Abstract
The postembryonic developmental program of the plant root system is plastic and allows changes in root architecture to adapt to environmental conditions such as water and nutrient availability. Among essential nutrients, phosphorus (P) often limits plant productivity because of its low mobility in soil. Therefore, the architecture of the root system may determine the capacity of the plant to acquire this nutrient. We studied the effect of P availability on the development of the root system in Arabidopsis. We found that at P-limiting conditions (<50 μm), the Arabidopsis root system undergoes major architectural changes in terms of lateral root number, lateral root density, and primary root length. Treatment with auxins and auxin antagonists indicate that these changes are related to an increase in auxin sensitivity in the roots of P-deprived Arabidopsis seedlings. It was also found that the axr1-3, axr2-1, and axr4-1 Arabidopsis mutants have normal responses to low P availability conditions, whereas the iaa28-1 mutant shows resistance to the stimulatory effects of low P on root hair and lateral root formation. Analysis of ethylene signaling mutants and treatments with 1-aminocyclopropane-1-carboxylic acid showed that ethylene does not promote lateral root formation under P deprivation. These results suggest that in Arabidopsis, auxin sensitivity may play a fundamental role in the modifications of root architecture by P availability.
P is an essential nutrient for plant growth, development, and reproduction. In many soils, P is the major limiting nutrient for agriculture. In acid soils, P forms insoluble compounds with aluminum and iron as well as organic matter, whereas in alkaline soils, it tightly binds calcium and magnesium to form sparingly soluble phosphates. Because of its reactivity, the total amount of P in the soil may be high, but unavailable for plant uptake (Holford, 1997).
The capacity of plants to access P under limiting conditions depends on important adaptive traits, including organic acid excretion, alteration of the pH of the rhizosphere, and increased ability to explore different layers of soil (Schachtman et al., 1998; López-Bucio et al., 2000). A primary adaptation to low P availability involves postembryonic developmental changes in the root system, which are directed toward enhancing P uptake. These include alterations in branching patterns, total root length, root hair elongation, and lateral root formation (Dinkelaker et al., 1995; Bates and Lynch, 1996; Borch et al., 1999).
Root hairs and lateral roots assist the acquisition of P by exploring a greater soil volume and by increasing the absorptive surface of the root. The formation of a highly branched root system, in response to nutrient starvation, may be a consequence of the canalization of carbon and energy resources to produce a root system capable of exploring large areas of the upper soil layer, where nutrient-rich patches are normally present (Stitt and Rudiger-Scheible, 1998).
Phytohormones such as auxin and ethylene are involved in altering primary root growth and in promoting root hair and lateral root formation (Torrey, 1976). It has been shown that application of natural and synthetic auxins increases lateral root formation, whereas auxin transport inhibitors reduce lateral root numbers (Torrey, 1950; Blakely et al., 1982; Muday and Haworth, 1994; Casimiro et al., 2001). It also has been demonstrated that polar auxin transport is essential for lateral root formation at high P (Reed et al., 1998). Ethylene may also play a role in lateral root development because auxins are thought to trigger ethylene production by roots, thus inhibiting primary root elongation with subsequent induction of lateral roots (for review, see Dolan, 1997).
Several mutants have been isolated that reinforce the connection between plant hormones and lateral root development. The tomato (Lycopersicon esculentum) mutant diageotropica (dgt), isolated for its unsupported horizontal growth of shoots, does not produce lateral roots. The defect in lateral root formation in dgt can be rescued by application of ethylene, but this effect seems to be related to a reduced auxin sensitivity (Zobel, 1974; Muday et al., 1995). The Arabidopsis mutant aberrant lateral root formation-4-1 (alf4-1) is unable to produce lateral roots and does not respond to exogenous auxins (Celenza et al., 1995). The auxin-resistant mutants axr1 and axr2 are agravitropic and produce fewer lateral roots than the wild type (Estelle and Somerville, 1987). Conversely, increased formation of lateral roots has been observed in the Arabidopsis mutants with elevated auxin content, including the rooty mutant and its alleles alf1 and superroot (sur1; Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995).
The close relationship between altered root growth and P deficiency suggests that phytohormones could be involved in the response to low P availability. Nutrient stress may affect hormone synthesis, transport, or sensitivity, and in this way alter root architecture. However, conclusive evidence is lacking because most studies have been carried out in crops and under conditions not easily amenable to physiological and molecular dissection (Lynch and Brown, 1997; Borch et al., 1999).
Several characteristics of Arabidopsis, including its small size, short generation time, and large collection of mutants make it useful for the study of root development and nutrient sensing at high resolution (Meyerowitz, 1987). It has been reported recently that P availability influences the development of the Arabidopsis root system favoring lateral root growth over primary root growth under suboptimal P conditions (Williamson et al., 2001). However, in this study, 100 μm was the lowest concentration of P tested, which is far higher than those generally present in soil. In an effort to more fully understand how P deficiency is perceived in plants and translated into a pathway for root development, we characterized to more detail the Arabidopsis lateral root response to P availability. Treatments were also established in which seedlings were grown at high P or low P in the presence of auxins, auxin transport inhibitors, ethylene, and cytokinins to determine the effect of these growth regulators on the responses of wild-type Arabidopsis to P availability. In addition, a suite of mutants in the auxin and ethylene response pathways were tested for their ability to correctly respond to P deficiency upon changes in root architecture to varied levels of phosphate.
RESULTS
Effect of P Availability on Growth of Arabidopsis Seedlings
To test the effect of P availability on the root and shoot biomass accumulation of Arabidopsis Columbia (Col-0) seedlings, a P dose growth response curve was constructed by growing plants in different P concentrations (1 μm to 1 mm) of soluble NaH2PO4. It was observed that root fresh weight decreases with increasing P concentrations and shoot fresh weight increases in higher concentrations of P (Table I). The root to shoot ratio decreases over 5-fold when Arabidopsis seedlings grown in 1 mm P are compared with seedlings grown in 1 μm P (Table I). It was also observed that at 25 μm P, the most significant change in shoot biomass occurs, whereas root fresh weight decreases at concentrations higher than 50 μm P. P content increased with the availability of this nutrient until the concentration reached 100 μm in the media (Table I). Similar responses to P deprivation have been reported for other plant species, including bean (Phaseolus vulgaris), tomato, and Brassica nigra (Christie and Moorby, 1975; Carswell et al., 1996).
Table I.
Effect of phosphate availability on Arabidopsis whole-plant growth and P accumulation
| P Treatment | Root Fresh Wt | Shoot Fresh Wt | Root:Shoot Ratio | P Content |
|---|---|---|---|---|
| mg | % | |||
| 0 | 0.043 ± 0.007∗ | 0.120 ± 0.009∗ | 0.360 + 0.027∗ | 0.13 + 0.02∗ |
| 1 μm | 0.048 ± 0.005∗ | 0.125 ± 0.012∗ | 0.384 + 0.024∗ | 0.19 + 0.03∗∗ |
| 25 μm | 0.041 ± 0.006∗ | 0.269 ± 0.016∗∗ | 0.152 + 0.012∗∗ | 0.31 + 0.04∗∗∗ |
| 50 μm | 0.042 ± 0.006∗ | 0.285 ± 0.014∗∗ | 0.147 + 0.007∗∗ | 0.45 + 0.04∗∗∗∗ |
| 100 μm | 0.022 ± 0.003∗∗ | 0.294 ± 0.016∗∗ | 0.074 + 0.009∗∗∗ | 0.64 + 0.04∗∗∗∗∗ |
| 1000 μm | 0.024 ± 0.004∗∗ | 0.299 ± 0.019∗∗ | 0.066 + 0.009∗∗∗ | 0.68 + 0.04∗∗∗∗∗ |
Wild-type (Col-0) seedlings were grown for 16 d on nutrient media with varying concentrations of soluble (NaH2PO4) phosphate, on vertically oriented agar plates. Roots and shoots were excised at the root-shoot junction and the fresh wt was determined on an analytical balance. Values shown represent the mean of five groups of 40 seedlings ± se. To determine the phosphate content of plants, 300 seedlings were harvested, dried, and total P assayed by a colorimetric method. Data show the mean ± se of two independent experiments. Asterisks are used to indicate means that differ significantly (P < 0.05).
Lateral Root Response to P Deficiency
Our results and previous work indicated that P availability regulates root development in Arabidopsis (Bates and Lynch, 1996; Narang et al., 2000; Williamson et al., 2001). To determine more closely the effect of P availability on the architecture of the Arabidopsis root system, seeds were germinated in vertically oriented petri dishes containing 0.1× Murashige and Skoog solid media with P concentrations ranging from 1 μm to 25 mm. Under these conditions, primary root length, number of lateral roots, and lateral root density were quantified for the Arabidopsis Col-0 ecotype. After 12 d of growth at low P concentration (1–10 μm), it was observed that Arabidopsis seedlings produce a highly branched root system with abundant lateral roots and a short primary root (Fig. 1, A and C). Under these growth conditions, primary and secondary roots had an abundance of long root hairs (Fig. 1C). At P concentrations of 100 μm or higher, Arabidopsis seedlings produce a long primary root with few lateral roots and short root hairs (Fig. 1B). It was observed that increasing P concentrations led to a longer primary root that reaches a maximum at 1 mm P and decreases in length at higher concentrations (Fig. 2A). It is interesting to note that the maximum change in root length (4-fold) was observed between P concentrations of 10 and 100 μm (Fig. 2A), and that lateral root number decreases up to 5-fold between 1 μm and 1 mm P (Fig. 2B). It has been reported previously that the distance between the primary root tip and the first lateral root decreases in seedlings grown at 100 μm P when compared with those grown at 500 μm P (Williamson et al., 2001). We found that at lower P concentrations (1–10 μm P), not only the distance between the primary root tip and the first lateral root, but also the site of lateral root formation is affected. At high P, lateral roots arose in close proximity to the root-shoot junction, whereas at low P, lateral root formation takes place closer to the primary root tip (Fig. 1, A and C).
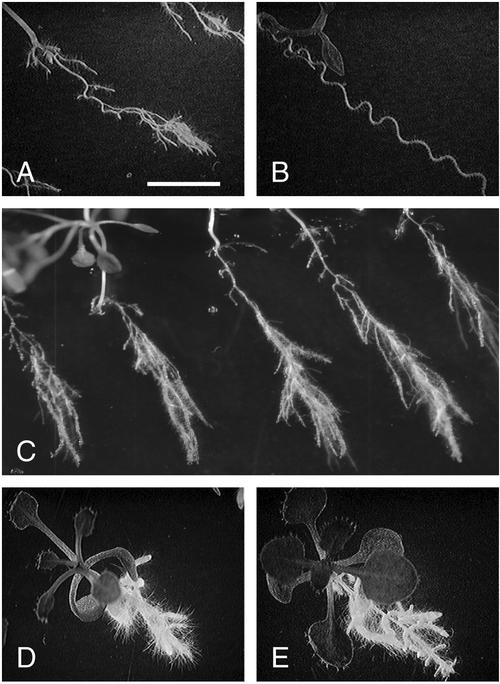
Figure 1.
Effect of phosphate availability and exogenous auxin on Arabidopsis root architecture. Wild-type Col-0 seedlings were grown in the presence of low (1 μm) or high (1 mm) soluble P on vertically oriented agar plates. A, Photograph of a 17-d-old plant grown at low P. B, Photograph of a 10-d-old seedling grown at high P. C, Close-up of 20-d-old low P-grown plants showing the zone of lateral root proliferation. D, Photograph of a 17-d-old seedling grown at low P in the presence of 5 × 10−8 m 2,4-dichlorophenoxyacetic acid (2,4-D). E, Photograph of a 17-d-old seedling grown at high P in the presence of 5 × 10−8 m 2,4-D. All photographs are at the same magnification. Bar = 1 cm.
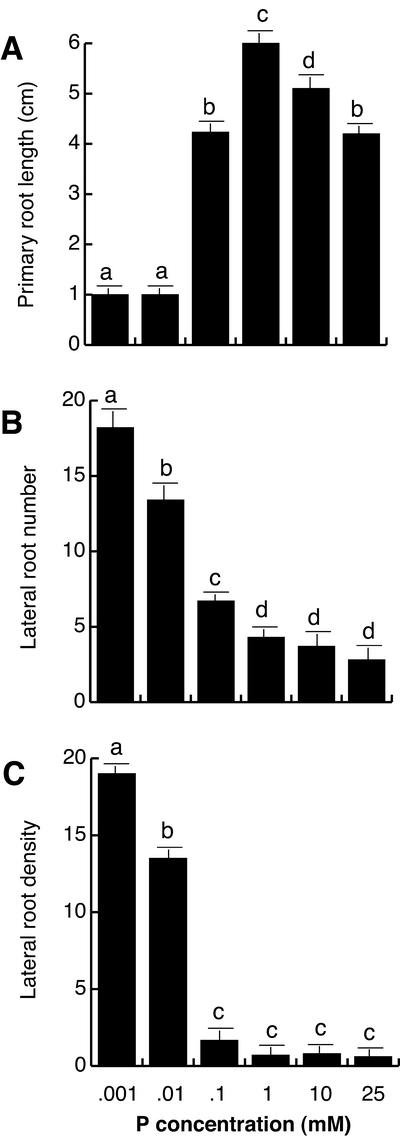
Figure 2.
The effect of phosphate availability on Arabidopsis root architecture. Wild-type Col-0 seedlings were grown for 17 d under a wide range of P concentrations, on vertically oriented agar dishes. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent the mean of 15 seedlings ± se. Different letters are used to indicate means that differ significantly (P < 0.05).
The density of lateral roots was also calculated by dividing the number of lateral roots by the length of the primary root to normalize for the effects of P availability on root length. Lateral root density decreased over 9-fold in plants grown at high P when compared with low P-grown plants (Fig. 2C) Lateral root density was similar in P concentrations ranging between 100 μm and 25 mm; however, it dramatically increased at P concentrations lower than 100 μm (Fig. 2C).
Arabidopsis Ecotypes Differ in Their Responsiveness to Low P Availability
To further characterize the effect of P availability on the formation of the Arabidopsis root system, we examined the root morphology of seedlings of four different Arabidopsis ecotypes (Col-0, Nossen [Nob], Rld, and Wassilewskija [Ws]). Seedlings were germinated in media containing low (1 μm) and high (1 mm) P concentrations. After 12 d of growth at low P concentration, it was observed that all Arabidopsis ecotypes produced a highly branched root system with abundant lateral roots and a short primary root. However, statistically significant differences in lateral root formation in response to low P were found among the Arabidopsis ecotypes included in these experiments. The ranking of lateral root density of the four accessions was found to be: Nob ≈ Col-0 > Rld > Ws (Table II).
Table II.
Effect of phosphate availability on Arabidopsis root development
| Ecotypes | Lateral Root (No.) | Primary Root Length | Lateral Root Densitya |
|---|---|---|---|
| cm | cm−1 | ||
| Low P | |||
| Col-0 | 31 ± 8.0∗ | 4.1 ± 1.2∗ | 9.7 ± 2.2∗ |
| Nob | 20 ± 6.0∗∗ | 2.2 ± 0.3∗∗ | 9.0 ± 2.7∗ |
| Ws | 19 ± 5.0∗∗ | 5.1 ± 1.4∗∗∗ | 4.0 ± 1.2∗∗ |
| Rld | 27 ± 5.0∗ | 4.8 ± 1.6∗∗∗ | 6.6 ± 1.8∗∗∗ |
| High P | |||
| Col-0 | 7.0 ± 1.6∗∗∗ | 6.9 ± 0.8∗∗∗∗ | 1.0 ± 0.2∗∗∗ |
| Nob | 6.0 ± 3.0∗∗∗ | 5.7 ± 0.8∗∗∗∗ | 1.0 ± 0.2∗∗∗ |
| Ws | 6.3 ± 1.9∗∗∗ | 6.0 ± 0.9∗∗∗∗ | 0.9 ± 0.3∗∗∗ |
| Rld | 5.3 ± 1.7∗∗∗ | 5.0 ± 1.0∗∗∗ | 1.0 ± 0.4∗∗∗ |
Arabidopsis ecotypes Col-0, Nob, Ws, and Rld were grown for 16 d on nutrient media containing low (1 μm) or high (1 mm) soluble P, on vertically oriented agar plates. Lateral roots were counted under a dissecting microscope. The reported values represent the mean of 15 seedlings ± se. Experiments were replicated three times with similar results. Asterisks are used to indicate means that differ significantly (P < 0.05).
Lateral root density was calculated by dividing the no. of lateral roots by the primary length for each plant.
Plants Grown at Low P Exhibit an Altered Sensitivity to Auxins
Exogenous auxin has been shown to inhibit the elongation of the primary root and to stimulate lateral root formation (Torrey, 1976; Blakely et al., 1982; Muday and Haworth, 1994; Casimiro et al., 2001). To test whether auxins can alter the root architectural responses to P availability, the effect of the synthetic auxin 2,4-D on the growth and development of the Arabidopsis (Col-0) root system was determined at low (1 μm) and high (1 mm) P levels. At low P conditions, 10−10 to 10−9 m 2,4-D produced a 60% to 70% reduction in primary root length when compared with untreated seedlings (Fig. 3A). Although these 2,4-D concentrations led to a significant (20%–30%) reduction in the number of lateral roots at low P (Fig. 3B), a 2- to 2.5-fold increase in lateral root density was observed (Fig. 3C). Higher 2,4-D concentrations (10−8 and 10−7 m) did not significantly change primary root length or lateral root density further in low P-grown seedlings (Fig. 3, A and C).
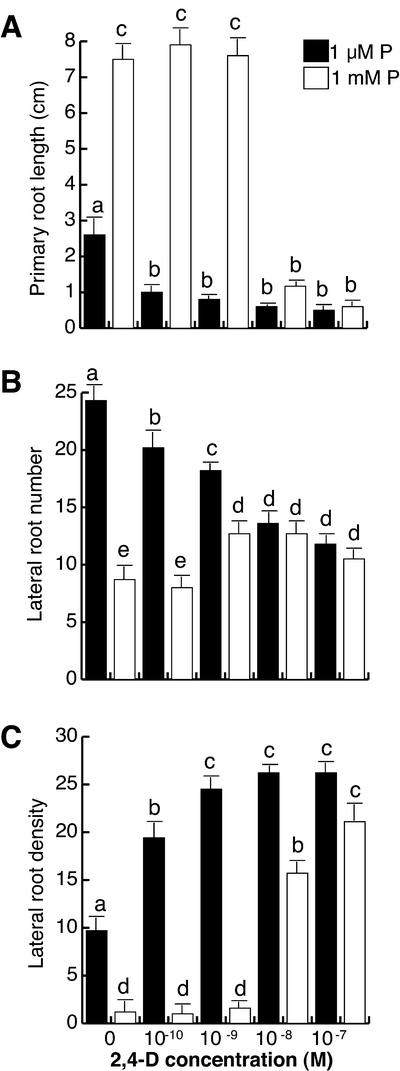
Figure 3.
The effect of exogenous auxin on Arabidopsis root development at low and high phosphate. Arabidopsis (Col-0) seedlings were grown for 16 d on nutrient medium containing low (1 μm) and high (1 mm) phosphate content and varying concentrations of the synthetic auxin 2,4-D. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent the mean of 15 seedlings ± se. Different letters are used to indicate means that differ significantly (P < 0.05).
In contrast to that observed for seedlings grown in 1 μm P, low 2,4-D concentrations (10−10– 10−9 m) did not have a significant effect on primary root length, lateral root number, or lateral root density on seedlings grown at 1 mm P (Fig. 3, A–C). Moreover, a 10- to 100-fold higher concentration of 2,4-D (10−8 m) was required to inhibit primary root growth, induce lateral root formation and increase lateral root density in seedlings grown under high P conditions (Fig. 3, A–C). At 10−7 m 2,4-D, seedlings on high P develop a highly branched root system similar to that observed in untreated low P-grown seedlings (Fig. 1, D and E). Similar results to those observed for 2,4-D were also obtained using indole acetic acid (IAA), a natural auxin. In this case, however, concentrations higher than 10−7 m IAA were required to induce lateral root formation in plants grown at high P (data not shown).
These results show that the effect of auxin upon primary root length and lateral root density increases in plants grown under P deprivation, as compared with those grown in high P (Fig. 3A). This indicates that low P conditions increase auxin sensitivity in the Arabidopsis root system. The fact that exogenous 2,4-D is able to mimic the low P response in high P-grown plants in terms of primary root elongation, lateral root number, and lateral root density would be consistent with the hypothesis that auxin synthesis or auxin sensitivity have an important role on the root architectural responses to P availability (Figs. 1E and 3, A–C).
Auxin Transport Influences the Root Architectural Response to Phosphate Availability
Lateral root development has been suggested to be under the control of polar auxin transport (Katekar and Geissler, 1977; Rubery, 1988). To determine whether polar auxin transport influences Arabidopsis P responses, we analyzed the effects of 2,3,5-triiodobenzoic acid (TIBA) on the architecture of the Arabidopsis root system at low and high P conditions. It was observed that TIBA treatment inhibited primary root elongation at low and high P conditions. However, this effect appeared to be more drastic in low P conditions: Primary root elongation decreased 2-fold in low P-treated seedlings grown in the presence of 10−7 m TIBA when compared with untreated controls, whereas a significant reduction of primary root elongation in seedlings grown at 1 mm P was only observed at concentrations of 10−5 m TIBA (Fig. 4A).
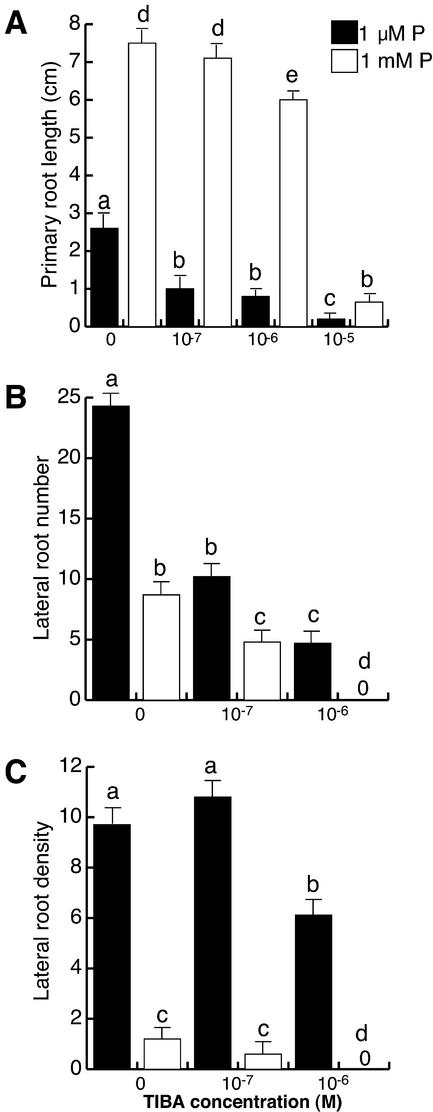
Figure 4.
The effect of auxin transport inhibition on Arabidopsis root development at low and high phosphate. Arabidopsis (Col-0) seedlings were grown for 16 d on nutrient medium containing low (1 μm) and high (1 mm) phosphate content and varying concentrations of TIBA. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent the mean of 15 seedlings ± se. Different letters are used to indicate means that differ significantly (P < 0.05).
Lateral root formation was similarly affected by treatments with 10−7 m TIBA at both high and low P concentrations, 41% and 50%, respectively (Fig. 4B). However, lateral root formation was completely abolished at 10−6 m TIBA in seedlings grown in 1 mm P, whereas at this concentration seedlings grown at low P still produce several lateral roots (Fig. 4B). As a consequence of these changes, the negative effect of TIBA on lateral root density at 1 μm P is significantly lower than at 1 mm P (Fig. 4C).
These results show that transport of auxins into the pericycle cells of the root is required for plants to correctly respond to P availability in terms of root architecture. The increased resistance of P-deprived seedlings to TIBA inhibition of lateral root formation, as compared with those grown in high P, could be explained by a higher sensitivity to auxin in P-deprived seedlings.
Effect of 1-Aminocyclopropane-1-Carboxylic Acid (ACC) and Zeatin on Root System Architecture at High and Low P Conditions
The balance between different phytohormones (i.e. cytokinins, ethylene, and auxins) rather than only auxin levels may alter components of the root P starvation rescue system (Martin et al., 2000; Rahman et al., 2001). Therefore, the effect of exogenous cytokinin (zeatin) and the ethylene precursor ACC on the Arabidopsis root system architecture was examined at low and high P conditions.
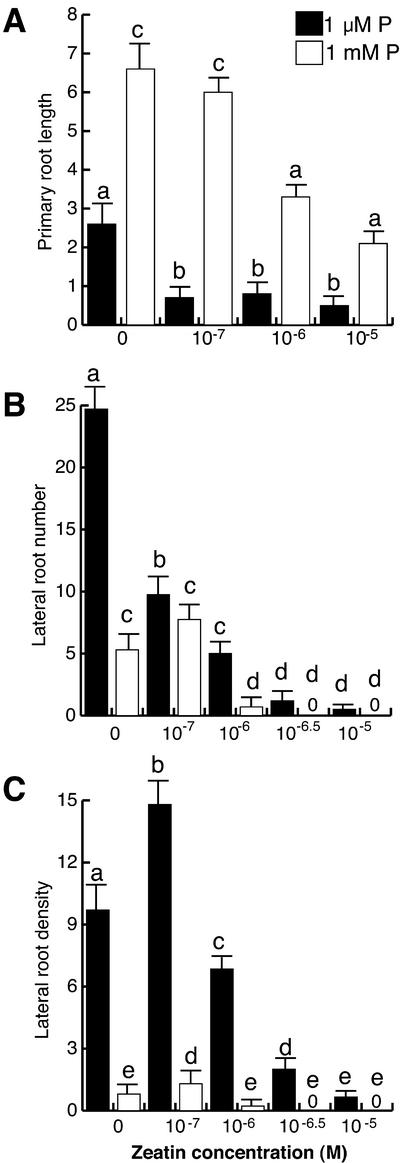
Zeatin drastically inhibited primary root elongation in low P seedlings at concentrations of 10−7 m and had a gradual effect on primary root elongation at high P conditions (Fig. 5A). Lateral root formation was also inhibited by zeatin at both low and high P conditions. However, there were no statistically significant differences between zeatin untreated plants grown at 1 mm P and low P-grown plants treated with 10−6 m of this hormone (Fig. 5B). Zeatin concentrations higher than 10−6 m completely block lateral root formation in both high and low P-grown seedlings (Fig. 5B). As expected, lateral root density was higher in P-deprived plants when compared with P-sufficient plants under treatments with increasing concentrations of zeatin (Fig. 5C).
Figure 5.
The effect of zeatin on Arabidopsis root development at low and high phosphate. Arabidopsis (Col-0) seedlings were grown for 16 d on nutrient medium containing low (1 μm) and high (1 mm) phosphate content and varying concentrations of zeatin. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent the mean of 15 seedlings ± se. Different letters are used to indicate means that differ significantly (P < 0.05).
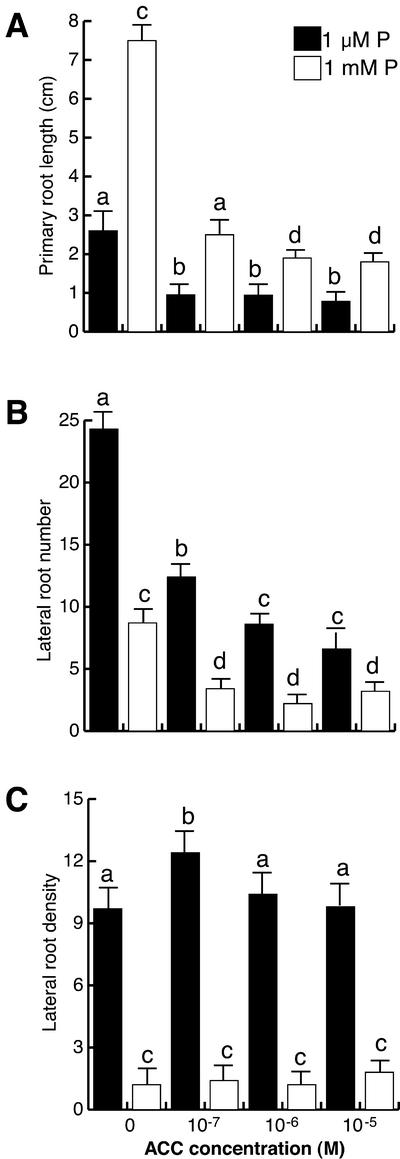
It was observed that ACC had a similar effect on the inhibition of primary root elongation at both 1 μm and 1 mm P, i.e. 66% and 67% inhibition at 10−7 m, respectively (Fig. 6A). ACC also inhibited lateral root formation in low and high P conditions in a similar way and was unable to completely suppress lateral root formation even at the highest concentration tested (10−5 m; Fig. 6B).
Figure 6.
The effect of ethylene on Arabidopsis root development at low and high phosphate. Arabidopsis (Col-0) seedlings were grown for 16 d on nutrient medium containing low (1 μm) and high (1 mm) phosphate content and varying concentrations of the ethylene precursor ACC. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent the mean of 15 seedlings ± se. Different letters are used to indicate means that differ significantly (P < 0.05).
The main difference between cytokinins and ACC treatments is that in contrast to zeatin that reduced lateral root density at both high and low P conditions, ACC had no effect on lateral root density at any of the concentrations tested (Figs. 5C and 6C).
Effect of P Availability on the Root System Architecture of Auxin and Ethylene Arabidopsis Mutants
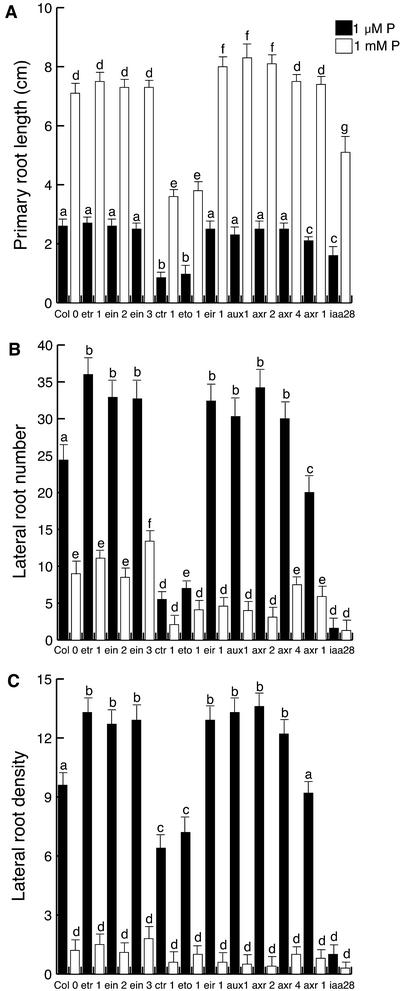
To further define the particular role of auxin and ethylene in the Arabidopsis root response to P availability, we tested the response to P availability of Arabidopsis mutants affected in the auxin and ethylene perception/signal transduction pathways. We tested Arabidopsis mutant lines affected in genes involved in auxin transport (aux1-7 and eir1-1; Picket et al., 1990; Roman et al., 1995), auxin response (axr1-3, axr2-1, axr4-2, and iaa28-1; Lincoln et al., 1990; Wilson et al., 1990; Hobbie and Estelle, 1995; Rogg et al., 2001), and the ethylene-signaling pathway (eto1, ctr1, etr1, ein2, ein3, and hls1; Guzmán and Ecker, 1990; Kieber et al., 1993; Chao et al., 1997). It was found that mutants affected in the auxin influx and efflux carriers, aux1-7 and eir1-1, respectively, produce a reduced number of lateral roots in high P conditions but retain a normal response to low P conditions (Fig. 7B). The axr1-3, axr2-1, and axr4-2 auxin-resistant mutants showed wild-type responses to high and low P levels, including the induction of lateral root formation at low P concentrations and the stimulation of primary root growth and inhibition of root hair elongation at high P concentrations (Figs. 7, A–C).
Figure 7.
Root development of wild-type Arabidopsis (Col-0), auxin-resistant, and ethylene perception mutants at low (1 μm) and high (1 mm) phosphate availability. A, Primary root length of 17-d-old seedlings. B, Lateral root number of 17-d-old seedlings. C, Lateral root density of 17-d-old seedlings. Values shown represent the mean of 16 seedlings ± se. Different letters are used to indicate means that differ significantly (P < 0.05).
The iaa28-1 mutant has been reported to be severely defective in lateral root formation and to be somewhat resistant to inhibition of root elongation by auxins, cytokinins, and ethylene (Rogg et al., 2001). Low P treatment that induces lateral root proliferation in other auxin-resistant mutants including axr1-3, axr2-1, and axr4-2 failed to rescue the inability of the iaa28-1 mutant to form lateral roots (Fig. 7A) and normal root hairs (data not shown). Interestingly, it was found that primary root elongation is inhibited by low P treatment in this mutant (Fig. 7B).
The ethylene-overproducing eto1 and the ethylene-constitutive triple-response ctr1 mutants were found to develop a short, hairy primary root with few lateral roots at both low and high P. In contrast, the ethylene insensitive mutants (etr1, ein2, and ein3) produce a short and highly branched root system in response to P deficiency and form a large primary root with few lateral roots at high P (Fig. 7, A–C). Lateral root density was significantly higher in all ethylene and auxin mutants at low P when compared with high P-grown plants, except for the iaa28-1 mutant, which had very low lateral root density values in both high and low P conditions (Fig. 7C).
DISCUSSION
P Availability Regulates Root Architecture in Plants
Nutrient distribution in natural soils is heterogeneous over time and space. For nutrients of limited mobility such as P, distribution is often stratified with higher concentrations in the upper soil layers in association with organic matter or by forming sparingly soluble Ca-P or Al-P compounds. In many native and cultivated plants including bean, lupin (Lupinus albus), tomato, and B. nigra, low P availability modifies important root architecture traits such as root branching, total root length, and root hair formation (Dinkelaker et al., 1995; Carswell et al., 1996; Borch et al., 1999). These adaptations are believed to lead toward enhancing the P uptake capacity of the root system. Despite the important implications for agriculture, little is known of the physiological and molecular events responsible for the efficient perception of P and the effect of P availability on the adaptive responses of the root system. In this work, we used Arabidopsis as a model system to further characterize the physiological and genetic basis by which P availability regulates plant growth and root architecture.
It has been observed that the pho2 Arabidopsis mutant shows an exaggerated root response, in terms of primary root elongation, at high P (Williamson et al., 2001). Because the pho2 mutant overaccumulates P in the shoot but not in the root, it was proposed that shoot phosphate homeostasis, rather than external P concentrations, plays an important role in regulating root system architecture (Williamson et al., 2001).
We observed that 25 μm P was sufficient to achieve maximum shoot growth. This growth effect correlated with a 2-fold increase in phosphate content. Although at higher P concentrations the seedlings accumulated more P, this did not result in a further increase in shoot growth. In contrast, root fresh weight and root system architecture were only affected when the seedlings were supplied with P concentrations of 100 μm or higher (Table I). These results indicate that P could alter plant development in two ways, one by acting as a nutrient which stimulates shoot biomass production, and the other by functioning as a regulatory signal mediating changes in root architecture. Consistent with this hypothesis, we found that the root of the pho2 mutant develops a similar response to P concentrations lower than 50 μm (a short and highly branched root system) to that seen in the wild-type parent (Col-0; J. López-Bucio and L. Herrera-Estrella, unpublished data).
Taken together, these results indicate that internal concentrations of P determine the rate of shoot growth and primary root elongation, whereas the effect of P upon root hair and lateral root formation seems to depend primarily on the external level of P rather than the P accumulated in the plant. The root architectural response to P deprivation should be of great adaptive significance because soil P levels seem to be in the range of 0 to 20 μm (Holford, 1997).
The phosphate-dependent changes we observed in the Arabidopsis root system are different from those reported for nitrate and micronutrient availability. In contrast to the dramatic changes observed at low and high P concentrations, nitrate concentrations over several orders of magnitude apparently have no effect on primary root elongation or lateral root formation. The effects of nitrate are more clearly observed or specific to the elongation of well-developed lateral roots (Zhang and Forde, 1998; Zhang et al., 1999).
An increase in the length and frequency of hairs on roots of Fe- and P-deficient Arabidopsis plants has also been documented (Schmidt et al., 2000). Hormone insensitivity in mutants and application of hormone antagonists to wild-type Arabidopsis plants inhibited the production of ectopic root hairs induced by Fe deficiency, but did not counteract the formation of extra hairs in response to P deprivation (Schmidt and Schikora, 2001). These results suggest that different pathways are involved in the regulation of root development by phosphate, nitrogen, and iron stress. Therefore, the root architectural responses to P availability seem to be highly specific (Bates and Lynch, 1996; Schmidt and Schikora, 2001; Williamson et al., 2001).
It is as yet unknown at which stage of root development P availability affects lateral root development. Using the DR5::uidA line of Arabidopsis that harbors a tandem of natural and highly active synthetic auxin response elements fused to the GUS reporter gene and shows strong GUS staining in the lateral root primordia (Ulmasov et al., 1997), we have observed that high P conditions arrest lateral root development after the lateral root meristem is formed but before it emerges from the primary root (J. López-Bucio and L. Herrera-Estrella, unpublished data). These preformed root meristems remain competent to form lateral roots at later stages.
The pattern of lateral root formation close to the root tip under low P conditions is reminiscent of roots with a damaged primary root meristem (Torrey, 1950). This could suggest that the effect of P deprivation on lateral root formation could be the result of damage in the primary root meristem. However, more recently it has been found that Arabidopsis lines with damaged primary root meristems because of the expression of a diphtheria toxin gene in the root cap form more lateral roots but have agravitropic roots (Tsugeki and Fedoroff, 1999). We have observed that low P Arabidopsis seedlings have normal gravitropism and that the primary root meristem is completely viable as determined by the expression of marker genes such as the DR5::uidA and the finding that upon transfer to high P conditions, the primary root rapidly reassumes growth (J. López-Bucio, M.F. Nieto-Jacobo, and L. Herrera-Estrella, unpublished data). Whether the increased formation of later roots in plants with damaged meristems is because of a change in auxin sensitivity or whether alteration in auxin sensitivity is triggered by damage and/or slow growth of the primary root in low P Arabidopsis seedlings remains to be determined.
P Availability Alters Hormone Sensitivity in the Root
To maximize the capability of an organ to expand or elongate, or to establish a particular developmental program such as root branching, plants have evolved mechanisms tightly coupled to the perception of environmental stimuli. Many of the plant responses to environmental factors are mediated by phytohormones, such as auxin and ethylene. To address the question of the role of phytohormones on the morphogenetic changes induced by P availability, we analyzed the effect of auxin, cytokinins, and ethylene on root architecture and lateral root formation at low and high P levels.
Treatment of high P-grown plants with 2,4-D inhibited primary root growth, induced formation of lateral roots and increased lateral root density. Moreover, 10−8 m 2,4-D was sufficient to reproduce the low P response in terms of lateral root density and inhibition of primary root growth (Figs. 1, D and E, and 3, A–C). Treatment of high P-grown plants with cytokinins also had an inhibitory effect on primary root growth, but in contrast to auxins, cytokinins inhibited lateral root formation and resulted in a reduction of lateral root density. These results suggest that under low P conditions, an increase in auxin synthesis and/or alterations in the polar transport of auxins mediate the changes in root system architecture. However, the finding that lateral root density in low P seedlings is affected by concentrations of 2,4-D 2 orders of magnitude lower than those required to have a similar effect on high P seedlings suggests that P-starved plants have a higher sensitivity to auxins (Fig. 3A). Therefore, changes in the auxin sensitivity of the root seem to be involved in the developmental response of the Arabidopsis root system to P starvation.
Auxin is synthesized in the young leaves of the shoot system and transported downward to the root through the vascular tissues (Casimiro et al., 2001). The formation and maintenance of auxin gradients are thought to occur through the action of a specific polar auxin transport system that requires active efflux of auxin (Estelle, 1998). Recently, polar auxin transport has been shown to be essential for lateral root development (Reed et al., 1998; Casimiro et al., 2001). We used TIBA, an auxin transport inhibitor, to gain knowledge of the participation of auxin transport on lateral root development in response to P availability. Primary root elongation in low P seedlings was more sensitive to TIBA than in high P seedlings (Fig. 4A); however, P-deprived plants showed lower sensitivity to the negative effect of TIBA on lateral root formation and lateral root density when compared with high P-grown plants (Fig. 4, B and C). Because it is known that auxins inhibit primary root elongation and stimulate lateral root formation, these observations appear somewhat paradoxical. However, it has recently been reported that treatment with auxin transport inhibitors results in suboptimal levels of auxins for lateral root initiation, but also in the accumulation of auxins in the root meristem (Casimiro et al., 2001). An increase in auxin sensitivity could explain why in low P seedlings, suboptimal levels of auxins resulting from TIBA treatment are sufficient to maintain lateral root formation. Moreover, increased auxin sensitivity together with an increased level of auxins in the root meristem could explain the enhanced TIBA inhibition of primary root elongation in low P seedlings.
Using the developmental changes that occur in the Arabidopsis root in response to high and low P, we have isolated Arabidopsis mutants that are unable to respond to P deprivation in terms of lateral root formation and inhibition of primary root elongation (J. López-Bucio, E. Hernández-Abreu, and L. Herrera-Estrella, unpublished data). Some of these mutants are partially auxin resistant at low P, but not at high P. These results also support the notion that the developmental response of the Arabidopsis root system to low P availability involves changes in auxin sensitivity.
Recently, it has been demonstrated that auxin also moves basipetally, from the root apex to the root-shoot junction (Rashotte et al., 2000). To date, it is not clear which of these auxin transport systems actually control lateral root formation (Casimiro et al., 2001). The use of TIBA demonstrates that auxin transport is required for roots to correctly respond to P availability. The close proximity of lateral roots to the root apex of plants grown under low P opens the possibility that basipetal transport of auxin could be involved in controlling the proliferation of lateral roots in response to P deficiency (Fig. 1B). However, because shoot apical synthesis of auxins is a large source of root auxins, an important role for acropetal transport of auxin cannot be excluded.
Root Architecture Responses to P Availability in Arabidopsis Mutants Affected in Auxin Signaling and Transport under Low P
To further elucidate some of the aspects of auxin transport/perception involved in the Arabidopsis response to low P availability, we analyzed the responses of Arabidopsis mutants with defects in the auxin signal transduction pathways. We found that auxin transport mutants (aux1-7 and eir1-1) form a reduced number of lateral roots in high P but retain a normal response to low P conditions (Fig. 7B). Our results suggest that the normal low P root response of auxin transport mutants is probably related to the increase in auxin sensitivity of P-deprived plants. We propose that the reduced level of auxin in the roots of auxin transport mutants is sufficient to induce xylem pole pericycle cells to form lateral roots at low P but not at high P levels, where higher concentrations of auxins are required to form a branched root system. The finding that the auxin-resistant mutants axr2-1, axr1-3, and axr4-1 also have normal responses to P deficiency indicates that the corresponding genes are not directly required by P-starved plants to develop abundant lateral roots (Fig. 7B).
Very recently, a new member of the Arabidopsis Aux/IAA gene family of transcription factors was reported. Plants with an iaa28 gain-of-function mutation are partially auxin resistant and show defects in lateral root formation and root hair development. Studies of the iaa28-1 mutant suggested that IAA28 normally represses transcription of genes that promote lateral root initiation in response to auxin signals (Rogg et al., 2001). We found that in contrast to other auxin-related mutants, the iaa28-1 mutant is unable to increase lateral root formation in response to low P conditions (Fig. 7B). However, the inhibition of primary root elongation by low P is still observed in this mutant (Fig. 7A). These results suggest the iaa28 mutant is inherently defective in lateral root formation and that the signaling pathway in which this mutant is affected is not involved in primary root inhibition in response to low P.
Is Ethylene Involved in the Arabidopsis Root Responses to P Availability?
Addition of the ethylene precursor ACC to low and high P-grown Arabidopsis seedlings inhibited lateral root formation (Fig. 6B) and primary root growth (Fig. 6A) but did not influence lateral root density (Fig. 6C). The effect of ACC on lateral root formation differs from the effect of auxins in that the first inhibits and the latter stimulates lateral root formation in high P seedlings and also because auxins stimulate lateral root density in high and low P-grown seedlings, whereas ACC does not alter root density in either case (see Figs. 3 and 6). These results suggest that although ethylene may regulate root development, it is not directly involved in the Arabidopsis lateral root response to low P conditions. In agreement with this, we observed that ethylene-insensitive (etr1, ein2, ein3, and hls1) mutants have normal responses in terms of lateral root formation and primary root inhibition when exposed to low P conditions, whereas ethylene-overproducing (eto1) and ethylene constitutive response (ctr1) mutants are less responsive (Fig. 7B). The negative effect of ACC in lateral root formation and the reduced production of lateral roots in response to low P conditions in the eto1 and ctr1 mutants suggest that ethylene may play a negative rather than a positive role in lateral root induction (Fig. 6B).
Iron and P deficiency has been shown to increase length and root hair number. Treatment with hormone antagonists and related mutants indicate that ethylene and auxin are essential for the development of extra root hairs in response to Fe deficiency, but are apparently not required for root hairs induced by P deficiency stress (Schmidt and Schikora, 2000). Considering the observed effects of ethylene on root hair and lateral root formation under P deficiency, it seems certainly possible that a P deficiency-specific stress signal may interact with components of an ethylene-independent pathway.
An increasing number of mutants have been identified that show defects in phosphate translocation (Poirier et al., 1991), P accumulation (Delhaize and Randall, 1995), and phosphatase production (Chen et al., 2000; Zakhleniuk et al., 2001). A thorough study of their physiology should provide a better understanding of gene function to phosphate availability. Toward this goal, the isolation of new P response mutants will help identify the processes and genes important in other key aspects of P nutrition, such as those with an impact on root architecture.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis ecotypes Col-0, Ws, Nob, and Rld were used. Seeds were soaked in distilled water for 30 min and surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach for 7 min. After five washes in distilled water, seeds were germinated and grown on petri plates containing sterile low P (1 μm NaH2PO4) or high P (1 mm NaH2PO4) in a modified Murashige and Skoog medium, pH 5.7, 0.5% (w/v) Suc, and 1% (w/v) agar. The basic medium contained 2.0 mm NH4NO3, 1.9 mm KNO3, 0.3 mm CaCl2.2H20, 0.15 mm MgSO4.7H20, 5 μm KI, 25 μm H3BO3, 0.1 mm MnSO4.H2O, 0.3 mm ZnSO4.7H20, 1 μm Na2MoO4.2H20, 0.1 μm CuSO4.5H20, 0.1 μm CoCl2.6H2O, 0.1 mm FeSO4.7H20, 0.1 mm Na2EDTA.2H20, inositol (10 mg L−1), and Gly (0.2 mg L−1).
Before experiments, plates were placed in darkness at 4°C for 48 h to promote and synchronize germination. Seeds were grown in petri dishes under a photoperiod of 16 h of light, 8 h of darkness, and temperature of 24°C for 14 to 16 d, until lateral roots were clearly visible. Plates were placed vertically at an angle of 65° to allow root growth along the surface of the agar and to allow the unimpeded growth of the hypocotyl into the air. For plant growth, we used a plant growth cabinet (Percival Scientific, Perry, IA). It is important to note that a relatively high light intensity (above 100 μE) is required to have a consistent root response because lateral root formation appears to be light regulated (J. López-Bucio, L. Sánchez-Calderón, and L. Herrera-Estrella, unpublished data).
Lateral Root and Root Length Measurements
Arabidopsis root systems were viewed with an AFX-II-A stereomicroscope (Nikon, Tokyo). All lateral roots observed at the 3× objective were taken into account for lateral root number data. Primary root length was determined for each root using a plastic rule.
Arabidopsis Growth Response to P Availability and P Determinations
A P dose growth response curve was constructed by growing Arabidopsis plants in six different P concentrations, from 0 P to 10 mm P. Sixteen days after germination, 300 Arabidopsis seedlings grown at each P treatment were harvested, washed with distilled water, placed in paper bags, and dried at 70°C for 48 h. Fresh and dry weights of shoots and roots were determined using an AE 50 analytical balance (Mettler Scientific, Highstown, NJ). Dry material was used for P determinations by a vanadate-molybdate colorimetric method (Hesse, 1971).
Mutant Strains
eto1-1, hls1-1, and ein2-1 (Guzmán and Ecker, 1990); ctr1-1 (Kieber et al., 1993); ein3-1 (Chao et al., 1997); eir1-1 (Roman et al., 1995); and axr2-1 (Wilson et al., 1990) were kindly provided by Dr. Plinio Guzmán (Departamento de Ingeniería Genética, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Irapuato Gto, Mexico). etr1-3 (Hua and Meyerowitz, 1998), aux1-7 (Picket et al., 1990), axr4-2 (Hobbie and Estelle, 1995), and axr1-3 (Lincoln et al., 1990) were kindly provided by Dr. Claire Grierson (School of Biological Sciences, University of Bristol, UK). The pho2 mutant (Delhaize and Randall, 1995) was kindly provided by Dr. Emmanuel Delhaize (Commonwealth Scientific and Industrial Research Organization, Plant Industry, Canberra, Australia). All these mutant lines are in the Col-0 background. The iaa28-1 mutant (Rogg et al., 2001), was kindly provided by Dr. Bonnie Bartel (Department of Biochemistry and Cell Biology, Rice University, Houston) and it is in the Ws ecotype.
Hormone Treatments
Low (1 μm NaH2PO4) and high (1 mm NaH2PO4) P nutrient medium was supplemented with 2,4-D, TIBA, zeatin, or ACC. Filter-sterilized compounds were added to cooled (50°C) molten medium and poured into plates. Chemicals were purchased from Sigma Chemical Co. (St. Louis).
Statistical Analysis
For all experiments, the overall data was statistically analyzed in the SPSS 10 program (SPSS, Chicago). Linear regressions of different phosphate concentrations were compared by the F ratio method. One-way ANOVA with a Tukey's Post Hoc test was used for testing differences in primary root length, lateral root number, and lateral root density under hormone treatments and the analysis of P response in mutants. Different letters are used to indicate means that differ significantly (P < 0.05).
ACKNOWLEDGMENTS
Antonio Vera-Nuñez and J. José Peña-Cabriales are thanked for their support and technical improvement for phosphate analyses. We are thankful to Plinio Guzmán, Claire Grierson, Bonnie Bartel, Nina Fedoroff, Manny Delhaize, and Tom Guilfoyle for kindly providing us mutant seed. We gratefully acknowledge Elena Alvarez-Buylla and Joseph Dubrovsky for critical reading of our manuscript.
Footnotes
This work was supported in part by the Consejo Nacional de Ciencia y Tecnología, Mexico (grant no. 31628–B), the European Commission (grant no. ICA–4–CT2000–30017), and by the Howard Hughes Medical Institute (grant no. Nbr55003677).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010934.
LITERATURE CITED
- Abeles FB. Ethylene in Plant Biology. New York: Academic Press; 1973. [Google Scholar]
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538. [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots: general methods, developmental stages, and spontaneous formation of laterals. Bot Gaz. 1982;143:341–352. [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999;22:425–431. [Google Scholar]
- Carswell C, Grant BR, Theodorou ME, Harris J, Niere JO, Plaxton WC. The fungicide phosphonate disrupts the phosphate starvation response in Brassica nigra seedlings. Plant Physiol. 1996;110:105–110. doi: 10.1104/pp.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE 3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chen DL, Delatorre CA, Bakker A, Abel S. Conditional identification of phosphate-starvation-response mutants in Arabidopsis thaliana. Planta. 2000;211:13–22. doi: 10.1007/s004250000271. [DOI] [PubMed] [Google Scholar]
- Christie EK, Moorby J. Physiological responses of semiarid grasses: I. The influence of phosphorus supply on growth and phosphorus adsorption. Aust J Agric Res. 1975;26:423–436. [Google Scholar]
- Delhaize E, Randall PJ. Characterization of a phosphate accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelaker B, Hengeler B, Marshner H. Distribution and function of proteoid roots and other root clusters. Bot Acta. 1995;108:183–200. [Google Scholar]
- Dolan L. The role of ethylene in the development of plant form. J Exp Bot. 1997;48:201–210. [Google Scholar]
- Estelle M. Polar auxin transport: new support for an old model. Plant Cell. 1998;10:1775–1778. doi: 10.1105/tpc.10.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle M, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse PR. Phosphorus. In: Hesse PR, editor. A Text Book of Soil Chemical Analysis. London: John Murray Ltd; 1971. pp. 254–300. [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Holford ICR. Soil phosphorus: its measurements and its uptake by plants. Aust J Soil Res. 1997;35:227–239. [Google Scholar]
- Hua J, Meyerowitz E. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Katekar GF, Geissler AE. Auxin transport inhibitors: III. Chemical requirements of a class of auxin transport inhibitors. Plant Physiol. 1977;60:826–829. doi: 10.1104/pp.60.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR 1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr 1 mutant of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Martínez de la Vega O, Guevara-García A, Herrera-Estrella L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat Biotechnol. 2000;18:450–453. doi: 10.1038/74531. [DOI] [PubMed] [Google Scholar]
- Lynch J, Brown KM. Ethylene and plant responses to nutritional stress. Physiol Plant. 1997;100:613–619. [Google Scholar]
- Martin AC, Del Pozo JC, Iglesias JC, Rubio V, Solano R, De la Peña A, Leyva A, Paz-Ares J. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J. 2000;24:559–567. doi: 10.1046/j.1365-313x.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM. Arabidopsis thaliana. Annu Rev Gen. 1987;21:93–111. doi: 10.1146/annurev.ge.21.120187.000521. [DOI] [PubMed] [Google Scholar]
- Muday GK, Haworth P. Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiol Biochem. 1994;32:193–203. [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Narang RA, Bruene A, Altmann T. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 2000;124:1786–1799. doi: 10.1104/pp.124.4.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picket FB, Wilson AK, Estelle M. The aux 1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J. A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 1991;97:1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Amakawa T, Goto N, Tsurumi S. Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant Cell Physiol. 2001;42:301–307. doi: 10.1093/pcp/pce035. [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday G. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000;122:481–490. doi: 10.1104/pp.122.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday G. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg LE, Lasswell J, Bartel B. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell. 2001;13:465–480. doi: 10.1105/tpc.13.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones and Their Role in Plant Growth and Development. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 341–362. [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SL. Phosphorus uptake by plants: from soil to cells. Plant Physiol. 1998;116:447–453. doi: 10.1104/pp.116.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Schikora A. Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiol. 2001;125:2078–2084. doi: 10.1104/pp.125.4.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Tittel J, Schikora A. Role of hormones in the induction of Fe deficiency responses in Arabidopsis roots. Plant Physiol. 2000;122:1109–1118. doi: 10.1104/pp.122.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Rudiger-Scheible W. Understanding allocation to shoot and root growth will require molecular information about which compounds act as signals for the plant nutrient status, and how meristem activity and cellular growth are regulated: opinion. Plant Soil. 1998;201:259–263. [Google Scholar]
- Torrey JG. Induction of lateral roots by indoleacetic acid and root decapitation. Am J Bot. 1950;37:257–264. [Google Scholar]
- Torrey JG. Root hormones and plant growth. Ann Rev Plant Physiol. 1976;27:435–459. [Google Scholar]
- Tsugeki R, Fedoroff NV. Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:12941–12946. doi: 10.1073/pnas.96.22.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle T. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AK, Picket FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene, and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Williamson L, Ribrioux S, Fitter A, Leyser O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001;126:1–8. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhleniuk OV, Raines CA, Lloyd JC. Pho 3: a phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta. 2001;212:529–534. doi: 10.1007/s004250000450. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde B. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde B. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Control of morphogenesis in ethylene-requiring tomato mutant, diageotropica. Can J Bot. 1974;52:735–741. [Google Scholar]