K, Hervé du Penhoat C. The three-dimensional structures of the pectic polysaccharides. Plant Physiol Biochem. 2000;38:37–55. [Google Scholar]
- Perrin RM, DeRocher AE, Bar-Peled M, Zeng WQ, Norambuena L, Orellana A, Raikhel NV, Keegstra K. Xyloglucan fucosyltransferase, an enzyme involved in plant cell wall biosynthesis. Science. 1999;284:1976–1979. doi: 10.1126/science.284.5422.1976. [DOI] [PubMed] [Google Scholar]
- Prade RA, Zhan DF, Ayoubi P, Mort AJ. Pectins, pectinases and plant-microbe interactions. Biotechnol Gen Eng Rev. 1999;16:361–391. doi: 10.1080/02648725.1999.10647984. [DOI] [PubMed] [Google Scholar]
- Reiter WD, Chapple C, Somerville CR. Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J. 1997;12:335–345. doi: 10.1046/j.1365-313x.1997.12020335.x. [DOI] [PubMed] [Google Scholar]
- Renard CM, Jarvis MC. A cross-polarization, magic-angle-spinning, 13C-nuclear-magnetic-resonance study of polysaccharides in sugar beet cell walls. Plant Physiol. 1999;119:1315–1322. doi: 10.1104/pp.119.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- Roberts K. The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol. 1994;6:688–694. doi: 10.1016/0955-0674(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Satoh S. Functions of the cell wall in the interactions of plant cells: analysis using carrot cultured cells. Plant Cell Physiol. 1998;39:361–368. [Google Scholar]
- Schols HA, Voragen AGJ. Complex pectins: structure elucidation using enzymes. In: Visser J, Voragen AGJ, editors. Pectin and Pectinases. Vol. 14. Amsterdam: Elsevier Science Publishing; 1996. pp. 3–19. [Google Scholar]
- Selvendran RR, March JF, Ring SG. Determination of aldoses and uronic acid content of vegetable fiber. Anal Biochem. 1979;96:282–292. doi: 10.1016/0003-2697(79)90583-9. [DOI] [PubMed] [Google Scholar]
- Skjøt M, Kauppinen S, Kofod LV, Fuglsang CC, Pauly M, Dalbøge H, Andersen LN. Functional cloning of an endo-arabinanase from Aspergillus aculeatus and its heterologous expression in A. oryzae and tobacco. Mol Gen Genomics. 2001;265:913–921. doi: 10.1007/s004380100489. [DOI] [PubMed] [Google Scholar]
- Sørensen SO, Pauly M, Bush M, Skjøt M, McCann MC, Borkhardt B, Ulvskov P. Pectin engineering: modification of potato pectin by in vivo expression of an endo-1,4-beta-d-galactanase. Proc Natl Acad Sci USA. 2000;97:7639–7644. doi: 10.1073/pnas.130568297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur BR, Singh RK, Handa AK. Chemistry and uses of pectin: a review. Crit Rev Food Sci Nutr. 1997;37:47–73. doi: 10.1080/10408399709527767. [DOI] [PubMed] [Google Scholar]
- Visser RGF, Stolte A, Jacobsen E. Expression of a chimeric granule-bound starch synthase-GUS gene in transgenic potato plants. Plant Mol Biol. 1991;17:691–699. doi: 10.1007/BF00037054. [DOI] [PubMed] [Google Scholar]
- Voragen AGJ, Pilnik W, Thibault J-F, Axelos MAV, Renard CMGC. Pectins. In: Stephen AM, editor. Food Polysaccharides and Their Applications. New York: Marcel Dekker; 1995. pp. 287–339. [Google Scholar]
- Weinstein J, Lee EU, McEntee K, Lai PH, Paulson JC. Primary structure of beta-galactoside alpha 2,6-sialyltransferase: conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- Willats WG, Marcus SE, Knox JP. Generation of a monoclonal antibody specific to (1→5)-alpha-l-arabinan. Carbohydr Res. 1998;308:149–152. doi: 10.1016/s0008-6215(98)00070-6. [DOI] [PubMed] [Google Scholar]
- Zablackis E, York WS, Pauly M, Hantus S, Reiter WD, Chapple CCS, Albersheim P, Darvill A. Substitution of l-fucose by l-galactose in cell walls of Arabidopsis mur1. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1808. [DOI] [PubMed] [Google Scholar]