Abstract
We generated transgenic tobacco (Nicotiana tabacum cv Xanthi) plants that contained only one to three enlarged chloroplasts per leaf mesophyll cell by introducing NtFtsZ1-2, a cDNA for plastid division. These plants were used to investigate the advantages of having a large population of small chloroplasts rather than a few enlarged chloroplasts in a leaf mesophyll cell. Despite the similarities in photosynthetic components and ultrastructure of photosynthetic machinery between wild-type and transgenic plants, the overall growth of transgenic plants under low- and high-light conditions was retarded. In wild-type plants, the chloroplasts moved toward the face position under low light and toward the profile position under high-light conditions. However, chloroplast rearrangement in transgenic plants in response to light conditions was not evident. In addition, transgenic plant leaves showed greatly diminished changes in leaf transmittance values under both light conditions, indicating that chloroplast rearrangement was severely retarded. Therefore, under low-light conditions the incomplete face position of the enlarged chloroplasts results in decreased absorbance of light energy. This, in turn, reduces plant growth. Under high-light conditions, the amount of absorbed light exceeds the photosynthetic utilization capacity due to the incomplete profile position of the enlarged chloroplasts, resulting in photodamage to the photosynthetic machinery, and decreased growth. The presence of a large number of small and/or rapidly moving chloroplasts in the cells of higher land plants permits more effective chloroplast phototaxis and, hence, allows more efficient utilization of low-incident photon flux densities. The photosynthetic apparatus is, consequently, protected from damage under high-incident photon flux densities.
During leaf development, chloroplasts in meristematic cells are differentiated from proplastids, which are the progenitors of various plastids found in the root and shoot meristem, embryos, endosperm, and in young developing leaves. Chloroplasts differentiated from proplastids undergo a secondary set of divisions that result in a large population of small chloroplasts in each mesophyll cell. As green photosynthetic plastids, chloroplasts typically measure 5 μm in diameter, are 1 to 2 μm thick, and occupy up to 70% of the surface area of a cell and approximately 20% of the total cell volume in mature leaf cells (Ellis and Leech, 1985). In the context of plant productivity and development of the photosynthetic surface area, and hence the size and number of leaf mesophyll cells, it is particularly important to understand what determines the ultimate chloroplast size and number in leaf cells and how chloroplast division is integrated with mesophyll cell development (Pyke, 1997).
Chloroplasts divide by a process of binary fission in which constriction of the envelope membranes occurs. This process is morphologically and genetically similar to bacterial cell division (Leech, 1976; Whatley, 1988). Recent genetic approaches for understanding chloroplast division and development using Arabidopsis clearly indicate a close similarity to the genetic control of prokaryotic cells. There are two main lines of research supporting this view. The first is based on genetic studies using a collection of Arabidopsis mutants with altered sizes and numbers of chloroplasts per cell, known as arc (accumulation and replication of chloroplast) mutants (Pyke and Leech, 1992). The second line of research was provided by transgenic Arabidopsis with fewer and larger chloroplasts in mesophyll cells, constructed by antisense suppression/sense expression of AtFtsZ (Osteryoung et al., 1998; Stokes et al., 2000).
Although considerable progress has been made in elucidating the molecular mechanisms of plastid binary fission in higher plants, an understanding of how cells control their plastid number is completely lacking (Pyke, 1999). A close correlation between the leaf mesophyll cell size and the number of chloroplasts within the cell clearly indicates that cell size is a primary determinant of the chloroplast number (Dean and Leech, 1982). Further, it appears that chloroplast division is initiated only after chloroplasts have attained a certain size (Ellis et al., 1983). Some arc mutants of Arabidopsis with greatly enlarged chloroplasts show continued chloroplast development with normal internal structure (Robertson et al., 1995, 1996). As a consequence, chloroplast division appears to be a process independent of chloroplast development. This implies that the chloroplast division event is an integral part of normal leaf cell development and that the evolution of higher plants led to each photosynthetic cell containing many small chloroplasts rather than a few large ones.
The question as to why the photosynthetic cells of higher plants contain so many small chloroplasts has not been addressed by detailed experimentation. Chloroplast positioning within these cells is controlled over a time period of minutes by the light supply (Trojan and Gabrys, 1996). Zurzycki (1957) hypothesized that chloroplast rearrangement as a function of different incident irradiance values maximizes use of limiting light and minimizes the chance of photodamage to the photosynthetic apparatus under excess light conditions. Chloroplast movement is believed to alleviate photodamage to PSII under high-light conditions (Park et al., 1996) and rapid rearrangement of chloroplasts can be facilitated by many small chloroplasts rather than by a few large ones in each cell. However, we know of no reports that address the question of chloroplast size in higher plant cells in relation to the effects of variation in the light supply at the subcellular, cellular, or whole-plant levels. Therefore, this study was intended to provide an experimental test of Zurzycki's hypothesis, which was based on a transgenic tobacco (Nicotiana tabacum cv Xanthi) line with fewer but enlarged chloroplasts in leaf mesophyll cells. Arabidopsis has become the definitive system in which to study plant biology over the last 10 years due to various advantages over other plants with respect to molecular genetics. However, Arabidopsis has not been widely utilized in research on photosynthesis, mainly due to difficulties in analyzing leaf photosynthetic gas exchanges and fractionating subcellular components. Therefore, we chose to use tobacco plants. Compared with wild types, NtFtsZ-overexpressed transgenic tobacco showed normal chloroplast development with a decreased capacity for chloroplast movement in response to varying light intensities. The presence of many small chloroplasts in each cell and/or a greater capacity for chloroplast phototaxis is probably an evolutionary adaptation that aids in efficient use of naturally fluctuating light intensities.
RESULTS
Transgenic Plants with Cells Containing a Few Enlarged Chloroplasts
Leaves of transgenic tobacco plants in which an NtFtsZ1-2 was overexpressed have one to three enlarged chloroplasts in each cell throughout development. In contrast, wild-type plants have many small chloroplasts that increase in number during development. The transgenic phenotypes are consistent with several arc mutants (Pyke and Leech, 1992) and FtsZ antisense/sense transgenic plants (Osteryoung et al., 1998; Stokes et al., 2000) of Arabidopsis.
Growth Characteristics and the Photosynthetic Apparatus
To characterize the growth performance of three independent transgenic lines that harbor a few enlarged chloroplasts, T1 and T2 plants were grown under three different light levels for 10 weeks in a greenhouse (Table I). Compared with wild-type plants, all transgenic lines grown under both low- and high-light conditions exhibited retarded growth, with comparable growth under medium light conditions. Based on plant height, stem thickness, fresh weight, and size of a fully expanded leaf, transgenic plants grew more slowly under both limiting and saturation light levels than wild-type plants (Table I). However, the Chl and protein contents and the Chl a/b ratio were similar in transgenic and wild-type plants. Transgenic plants grown under high-light conditions had a lower Chl content and a higher Chl a/b ratio per unit area, suggesting photoinhibitory damage. These growth characteristics of transgenic plants under both low- and high-light levels in a greenhouse were also observed in plants under growth chamber conditions (data not shown).
Table I.
Plant growth characteristics
| Treatment | PH | ST | LA | FW | Chl | Chl a/b | Protein |
|---|---|---|---|---|---|---|---|
| HL | |||||||
| WT | 69.37 ± 3.81 | 1.56 ± 0.09 | 499.13 ± 71.29 | 13.41 ± 1.91 | 0.37 ± 0.030 | 3.70 ± 0.13 | 1.55 ± 0.10 |
| FtsZ | 36.56 ± 6.65 | 1.22 ± 0.15 | 341.22 ± 89.74 | 9.09 ± 2.54 | 0.27 ± 0.006 | 4.22 ± 0.12 | 1.54 ± 0.16 |
| GUS | 60.12 ± 5.54 | 1.57 ± 0.15 | 516.17 ± 50.95 | 13.87 ± 1.36 | 0.36 ± 0.003 | 3.65 ± 0.14 | 1.49 ± 0.20 |
| ML | |||||||
| WT | 48.23 ± 5.01 | 0.85 ± 0.09 | 200.75 ± 47.02 | 4.89 ± 1.14 | 0.31 ± 0.010 | 3.30 ± 0.05 | 1.23 ± 0.02 |
| FtsZ | 40.24 ± 3.23 | 0.80 ± 0.06 | 186.88 ± 33.72 | 4.53 ± 0.82 | 0.30 ± 0.009 | 3.47 ± 0.01 | 1.40 ± 0.09 |
| GUS | 47.40 ± 3.50 | 0.86 ± 0.07 | 218.44 ± 55.08 | 5.32 ± 1.34 | 0.33 ± 0.011 | 3.43 ± 0.02 | 1.19 ± 0.01 |
| LL | |||||||
| WT | 29.20 ± 5.05 | 0.47 + 0.09 | 110.21 ± 17.73 | 2.47 ± 0.39 | 0.20 ± 0.005 | 2.91 ± 0.02 | 0.48 ± 0.04 |
| FtsZ | 8.80 ± 3.14 | 0.28 ± 0.06 | 42.27 ± 14.22 | 0.92 ± 0.33 | 0.19 ± 0.005 | 2.98 ± 0.05 | 0.48 ± 0.05 |
| GUS | 26.62 ± 9.63 | 0.48 ± 0.08 | 92.10 ± 20.42 | 2.07 ± 0.45 | 0.20 ± 0.004 | 2.99 ± 0.01 | 0.52 ± 0.05 |
PH, Plant height (cm). ST, Stem thickness (cm). LA, The youngest fully expanded leaf size (estimated by leaf area, cm2). FW, Fresh wt (g−1 leaf of the youngest fully expanded leaf. Chl a/b, Chlorophyll a/b ratio. Chl, Chlorophyll content (mmol m−2). Protein, The soluble protein content (g m−2). Chl a/b, Chl and protein were measured on the fully expanded leaves, third to fifth from the apex of the plants. WT, Wild-type plants. FtsZ, Average of three independent FtsZ1-2 overexpressed transgenic tobacco plants. GUS, GUS-expressed transgenic tobacco plants. HL, High light (700–1,500 μmol photons m−2 s−1). ML, Medium light (200–400 μmol photons m−2 s−1). LL, Low light (30–150 μmol photons m−2 s−1). Mean values (±se) for eight to 15 plants are shown.
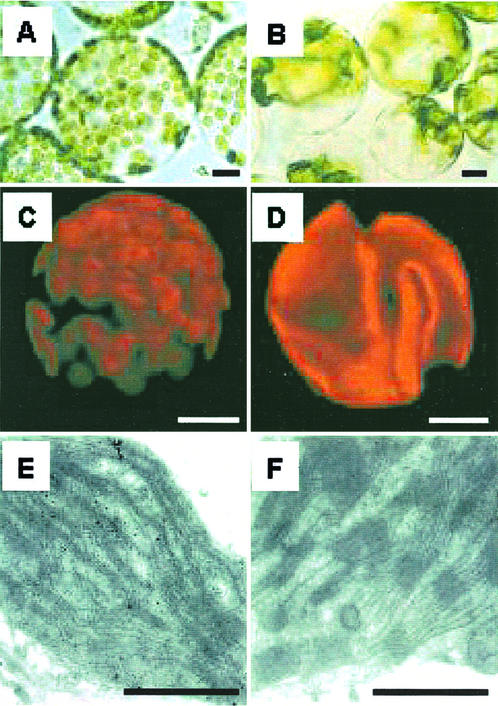
Compared with wild-type cells, protoplasts isolated from transgenic plant leaf tissues contain between one and three enlarged chloroplasts per cell (Fig. 1, A and B). These transgenic plant chloroplasts are much larger than wild-type plant chloroplasts and have variable shapes (Fig. 1, C and D). This low number of chloroplasts per cell is maintained throughout leaf development. Electron micrographs of chloroplasts from wild-type and transgenic plants grown under high-light conditions were examined. Both types of chloroplasts showed thylakoids differentiated into grana and non-stacked membrane regions with a similar extent of grana membrane stacking (Fig. 1, E and F). Unlike the wild-type chloroplasts, highly extended bundles of microtubule-like structures in the stroma were observed in electron micrographs of transgenic chloroplasts (data not shown).
Figure 1.
Light (A and B), confocal (C and D), and ultramicroscopic (E and F) photomicrographs of protoplasts, chloroplasts, and internal chloroplast structures from wild-type (A, C, and E) and transgenic (B, D, and F) tobacco. The bars in A through D and E through F are 10 and 1 μm, respectively.
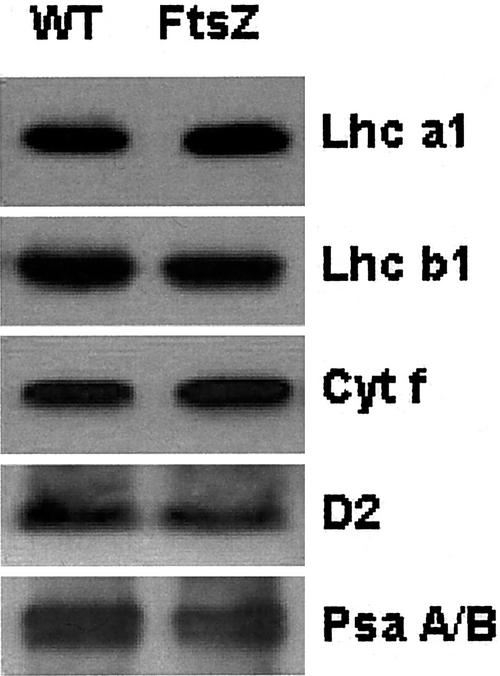
The contents of the PSII reaction center D2 polypeptide Cytf, the PSI reaction center heterodimer (PsaA/PsaB), and light-harvesting pigments Lhcb1 and Lhcb2 were examined on a Chl basis using immunoblotting (Fig. 2). The relative Rubisco content also was determined spectrophotometrically on a soluble protein basis (data not shown). The photosynthetic components of transgenic plants that were examined showed no significant changes compared with the wild type.
Figure 2.
Representative immunoblots of the light harvesting Chl pigments of the two photosystems (Lhca1 and Lhca2), the PSII reaction center D2 protein, the PSI reaction center PsaA/B heterodimer, and Cytochrome f (Cyt f) from isolated thylakoid membranes.
Chloroplast Movements and Photosynthesis
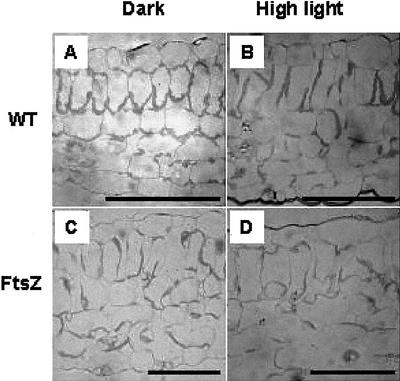
To investigate differences in chloroplast rearrangement between the two plant types, chloroplast movements were determined microscopically and by changes in the amount of light transmitted through the leaf tissue. Light micrographs of wild-type and transgenic leaf sections were examined before and after illumination treatments. High-light treatment induced significant changes in chloroplast arrangement in wild-type plants relative to transgenic plants (Fig. 3). Wild-type chloroplasts in leaves pretreated in the dark were mainly located along walls perpendicular to the light direction (face position). Exposure to high photon flux densities induced chloroplast movement from the face position to the profile position where chloroplasts were aligned with the cell walls parallel to the light direction. In contrast, rearrangement of transgenic tobacco chloroplasts in response to light conditions was not evident (Fig. 3, C and D).
Figure 3.
Comparison of light photomicrographs of cross sections of tobacco leaves, wild-type (A and B), and transgenic (C and D) plants. Leaf sections were taken before (A and C) and after (B and D) illumination under high light (750 μmol m−2 s−1) for 30 min. The bar represents 100 μm.
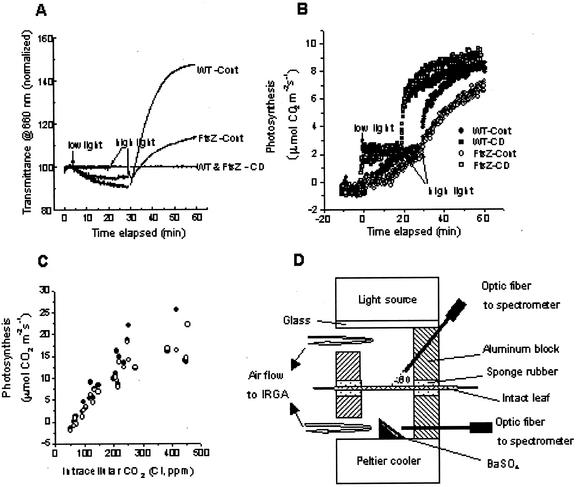
Transmittance changes have been described by the sieve effect and provide a convenient and nondestructive method for monitoring chloroplast movement. Hence, chloroplast rearrangement in transgenic plants was also investigated by leaf trans-mittance changes. Leaves maintained in the dark for 20 min were first illuminated with low light (40 μmol m−2 s−1) for 30 min, followed by high light (750 μmol m−2 s−1) for 30 min (Fig. 4A). Leaf transmittance in wild-type tissues decreased under low light, then increased rapidly under high-light illumination. Changes in transmittance under both irradiances were completed within 30 min, resulting in changes in leaf absorbance estimated either at 660 nm or in the 400- to 700-nm range (Table II). Contrary to the typical traces for changes in leaf transmittance, transgenic leaves showed greatly diminished changes in leaf transmittance under both irradiance levels. These changes in leaf transmittance are indicative of chloroplast movement because leaf tissues of both types treated with the actin antagonist cytochalasin D did not show any significant changes in transmittance. Therefore, the diminished changes in leaf transmittance observed in transgenic plant tissue strongly suggest that chloroplast rearrangement is severely retarded, consistent with the results shown in Figure 3.
Figure 4.
Changes in leaf transmittance (A) and CO2 exchange rates (B) in tobacco leaves measured at 660 nm. Dark-adapted intact leaves were first illuminated under low light (40 μmol m−2 s−1), then immediately followed by high light (750 μmol m−2 s−1). WT-Cont, Wild type treated with DW; FtsZ-Cont, transgenic plant treated with DW; WT & FtsZ-CD, wild-type and transgenic plants treated with 5 μm cytochalasin D; wild type (▪ and ●); transgenic plant (□ and ○). C, Net photosynthesis (Pn) versus intracellular CO2 concentration (Ci) in attached leaves of the wild-type (●) and transgenic (○) tobacco plants. The light intensity for CO2 exchange was 750 μmol m−2 s−1 and leaf temperature was 25°C ± 1°C. D, Schematic diagram of a Parkinson leaf chamber modified for the simultaneous measurement of optical properties and gas exchange. Two optic fibers were inserted into the chamber to collect lights reflected directly from an intact sample and from a small block coated with barium sulfate mounted on the bottom of the chamber, respectively.
Table II.
Leaf optical properties
| Optical Property | 660 nm
|
400–700 nm
|
||
|---|---|---|---|---|
| WT | FtsZ | WT | FtsZ | |
| Transmittance | ||||
| LL | 0.094 ± 0.007 | 0.105 ± 0.0089 | 0.029 ± 0.003 | 0.040 ± 0.006 |
| HL | 0.120 ± 0.014 | 0.106 ± 0.0084 | 0.066 ± 0.014 | 0.049 ± 0.008 |
| Reflectance | ||||
| LL | 0.120 ± 0.001 | 0.154 ± 0.011 | 0.090 ± 0.004 | 0.107 ± 0.007 |
| HL | 0.130 ± 0.001 | 0.143 ± 0.012 | 0.088 ± 0.003 | 0.098 ± 0.008 |
| Absorptance | ||||
| LL | 0.778 ± 0.006 | 0.742 ± 0.019 | 0.881 ± 0.003 | 0.853 ± 0.012 |
| HL | 0.749 ± 0.014 | 0.751 ± 0.019 | 0.846 ± 0.012 | 0.853 ± 0.015 |
WT, Wild-type plants. FtsZ, FtsZ1-2-overexpressed transgenic tobacco plants. Leaf transmittance, reflectance, and absorptance were measured from leaves of plants grown under medium-light conditions. Leaf transmittance and reflectance measured at 660 nm in the visible light range (400–700 nm, mean of the 92 measurements between 399.89 and 700.05 nm, each one-half band width = about 2.5 nm) were measured after 30 min of illumination under low light (LL; 40 μmol m−2 s−1) and high light (HL; 750 μmol m−2 s−1), respectively. Mean values (±se) for four to six leaves are shown.
Photosynthetic induction responses based on leaf CO2 exchange rates under limiting and saturating irradiances at a saturating CO2 pressure (850 μL L−1) were simultaneously measured with leaf transmittance values (Fig. 4, B and D). Upon illumination of wild-type plant leaves under low light (40 μmol m−2 s−1) following a 10-min dark period, there was a slight rise of the photosynthetic CO2 uptake rate, followed by a lag period and a gradual increase to steady-state conditions with a half maximum rate after 10 min of illumination. Compared with the photosynthetic induction curve under low-light conditions, illumination of leaves under saturating light following low light showed similar photosynthetic induction kinetics without any significant lag period. Transgenic plants showed three-phase induction kinetics with an extended lag period, and steady-state photosynthetic rates similar to the wild type (Fig. 4B).
Measurements of photosynthetic rates (Pn) at a saturating light were supplemented by experiments at CO2 levels equal to, above (700 and 500 μL L−1), and below (200, 125 and 50 μL L−1) the present atmospheric CO2 value of approximately 360 μL L−1 in postinduction light regimes. Results for net photosynthetic CO2 exchange rates, Pn as a function of the intercellular space CO2 (Ci) for a number of wild and transgenic lines (Fig. 4C), showing no significant differences in the photosynthetic carbon assimilation between wild and transgenic plants.
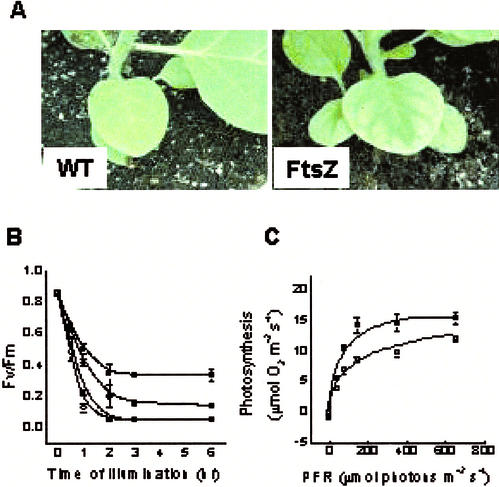
Photoinhibition of Photosynthesis
Differences in susceptibility to high-light conditions between wild-type and transgenic tobacco plants grown under low-light conditions were measured. Control (distilled water [DW] treated) or lincomycin-treated tobacco leaf discs were floated on water and illuminated at 1,600 μmol m−2s−1 for various periods up to 6 h. Photoinhibition was assayed by the Chl fluorescence ratio (Fv/Fm), a measure of the quantum efficiency of PSII photochemistry (Fig. 5B). Lincomycin was used to prevent replacement of damaged D1 proteins during light treatment and allowed us to assess the gross photoinhibition of PSII. With an increasing treatment time, there was an abrupt decline in the Fv/Fm ratio during the first 2 h of light treatment, followed by a phase of constant low values. Clearly, the Fv/Fm ratio of wild-type plants was higher than transgenic plants throughout the experimental period, showing that the PSII reaction centers of transgenic plants are more susceptible to photoinhibition. Compared with the control, lincomycin treatment accelerated photoinhibition of PSII, especially in wild-type plants. However, the PSII units of transgenic plants were more susceptible to photoinhibitory light treatment than wild-type plants. As expected, plants grown under high-light conditions in a greenhouse often showed the photoinhibitory symptom of photobleaching at the leaf blade (Fig. 5A). When light response curves of photosynthetic O2 evolution were measured using plants that did not show any visible symptoms, as shown in Figure 5A, the photosynthetic rate of transgenic plants was approximately 40% lower than wild-type plants under all light regimes (Fig. 5C), clearly indicating that the photosynthetic machinery of the transgenic plants is more susceptible to photodamage.
Figure 5.
A, Photographs showing photoinhibitory damage in leaves of transgenic (FtsZ) tobacco. B, Photoinhibition of photosystem II estimated by the Chl fluorescence ratio Fv/Fm as a function of the high-light illumination period in leaf discs of wild-type (▪ and ●) and transgenic (□ and ○) plants grown for 10 weeks under low light. Lincomycin (● and ○) treatment was applied for inhibition of the photodamaged PSII reaction center. C, Light response curve of photosynthetic O2 evolution in leaf discs of the wild-type (▪) and transgenic (□) tobacco plants grown under high-light conditions. O2 evolution in leaf discs were measured at 5% (v/v) CO2 concentration at 25°C ± 1°C. Mean values (±se) for 15 leaf discs are shown.
DISCUSSION
Why higher plant chloroplasts divide rather than simply expand is unknown. Two different types of plants, one with many small chloroplasts and the other with a few enlarged chloroplasts per leaf mesophyll cell, were systematically compared under varying levels of incident light. Transgenic tobacco plants with a few enlarged chloroplasts per cell were generated by overexpression of the NtFtsZ gene. The overall growth of transgenic plants was retarded under both low- and high-light conditions and was comparable under medium-light conditions (Table I). However, Arabidopsis FtsZ transgenic plants and arc mutants with a few enlarged chloroplasts per cell are not distinguishable from wild-type plants in outward appearance and growth (Pyke et al., 1994; Robertson et al., 1995, 1996; Osteryoung et al., 1998). In this respect, transgenic tobacco plants are different from the arc mutants and transgenic plants of Arabidopsis. Considering the observed plant productivity and leaf mesophyll functionality in photosynthesis, these differences in growth patterns imply that cells with many small and/or rapidly moving chloroplasts derive certain benefits in absorption and utilization of light energy that are not available to cells with a few gigantic and/or slowly moving chloroplasts. Plant growth ultimately depends on leaf photosynthesis, which is determined by the rate of excitation of the photosystem and utilization of this excitation energy through electron transport and metabolism, as constrained by the supply of carbon dioxide to Rubisco (Huner et al., 1998). Therefore, although there are differences in assimilation allocation among organs between wild-type and transgenic plants, some of the differences in plant growth arise from changes in either the photosynthetic structure or the functional performance of the photosynthetic apparatus (Slatyer, 1970). To clarify this point, we first examined the ultrastructure of the chloroplast and the relative stoichiometry of various photosynthetic components (Figs. 1 and 2). The ultrastructure of the chloroplast, including the degree of grana stacking that is involved in adjustment of the photosynthetic apparatus to a fluctuating light environment, is common to both wild-type and transgenic plants. Further, quantification of the photosynthetic components involved in light energy absorption and electron transport, carbon dioxide fixation, and carbon assimilation rates clearly indicates that all of the photosynthetic components measured are similar in the two plant types. All ultrastructural, biochemical, and gas exchange data indicate strongly against a growth difference being due to a change in the composition of the chloroplasts induced by inhibiting chloroplast division.
Despite similar photosynthetic structures and functions at the subchloroplast level, differences were observed at the whole organism level. The growth performance of transgenic plants with a few enlarged chloroplasts was inferior to plants with many small chloroplasts. To determine whether inefficient light absorption is responsible for this observed difference in growth performance, we measured the physical antenna size of the two photosystems and the rearrangement ability of the chloroplasts. The rate of light absorption per reaction center is determined by the physical antenna size of each photosystem (Mauzerall and Greenbaum, 1989; Huner et al., 1998) and the arrangement of chloroplasts (Haupt and Scheuerlein, 1990; Augustynowicz and Gabrys, 1999). The Chl contents, the Chl a/b ratio, and western-blot data for Lhca1 and Lhcb1, which are parameters often used to describe the physical antenna size, were comparable between the two types (Table I; Fig. 2). However, dramatic differences in chloroplast rearrangement and leaf optical properties between wild-type and transgenic plants were observed (Figs. 3 and 4A; Table II). This indicates involvement of chloroplast movement in the observed differences in plant growth. Decreased growth is probably due to decreased light absorption (limiting conditions) and an increased susceptibility to photoinhibition (high-light conditions). Under limiting light, photosynthesis is often limited by the rate of light absorption, which is, in turn, dependent on the physical antenna size. However, the fact that the physical antenna sizes are comparable between the two types of plants indicates that the observed differences in growth performance under low-light conditions result from leaf optical changes at the whole plant level, not at the leaf level.
In productive agricultural ecosystems, the values of the leaf area index and the ratio of photosynthetic leaf area to covered ground area fall in the range of 3 to 5 (Hopkins, 1999). This means that utilization of light by leaves in the canopy is an important factor in plant productivity. At the whole plant level, a lowered transmittance in the outermost leaves of transgenic plants might result in a limitation of light available for photosynthesis to inner leaves in the canopy, especially when the light supply is the limiting factor for photosynthesis in these leaves. Because the availability of light for plants growing under light-limiting conditions is the most important factor determining photosynthetic activity, a lowered trans-mittance results in decreased primary productivity. In addition, the outermost leaves of transgenic plants exposed to varying light intensities would be subject to an increased potential for photoinhibition. This view is partly supported by the greater susceptibility of photosystem II of transgenic plants to high light compared with wild-type plants, and the lowered photosynthetic O2 evolution rate under all light regimes (Fig. 5). Thus, the observed poor growth of transgenic plants may be attributable to reduced photon absorption by shaded leaves and a greater tendency for photoinhibition in the outermost leaves. However, it must be borne in mind that allocation of photosynthate to production of new photosynthetic machinery (leaf area), or to other sinks in the plant, is also a determining factor for how photosynthesis on a leaf area basis relates to the plant growth rate (Slatyer, 1970). Besides the allocation of photosynthate, we do not exclude the possibility that poor growth can be related to a reduced surface area to volume ratio of enlarged chloroplasts, and affected proplastid division in growing zones (Robertson et al., 1995). However, in this study, growth performance of transgenic tobacco plants grown under medium-light conditions was comparable with wild-type plants, eliminating the possibility of impaired growth due to a decreased surface area to volume ratio.
The simplest assumption is that the cellular level responses of photosynthesis related to differences in chloroplast movement and distribution are the major causes of the slower growth of transgenic plants. These observations are consistent with reports of various species that exhibit chloroplast movement, supporting Zurzycki's hypothesis of a dual function for chloroplast movement in ensuring maximum light absorption under limiting light and protecting chloroplasts from photodamage (Zurzycki, 1957).
In interpreting the effects of restricting FtsZ expression in tobacco, we can rely on comparative biology, looking to multicellular organisms that naturally have only one chloroplast per cell compared with organisms containing many chloroplasts in each cell. Particular reference is made to the speed at which chloroplasts respond phototactically and the extent to which a single large chloroplast can restrict the inorganic carbon supply to Rubisco, relative to many small chloroplasts per cell.
Examples of organisms from the embryophytes and the closely related charophycean algae that naturally have only one plastid per cell are the algae Coleochaete pulvinata, Klebsormidium flaccidum (formerly Hormidium), and Mougeotia sanfordiana (Van den Hoek et al., 1995), the anthocerote bryophytes (hornworts), and some leaf cells in Selaginella spp. Of these, Klebsomormidium, Mougeottia, and Selaginella show phototactic plastid movements, whereas the time necessary for the phototactic repositioning of the plastid to occur may be somewhat longer than in embryophyte cells with many small plastids (Haupt and Scheuerlein, 1990; Augustynowicz and Gabrys, 1999). The difference is much less than that between wild-type and NtFtsZ-overexpressed tobacco plants, indicating that the few, large, slowly responding chloroplasts of the transgenic plants probably have restricted phototactic movement for a reason other than their large size.
The data presented here and reported earlier (Chow, 1994; Park et al., 1996) indicate that chloroplast movement is an effective photoprotection mechanism in land plants in both exposed (tobacco) and shaded forest floor (Tradescantia albiflora) habitats. Large-scale nastic movements can also serve in photoprotection, e.g. in the leaflet folding of forest floor species of Oxalis (Raven, 1989). It is possible that the stem movements and leaf rolling of some Selaginella spp. that grow exposed to full sun in the dry summer are photoprotective responses. Movement and rolling are absent in the rainy winter season when there is less light (e.g. Selaginella tamariscina). It is not yet clear, however, whether the bending and rolling is mainly a response to excess light or insufficient water. The involvement of phototactic chloroplast movement in photoprotection is also found in benthic aquatic plants.
Some algae (e.g. M. sanfordiana) with a single plank-like plastid in each have parts of the plastid at least 10 μm from the plasmalemma; however, this may not impact on inorganic carbon supply to Rubisco because these algae, unlike tobacco, have an inorganic carbon concentrating mechanism. Any problems with CO2 diffusion in transgenic tobacco for increased FtsZ expression can be related to the larger radial dimension of a few enlarged chloroplasts, and especially to the large distance between the plasmalemma and the chloroplast envelope.
In conclusion, based on transgenic tobacco plants with a few enlarged chloroplasts, we suggest that natural selection pressure favors cells with many small chloroplasts over those with a few enlarged chloroplasts by efficient utilization of radiant energy and minimization of photodamage under varying light conditions, as suggested by Zurzycki (1957).
MATERIALS AND METHODS
Transgenic Tobacco (Nicotiana tabacum cv Xanthi) and Plant Growth
NtFtsZ1-2 (FtsZ1-2 of tobacco) was overexpressed in tobacco under control of the cauliflower mosaic virus promoter. Three independent transgenic tobacco plants were confirmed by Southern, northern, and western blotting (data not shown). Northern- and western-blot analyses showed that FtsZ1-2 and FtsZ1-2 in transgenic plants were overexpressed about 3 to 12 times more than wild type. Transgenic plants consisted of two distinct phenotype classes. The first had a few enlarged chloroplasts and the second had one gigantic chloroplast with several normal-sized chloroplasts in mature cells. Plant growth comparisons were based on the T1 and T2 progeny of three independent transgenic plants with one to three enlarged chloroplasts, wild-type plants, and transgenic plants expressing GUS (a parallel control) grown for 10 weeks in a greenhouse. Typical temperatures reached 32°C and irradiance during the day ranged from 750 to 1,500 μmol m−2 s−1. The maximum irradiance during the day was defined arbitrarily as 100% (high light), 20% (medium light), and 5% (low light). Relative growth irradiances were obtained using layers of shade cloth. T1 plants containing between one and three greatly enlarged chloroplasts were selected under a light microscope and the fully expanded leaves, third to fifth position from the apex, of the T1 plants used for further experiments.
Inhibitor Treatments
Leaf petioles were cut under water and excised leaves were transferred to 10-mL Falcone tubes containing either 5 μm cytochalasin D (actin antagonist; Sigma, St. Louis) or 1 mg mL−1 lincomycin (protein synthesis inhibitor; Sigma) for 10 h under dim light (10 μmol m−2 s−1).
Light, Confocal, and Electron Microscopy
Light and confocal micrographs were measured on protoplasts from the leaves from 6-week-old plants in the growth chamber. Electron micrographs of chloroplasts from wild-type and transgenic plants grown for 10 weeks under high light in the greenhouse were examined. Light microscopic observations for the chloroplast movement were taken from tissue pieces approximately 1 mm long and 0.5 mm thick from leaves grown for 10 weeks under the medium-light greenhouse condition. Before high-light (750 μmol m−2 s−1) treatment for 30 min, leaves were fed either with 5 μm cytochalasin D or distilled water for about 10 h under dim light through cut petioles. Leaf sections were fixed immediately in ice-chilled 3% (v/v) glutaraldehyde for 1 h under high light to prevent chloroplast rearrangement during fixation (Park et al., 1996). Leaf sections were stained with toluidine blue, then observed and photographed. For confocal microscopic observations, leaf protoplasts were isolated by incubation from leaves and placed in an enzyme digestion solution (2% [w/v] cellulase Onozuka R-10, 1% [w/v] Macerase R-10, 0.6 m mannitol, and filter-sterilized 5 mm MES) overnight. Protoplasts purified by filtering through a mesh, centrifugation, and washing, and were embedded in 0.6% (w/v) agarose. Images of Chl autofluorescence were observed with an LSM 410 confocal microscope (Zeiss, Overkochen, Germany) and photographed. For electron microscopic observations, leaf tissues were fixed in phosphate-buffered 3% (v/v) glutaraldehyde, followed by 1% (w/v) OsO4, dehydrated, and embedded in Spurr's resin. Thin sections were stained with lead citrate and examined with a Zeiss EM 912 Omega at 80 kV.
Determination of the Photosynthetic Components
Relative leaf Rubisco contents from wild-type and transgenic tobacco plants were determined spectrometrically after formamide extraction of Coomassie Brilliant Blue R-250-stained subunit bands separated by SDS-PAGE (Makino et al., 1997). Soluble protein contents were measured using protein assay reagents according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA). Thylakoidal protein components were measured immunochemically after isolation of thylakoid membrane proteins. Thylakoid proteins were prepared from protoplasts using methods modified from Gray et al. (1996). Thylakoid membranes were isolated from the protoplasts of the fully expanded leaves, third to fifth position from the apex, of the plants grown for 10 weeks under the medium-light condition in the greenhouse. Protoplasts were broken in 10 mm sodium phosphate buffer containing 5 mm NaCl and 5 mm MgCl2. Thylakoid membranes were resuspended in 10 mm Tricine-NaOH, pH 7.0; 300 mm Suc; and 5 mm MgCl2. Chl contents were determined in 80% (v/v) acetone by the method of Porra et al. (1989). For protein gel blots, membranes were solubilized in 60 mm Tris-HCl (pH 7.8), 12% (w/v) Suc, 2% (w/v) SDS, 1 mm EDTA, and 58 mm dithiothreitol. Protein gel electrophoresis was performed according to Laemmli (1970). Separated proteins were electrophoretically transferred to Immobilon-P (Millipore, Bedford, MA). Immunodetection by chemiluminescence using antibodies was performed according to the manufacturer's instructions (ECL + Plus; Amersham-Pharmacia Biotech, Uppsala). Antibodies against Lhca1 and Lhcb1, Psa A/B, D2, and Cytf were provided by Drs. Stefan Jansson (Umea, Sweden), Kintake Sonoka (Tokyo), MinKyun Kim (Suwon, Korea), and Amane Makino (Tohoku, Japan), respectively.
Leaf Gas Exchange and Optical Property
A Parkinson leaf chamber (broad type, ADC, Hoddesdon, Hertfordshire, UK) was modified for simultaneous measurements of the CO2 exchange rate and of the transmittance and reflectance in the range of 400 to 700 nm (Fig. 4D) for sample leaves from 10-week-old plants under the medium-light condition in the greenhouse. One of two optic fibers was inserted through the upper lid of the chamber at an angle of 60° to the plane of the lid to collect and guide the light reflected directly from the upper surface of the leaf. The other optic fiber was inserted at right angles through the lower lid of the chamber to collect and guide the light that had traversed the leaf and then been diffused from a small triangular cross section block coated with barium sulfate located at the bottom of the chamber with the face at 45° to the leaf surface. Transmittance was determined by dividing the photon flux density detected by the lower probe with the leaf in place by that detected in the absence of the leaf. Reflectance was measured by dividing the photon flux density detected by the upper probe with the leaf in place by that measured when the leaf was replaced by a 1-mm-thick plastic panel coated with barium sulfate. The spectral intensity of the light collected and guided by the optic fibers was measured with a silicon photodiode detector (MMS, Zeiss). CO2 exchange rates at saturating CO2 (850 μL L−1) were measured with a steady-state gas exchange system (LCA2, ADC). Incident photon flux densities (40 and 750 μmol m−2 s−1) from a halogen lamp (12W DC, Philips, Eindhoven, The Netherlands) were provided with neutral density filters. CO2 exchange rates for Pn verus Ci curve were measured at a saturating light intensity (750 μmol m−2 s−1). Air containing various concentrations of CO2 (50, 125, 200, 360, 500, and 850 μL L−1) and preconditioned to 40% relative humidity was supplied to the chamber at 250 mL min−1. The leaf temperature was maintained at 25 ± 1°C with a Peltier cooler (Sungjoo Electronics, Seoul) and a temperature controller (MX7, Hanyong Co., Seoul).
Determination of Chl Fluorescence Parameters and Photosynthetic O2 Evolution
The maximum efficiency of PSII was estimated from the Chl fluorescence ratio Fv/Fm at room temperature. After photoinhibitory light treatments at 1,600 μmol m−2 s−1 for varying durations, leaf discs from plants grown for 10 weeks under low-light conditions in a growth chamber were dark treated for 30 min in leaf clips of a Plant Efficiency Analyzer (Hansatech, King's Lynn, UK). Light-response curves of photosynthetic O2 evolution during illumination were determined with a leaf disc O2 electrode (Oxygraph system, Hansatech) using plants grown under high-light conditions in a greenhouse. Various irradiances were provided using neutral density filters while the temperature was kept constant at 25°C at 5% (v/v) CO2.
ACKNOWLEDGMENTS
We thank Dr. Wah Soon Chow for critical review of the manuscript. We also thank the Korea Basic Science Institute for preparation of microscopic images.
Footnotes
This work was supported by the Ministry of Science and Technology in Korea (grant no. FGM0040012 to J.R.L.); in part by the Korea Science and Engineering Foundation (grant through the Plant Metabolism Research Center to J.R.L.); in part by the Crop Functional Genomics Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea (grant no. CGM0400111 to J.R.L.); in part by the Korea Science and Engineering Foundation through the Agricultural Plant Stress Research Center at Chonnam National University (to Y.I.P.); and in part by the Korea Science and Engineering Foundation (grant no. 971–0511–059–2 to K.H.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.000588.
LITERATURE CITED
- Augustynowicz J, Gabrys H. Chloroplast movements in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant Cell Environ. 1999;22:1239–1248. [Google Scholar]
- Chow WS. Photoprotection and photoinhibitory damage. Adv Mol Cell Biol. 1994;10:151–196. [Google Scholar]
- Dean C, Leech RM. Genome expression during normal leaf development: I. Cellular and chloroplast numbers and DNA, RNA and protein levels in tissues of different ages within a seven-day old leaf. Plant Physiol. 1982;69:904–910. doi: 10.1104/pp.69.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JR, Jellings AJ, Leech RM. Nuclear DNA content and the control of chloroplast replication in wheat leaves. Planta. 1983;157:376–380. doi: 10.1007/BF00397411. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Leech RM. Cell size and the chloroplast size in relation to chloroplast replication in light-grown wheat leaves. Planta. 1985;165:120–125. doi: 10.1007/BF00392220. [DOI] [PubMed] [Google Scholar]
- Gray GR, Savitch LV, Ivanov AG, Huner NPA. Photosystem II excitation pressure and development of resistance to photoinhibition. Plant Physiol. 1996;110:61–71. doi: 10.1104/pp.110.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt W, Scheuerlein R. Chloroplast movement. Plant Cell Environ. 1990;13:596–614. [Google Scholar]
- Hopkins WG. Introduction to Plant Physiology. Ed 2. New York: John Wiley & Sons, Inc.; 1999. pp. 261–263. [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leech RM. The replication of plastids in higher plants. In: Yeoman MM, editor. Cell Division in Higher Plants. London: Academic Press; 1976. pp. 135–159. [Google Scholar]
- Makino A, Sato T, Nakano H, Mae T. Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta. 1997;203:390–398. [Google Scholar]
- Mauzerall D, Greenbaum NL. The absolute size of a photosynthetic unit. Biochim Biophys Acta. 1989;974:118–140. [Google Scholar]
- Osteryoung KW, Stokes KD, Rutherford SM, Percival AL, Lee WY. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ. Plant Cell. 1998;10:1991–2004. doi: 10.1105/tpc.10.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y-I, Chow WS, Anderson JM. Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol. 1996;111:867–875. doi: 10.1104/pp.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Pyke KA. The genetic control of plastid division in higher plants. Am J Bot. 1997;84:1017–1027. [PubMed] [Google Scholar]
- Pyke KA. Plastid division and development. Plant Cell. 1999;11:549–556. doi: 10.1105/tpc.11.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. Chloroplast division and expansion is radically altered by nuclear mutations in Arabidopsis thaliana. Plant Physiol. 1992;99:1005–1008. doi: 10.1104/pp.99.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Rutherford SM, Robertson EJ, Leech RM. arc6, fertile Arabidopsis mutant with only two mesophyll cell chloroplasts. Plant Physiol. 1994;106:1169–1177. doi: 10.1104/pp.106.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. Fight or flight: the economies of repair and avoidance of photoinhibition. Funct Ecol. 1989;3:5–19. [Google Scholar]
- Robertson EJ, Pyke KA, Leech RM. arc6, an extreme chloroplast division mutant of Arabidopsis also alters proplastid proliferation and morphology in shoot and root apices. J Cell Sci. 1995;108:2937–2944. doi: 10.1242/jcs.108.9.2937. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Rutherford SM, Leech RM. Characterization of chloroplast division using the Arabidopsis mutant arc5. Plant Physiol. 1996;112:149–159. doi: 10.1104/pp.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatyer RO. Comparative photosynthesis, growth and transpiration of two species of Atriplex. Planta. 1970;93:175–189. doi: 10.1007/BF00387639. [DOI] [PubMed] [Google Scholar]
- Stokes KD, McAndrew RS, Figueroa R, Vitha S, Osteryoung KW. Chloroplast division and morphology are differentially affected by overexpression of FtsZ1 and FtsZ2 genes in Arabidopsis. Plant Physiol. 2000;124:1668–1677. doi: 10.1104/pp.124.4.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan A, Gabrys H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoek C, Mann DG, Jahns HM. Algae. An Introduction to Phycology. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- Whatley JM. Mechanisms and morphology of plastid division. In: Boffey SA, Lloyd D, editors. Division and Segregation of Organelles. Cambridge, UK: Cambridge University Press; 1988. pp. 63–84. [Google Scholar]
- Zurzycki J. The destructive effect of light on the photosynthetic apparatus. Acta Soc Bot Pol. 1957;26:157–175. [Google Scholar]