Abstract
Chicory (Cichorium intybus) is known to contain guaianolides, eudesmanolides, and germacranolides. These sesquiterpene lactones are postulated to originate from a common germacranolide, namely (+)-costunolide. Whereas a pathway for the formation of germacra-1(10),4,11(13)-trien-12-oic acid from farnesyl diphosphate had previously been established, we now report the isolation of an enzyme activity from chicory roots that converts the germacrene acid into (+)-costunolide. This (+)-costunolide synthase catalyzes the last step in the formation of the lactone ring present in sesquiterpene lactones and is dependent on NADPH and molecular oxygen. Incubation of the germacrene acid in the presence of 18O2 resulted in the incorporation of one atom of 18O into (+)-costunolide. The label was situated at the ring oxygen atom. Hence, formation of the lactone ring most likely occurs via C6-hydroxylation of the germacrene acid and subsequent attack of this hydroxyl group at the C12-atom of the carboxyl group. Blue light-reversible CO inhibition and experiments with cytochrome P450 inhibitors demonstrated that the (+)-costunolide synthase is a cytochrome P450 enzyme. In addition, enzymatic conversion of (+)-costunolide into 11(S),13-dihydrocostunolide and leucodin, a guaianolide, was detected. The first-mentioned reaction involves an enoate reductase, whereas the formation of leucodin from (+)-costunolide probably involves more than one enzyme, including a cytochrome P450 enzyme.
Chicory (Cichorium intybus), also known as French endive, Witloof, and succory is probably a native of Europe and Asia. At present, this composite plant is mainly cultivated for its roots (C. intybus var sativum) that contain high amounts of inulin (a Fru polymer) or for its sprouts (C. intybus var foliosum Hegi) that are a well-known salad crop (Vogel et al., 1994; Kruistum, 1997; Westerdijk, 2000). The white sprouts, which are grown in the dark, have a slightly bitter taste that is associated with the presence of sesquiterpene lactones. These compounds occur throughout the plant, though at highest levels in the roots (0.42% dry weight), and act as deterrents toward insects (Rees and Harborne, 1985; Price et al., 1990).
Sesquiterpene lactones are a major class of plant secondary metabolites that are mainly found in the Asteraceae but also occur infrequently in other high plant families and lower plants (Seigler, 1998). The majority of the more than 4,000 known different structures has a guaiane, eudesmane, or germacrane framework. Also the sesquiterpene lactones of chicory belong either to the guaianolides, eudesmanolides, or germacranolides (Fig. 1; Seto et al., 1988; van Beek et al., 1990). (+)-Costunolide (Fig. 2, 15) is structurally the simplest of all germacranolides and is generally accepted as the parent compound of the three mentioned types of sesquiterpene lactones (Geissman, 1973; Fischer et al., 1979; Seaman, 1982; Fischer, 1990).
Figure 1.
The sesquiterpene lactones of chicory: the guaianolides lactucin (1), 8-deoxylactucin (2), lactucopicrin (3), 11(S),13-dihydrolactucin (4), 11(S),13-dihydro-8-deoxylactucin (5), and 11(S),13-dihydrolactucopicrin (6); the eudesmanolides cichoriolide A (7) and sonchuside C (8); and the germacranolides sonchuside A (9) and cichorioside (10). The major sesquiterpene lactones in chicory are compounds 1 through 3.
Figure 2.
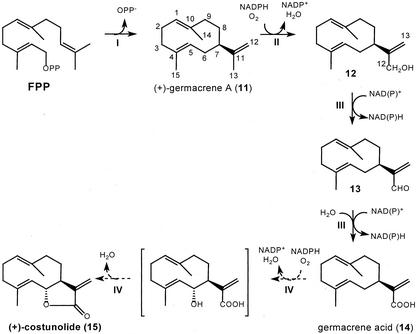
Proposed biosynthetic route for (+)-costunolide, a postulated key intermediate in sesquiterpene lactone biosynthesis. I, Cyclization of farnesyl diphosphate (FPP) to (+)-germacrene A (11) by (+)-germacrene A synthase (a sesquiterpene synthase). II, Hydroxylation of the isopropenyl side chain by (+)-germacrene A hydroxylase, a cytochrome P450 enzyme. III, Oxidation of germacra-1(10),4,11(13)-trien-12-ol (12) via germacra-1(10),4,11(13)-trien-12-al (13) to germacra-1(10),4,11(13)-trien-12-oic acid (14) catalyzed by NAD(P)+-dependent dehydrogenase(s). IV, Postulated hydroxylation at the C6-position of germacratrien-12-oic acid (14) and subsequent lactonization will yield (+)-costunolide (15).
Our studies with chicory roots have made it apparent that its sesquiterpene lactones are derived from (+)-germacrene A (11; de Kraker et al., 1998). This sesquiterpene olefin is oxidized through germacra-1(10),4,11(13)-trien-12-ol (12) and germacra- 1(10),4,11(13)-trien-12-al (13) into germacra-1(10), 4,11(13)-trien-12-oic acid (14; Fig. 2; de Kraker et al., 2001a). Formation of (+)-costunolide (15) from the germacrene acid is expected to be the next step, but this has not yet been demonstrated. It has been postulated to occur via hydroxylation at the C6-position by a cytochrome P450 enzyme, after which lactonization yields (+)-costunolide (Geissman, 1973; Fischer et al., 1979; Seaman, 1982; Fischer, 1990). Germacrene acid (14) was only very recently isolated from fresh costus roots in quantities that make it possible to investigate this last crucial step in the formation of the lactone ring present in sesquiterpene lactones (de Kraker et al., 2001b).
(+)-Costunolide (15) is also considered to be a branching point in the biosynthesis of sesquiterpene lactones from where pathways for the formation of guaianolides, eudesmanolides, and germacranolides divide. It has been postulated by various authors that cyclization of (+)-costunolide to either guaianolides or eudesmanolides is mediated by C4-C5 epoxidation (i.e. via parthenolide [22]) or C1-C10 epoxidation, respectively (Brown et al., 1975; Fischer, 1990; Teisseire, 1994; Piet et al., 1995). As an alternative, the possibility of a C3-hydroxylation of the germacranolide for formation of a guaianolide has been suggested (Piet et al., 1996). This article will mainly focus on the formation of (+)-costunolide (15) from germacrene acid (14), but some attention is also paid to the subsequent conversions of (+)-costunolide.
RESULTS
Conversion of Germacrene Acid into Sesquiterpene Lactones
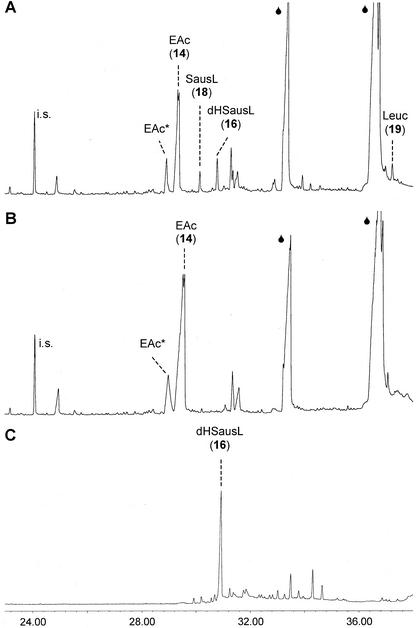
Gas chromatography-mass spectroscopy (GC-MS) analyses of the pentane-ether extract from the incubation of a 20,000g chicory root supernatant with germacra-1(10),4,11(13)-trien-12-oic acid (14) and NADPH revealed three products that were detected neither in incubations without NADPH nor in incubations with boiled supernatant (Fig. 3, A and B). The major peak co-eluted with a standard of (+)-costunolide (15) that at an injection port temperature of 320°C is detected as its Cope rearrangement product dehydrosaussurea lactone (16; Fig. 3C). At this high injection port temperature, germacrene acid, the substrate, is also Cope rearranged, and this results inthe formation of elemene acid (21) and diastereomeric elemene acid, which elute at different retention times (de Kraker et al., 2001b).
Figure 3.
A, GC-MS analyses of the products formed in the incubation of a 20,000g supernatant from chicory roots with NADPH and germacra-1(10),4,11(13)-trien-12-oic acid (14) displays peaks of dehydrosaussurea lactone (dHSausL [16]), saussurea lactone (SausL [18]), and leucodin (Leuc [19]). B, These products are not observed in the absence of NADPH. Germacrene-1(10),4,11(13)-trien-12-oic acid (14) is observed as elema-1,3,11(13)-trien-12-oic acid (EAc) plus its diastereomer (EAc*); the internal standard (i.s.) is 1 nmol of cis-nerolidol. The huge fronting peaks ( ) are fatty acids (palmitic and linoleic acid). C, The standard of 0.5 mm costunolide (15) in ethanol yields a tailing peak of dehydrosaussurea lactone (dHSausL [16]).
) are fatty acids (palmitic and linoleic acid). C, The standard of 0.5 mm costunolide (15) in ethanol yields a tailing peak of dehydrosaussurea lactone (dHSausL [16]).
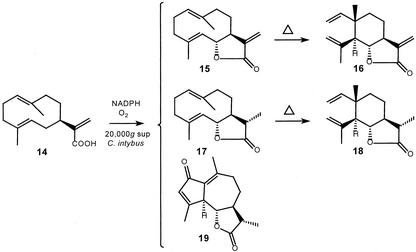
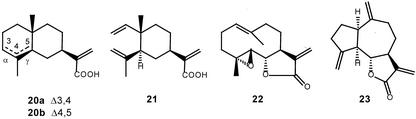
The two other products were identified as 11(S),13-dihydrocostunolide (17), which is Cope rearranged into saussurea lactone (18), and leucodin (19; Fig. 4). Both 11(S),13-dihydrocostunolide and leucodin are enzymatically formed from (+)-costunolide (15), because they also appeared in incubations of (+)-costunolide with the 20,000g supernatant and NADPH (vide infra). It cannot be excluded that even more products are formed during the incubation of germacrene acid, because higher oxygenated sesquiterpene lactones are likely not volatile enough for detection by GC. Furthermore, the presence of fatty acids in chicory extracts (Sannai et al., 1982) complicates the GC-analysis, because they yield big peaks under which smaller product peaks may “disappear.” Similar incubations with the 20,000g supernatant of the substrate analogs α- and γ-costic acid (Fig. 5; 20) and elema-1,3,11(13)-trien-12-oic acid (Fig. 5; 21) did not result in any product formation.
Figure 4.
Products formed from germacra-1(10),4,11(13)-trien-12-oic acid (14) in the presence of NADPH and oxygen by a 20,000g supernatant from chicory roots. Leucodin (19) is detected as such, whereas costunolide (15) and 11,13-dihydrocostunolide (17) are detected as their Cope rearrangement products dehydrosaussurea lactone (16) and saussurea lactone (18), respectively.
Figure 5.
Various substrates that were also incubated with the 20,000g supernatant and NADPH. A mixture of α-costic acid (20a) and γ-costic acid (20b) or elema-1,3,11(13)-trien-12-oic acid (21) may serve as substrate analogs of germacrene acid (14). Parthenolide (22) and dehydrocostus lactone (23) serve as model compounds for conversions that might occur during chicory sesquiterpene lactone biosynthesis after formation of (+)-costunolide.
Characterization of the (+)-Costunolide Synthase
For characterization of the (+)-costunolide synthase the response of the GC-MS to different concentrations of (+)-costunolide (15) should preferably be linear. Yet, at the injection port temperature of 320°C we used, the GC-trace of costunolide (Fig. 3C) does not show a sharp peak of dehydrosaussurea lactone (16) but a tailing peak that contains minor peaks of costunolide-related products like α- and β-cyclocostunolide. (+)-Costunolide is apparently more resistant to Cope rearrangement than, for instance, germacrene acid (14). In literature, it has also been noted that a lactone ring has a strong influence on the thermal stability of germacrenes and that Cope rearrangement of (+)-costunolide is reversible (Jain et al., 1970; Minnaard, 1997). Grieco and Nishazawa (1977) even used this thermal equilibration to prepare (+)-costunolide from dehydrosaussurea lactone (16) on a preparative GC. Despite the lower susceptibility of (+)-costunolide to Cope rearrangement, a lowered injection port temperature (200°C) still resulted in Cope rearrangement of (+)-costunolide during its migration through the GC-column and yielded a broad hump (similar to the one shown for germacrene aldehyde in de Kraker et al., 2001b). Although of poor quality, the dehydrosaussurea lactone GC-peak visualized in Figure 3C proved to be linear with the injected (+)-costunolide concentration in a range of 5 to 50 μm. Costunolide concentrations below 5 μm, i.e. 0.25 nmol in the 1-mL incubation mixture, were not measurable.
A more serious problem for characterization of the (+)-costunolide synthase is that, almost certainly, all subsequent conversion products of (+)-costunolide have not been detected, let alone quantified. Hence, the given enzyme activities are a summation of the peaks of dehydrosaussurea lactone (16), saussurea lactone (18), and leucodin (19) and are consequently more an indication than an absolute value of (+)-costunolide synthase activity. Quantitative measurement of the elemene acid peak (substrate peak) area was not an option, because it is not linear with the concentration and, more generally, acid peaks in GC-measurements are of poor quality.
Table I shows that (+)-costunolide synthase is dependent on oxygen and NADPH, whereas NADH was much less effective as a reductant. This suggests the involvement of a cytochrome P450 enzyme, which was confirmed by the effect of various established cytochrome P450 inhibitors (West, 1980; Mihaliak et al., 1993). In the presence of cytochrome c (100 μm), no enzymatic products of germacrene acid (14) were measured; 100 μm miconazole reduced the amount of measurable products with 71%, 100 μm aminobenzotriazole with 44%, 1 mm metyrapone with 78%, and 100 μm clotrimazole with 97%.
Table I.
Requirements for (+)-costunolide synthase activity
| Addition | Percentage Product Formation ± sd |
|---|---|
| None | 0 ± 0 |
| 1 mm NADPH | 100 ± 8a |
| 1 mm NADH | 7 ± 2 |
| 1 mm NADPH + 1 mm NADH | 109 ± 23 |
| 1 mm NADP++ NAD+ | 1 ± 1 |
| 1 mm NADPH + argon atmosphere | 0 ± 0 |
One hundred percent enzyme activity corresponds to the summation of a dehydrosaussurea lactone (16), saussurea lactone (18), and leucodin (19) peak size of 0.35, 0.19, and 0.09 × internal standard (1 nmol of cis-nerolidol), respectively.
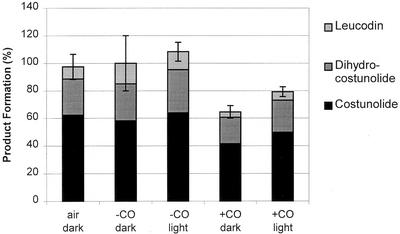
The strongest proof for the involvement of a cytochrome P450 enzyme is blue light-reversible inhibition of enzyme activity by CO (West, 1980; Mihaliak et al., 1993). The results depicted in Figure 6 show an inhibitory effect of CO on the produced sum of (+)-costunolide (15), 11(S),13-dihydrocostunolide (17), and leucodin (19), which could to some extent be reversed by blue light.
Figure 6.
Demonstration of blue-light reversible CO inhibition of the costunolide synthase. Product formation in the presence of 90% (v/v) N2 + 10% (v/v) O2 (labeled as −CO) is set at 100% and this activity corresponds to a peak size of dehydrosaussurea lactone (16) (costunolide), saussurea lactone (18) (dihydrocostunolide), and leucodin (19) of 0.58, 0.27, and 0.15 × internal standard (1 nmol of cis-nerolidol) respectively. The inhibition in the presence of 90% (v/v) CO + 10% (v/v) O2 (labeled as +CO) was reversed to some extent by irradiation with blue light (450 nm). All incubations were done in the absence of FAD and FMN.
Characterization of the Subsequent Conversions of (+)-Costunolide
Incubation of (+)-costunolide with a 20,000g chicory root supernatant in the presence of NADPH yielded 11(S),13-dihydrocostunolide (17) and leucodin (19). No other products were detected by GC-MS analysis of the incubation, but comparison of the decrease in peak height of dehydrosaussurea lactone (16) with the intensity of the product peaks strongly suggested the formation of other products that are not detected by GC. The enzyme activity that catalyzes the reduction of the 11(S),13-exocyclic double bond of (+)-costunolide was not capable of doing the same with the model compound dehydrocostus lactone (Fig. 5; 23), i.e. it did not yield 11(S),13-dihydro-dehydrocostus lactone. Nevertheless, incubation of dehydrocostus lactone in the presence of NADPH and a chicory root extract gave a new unknown product that had a shorter retention time, the same molecular mass, and exhibited a very similar mass spectrum as dehydrocostus lactone. It is probably the result of a double bond isomerization somewhere in the molecule. Incubation of parthenolide (Fig. 5; 22)—a postulated intermediate of guaianolide biosynthesis—did not yield leucodin (19), but conversion of parthenolide into other non-GC-MS-measurable sesquiterpene lactones cannot be excluded. Parthenolide itself disintegrates upon GC-analysis in a number of compounds; for this reason, no quantitative determination could be done of the amount of parthenolide present after incubation.
To test which type(s) of enzymes might catalyze the formation of 11(S),13-dihydrocostunolide (17) and leucodin (19), the pyridine nucleotide cofactors were varied (Table II). Formation of both compounds was dependent on NADPH, but a part of the (+)-costunolide reductase activity was retained in the absence of any cofactor.
Table II.
Pyridine nucleotide cofactor dependency of 11(S),13-dihydrocostunolide and leucodin biosynthesis from (+)-costunolide
| Pyridine Nucleotide Cofactor | Percentage Product Formation ± sd
|
|
|---|---|---|
| Dihydrocostunolide (17) | Leucodin (19) | |
| 1 mm NADPH | 100 ± 3a | 100 ± 10a |
| None | 15 ± 1 | 0 ± 0 |
| 1 mm NADH | 14 ± 2 | 0 ± 0 |
| 1 mm NADPH + 1 mm NADH | 107 ± 4 | 65 ± 19 |
| 1 mm NADP++ 1 mm NAD+ | 18 ± 3 | 0 ± 0 |
One hundred percent product formation corresponds to a saussurea lactone (18) and leucodin (19) peak size of 1.34 and 0.42 × internal standard (1 nmol of cis-nerolidol), respectively.
Table III shows that the formation of leucodin (19) from (+)-costunolide (15) is dependent on oxygen, whereas the formation of 11(S),13-dihydrocostunolide (17) is not. Formation of leucodin was strongly inhibited by CO, which suggests the involvement of a cytochrome P450 enzyme. The reaction was also inhibited by all of the tested cytochrome P450 inhibitors except amino-benzotriazole. Interestingly, the measured amount of 11(S),13-dihydrocostunolide was raised in the presence of carbon monoxide or the absence of oxygen. This indicates that in these incubations, at least a part of the successive (hypothetical) conversion of 11(S),13-dihydrocostunolide to higher oxygenated sesquiterpene lactones is inhibited and that some of the involved reactions are possibly cytochrome P450 catalyzed. It is somewhat contradictory that the detected amount of 11(S),13-dihydrocostunolide was hardly effected or even lowered when cytochrome P450-inhibitors had been added to the incubations. Perhaps each individual inhibitor effects only some of the successive cytochrome P450-catalyzed reactions, whereas CO and cytochrome c inhibit all cytochrome P450-catalyzed reactions. It cannot be excluded that the inhibitors themselves influence the formation of 11(S),13-dihydrocostunolide as well, because they were also able to inhibit the (+)-germacrene A synthase (de Kraker et al., 2001a).
Table III.
Inhibition of 11(S),13-dihydrocostunolide and leucodin biosynthesis from (+)-costunolide
| Assay Conditionsa | Percentage Product Formation ± sd
|
|
|---|---|---|
| Dihydrocostunolide (17) | Leucodin (19) | |
| Air (≈80% [v/v] N2+ 20% [v/v] O2) | 100 ± 9b | 100 ± 33b |
| 80% (v/v) CO + 20% (v/v) O2 | 140 ± 4 | 8 ± 1 |
| Argon | 134 ± 6 | 0 ± 0 |
| Control | 100 ± 3c | 100 ± 10c |
| Cytochrome c (100 μm) | 105 ± 5 | 0 ± 0 |
| DMSO (1%) (solvent control) | 100 ± 5d | 100 ± 10d |
| Metyrapone (100 μm) | 87 ± 11 | 70 ± 16 |
| Clotrimazole (10 μm) | 119 ± 9 | 0 ± 0 |
| Miconazole (10 μm) | 100 ± 5 | 0 ± 0 |
| Aminobenzotriazole (10 μm) | 75 ± 8 | 86 ± 33 |
All incubations were carried out in the presence of 1 mm NADPH-regenerating system and flavins.
One hundred percent product formation corresponds to a saussurea lactone (18) and a leucodin (19) peak size of 1.63 and 0.34 × internal standard (1 nmol of cis-nerolidol), respectively.
One hundred percent product formation corresponds to a saussurea lactone (18) and a leucodin (19) peak size of 1.34 and 0.42 × internal standard (1 nmol of cis-nerolidol), respectively.
One hundred percent product formation corresponds to a saussurea lactone (18) and a leucodin (19) peak size of 1.43 and 0.18 × internal standard (1 nmol of cis-nerolidol), respectively.
Incorporation of Oxygen-18
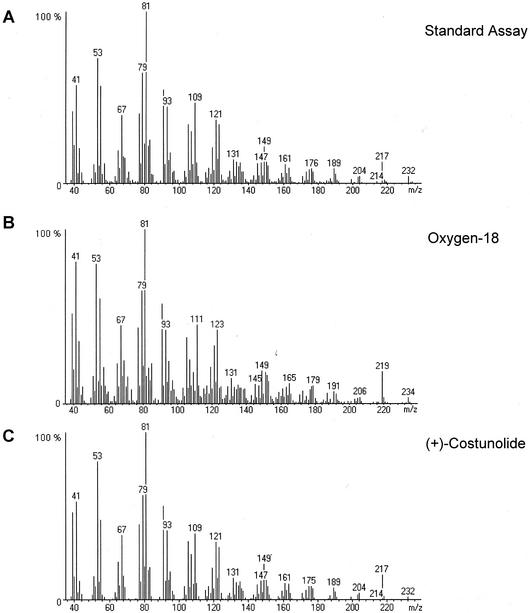
Incubation of germacrene acid (14) in the presence of 18O2 led to the incorporation of one atom of 18O into (+)-costunolide (15). As a result, the ion peak in the mass spectrum of dehydrosaussurea lactone (16) was shifted from 232 to 234 atomic mass units (amu; Fig. 7). Similar changes were observed in the mass spectrum of saussurea lactone (18), the Cope rearrangement product of 11(S),13-dihydrocostunolide (17; data not shown). The ion peak of this compound was shifted from 234 to 236 amu, whereas the [M-Me]+ peak was shifted from 219 to 221 amu. Genuine costunolide (15) and dehydrosaussurea lactone (16) have an almost similar mass spectrum (Jain et al., 1971), and the small peak of 204 amu in their mass spectra arises from the loss of carbon monoxide from the lactone moiety of the molecular ion (Sathe et al., 1969, 1971). Because in the 18O2-labeling experiment, the ion peak of 204 amu from dehydrosaussurea lactone is shifted to 206 amu (Fig. 7)—i.e. carbon monoxide expulsion does not result in the loss of the 18O-label—it is most likely the ring oxygen atom of costunolide that has been labeled.
Figure 7.
Mass spectra of dehydrosaussurea lactone (16) originating from (+)-costunolide (15) that has been produced from germacrene acid (14) under standard assay conditions (A), in the presence of 18O2 (B), or that originates from a standard of (+)-costunolide (C).
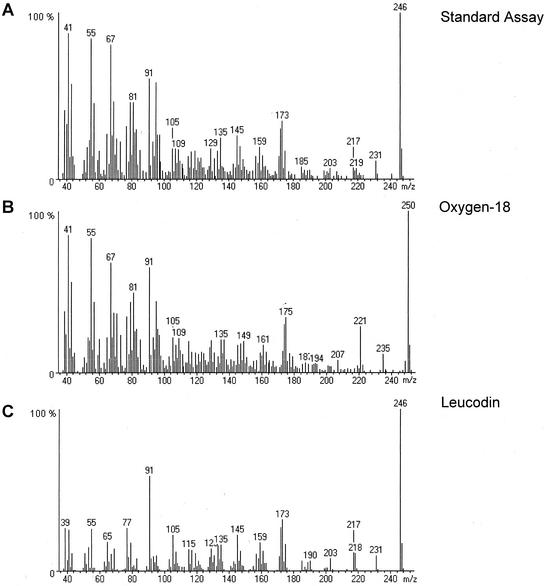
The mass spectra of leucodin (19) showed the incorporation of two atoms of 18O because the mass of the ion peak was shifted with 4 units from 246 to 250 amu (Fig. 8). Unfortunately, in the enzyme assay, the GC-peak of leucodin is superpositioned on the tailing peak of linoleic acid (Fig. 3A), which obscures the mass spectrum of leucodin particular in the lower mass (m/z) range. In general, bubbling of the enzyme assay with 18O2 did not have any effect on the detected amounts of enzymatic products, i.e. it does not effect the enzyme activities.
Figure 8.
Mass spectra of leucodin (19) produced from germacrene acid (14) in an enzyme assay under standard conditions (A) or in the presence of 18O2 (B). C, Mass spectrum of the leucodin standard.
DISCUSSION
(+)-Costunolide
The present results show that chicory roots contain an enzyme that converts germacra-1(10),4,11(13)-trien-12-oic acid (14) into (+)-costunolide (15), yielding the lactone ring present in sesquiterpene lactones. This step is the final proof for the postulated pathway depicted in Figure 2 from farnesyl diphosphate to (+)-costunolide via (+)-germacrene A (11), germacra- 1(10),4,11(13)-trien-12-ol (12), and germacra-1(10),4, 11(13)-trien-12-oic acid (14; Geissman, 1973; Fischer et al., 1979; Fischer, 1990; de Kraker et al., 2001a).
The (+)-costunolide synthase is a cytochrome P450 enzyme that is dependent on NADPH and O2 and is accordingly inhibited by various established cytochrome P450 inhibitors. Blue light-reversible CO inhibition of the (+)-costunolide synthase could be demonstrated as well, although the results are somewhat obscured by subsequent enzymatic conversions of (+)-costunolide. Incubation of germacrene acid (14) in the presence of 18O2 showed the incorporation of one atom of 18O into (+)-costunolide, another typical feature of cytochrome P-450 enzymes (West, 1980; Mihaliak et al., 1993). This incorporation of one atom of oxygen inside the ring also validates that germacrene acid (14) is first hydroxylated at the C6-position (Fischer et al., 1979), after which this hydroxyl group attacks, possibly enzyme-mediated, the carboxyl group at C12 (Fig. 9). After lactonization, the oxygen isotope is incorporated in the lactone, inside the ring. The obtained result rules out an alternative mechanism for formation of the lactone ring in which hydride abstraction at C6 (by an oxidase) is followed by an internal quenching of the resultant C6 carbocation by the carboxyl group. In such a case both oxygen atoms present in costunolide (15) would have arisen from the carboxyl group of germacrene acid (14) and no 18O would have been incorporated.
Figure 9.
Mechanism for the enzyme catalyzed formation of (+)-costunolide (15) from germacrene acid (14) that results in the incorporation of one atom of 18O from 18O2.
The (+)-costunolide synthase is not capable of converting the substrate analogs α-costic acid (20a) and elema-1,3,11(13)-trien-12-oic acid (21), which is not unexpected because the C6-position is not allylic. However, γ-costic acid (20b)—in which the C6-position is allylic—was not converted either, so apparently the geometry of the cyclodecadiene ring system is also required for the reaction catalyzed by the (+)-costunolide synthase.
Germacranolides, Guaianolides, and Eudesmanolides
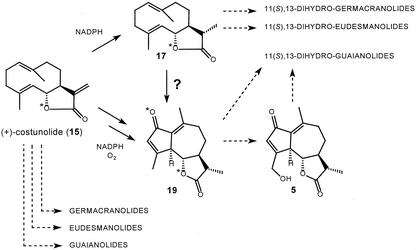
The present results clearly show that (+)-costunolide (15) is the intermediate sesquiterpene lactone in the biosynthesis of the guaianolides, and germacranolides present in chicory (Fig. 1), and presumably the eudesmanolides as well. It is reasonable to assume that this conclusion is also valid for other plant species that contain these skeletal types of sesquiterpene lactones. Although the used GC-MS technique is not the appropriate method to measure higher oxygenated sesquiterpene lactones, some information was obtained about the steps between (+)-costunolide and the final products of sesquiterpene lactone biosynthesis in chicory. Incubations of (+)-costunolide and a 20,000g supernatant in the presence of NADPH and oxygen yielded 11(S),13-dihydrocostunolide (17) and leucodin (19; Fig. 10).
Figure 10.
(+)-Costunolide (15) is enzymatically converted into 11(S),13-dihydrocostunolide (17) and leucodin (19) in the presence of NADPH and O2. Incubation of germacrene acid (14) in the presence of 18O2 gives incorporation at positions marked with an asterisk. It is unclear whether leucodin (19) is formed via 17. Both (+)-costunolide and 11(S),13-dihydro-costunolide are likely precursors for other sesquiterpene lactones present in chicory. Notably, leucodin (19) is only one hydroxylation away from 11(S),13-dihydro-8-deoxylactucin (5), a minor sesquiterpene lactone of chicory.
The formation of 11(S),13-dihydrocostunolide is not dependent upon oxygen but is strongly enhanced in the presence of NADPH, whereas some enzyme activity (15%) is retained in the absence of NADPH. The reduction of the C11-C13 exocyclic double bond is comparable with the type of reactions catalyzed by enoate reductases. This is a group of iron-sulfur flavoproteins that are involved in fatty acid biosynthesis and can be found in many micro-organisms such as Clostridia sp. and baker's yeast, but they have also been observed in plant cell cultures such as that of tobacco. They catalyze the reduction of double bonds that are “activated” by an electron-withdrawing substituent, like the olefinic bond of α,β-unsaturated carboxylic acids and esters, under anaerobic conditions in the presence of reducing agents (generally NADH; Holland, 1992; Faber, 2000). An enoate reductase type of reaction has also been described for a cell-free system of peppermint (Mentha piperita) leaves. It contains a so-called terpenone-Δ4.8-reductase that converts the isopropylidene side chain of (+)-pulegone, which is positioned next to a keto group, into an isopropyl side chain (Battaile et al., 1968; Croteau and Venkatachalam, 1986). This NADPH-catalyzed reaction involved in monoterpene metabolism is thought to occur stereoselectively, and distinct terpenone-Δ4.8-reductases yield either (−)-menthone or (+)-isomenthone (Croteau et al., 1991).
Similar to the stereoselective reactions catalyzed by enoate reductases of microorganisms and the terpenone-Δ4.8-reductase, reduction of the C11-C13 exocyclic double bond of (+)-costunolide yields only one isomer, i.e. the 11(S),13-stereoisomer of dihydrocostunolide. This compound has the same stereochemistry that is present in the 11,13-dihydro sesquiterpene lactones (4–6 and 8) of chicory (Seto et al., 1988; van Beek et al., 1990). This notwithstanding, some plant species do contain C11-epimers, like achillin, which is the 11(R)-epimer of leucodin (Martínez et al., 1988; Ho et al., 1998), indicating that a stereoselective enzyme that synthesizes these C11-epimers should also exist. The enzyme exhibits at least some substrate specificity because the C11-C13 exocyclic double bond of dehydrocostus lactone (6) was not reduced. The detected formation of leucodin (19) proves that guaianolides originate from (+)-costunolide (15). Interestingly, leucodin is only one hydroxylation away from 11(S),13-dihydro-8-deoxylactucin (5; Fig. 10), a minor sesquiterpene lactone of chicory (van Beek et al., 1990). The conversion of (+)-costunolide into leucodin most likely involves more than one enzyme, but whether leucodin originates from 11(S),13-dihydrocostunolide (17) was not investigated. Parthenolide (5) was shown not to be involved in leucodin biosynthesis, but it still cannot be excluded that 11(S),13-dihydroparthenolide is. Various authors have suggested that either such a 4,5-epoxide or a C3-hydroxyl group is necessary to direct the cyclization of (+)-costunolide toward a guaiane framework (Brown et al., 1975; Fischer, 1990; Teisseire, 1994; Piet et al., 1995, 1996).
Formation of leucodin from (+)-costunolide is oxygen dependent and it could be inhibited by the addition of cytochrome P450 inhibitors or CO, whereas formation of 11(S),13-dihydrocostunolide could not. Furthermore, experiments with 18O2 demonstrated that the oxygen atom of the keto group in leucodin also originates from molecular oxygen. Taken together, these observations at least suggest the involvement of a cytochrome P450-enzyme in leucodin biosynthesis (West, 1980; Mihaliak et al., 1993).
We assume that 11(S),13-dihydrocostunolide (17) is the precursor of all 11(S),13-dihydro-sesquiterpene lactones (4–6 and 8–10) present in chicory, including 11(S),13-dihydro-8-deoxylactucin (5; Fig. 10). However, in the major sesquiterpene lactones of chicory, the guaianolides 1 and 3 (van Beek et al., 1990), the C11-C13 bond is unsaturated and these compounds are most likely formed from (+)-costunolide without the intermediacy of 11(S),13-dihydrocostunolide. Perhaps some of these sesquiterpene lactones were already formed during the experiments, but unfortunately these compounds cannot be detected in GC-MS measurement because of their polarity and reduced volatility. The detection and analysis of such higher oxygenated compounds in enzymatic reaction mixtures will need derivatization and/or the use of other chromatographic techniques like HPLC.
MATERIALS AND METHODS
Materials
Fresh roots of cultivated chicory (Cichorium intybus L. cv Focus) were harvested during late summer and obtained from a grower (J. de Mik) in Veenendaal, The Netherlands. The chicory roots were cut into small pieces, frozen in liquid nitrogen, and stored at −80°C. Germacra-1(10),4,11(13)-trien-12-oic acid (14), (+)-costunolide (15), and dehydrocostus lactone (23) were isolated from costus roots (de Kraker et al., 2001b). The synthesis of a mixture of α- and γ-costic acid (20) and the synthesis of elema-1,3,11(13)-trien-12-oic acid (21) have previously been described (de Kraker et al., 2001b, 2001a; respectively). The germacrene acid, costic acid, and elemene acid were dissolved in tert-butyl methyl ether at 15 mm concentrations. (+)-Costunolide, dehydrocostus lactone, and parthenolide (22; Sigma, St. Louis) were dissolved in ethanol at 10 mm concentrations. A sample of leucodin (19) was kindly provided both by Prof. M. Ando (Niigata University, Niigata City, Japan; Ando et al., 1994) and Dr. Shi Yong Ryu (Korea Research Institute of Chemical Technology, Yusung, Taejon, Korea), who also provided its C11-epimer achillin (Ho et al., 1998). 11,13-Dihydro-dehydrocostus lactone (mokko lactone) was a gift of Prof. Y. Asakawa (Tokushima Bunri University, Tokushima, Japan). cis-Nerolidol was purchased from Fluka (Buchs, Switzerland); ether (diethyl ether) and pentane were redistilled before use.
A GC-standard of 11(S),13-dihydro-costunolide (17) was prepared from 2 mg of (+)-costunolide (15) that was dissolved in 1.5 mL of ethyl acetate and stirred with 1.5 mg of NaBH4 at 0°C, a procedure described for the reduction of the C11-C13 exocyclic double bond of various sesquiterpene lactones (Asakawa et al., 1980; Asakawa, 1982; Seto et al., 1988). After 45 min, the reaction was stopped by the addition of 1% (w/w) HAc and an extra 2 mL of ethyl acetate. The organic phase was filtered through a glass-wool plugged (dimethyl chlorosilane-treated glass wool, Chrompack, Raritan, NJ) Pasteur pipette that contained 0.45 g of silica and a little anhydrous MgSO4. GC-MS analysis of the filtrate showed that one-half of the (+)-costunolide was converted into 11(S),13-dihydro-costunolide, whereas no trace of its C11-epimer was detected.
Upon request, small samples of the sesquiterpenes used and the crude enzyme preparations studied will be made available in a timely manner for non-commercial research.
Enzyme Isolation and Assay for (+)-Costunolide Synthase Activity
A cell free extract of chicory roots was prepared from the frozen material in the same way as described for the isolation of the germacrene A hydroxylase (de Kraker et al., 2001a), but MgCl2 was omitted from the extraction buffer. The prepared 20,000g supernatant was desalted with an Econo-Pac10 DG column (Bio-Rad, Hercules, CA) to an assay buffer containing 25 mm Tris (pH 7.5), 1 mm ascorbic acid, 5 μm FAD, 5 μm FMN, and 10% (v/v) glycerol. Dithiothreitol was omitted from the assay buffer, because the SH-groups present in dithiothreitol may undergo a Michael-type addition to the C11-C13 exocyclic double bond of (+)-costunolide (Kupchan et al., 1970). A 1-mL aliquot of the desalted supernatant was incubated in the presence of 45 μm germacrene acid (14) and a 1 mm NADPH-regenerating system, which consists of 1 mm NADPH, 5 mm Glc-6-phosphate, and 1.2 IU Glc-6-phosphate dehydrogenase (all from Sigma). Incubations were also done with boiled desalted supernatant and in the absence of NADPH. The experiments were repeated with elema-1,3,11(13)-trien-12-oic acid (21) and with a mixture of α- and γ-costic acid (20), which may serve as substrate analogs for the germacrene acid (14). After 1 h of incubation at 30°C, the reactions were stopped by storage in the freezer at −20°C.
The incubations were extracted thrice with 1 mL of 20% (v/v) ether in pentane, after the addition of 5 μL of a 0.2 mm cis-nerolidol solution in ethanol that serves as internal standard (response factor of [+]-costunolide [i.e. generated peak of dehydrosaussurea lactone] relative to cis-nerolidol is 0.15). The organic phase was filtered through a glass-wool plugged Pasteur pipette that contained 0.45 g of silica and a little anhydrous MgSO4. The column was washed with 1.5 mL of ether, and the extract was carefully concentrated to approximately 50 μL under a stream of nitrogen. Samples of 2 μL were analyzed by GC-MS using an injection port temperature of 320°C to provoke Cope rearrangement of (+)-costunolide (15). Mass spectra were recorded at 70 eV scanning from 35 to 300 amu; the GC oven temperature was programmed as described previously (de Kraker et al., 1998).
Characterization of (+)-Costunolide Synthase Activity
To determine whether the formation of (+)-costunolide (15) from germacrene acid (14) was catalyzed by a cytochrome P450-enzyme, the effect of various established cytochrome P450-inhibitors (cytochrome c, metyrapone, clotrimazole, micozanole, and amino-benzotriazole) on this reaction was tested, as well as the effect of CO or an argon atmosphere. The cofactor dependence was also investigated, i.e. NAD(P)+ or NADH was added instead of NADPH. Experiments were carried out in a similar manner as described for the germacrene A hydroxylase (de Kraker et al., 2001a), using a 20,000g supernatant and 5 μL of 0.2 mm cis-nerolidol as internal standard. Blue-light reversal of CO inhibition was investigated with gas mixtures of 10% (v/v) O2 + 90% (v/v) N2 (blank) and 10% (v/v) O2 + 90% (v/v) CO.
The origin of the oxygen incorporated in the lactone ring of (+)-costunolide was investigated with 18O2 (99% pure; Icon Services, Mt. Marlon, NY). One milliliter of incubation mixture in a (vented) septum-capped 4.5-mL vial was first bubbled with nitrogen to remove air and subsequently bubbled with 18O2. The mass spectra of the compounds produced were compared with those formed in the standard enzyme assays under air.
Investigation of Subsequent Conversions of (+)-Costunolide
To investigate the conversion (+)-costunolide (15), this compound was incubated at 30 μm concentrations in the same manner as described for germacrene acid (14). Parthenolide (22; 20 μm) was incubated as well, because it might be an intermediate in the formation of guaianolides. Dehydrocostus lactone (23; 20 μm) was incubated to investigate the reduction of the C11-C13 exocyclic double bond in sesquiterpene lactones of chicory. The effect of cytochrome P450 inhibitors and CO on the conversion of (+)-costunolide was studied, just as the effect of various pyridine nucleotide cofactors and an argon atmosphere.
ACKNOWLEDGMENTS
We thank Prof. M. Ando (Niigata University, Niigata City, Japan; Ando et al., 1994) and Dr. S.Y. Ryu (Korea Research Institute of Chemical Technology, Yusung, Taejon) for the gift of leucodin and Prof. Y. Asakawa (Tokushima Bunri University, Tokushima, Japan) for the gift of dihydro-dehydrocostus lactone. We also thank J. de Mik for the gift of the chicory roots and F.W.A. Verstappen and M. Schurink for their useful suggestions and technical assistance.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010957.
LITERATURE CITED
- Ando M, Ibayashi K, Minami N, Nakamura T, Isogai K. Studies on the synthesis of sesquiterpene lactones, 16. The synthesis of 11β,13-dihydrokauniolide, estafiatin, isodehydro-costuslactone, 2-oxodesoxyligustrin, arborescin, 1,10-epiaborescin, 11β,13-dihydroludartin, 8-desoxy-11β,13-dihydrorupicolin B, 8-deoxyrupicolin B, 3,4-epiludartin, ludartin, kauniolide, dehydroleucodin and leucodin. J Nat Prod. 1994;57:443–445. [Google Scholar]
- Asakawa Y. Chemical constituents of Hepaticae. In: Herz W, Grisebach H, Kirby GW, editors. Progress in the Chemistry of Organic Natural Products. Vol. 42. Vienna: Springer Verlag; 1982. [Google Scholar]
- Asakawa Y, Matsuda R, Takemoto T. Mono and sesquiterpenoids from Wiesnerella denudate. Phytochemistry. 1980;19:567–569. [Google Scholar]
- Battaile J, Alice J, Burbott, Loomis WD. Monoterpene interconversions: metabolism of pulegone by a cell-free system from Mentha piperita. Phytochemistry. 1968;7:1159–1163. [Google Scholar]
- Brown ED, Sutherland JK, Sam TW (1975) Medium-ring 1,5-dienes: Part III. Cyclisation of germacra-1(10),4,7-(11)-triene oxides. J Chem Soc Perkin Trans I: 2332–2336
- Croteau R, Karp F, Wagschal KC, Satterwhite DM, Hyat DC, Skotland CB. Biochemical characterization of a spearmint mutant that resembles peppermint in monoterpene content. Plant Physiol. 1991;96:744–752. doi: 10.1104/pp.96.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Venkatachalam KV. Metabolism of monoterpenes: demonstration that (+)-cis-isopulegone, not piperitenone is the key intermediate in the conversion of (−)-isopiperitenone to (+)-pulegone in peppermint (Mentha piperita) Arch Biochem Biophys. 1986;249:306–315. doi: 10.1016/0003-9861(86)90007-x. [DOI] [PubMed] [Google Scholar]
- de Kraker J-W, Franssen MCR, de Groot A, Dalm MCF, Bouwmeester HJ. Biosynthesis of germacrene A carboxylic acid in chicory roots: demonstration of a cytochrome P450 (+)-germacrene A hydroxylase and NADP+-dependent sesquiterpenoid dehydrogenase(s) involved in sesquiterpene lactone biosynthesis. Plant Physiol. 2001a;125:1930–1940. doi: 10.1104/pp.125.4.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kraker J-W, Franssen MCR, de Groot A, König WA, Bouwmeester HJ. (+)-Germacrene A biosynthesis: the committed step in the biosynthesis of sesquiterpene lactones in chicory. Plant Physiol. 1998;117:1381–1392. doi: 10.1104/pp.117.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kraker J-W, Franssen MCR, de Groot A, Shibata T, Bouwmeester HJ. Germacrenes from fresh costus roots. Phytochemistry. 2001b;58:481–487. doi: 10.1016/s0031-9422(01)00291-6. [DOI] [PubMed] [Google Scholar]
- Faber K. Biotransformations in Organic Chemistry: A Textbook. Ed 4. Berlin: Springer Verlag; 2000. pp. 206–213. [Google Scholar]
- Fischer NH. Sesquiterpene lactones: biogenesis and biomimetic transformations. In: Towers G, Towers H, editors. Biochemistry of the Mevalonic Acid Pathway to Terpenoids. New York: Plenum Press; 1990. pp. 161–201. [Google Scholar]
- Fischer NH, Olivier EJ, Fischer HD. The biogenesis and chemistry of sesquiterpene lactones. In: Herz W, Grisebach H, Kirby GW, editors. Progress in the Chemistry of Organic Natural Products. Vol. 38. Vienna: Springer Verlag; 1979. [Google Scholar]
- Geissman TA. The biogenesis of sesquiterpene lactones of the compositae. In: Runeckles VC, Marby TJ, editors. Recent Advances in Phytochemistry. Vol. 6. New York: Academic Press; 1973. pp. 65–95. [Google Scholar]
- Grieco PA, Nishazawa M. Total synthesis of (+)-costunolide. J Org Chem. 1977;42:1717–1720. [Google Scholar]
- Ho C, Choi EJ, Yoo GS, Kim K-M, Ryu SY. Desacetylmatricin, an anti-allergic component from Taraxacum platycarpum. Planta Med. 1998;64:577–578. doi: 10.1055/s-2006-957520. [DOI] [PubMed] [Google Scholar]
- Holland HL. Organic Synthesis with Enzymes. New York: VCH Publishers; 1992. pp. 29–31. [Google Scholar]
- Jain TC, Banks CM, McCloskey JE. Dehydrosaussurea lactone and reversibility in the germacranolide-Cope reaction. Tetrahedron Lett. 1970;11:841–844. [Google Scholar]
- Jain TC, McCloskey JE, Banks CM. Germacranolide-Cope reaction under electron-impact. Org Mass Spectrom. 1971;5:751–755. [Google Scholar]
- Kruistum G. Witlof en roodlof, teelthandleiding nr. 79. Praktijkonderzoek voor de akkerbouw en vollegronds-groenteteelt. The Netherlands: Lelystad; 1997. [Google Scholar]
- Kupchan SM, Fessler DC, Eakin MA, Giacobbe TJ. Reactions of alpha methylene lactone tumor inhibitors with model biological nucleophils. Science. 1970;168:376–377. doi: 10.1126/science.168.3929.376. [DOI] [PubMed] [Google Scholar]
- Martínez M, Muñoz-Zamora A, Joseph-Nathan P. Conformational analysis of achillin and leukodin. J Nat Prod. 1988;51:221–228. [Google Scholar]
- Mihaliak CA, Karp F, Croteau R. Cytochrome P-450 terpene hydroxylases. In: Lea PJ, editor. Methods in Plant Biochemistry, Enzymes of Secondary Metabolism. Vol. 9. London: Academic Press; 1993. pp. 261–279. [Google Scholar]
- Minnaard AJ. Germacrene sesquiterpenes: synthesis and role in biosynthesis. PhD thesis. The Netherlands: Wageningen University; 1997. [Google Scholar]
- Piet DP, Franssen MCR, de Groot Ae. Biotransformation of allylically activated (E,E)-cyclodeca-1,6-dienols by Cichorium intybus. Tetrahedron. 1996;52:11273–11280. [Google Scholar]
- Piet DP, Schrijvers R, Franssen MCR, de Groot Ae. Biotransformation of germacrene epoxides by Cichorium intybus L. Tetrahedron. 1995;51:6303–6314. [Google Scholar]
- Price KR, DuPont MS, Shepherd R, Chan HW-S, Fenwick GR. Relationship between the chemical and sensory properties of exotic salad crops: colored lettuce (Lactuca sativa) and chicory (Cichorium intybus) J Sci Food Agric. 1990;53:185–192. [Google Scholar]
- Rees BS, Harborne JB. The role of sesquiterpene lactones and phenolics in the chemical defense of the chicory plant. Phytochemistry. 1985;24:2225–2231. [Google Scholar]
- Sannai A, Fujimori T, Kato K. Studies on flavor components of roasted chicory root. Agric Biol Chem. 1982;46:429–433. [Google Scholar]
- Sathe RN, Deshpande NR, Kulkarni GH, Kelkar GR, Das KG. Correlation of structure and fragmentation modes of costunolide and its derivatives. Org Mass Spectrom. 1971;5:197–202. [Google Scholar]
- Sathe RN, Kulkarni GH, Kelkar GR, Das KG. Terpenoids—CXXXVII: fragmentation of costunolide and its derivatives under electron-impact. Org Mass Spectrom. 1969;2:935–945. [Google Scholar]
- Seaman FC. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot Rev. 1982;48:124–595. [Google Scholar]
- Seigler DS. Plant Secondary Metabolism. Norwell, MA: Kluwer Academic Publishers; 1998. pp. 367–398. [Google Scholar]
- Seto M, Miyase T, Umehara K, Ueno A, Hirano Y, Otani N. Sesquiterpene lactones from Cichorium endivia L. and C. intybus L. and cytotoxic activity. Chem Pharm Bull. 1988;36:2423–2429. doi: 10.1248/cpb.36.2423. [DOI] [PubMed] [Google Scholar]
- Teisseire PJ. Chemistry of Fragrant Substances. New York: VCH Publishers; 1994. pp. 193–289. [Google Scholar]
- van Beek TA, Maas P, King BM, Leclercq E, Voragen AGJ, de Groot Ae. Bitter sesquiterpene lactones from chicory roots. J Agric Food Chem. 1990;38:1035–1038. [Google Scholar]
- Vogel G, Hartman HD, Krahnstöver K. Handbuch des speziellen Gemüsebaues. Stuttgart, Germany: Ulmer; 1994. pp. 84–144. [Google Scholar]
- West CA. Hydroxylases, monooxygenases, and cytochrome P-450. In: Davis DD, editor. The Biochemistry of Plants. Vol. 2. London: Academic Press; 1980. pp. 317–342. [Google Scholar]
- Westerdijk CA. Teelt van cichorei, teelthandleiding nr. 90. Praktijkonderzoek voor de akkerbouw en vollegronds-groenteteelt. The Netherlands: Lelystad; 2000. [Google Scholar]