Abstract
In the Rhizobium-legume symbiosis, compatible bacteria and host plants interact through an exchange of signals: Host compounds promote the expression of bacterial biosynthetic nod (nodulation) genes leading to the production of a lipochito-oligosaccharide signal, the Nod factor (NF). The particular array of nod genes carried by a given species of Rhizobium determines the NF structure synthesized and defines the range of legume hosts by which the bacterium is recognized. Purified NF can induce early host responses even in the absence of live Rhizobium One of the earliest known host responses to NF is an oscillatory behavior of cytoplasmic calcium, or calcium spiking, in root hair cells, initially observed in Medicago spp. and subsequently characterized in four other genera (D.W. Ehrhardt, R. Wais, S.R. Long [1996] Cell 85: 673–681; S.A. Walker, V. Viprey, J.A. Downie [2000] Proc Natl Acad Sci USA 97: 13413–13418; D.W. Ehrhardt, J.A. Downie, J. Harris, R.J. Wais, and S.R. Long, unpublished data). We sought to determine whether live Rhizobium trigger a rapid calcium spiking response and whether this response is NF dependent. We show that, in the Sinorhizobium meliloti-Medicago truncatula interaction, bacteria elicit a calcium spiking response that is indistinguishable from the response to purified NF. We determine that calcium spiking is a nod gene-dependent host response. Studies of calcium spiking in M. truncatula and alfalfa (Medicago sativa) also uncovered the possibility of differences in early NF signal transduction. We further demonstrate the sufficiency of the nod genes for inducing calcium spiking by using Escherichia coli BL21 (DE3) engineered to express 11 S. meliloti nod genes.
The Rhizobium-legume interaction initiates the development of a novel organ on the root of the host plant, the nodule, and its colonization by the bacteria, resulting in a nitrogen-fixing symbiosis. Within the first 12 to 24 h, bacteria trigger a series of microscopically visible morphological changes. In the epidermis, altered growth of root hair cells (root hair deformation) is followed by root hair curling. Bacteria concurrently induce renewed cortical cell division that will lead to the formation of a root nodule. Invasion structures, called infection threads, initiate within curled root hairs and grow into the developing nodule. Bacteria are eventually released from infection threads into the cells of the nodule, where they begin fixing nitrogen. Thus, in a compatible interaction, Rhizobium elicits root hair deformation and curling, infection thread development, and cell division in the root cortex leading to nodule formation. These morphological responses are considered to be the hallmarks of nodulation.
Nodulation occurs only when compatible species of legumes and Rhizobium come into contact. Thus, Sinorhizobium meliloti interacts with Medicago spp. but not Vicia spp., which in turn form nodules in the presence of Rhizobium leguminosarum bv viciae. The specificity of the interaction is based on a reciprocal exchange of signals between symbiotic partners. The host plant secretes compounds, often flavonoids, that act in concert with bacterial transcriptional regulators to promote the expression of bacterial nod (nodulation) genes. These genes, in turn, encode biosynthetic enzymes responsible for the assembly of a lipo-chitooligosaccharide signal, called Nod factor (NF), that triggers morphogenetic changes in the receptive host. NF is required for nodulation: Bacteria that fail to synthesize NF because of mutations in nod genes fail to elicit the morphological responses associated with nodulation.
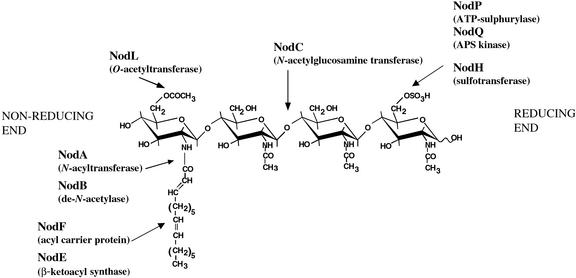
NFs isolated from loss-of-function bacterial nod mutants correspond to the predicted structure based on known nod gene function (Roche et al., 1991; Demont et al., 1993; Ardourel et al., 1994; Debellé et al., 1996). The nod genes are divided into two categories, common and host specific. The common nod genes, nodABC, are required for the synthesis of the N-acetylglucosamine backbone and attachment of the lipid moiety at the nonreducing end of NF (Fig. 1). These genes are required for host responses and can be exchanged between Rhizobium without affecting the range of legumes nodulated (Dénarié et al., 1996). Host range of a given Rhizobium is determined by its array of host-specific nod genes, and the exact structure of the resultant NF. Host-specific nod genes contribute to the further modification of the reducing and nonreducing ends of the NF lipo-chitooligosaccharidic backbone. S. meliloti carries six host-specific nod genes with distinct functions in NF modification: nodF, nodE, nodL, nodP, nodQ, and nodH. The NFs produced by wild-type S. meliloti, which nodulates Medicago spp., have a C16:2 lipid tail, whose synthesis requires NodF and NodE, at the nonreducing end as well as a 6-O-acetyl modification, attached by NodL (Demont et al., 1993; Ardourel et al., 1995). There is a single modification at the reducing end of S. meliloti NF, a 6-O-sulfate that requires the activity of NodH, NodP, and NodQ (Roche et al., 1991).
Figure 1.
S. meliloti NF structure and Nod protein function. Each Nod protein is encoded by an equivalently named nod gene. NodA, NodB, and NodC are common to all rhizobia. The remaining Nod proteins are responsible for the modifications of NF that confer activity on selected legume species.
The ability of S. meliloti NFs to induce the morphological responses associated with nodulation in its hosts alfalfa (Medicago sativa) and Medicago truncatula is dependent on the presence of all the substituents of the NF (Dénarié et al., 1996; Catoira et al., 2000, 2001). Whereas loss of common nod genes leaves S. meliloti unable to induce any nodulation response, loss of host-specific nod genes alters the host range of the bacteria (Dénarié et al., 1996). Thus, a nodH S. meliloti mutant no longer nodulates alfalfa, but gains activity on vetch (Vicia sativa), a legume outside of S. meliloti's normal host range (Faucher et al., 1989). This is the most dramatic example of a host range effect: nodH mutants produce NFs that differ from wild type only in that they are not sulfated at the reducing end and yet fail to trigger any morphological response in alfalfa, similar to bacterial mutants that cannot synthesize NF (Roche et al., 1991). Mutations in nodL, nodF, and nodE that affect the O-acetyl or the N-linked fatty acid modification at the nonreducing end lead to delayed and reduced nodulation in Medicago hosts, but still provoke all the morphological responses associated with nodulation (Debellé et al., 1986; Swanson et al., 1987; Ardourel et al., 1994). However, when these mutations are combined in a single strain, the nodulation defect is much more severe: A nodFnodL double mutant fails to elicit nodule development, cortical cell division, and infection thread formation, although the bacteria still induce root hair deformation in M. truncatula and M. sativa (Ardourel et al., 1994).
In purified form, NF is sufficient to trigger early morphological responses, such as root hair deformation, root hair branching, and cortical cell division, but not further responses, such as “shepherd's crook” root hair curling and infection thread formation. These microscopically visible responses are presumably the downstream result of changes in cell activity and gene expression initiated in the host root upon NF perception. The availability of pure and structurally characterized NF led to a search for events triggered within minutes of signal application that might be involved in signal transduction. Studies with S. meliloti NF have shown that a series of physiological changes are induced in root hairs within minutes (Ehrhardt et al., 1992; Felle et al., 1996, 1998, 1999), including rapid cytoplasmic alkalinization and fluxes in calcium, chloride, and potassium in alfalfa root hairs. The most widely examined NF-induced response occurs within an average of 10 min: Cytoplasmic oscillations of calcium, or calcium spiking, have been documented in root hairs of alfalfa, M. truncatula, vetch, pea (Pisum sativum), Melilotus albus, and Lotus japonicus (Ehrhardt et al., 1996; Wais et al., 2000; Walker et al., 2000; E. Engstrom, D.W. Ehrhardt, J.M. Harris, R.J. Wais, and S.R. Long, unpublished data). This response is robust and sensitive, occurring in more than 80% of root hairs examined in Medicago hosts in response to as little as 1 pm NF (Ehrhardt et al., 1996; Wais et al., 2000; Oldroyd et al., 2001a).
The suite of ion fluxes and behaviors triggered by purified NFs in root hairs precedes by hours, or days, the known morphological responses. It is hypothesized that these very early events are part of the initial signal perception and transduction pathway in nodulation, based on the ability of NFs to induce them and on their timing relative to other host responses. Further criteria for a response associated with nodulation are whether it is activated by live bacteria and whether or not it shows the same NF structural specificity as the later morphological responses. In the following study, we have addressed these questions for the calcium spiking response. We show that live Rhizobium elicit a robust calcium spiking response indistinguishable from the NF-induced response. The use of living S. meliloti bacteria made it possible to examine the dependence of the response on bacterial nod genes and compare the nod gene requirements for calcium spiking with those for the later morphological responses associated with nodulation. In so doing, we found that the requirements for calcium spiking are not as stringent as those for complete nodulation; rather, they are correlated with early epidermal responses such as root hair deformation.
RESULTS
S. meliloti Wild-Type Strain Rm1021 Causes Calcium Spiking in M. truncatula Root Hairs
Ion fluxes are triggered in host root hairs within minutes of exposure to NFs purified from compatible Rhizobium (Downie and Walker, 1999). The ability of live bacteria to provoke similar behaviors has not been established. We tested whether live Rhizobium can trigger calcium spiking by applying S. meliloti Rm1021 to M. truncatula root hairs injected with the calcium-sensitive dye Oregon Green-dextran. Dye fluorescence, a relative measure of cytoplasmic calcium concentration, was monitored in root hairs exposed to bacteria and compared with fluorescence patterns seen in root hairs exposed to purified NF (Fig. 2A). Live bacteria trigger a calcium spiking response that is indistinguishable from the NF-induced response (Fig. 2). Rhizobium-induced calcium spiking could be maintained for at least 4 h once initiated, similar to experiments with purified NFs (data not shown; Ehrhardt et al., 1996). Quantitative comparison of NF- and Rm1021-induced calcium spiking revealed no significant difference between treatments in three parameters evaluated: percentage of root hairs in which calcium spiking is induced, lag time to the onset of calcium spiking, and calcium spiking frequency (Table I). Thus, within the limits of the assay, Rm1021 triggers the same calcium spiking behavior as purified S. meliloti NFs.
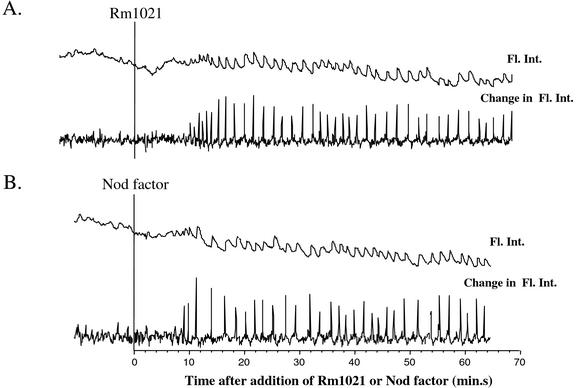
Figure 2.
Rm1021 causes calcium spiking in M. truncatula. A, Rm1021-induced calcium spiking in M. truncatula. B, NF-induced calcium spiking in M. truncatula. A and B show representative traces of calcium spiking. Top trace is the fluorescence intensity corrected for background fluctuations. Bottom trace in each case shows the change in fluorescence intensity from one time point to the next [X(n + 1) − Xn]. Bacteria were prepared as described in “Materials and Methods.” One nanomolar NF or 108 Rm1021 bacteria were added at vertical line. Fl. Int, Fluorescence intensity.
Table I.
Comparison of M. truncatula calcium response induced by NF versus live S. meliloti cells
| Treatment | No. of Cells Spiking/Total No. of Cells (No. of Plants) | Cells Spiking | Laga | Periodb |
|---|---|---|---|---|
| % | min | s | ||
| Nod factor (1 nm) | 36 /48 (14) | 83.3 | 11.1 ± 3.4 | 100 ± 30 |
| Rm1021 Cells (108 cells) | 44 /52 (11) | 86.8 | 11.5 ± 5.3 | 110 ± 30 |
The lag was calculated by finding the first peak in the data for each root hair after application of NF or live cells and measuring the time interval.
The period of spiking was calculated for each root hair by measuring the time interval between neighboring peaks. Stretches of data where no spiking was observed for more than 3 min were not included in the calculation.
S. meliloti Common nodABC Genes Are Required to Trigger Calcium Spiking
The ability of live Rhizobium to trigger calcium spiking allowed us to use bacterial mutants to examine which nod genes are required to activate this response. We first tested the requirement for common nod genes, nodABC, which are necessary to elicit all known plant nodulation responses (Debellé et al., 1986; Swanson et al., 1987). The nodABC mutant SL44 and the nodA mutant GMI3253 should produce no NF and NFs that lack a lipid tail, respectively (Fisher et al., 1988; Debellé et al., 1996). Both strains failed to trigger calcium spiking in M. truncatula root hairs (Table II, Fig. 3). These root hairs were then challenged with NF purified from Rm1021 to demonstrate that the cells were capable of initiating calcium spiking. The deletion in SL44 spans nodD1, a transcriptional regulator of nod gene expression. We found that a nodD1 mutant retained the ability to trigger calcium spiking (data not shown), indicating that the behavior of SL44 is because of the absence of the biosynthetic nodABC genes. Thus, calcium spiking shows the same dependence on common nod genes as the known nodulation responses. The requirement for common nod genes indicates that there is no nod gene-independent signal in Rhizobium that can induce, or interfere with, calcium spiking.
Table II.
Calcium oscillation response induced by S. meliloti nod mutants
| Strain | Relevant Genotype | No. of Cells Spiking/Total Cells (No. of Plants) | Inferred NFa |
|---|---|---|---|
| Rm1021 | Wild-type S. meliloti | 44/52 (11) | NodRmIV (Ac, C16:2, S) |
| SL44 | nodD1ABC− | 0/12 (4) | Noneb |
| GMI3253 | nodA− | 0/14 (3) | NodRmIV (Ac, S) |
| JAS108 | nodF− | 15/17 (4) | NodRmIV (Ac, C18:1, S) |
| RJW13 | nodL− | 12/12 (4) | NodRmIV (C16:2, S) |
| RJW14 | nodF− nodL− | 17/25 (6) | NodRmIV (C18:1, S) |
| JT210 | nodH− | 0/13 (4) | NodRmIV (Ac, C16:2)b |
NF structures are either inferred or shown as determined biochemically for mutant strains in Rm1021 or Rm2011 backgrounds.
Inferred NF structures.
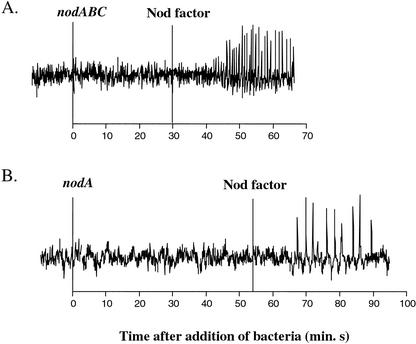
Figure 3.
Calcium spiking response to common nod mutants. A, nodABC strain SL44. B, nodA strain GMI3253. Representative traces of the change in fluorescence in root hairs responding to bacterial mutants lacking common nod genes. Bacteria were added at the vertical line (time = 0 min). Purified NF was added back to verify that the root hair was capable of initiating calcium spiking.
nod Genes Are Sufficient to Confer upon Escherichia coli the Ability to Trigger Calcium Spiking in M. truncatula
The ability to synthesize NF, as defined by the requirement for common nod genes, is necessary for S. meliloti to trigger calcium spiking in M. truncatula. Therefore, we examined whether the ability to make NF was sufficient to allow non-rhizobial bacteria to elicit calcium spiking. To test this, S. meliloti common and host-specific nod genes were introduced into E. coli, a species that does not normally interact symbiotically with legumes. The common nodABC and/or the host-specific nodPQ1, nodH, and nodFEG genes were introduced into E. coli strain BL21 (DE3). We postulated that T7 RNA polymerase in this strain might permit expression from Rhizobium promoters, based on the observation that Rhizobium RNA polymerase recognizes T7 promoters (see “Materials and Methods”). We tested the ability of each of the E. coli strains to induce root hair deformation, a known nodulation response, and calcium spiking. We found that E. coli carrying both common and host-specific nod genes induces abundant root hair deformation and calcium spiking (Fig. 4, A and B). In contrast, strains carrying only the common nod genes, the host-specific nod genes, or lacking all nod genes failed to elicit root hair deformation or calcium spiking (Fig. 4, A and C–E). These results demonstrate that, in addition to being necessary in S. meliloti, 11 NF biosynthetic genes are sufficient to enable an incompatible bacterium to trigger calcium spiking, as well as root hair deformation. Furthermore, these data suggest that no dedicated secretion or transport system is required for delivery of S. meliloti NF to the plant to elicit these early host responses.
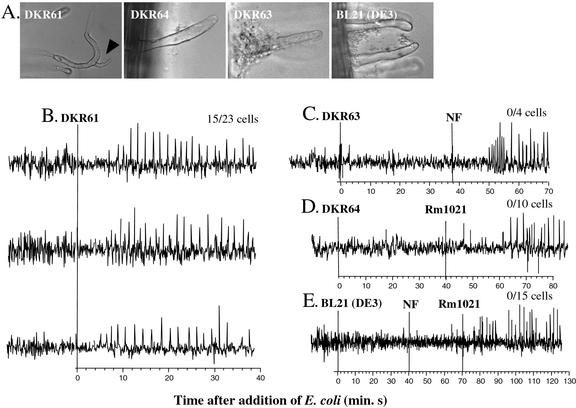
Figure 4.
E. coli carrying 11 nod genes can trigger calcium spiking in M. truncatula. A, Root hair deformation response to DKR61 (carrying common and host-specific nod genes), DKR64 (carrying host-specific nod genes), DKR63 (carrying common nod genes), and BL21 (DE3). Black arrowhead marks a branched root hair. B, Calcium spiking response of three representative root hairs to DKR61 [BL21 (DE3) pRmE2 pRmJT5]. C, Calcium spiking response to DKR63 [BL21 (DE3) pRmE2]. D, Calcium spiking response to DKR64 [BL21 (DE3) pRmJT5]. E, Calcium spiking response to E. coli BL21 (DE3). Root hair deformation (A) and calcium spiking phenotypes (B–E) elicited by E. coli BL21 (DE3) strains carrying common and/or host-specific nod genes. Root hair deformation assays were scored 48 h after inoculation with bacteria, as described in “Materials and Methods.” In B through E, E. coli cells were added at time = 0 min, marked by the solid vertical line, and NF and/or Rm1021 cells were added subsequently as a positive control.
Host-Specific nod Genes Influence the Induction of Calcium Spiking
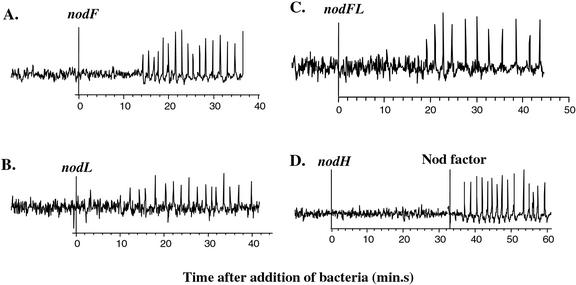
The host-specific nod genes nodFE, nodL, nodPQ, and nodH function in the modification of NF. When these genes are mutated, the ability of Rhizobium to form nodules on host plants is delayed and often impaired. We tested the effect of mutations in each of these nod genes on the ability of bacteria to trigger calcium spiking. We first examined the requirement for the NodF, NodE, and NodL functions that modify the nonreducing end of NF. NodF and NodE are required for the synthesis of the multiply unsaturated fatty acid, whereas NodL is required for the attachment of an O-acetyl group at the nonreducing end (Fig. 1). We found that, individually, nodF, nodE, and nodL genes are not required for the ability of S. meliloti to trigger calcium spiking. JAS108, a nodF::Tn5 mutant, induced calcium spiking in 15 of 17 cells on four plants (Fig. 5A; Table II). Similar results were obtained with a nodE mutant (data not shown). RJW13, carrying a nodL::Tn5, elicited calcium spiking in all of the 12 cells tested on four plants (Fig. 5B; Table II). We also found that RJW14, carrying a double nodFnodL mutation, induced calcium spiking in 17 of 25 cells on six plants (Fig. 5C; Table II). These results indicate that absence of the multiply unsaturated fatty acid modification or the O-acetyl modification or both modifications does not abolish the activity of S. meliloti NFs with respect to calcium spiking in M. truncatula. nodFnodL mutants trigger early morphological responses in M. truncatula (Ardourel et al., 1994; Catoira et al., 2001). Thus, in this host, calcium spiking appears to have the same structural specificity as root hair deformation, root hair curling, and cortical cell activation.
Figure 5.
Calcium spiking response to host-specific nod mutants. A, nodF strain JAS108. B, nodL strain RJW13. C, nodFL strain RJW14. D, nodH strain JT210. Representative traces of the change in fluorescence in root hairs responding to bacterial mutants lacking common nod genes. Bacteria were added at time = 0 min. Purified NF was added back to verify that the root hair was capable of initiating calcium spiking.
We then examined whether modification of the reducing end of NF, dependent on nodH and nodPQ genes, is necessary for the ability of bacteria to trigger calcium spiking. In the absence of NodH, NodP, and NodQ, S. meliloti NF lacks the O-linked sulfate modification on the reducing end (Fig. 1). A nodH::Tn5 strain, JT210, was tested and failed to induce calcium spiking in 13 root hairs on four separate M. truncatula plants (Fig. 5D; Table II). Similar results were seen in tests of a nodPQ− derivative of Rm1021, which lacks both nodPQ1 and nodPQ2 (data not shown). In all of these experiments, subsequent application of either purified NF or live Rm1021 cells induced normal calcium spiking in the target root hairs, showing they were capable of response and that the inactive bacterial cells did not inhibit the host calcium response.
R. leguminosarum bv viciae and S. meliloti Strains That Produce High Levels of Unsulfated NFs Can Trigger Calcium Spiking in M. truncatula
Loss of NF sulfation because of mutation in nodH or nodPQ leads to an inability of bacteria to trigger host responses. This phenotype is attributed to the lack of activity of unsulfated NFs produced by such mutants. However, it has been noted that significantly less NF is recovered from nodH mutants than wild-type cells (Roche et al., 1991), raising the possibility that JT210, and other nodH mutants of S. meliloti, fail to trigger calcium spiking because of an inability to efficiently produce or deliver NF. To address this possibility, we tested an S. meliloti nodH derivative engineered to overexpress nod genes. It has been shown that unsulfated NFs can be recovered from culture supernatants of nodH strains carrying extra copies of nodD, encoding transcriptional activators of the biosynthetic nod genes (Roche et al., 1991). RJW25, a nodH::Tn5 strain carrying a plasmid containing nodD3 under the control of the strong constitutively active trp promoter, was tested for its ability to induce calcium spiking in M. truncatula. We found that this strain induces calcium spiking: 19 of 22 cells on five plants showed a response.
In the canonical model for host specificity, based on S. meliloti-alfalfa interactions, the sulfate substitution on the reducing end is absolutely required for the NF's activity (Roche et al., 1991). The ability of RJW25 to elicit calcium spiking in M. truncatula could indicate a difference in the structural specificity of the calcium spiking response from known nodulation responses. Alternatively, M. truncatula and alfalfa may respond differently to nodH bacteria. To address this issue, we tested whether RJW25 cells trigger calcium spiking in alfalfa root hairs. None of the 15 cells tested on four alfalfa plants initiated calcium spiking in response to this strain. These results indicate that the NF structural requirements for the calcium spiking response are consistent with those for host morphological responses in alfalfa, and that M. truncatula and alfalfa differ in their requirement for nodH.
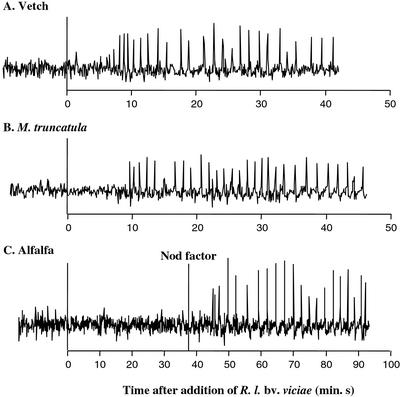
To confirm this result, we tested a second strain that produces unsulfated NFs, R. leguminosarum bv viciae wild-type strain A34. This species produces NFs that differ from wild-type S. meliloti NFs in that they lack the sulfate modification at the reducing end and are modified by a longer multiply unsaturated fatty acid (C18:4) at the nonreducing end. R. leguminosarum bv viciae nodulates Vicia spp. but not Medicago spp. (Faucher et al., 1989). Furthermore, NF purified from R. leguminosarum bv viciae elicits calcium spiking in vetch root hairs but not alfalfa root hairs (Ehrhardt et al., 1996). We tested whether R. leguminosarum bv viciae A34 triggers calcium spiking in the root hairs of its host, vetch, and found that nine of 14 cells in three plants tested showed calcium spiking (Fig. 6A). Thus, the ability to trigger calcium spiking in host root hairs is not limited to S. meliloti. The response elicited by R. leguminosarum bv viciae bacteria was not obviously different from that induced by the purified R. leguminosarum bv viciae NFs (data not shown; D.W. Ehrhardt, J.A. Downie, and S.R. Long, unpublished data). We tested the calcium response of M. truncatula and alfalfa to R. leguminosarum bv viciae A34. As seen with RJW25, R. leguminosarum bv viciae triggered a response in M. truncatula plants (24 of 32 cells on four plants; Fig. 6B) but not alfalfa (0 of 21 on three plants; Fig. 6C). The results with R. leguminosarum bv viciae cells reflect the same difference in specificity between alfalfa and M. truncatula that was observed with RJW25 and are consistent with previous work demonstrating that R. leguminosarum bv viciae fails to induce nodulation responses in alfalfa.
Figure 6.
R. leguminosarum bv viciae triggers calcium spiking in vetch and M. truncatula but not alfalfa. Representative traces of calcium response elicited by R. leguminosarum bv viciae strain A34 in vetch (A), M. truncatula (B), and alfalfa (C). Bacteria were added at time = 0 min in all cases. For alfalfa, where A34 cells failed to induce a response, purified NF was added back to verify the root hair's ability to initiate calcium spiking.
Calcium Spiking in Alfalfa Shows the Same Host Specificity as Known Nodulation Responses
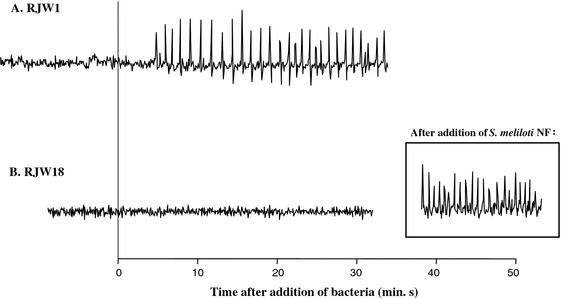
Faucher et al. (1989) demonstrated that transfer of S. meliloti host-specific nod genes into R. leguminosarum bv viciae allows this strain to induce root hair deformation and cortical cell division in alfalfa, whereas wild-type R. leguminosarum bv viciae elicits no nodulation response in alfalfa. Knowing that wild-type R. leguminosarum bv viciae does not trigger calcium spiking in alfalfa root hairs, we tested whether the transfer of S. meliloti host-specific nod genes nodH, nodPQ1, and nodFEG into R. leguminosarum bv viciae A34 would allow this species to induce calcium spiking in alfalfa. RJW1, R. leguminosarum bv viciae carrying S. meliloti host-specific nod genes, elicited calcium spiking in alfalfa root hairs (22 of 22 cells on five plants, Fig. 7A). This response is nodH dependent: RJW18, which differs from RJW1 only in that it lacks nodH, failed to trigger calcium spiking in alfalfa (0 of 10 cells on two plants). Both RJW1 and RJW18 retain the ability to elicit calcium spiking in vetch (data not shown), indicating that the presence of S. meliloti nod genes does not suppress R. leguminosarum bv viciae activity on its host plant. Thus, the same S. meliloti host-specific nod genes that confer the ability to elicit root hair deformation and cortical cell division enable R. leguminosarum bv viciae to induce calcium spiking in alfalfa.
Figure 7.
S. meliloti host-specific nod genes confer upon R. leguminosarum bv viciae the ability to trigger calcium spiking in alfalfa. Representative traces of change in fluorescence in alfalfa root hairs exposed to: A, RJW1 (A34 pRmJT5); or B, RJW18 (A34 pRmS210). In B, the ability of the root hair to initiate calcium spiking is demonstrated after exposure to wild-type S. meliloti NF.
Purified Unsulfated NF Triggers Calcium Spiking in M. truncatula and Alfalfa at the Same Concentration
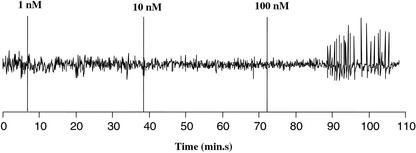
Experiments with RJW25 and A34 introduce additional variables, such as overexpression or the presence of an incompatible bacterium, that might affect the observed plant response. It has been shown that purified unsulfated NF isolated from nodH− S. meliloti, or nodH NF, can elicit calcium spiking in M. truncatula if presented at high dose (Oldroyd et al., 2001b). At 1 nm, the standard concentration at which wild-type NF is used, the nodH NF does not trigger calcium spiking, but when the dose is raised to 100 nm, this altered NF elicits a response comparable with that elicited by wild-type NF. We examined the ability of purified unsulfated S. meliloti NF to trigger calcium spiking in alfalfa to determine whether M. truncatula and alfalfa are differentially sensitive to this NF. We tested the ability of nodH NF to trigger calcium spiking in alfalfa at 1, 10, and 100 nm doses. Similar to observations in M. truncatula, unsulfated NF elicits calcium spiking at 100 nm in alfalfa but not at 1 or 10 nm (Fig. 8). Thus, although alfalfa and M. truncatula differ in their response to bacteria producing unsulfated NFs, the plants show no difference in sensitivity to purified unsulfated S. meliloti NF.
Figure 8.
Calcium spiking response of alfalfa to nodH-derived NF. Representative trace of alfalfa root hair response to increasing doses of unsulfated S. meliloti NF.
DISCUSSION
NF elicits a series of rapid responses in host root hairs, ranging from ion fluxes to cytoskeletal rearrangements and including cytoplasmic calcium spiking (for review, see Downie and Walker, 1999; Cardenas et al., 2000). Calcium spiking is the first of these responses to be studied using live bacteria. We have demonstrated that calcium spiking is a feature of the interaction between at least three different bacterium host pairs, suggesting that it is common among legume species and likely involved in nodulation. In the S. meliloti-M. truncatula interaction, an analysis of calcium spiking with respect to several parameters showed that wild-type bacteria and purified NF trigger the same response. In addition, S. meliloti and R. leguminosarum bv viciae elicit a calcium spiking response in their respective hosts as rapidly as purified NFs. Thus, the context in which wild-type NF is delivered, whether in purified form or by live bacteria, does not seem to affect NF perception as it relates to calcium spiking.
The nod Genes Are Necessary and Sufficient to Trigger Calcium Spiking
NF and the common nod genes required for synthesis are tightly correlated with the ability of Rhizobium to induce nodulation responses in host roots. We observed the same correlation for the calcium spiking response: Absence of the activities necessary for the assembly of the lipochito-oligosaccharide backbone abolishes the ability of bacteria to elicit the calcium behavior. Experiments using E. coli strains engineered to express S. meliloti nod genes further support the idea that the calcium spiking response is triggered by NF and that the genetic requirements for the ability to elicit this root hair response are the same as the requirements for root hair deformation, a known nodulation response. Tests of S. meliloti nod mutants and E. coli strains carrying nod genes establish that the genes are both necessary and sufficient for the ability to elicit calcium spiking. The nod gene dependence strongly suggests that NF is the critical signal needed to trigger calcium spiking, just as it is the principal signal in nodule development. Thus, calcium spiking is likely to be associated with initial events in the symbiosis, such as NF perception or signaling.
Calcium Spiking Is Only Partially Structurally Selective, Similar to Other Early Host Responses
Using S. meliloti nod mutants, we were able to examine the structural specificity of calcium spiking in a context that allowed comparison to known nodulation responses provoked by these same strains. We found that the calcium spiking response in M. truncatula does not show the same stringent requirement for S. meliloti host-specific nod genes as does full nodulation. Mutations leading to structural changes in the N-linked fatty acid and/or loss of the acetyl moiety at the nonreducing end of the NF did not abolish the ability of bacteria to trigger calcium spiking. These results are consistent with the findings of a recent study of M. truncatula calcium spiking in which it was shown that NFs purified from S. meliloti nodF, nodL or nodFnodL mutants were effective over similar concentration ranges as NFs derived from wild-type S. meliloti (Oldroyd et al., 2001b). Mutations in S. meliloti nodF and nodL genes disrupt the ability to elicit nodule development and infection thread formation but do not affect the ability to trigger calcium spiking. These results with a bacterial mutant that separates early root hair responses from cortical responses and infection events allowed us to correlate the calcium spiking response with the very earliest responses in nodulation. Similarly, in studies of the calcium spiking response in nodulation mutants of M. truncatula and pea, the calcium behavior is associated with early events such as root hair deformation (Wais et al., 2000; Walker et al., 2000). Thus, the ability to trigger a calcium spiking response is not sufficient to lead to full nodulation.
The hallmark of a true nodulation response is its selective activation by bacteria producing particular NF structures. Structural specificity is a significant component of host range determination. This is exemplified by the strict requirement for NF sulfation in the S. meliloti-alfalfa symbiosis (Faucher et al., 1988, 1989). Transfer of S. meliloti host-specific nod genes into R. leguminosarum bv viciae allowed this normally incompatible species to elicit early nodulation responses in alfalfa. We were able to demonstrate the same nod gene dependence for the calcium spiking response in alfalfa: Transfer of host-specific S. meliloti nod genes into R. leguminosarum bv viciae conferred the ability to trigger calcium spiking in alfalfa root hairs and, similar to the host responses observed by Faucher et al. (1989), this ability was dependent on the presence of the S. meliloti nodH gene. Thus, in alfalfa, calcium spiking shows the same sensitivity to bacterial host range as has been observed for early known nodulation responses.
The Sensitivity of Calcium Spiking Led to the Detection of Differences in the Behavior of nodH S. meliloti Mutants
Experiments with nodH mutants uncovered a behavior that suggests that, in S. meliloti, the sulfate modification may affect some aspect of NF production or export. Our finding that a nodH mutant engineered to overexpress nod genes triggers calcium spiking in M. truncatula is consistent with prior reports that unsulfated NFs are recovered in lower than expected quantities based on NF yield from wild-type bacteria (Roche et al., 1991). The decrease in NF production does not appear to be because of reduced nod gene expression in nodH mutants (Faucher et al., 1988). We also found that nodH− strains failed to elicit calcium spiking in vetch, which responds to unsulfated NFs purified from nodH S. meliloti or wild-type R. leguminosarum bv viciae strains at doses as low as 1 pm (data not shown; D.W. Ehrhardt, J.A. Downie, and S.R. Long, unpublished data). Taken together, these results indicate that some aspect of NF synthesis, stability, secretion, or delivery is altered in the S. meliloti nodH mutants.
Specialized secretion systems for NF delivery have been proposed (Downie, 1998), that could account for the ability of live bacteria to elicit host responses not seen with purified NF. Several candidate secretory proteins have been identified in Rhizobium. Mutation of genes such as nodIJ in R. leguminosarum species can cause delayed nodulation (Downie, 1998). Similar mutations in S. meliloti, however, have no effect on nodulation kinetics (Jacobs et al., 1985). S. meliloti wild-type NF may have chemical properties that allow it to be exported without specialized mechanisms: 11 nod genes, all with roles in NF synthesis, were sufficient to allow E. coli to trigger root hair deformation and calcium spiking, indicating that there was no required specialized delivery system for the signal. Thus, it is possible that different species of Rhizobium have developed mechanisms of secretion or delivery optimized for the chemical properties of their specific NF. It has been noted that in R. leguminosarum bv viciae, NFs modified by NodX, containing an acetyl substitution at the reducing end, partition to a membrane fraction in biochemical tests and are more difficult to purify than the unmodified NFs (Firmin et al., 1993). More hydrophobic molecules, such as NFs that have unmodified reducing ends, may be recognized by and depend on specialized secretion mechanisms for transport outside the bacterium. This could explain the observed behavior of S. meliloti that normally produces a sulfated NF and may not need to rely on a specialized secretion mechanism. When nodH is mutated, the bacteria produce a more hydrophobic unsulfated NF that may not exit the cells efficiently. Whether bacteria rely on specialized systems to deliver the correct NF to the host plant, or on the lack of a secretion system to prevent incomplete NFs from reaching a host plant, both strategies may ensure that only the correct structure is presented to the host plant. This could be particularly important to prevent incomplete NFs from triggering partial, nonproductive responses in host legumes.
The Quantitative Nature of Calcium Spiking Allows for a Comparison of the Responsiveness of Closely Related Hosts
Studies of the structural specificity of nodulation responses in Medicago-S. meliloti have historically focused on alfalfa (Debellé et al., 1986; Swanson et al., 1987; Faucher et al., 1988, 1989; Ardourel et al., 1994). M. truncatula, a close relative of alfalfa, recently has been developed as a more tractable model organism for genetic analysis of nodulation (Cook, 1999). These species have the same overall nodulation phenotype in response to bacterial nod mutants; in particular, nodFnodL and nodH mutants of S. meliloti fail to induce nodules on both plants (Ardourel et al., 1994). However, there is a growing body of evidence indicating that, upon closer study, the two species differ in their degree of responsiveness to bacterial mutants. For instance, nodFnodL S. meliloti induces root hair curling in M. truncatula but not in alfalfa (Ardourel et al., 1994; Catoira et al., 2001). It has also been noted that, at very low frequency, nodH S. meliloti can even induce nodule initiation in M. truncatula plants (J.M. Harris, D.H. Keating, and S.R. Long, unpublished data). Our comparison of calcium spiking in M. truncatula and alfalfa treated with R. leguminosarum bv viciae or nodH S. meliloti shows that there is a quantifiable difference in the responsiveness of these two legume species to bacteria producing unsulfated NFs. Thus, even if two species exhibit the same overall nodulation phenotype, they may not exhibit the same phenotype for every response associated with nodulation. These differences in specificity may relate to underlying differences in signal reception or initial transduction events and may prove useful in future studies of candidate host-signaling proteins.
Studies with Live Bacteria Can Reveal Behavior Not Seen with Purified NF
In attempting to determine whether the observed difference in calcium spiking response between alfalfa and M. truncatula treated with nodH S. meliloti lay in their respective sensitivities to unsulfated NF, we uncovered an apparent contradiction. Whereas M. truncatula responds to nodH S. meliloti and alfalfa does not, NF purified from these bacteria triggers calcium spiking at 100 nm in both hosts (Oldroyd et al., 2001b). Thus, it would appear that these two hosts, which show different responses to nodH S. meliloti, are nonetheless comparably sensitive to the NF derived from this strain. One possible explanation for these data is that M. truncatula and alfalfa differ by less than an order of magnitude in their sensitivity to unsulfated NF. A finer scale study of the dose response of both hosts to unsulfated NF might reveal a threshold concentration at which calcium spiking is activated in M. truncatula but not in alfalfa. If this is the case, then the current assay conditions are such that the nodH S. meliloti inoculum exposes the plants to the precise threshold concentration of unsulfated NF necessary to produce a response in one host but not the other. If the differing behavior of M. truncatula and alfalfa is not caused by a difference in structural specificity, then there remains the possibility that the context in which the plant perceives NF affects its response. Differential responses to bacteria versus purified NFs are well documented, and have even been noted in the ability of nodH S. meliloti but not nodH NFs to trigger cortical cell activation in Medicago hosts (Vernoud et al., 1999); however, our observation is unusual in that purified nodH NF elicits a response that is not triggered by live bacteria. To our knowledge, this is the first indication that the way in which NF is delivered can alter the calcium spiking response. There could also be a secondary component in alfalfa that modulates the host response to NF, but such a component would only be significant in interactions involving mutant S. meliloti. As evidenced by this study, tests with live bacteria introduce additional variables into the characterization of host responses. However, without such studies, it is not possible to fully understand the role of early host responses in the context of nodulation, a process that requires the presence of both symbiotic partners.
MATERIALS AND METHODS
Plant Growth and Preparation
Seeds of Medicago truncatula cv Jemalong (Purkiss Seeds, Armidale, Australia) were surface-sterilized in 70% (v/v) ethanol for 45 min followed by incubation in 5.25% (v/v) sodium hypochlorite for 45 min. Alfalfa (Medicago sativa GT13R) seeds (ABI, Nampa, ID) were treated with 70% (v/v) ethanol for 10 min, followed by 30 min in 5.25% (w/v) sodium hypochlorite. Vetch (Vicia sativa nigra) seeds were scarified in concentrated sulfuric acid for 15 min, then surface sterilized in 5.25% (w/v) sodium hypochlorite for 10 min. All seeds were imbibed overnight at room temperature and then stored up to 7 d under water at 4°C until use. M. truncatula and alfalfa seeds were germinated inverted in plastic petri dishes in the dark and then transferred to plates containing buffered nodulation medium (BNM; Ehrhardt et al., 1992) with 1.2% (w/v) purified agar (Sigma, St. Louis). Vetch seeds were germinated in plates containing 1% (w/v) agar in water to help the seeds adhere to the plate. M. truncatula seedlings were grown on BNM containing 0.1 μm l-α-(2-aminoethoxyvinyl)Gly (Sigma). For cellular calcium assays, 2-d-old seedlings were prepared as described by Ehrhardt et al. (1996) with the following modifications. Two to three plants were set up on a 48- × 60-mm coverslip (no. 1) in a bath of approximately 2 mL of BNM [no l-α-(2-aminoethoxyvinyl)Gly]. For root hair deformation assays, 6-d-old plants were inoculated on plates with 10 μL of bacteria resuspended in BNM at an optical density at a wavelength of 600 nm (OD600) of 0.5. The drop was placed at the very tip of the growing root. Root hair deformation was scored 36 to 48 h after inoculation.
Bacterial Strain Construction and Growth Conditions
Strains tested for calcium spiking were grown in Tryptone-Yeast extract liquid medium (Beringer, 1974), under appropriate antibiotic selection. Antibiotics were used at the following concentrations: 500 μg mL−1 streptomycin, 50 μg mL−1 spectinomycin, 50 μg mL−1 neomycin, and 10 μg mL−1 tetracycline. For calcium spiking and root hair deformation assays, bacterial cultures were grown to mid- or late-log phase, then cells were spun at 20,000g for 1 min, washed, and resuspended in BNM at an OD600 of 0.5. In the calcium spiking assay, 100 μL of the resuspended cells was added to the 2-mL bath containing the injected plants. Approximately 108 cells, determined by counting colony-forming units from serial dilution assays, were added to the bath.
All Sinorhizobium meliloti strains were tested in the Rm1021 background. Strains RJW13 and RJW14 (Table III) were constructed by cotransduction with phage N3 of the marked nodL::Tn5 and the nodF deletion from GMI3032 (Ardourel et al., 1994) or GMI6436 (Ardourel et al., 1995) into JAS115 containing a nodE::Tn5-233. The average transduced segment is approximately 160 kb, sufficient for cotransduction of both the nodF deletion and the nodL::Tn5 (Martin and Long, 1984). Colonies were selected for growth on neomycin and then screened for loss of spectinomycin resistance. This allowed us to select for transductants that carried the nodF deletion from GMI3032 in the case of the nodFnodL strain, or the comparable region, with an intact nodF from GMI6436 in the case of the nodL strain. The nodF deletion was confirmed in RJW14 by sequencing. RJW23 and RJW25 were made by introducing, via triparental mating, with helper plasmid pRK600 (Glazebrook and Walker, 1991), pRmE65, carrying nodD3 under the control of the trp promoter, into 912T (Table III). 912T carries a nodH::Tn5 in an Rm1021 background.
Table III.
Strains and plasmids
| Strain or Plasmid | Relevant Characteristics | Source or Reference |
|---|---|---|
| S. meliloti | ||
| Rm1021 | Wild type, SU47 str21 | Meade et al. (1982) |
| SL44 | Rm1021 ΔnodD1ABC | Fisher et al. (1988) |
| GMI3253 | Rm1021 ΔnodA | Debellé et al. (1996) |
| JAS108 | Rm1021 nodF∷Tn5-233, Spr a | J.A. Swanson (unpublished data) |
| JAS115 | Rm1021 nodE∷Tn5-233, Spr | J.A. Swanson (unpublished data) |
| GMI6436 | Rm2011 nodL∷Tn5, Nmr b | Ardourel et al. (1995) |
| RJW13 | Rm1021 nodL∷Tn5, Nmr | This study |
| JT210 | Rm1021 nodH∷Tn5, Nmr | Swanson et al. (1987) |
| 912T | Rm1021 nodH∷Tn5, Nmr | J. Ogawa (unpublished data) |
| RJW25 | 912T pRmE65 | This study |
| GMI3032 | Rm2011 ΔnodF nodL∷Tn5, Nmr | Ardourel et al. (1994) |
| RJW14 | Rm1021 ΔnodF nodL∷Tn5, Nmr | This study |
| R. leguminosarum bv viciae | ||
| A34 | Wild type, 8401 pRL1JI | Downie et al. (1983) |
| RJW1 | A34 pJT5 | This study |
| RJW18 | A34 pRmS210 | This study |
| E. coli | ||
| BL21 DE3 | F−ompT, hsdSR (rB−mB−) gal dcm (DE3) | Studier and Moffatt (1986) |
| DKR 61 | BL21 DE3 pRmE2 pRmJT5 | This study |
| DKR 63 | BL21 DE3 pRmE2 | This study |
| DKR 64 | BL21 DE3 pRmJT5 | This study |
| Plasmids | ||
| pRmE65 | nodD3 Expressed under control of trp promoter in pTE3 | Fisher et al. (1988) |
| pRmJT5 | nodFEGHPQ1 syrM nodD3 in pLAFR1 | Swanson et al. (1987) |
| pRmS210 | nodH∷Tn5 in pJT5 | Swanson et al. (1987) |
| pRmE2 | nodABC in pAD10 | Egelhoff and Long (1985) |
| pRK600 | Helper plasmid derived from pRK290 | Glazebrook and Walker (1991) |
| Phage | ||
| N3 | S. meliloti-transducing phage | Martin and Long (1984) |
Spr, Spectinomycin resistant.
Nmr, Neomycin resistant.
Rhizobium leguminosarum bv viciae strains tested in this study were derived from the wild-type strain A34 (Downie et al., 1983). RJW1 carries pRmJT5 (Table III) introduced into A34 by triparental mating. RJW18 carries plasmid pRmS210, derived from pRmJT5 and containing a nodH::Tn5 (Fisher et al., 1987b).
Escherichia coli strains were derived from BL21 (DE3) (Studier and Moffatt, 1986), which contains a T7 RNA polymerase under control of the lacUV5 promoter. Studies of nodulation responses by heterologous bacteria such as E. coli have been limited by a lack of expression of nod genes, as judged by a lack of activity of nod-lac fusions in E. coli backgrounds (Fisher et al., 1987a). A previous report demonstrating that the T7 promoter is recognized in R. leguminosarum (Ritsema et al., 1994) led us to test whether the converse is true; that is, strains that express T7 polymerase have an increased ability to recognize nod gene promoters. We introduced plasmid pRmE2, containing nodABC under control of the lac promoter, and pRmJT5, carrying the S. meliloti host specificity genes under their native promoters, into BL21 (DE3). The resultant strain, DKR61, elicits root hair deformation, a host nodulation response. Other E. coli strains into which Rhizobium genes were introduced did not show the same activity, suggesting that BL21 (DE3) seems better able to express S. meliloti nod genes (D.H. Keating and S.R. Long, unpublished data). Two further BL21 (DE3)-derived strains were constructed as controls for DKR61: DKR63 and DKR64 carry pRmE2 and pJT5, respectively.
NFs
S. meliloti wild-type NF, NodRmIV(Ac, C16:2, 5), was purified as described previously (Ehrhardt et al., 1996). Unsulfated NF from nodH S. meliloti, NodRmIV(Ac, C16:2), was the kind gift of J. Dénarié (Laboratoire de Biologie Moléculaire des Relations Plantes-Microorganismes, CNRS-INRA, Castanet-Tolosan, France). Because of the tendency of unsulfated nodH S. meliloti NFs NFs to partition onto glass and plastic surfaces (J.A. Downie, personal communication), 0.01% (w/v) CHAPS was added to the buffer in experiments using these structures.
Calcium Oscillation Assay
Calcium imaging was carried out as previously described (Wais et al., 2000). Only root hairs that showed active cytoplasmic streaming after dye injection were chosen for subsequent imaging. In all experiments, root hairs were imaged for at least 30 min after addition of NF or bacteria. Fluorescence data were corrected and transformed as described previously (Wais et al., 2000). In brief, background-corrected fluorescence data were transformed using the function Y = X(n + 1) − Xn, where Y is the point-to-point change in fluorescence, and X(n + 1) and Xn are the intensity measurements at timepoints (n) and (n + 1). For comparison of NF and Rm1021 treatments, lag time and period of the calcium spiking were calculated as previously described (Wais et al., 2000). Unless indicated otherwise in the results, purified NF treatments were performed at a final concentration of 1 nm. In determining when a given bacterial strain was unable to trigger calcium spiking, we first exposed plants to the test strain and, if no calcium spiking response was seen, we added back either purified NF or wild-type bacteria as a positive control. The total number of root hairs tested with bacterial strains that do not elicit calcium spiking, therefore, reflects the number of root hairs that showed calcium spiking to the control treatment but not the test strain.
It was noted that Rm1021 does not require pretreatment with plant flavonoids to trigger calcium spiking and that this behavior is because of the production, in Rm1021, of a low level of NF even in the absence of plant flavonoids (R.J. Wais and S.R. Long, unpublished data). As a control, all Rm1021-derived nod mutants that failed to elicit calcium spiking were also grown in the presence of luteolin and tested for subsequent ability to induce calcium spiking. In no case did an Rm1021-derived strain that failed to induce calcium spiking without luteolin treatment show an ability to trigger calcium spiking after luteolin treatment. R. leguminosarum bv viciae cells were grown in the presence of 3 μm eriodyctiol to induce nod gene expression. A34 cells not pretreated with eriodyctiol failed to induce calcium spiking in root hairs during a 30-min period of observation (data not shown).
ACKNOWLEDGMENTS
We thank Jean Dénarié for his generous gift of NodRmIV (Ac, C16:2) unsulfated S. meliloti NF; David Ehrhardt, Robert Fisher, Giles Oldroyd, and Sidney Shaw for helpful discussions; Raka Mitra for the calcium spiking analysis software; and Sidney Shaw for help with digital imaging and microscopy.
Footnotes
This work was supported in part by the Department of Energy (grant no. DE–FG03–90ER200210). S.R.L. is an investigator of the Howard Hughes Medical Institute.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010690.
LITERATURE CITED
- Ardourel M, Demont N, Debelle F, Maillet Debelle, de Billy F, Prome JC, Denarie J, Truchet G. Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell. 1994;6:1357–1374. doi: 10.1105/tpc.6.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardourel M, Lortet G, Maillet F, Roche P, Truchet G, Prome JC, Rosenberg C. In Rhizobium meliloti, the operon associated with the nod box n5 comprises nodL, noeA and noeB, three host-range genes specifically required for the nodulation of particular Medicago species. Mol Microbiol. 1995;17:687–699. doi: 10.1111/j.1365-2958.1995.mmi_17040687.x. [DOI] [PubMed] [Google Scholar]
- Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Cardenas L, Holdaway-Clarke TL, Sanchez F, Quinto C, Feijo JA, Kunkel JG, Hepler PK. Ion changes in legume root hairs responding to Nod factors. Plant Physiol. 2000;123:443–452. doi: 10.1104/pp.123.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R, Galera C, de Billy F, Penmetsa RV, Journet EP, Maillet F, Rosenberg C, Cook D, Gough C, Dénarié J. Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell. 2000;12:1647–1666. [Google Scholar]
- Catoira R, Timmers ACJ, Maillet F, Galera Maillet, Penmetsa RV, Cook D, Dénarié J, Gough C. The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development. 2001;128:1507–1518. doi: 10.1242/dev.128.9.1507. [DOI] [PubMed] [Google Scholar]
- Cook DR. Medicago truncatula: a model in the making! Curr Opin Plant Biol. 1999;2:301–304. doi: 10.1016/s1369-5266(99)80053-3. [DOI] [PubMed] [Google Scholar]
- Debellé F, Plazanet C, Roche P, Pujol C, Roche P, Savagnac A, Rosenberg C, Prome JC, Dénarié J. The NodA proteins of Rhizobium meliloti and Rhizobium tropici specify the N-acylation of Nod factors by different fatty acids. Mol Microbiol. 1996;22:303–314. doi: 10.1046/j.1365-2958.1996.00069.x. [DOI] [PubMed] [Google Scholar]
- Debellé F, Rosenberg C, Vasse J, Maillet F, Martinez E, Denarie J, Truchet G. Assignment of symbiotic developmental phenotypes to common and specific nodulation (nod) genetic loci of Rhizobium meliloti. J Bacteriol. 1986;168:1075–1086. doi: 10.1128/jb.168.3.1075-1086.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demont N, Debelle F, Aurelle H, Denarie J, Prome JC. Role of the Rhizobium meliloti nodF and nodE genes in the biosynthesis of lipo-oligosaccharidic nodulation factors. J Biol Chem. 1993;268:20134–20142. [PubMed] [Google Scholar]
- Dénarié J, Debellé F, Prome JC. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu Rev Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- Downie JA. Functions of rhizobial nodulation genes. In: Spaink HP, Kondorosi A, Hooykaas PJJ, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 387–402. [Google Scholar]
- Downie JA, Hombrecher G, Ma Q-S, Knight CD, Wells B, Johnston AWB. Cloned nodulation genes of Rhizobium leguminosarum determine host-range specificity. Mol Gen Genet. 1983;190:359–365. [Google Scholar]
- Downie JA, Walker SA. Plant responses to nodulation factors. Curr Opin Plant Biol. 1999;2:483–489. doi: 10.1016/s1369-5266(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Egelhoff TT, Long SR. Rhizobium meliloti nodulation genes: identification of nodABC gene products, purification of nodA protein and expression of nodA in Rhizobium meliloti. J Bacteriol. 1985;164:591–599. doi: 10.1128/jb.164.2.591-599.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Faucher C, Camut S, Dénarié J, Truchet G. The nodH and nodQ host range genes of Rhizobium meliloti behave as avirulence genes in Rhizobium leguminosarum biovar viciae and determine changes in the production of plant-specific extracellular signals. Mol Plant-Microbe Interact. 1989;2:291–300. [Google Scholar]
- Faucher C, Maillet F, Vasse J, Rosenberg C, van Brussel AA, Truchet G, Dénarié J. Rhizobium meliloti host range nodH gene determines production of an alfalfa-specific extracellular signal. J Bacteriol. 1988;170:5489–5499. doi: 10.1128/jb.170.12.5489-5499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996;10:295–301. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J. 1998;13:455–463. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Elevation of the cytosolic free (Ca2+) is indispensable for the transduction of the Nod factor signal in alfalfa. Plant Physiol. 1999;121:273–279. doi: 10.1104/pp.121.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmin JL, Wilson KE, Carlson RW, Davies AE, Downie JA. Resistance to nodulation of cv. Afghanistan peas is overcome by nodX, which mediates an O-acetylation of the Rhizobium leguminosarum lipo-oligosaccharide nodulation factor. Mol Microbiol. 1993;10:351–360. doi: 10.1111/j.1365-2958.1993.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Fisher RF, Brierley HL, Mulligan JT, Long SR. Transcription of Rhizobium meliloti nodulation genes: identification of a nodD transcription initiation site in vitro and in vivo. J Biol Chem. 1987a;262:6849–6855. [PubMed] [Google Scholar]
- Fisher RF, Egelhoff TT, Mulligan JT, Long SR. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 1988;2:282–293. doi: 10.1101/gad.2.3.282. [DOI] [PubMed] [Google Scholar]
- Fisher RF, Swanson JA, Mulligan JT, Long SR. Extended region of nodulation genes in Rhizobium-Meliloti 1021. II. Nucleotide sequence transcription start sites and protein products. Genetics. 1987b;117:191–202. doi: 10.1093/genetics/117.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Walker GC. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 1991;204:398–418. doi: 10.1016/0076-6879(91)04021-f. [DOI] [PubMed] [Google Scholar]
- Jacobs TW, Egelhoff TT, Long SR. Physical and genetic map of a Rhizobium meliloti nodulation gene region and nucleotide sequence of nodC. J Bacteriol. 1985;162:469–476. doi: 10.1128/jb.162.2.469-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MO, Long SR. Generalized transduction in Rhizobium meliloti. J Bacteriol. 1984;159:125–129. doi: 10.1128/jb.159.1.125-129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GED, Engstrom EM, Long SR. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001a;13:1835–1849. doi: 10.1105/TPC.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GED, Mitra RM, Wais RJ, Long SR. Evidence for structurally specific negative feedback in the Nod factor signal transduction pathway. Plant J. 2001b;28:191–199. doi: 10.1046/j.1365-313x.2001.01149.x. [DOI] [PubMed] [Google Scholar]
- Ritsema T, Geiger O, Van Dillewijn P, Lugtenberg BJJ, Spaink HP. Serine residue 45 of nodulation protein NodF from Rhizobium leguminosarum bv. viciae is essential for its biological function. J Bacteriol. 1994;176:7740–7743. doi: 10.1128/jb.176.24.7740-7743.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P, Debelle F, Maillet F, Lerouge Maillet, Faucher C, Truchet G, Denarie J, Prome JC. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell. 1991;67:1131–1143. doi: 10.1016/0092-8674(91)90290-f. [DOI] [PubMed] [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Tu JK, Ogawa JM, Sanga R, Fisher R, Long SR. Extended region of nodulation genes in Rhizobium meliloti 1021: I. Phenotypes of Tn5 insertion mutants. Genetics. 1987;117:181–189. doi: 10.1093/genetics/117.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Journet E-P, Barker DG. MtENOD20, a nod factor-inducible molecular marker for root cortical cell activation. Mol Plant-Microbe Interact. 1999;12:604–614. [Google Scholar]
- Wais RJ, Galéra C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Dénarié J, Long S. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA. 2000;97:13407–13412. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SA, Viprey V, Downie JA. Dissection of nodulation signalling using pea mutants defective for calcium spiking induced in root hairs by Nod factors and chitin oligomers. Proc Natl Acad Sci USA. 2000;97:13413–13418. doi: 10.1073/pnas.230440097. [DOI] [PMC free article] [PubMed] [Google Scholar]