Abstract
Isoprene (2-methyl-1,3-butadiene) protection against effects of singlet oxygen was investigated in Myrtus communis and Rhamnus alaternus. In M. communis, singlet oxygen produced in the leaves by Rose Bengal (RB) led to a 65% decrease in net assimilation rates within 3 h, whereas isoprene emission rates showed either a 30% decrease at ambient CO2 concentrations or a 70% increase under high CO2. In both cases, these changes led to an increase in calculated internal isoprene concentrations. The isoprene protection effect was directly demonstrated by fumigation of young (non-emitting) leaves, treated with RB or bromoxynil (simulating photoinhibition). There was 42% and 29% reduction in the damage to net assimilation compared with non-fumigated leaves for RB or bromoxynil, respectively. In R. alaternus, similar effects of RB on net assimilation were observed, and additional fluorescence measurements showed a significantly smaller decrease in Fv/Fm in isoprene-fumigated young leaves treated with RB (from 0.78 to 0.52), compared with non-fumigated leaves (from 0.77 to 0.27). The internal isoprene concentrations used in this study and possible rate of 1O2 production in leaves indicate that the protective effects observed should be beneficial also under natural conditions.
Isoprene (2-methyl-1,3-butadiene) is emitted by a variety of plant species (Harley et al., 1999; Kesselmeier and Staudt, 1999). It influences the trace-gas composition of the troposphere by reacting with OH radicals and NOX to generate tropospheric ozone (Trainer et al., 1987; Chameides et al., 1988; Ryerson et al., 2001). Isoprene also affects the oxidizing capacity of the atmosphere by scavenging OH radicals (Jacob and Wofsy, 1988) and may indirectly influence atmospheric methane accumulation. Models predicting ozone concentrations such as that of Trainer et al. (1987) use emission rates and inventories of isoprene, as well as other volatile organic compounds, and must consider physiological and environmental influences on emission rates.
Isoprene production can consume a few percent of the carbon fixed in photosynthesis but, despite much research, the role of isoprene emission is not fully understood. It was suggested to be protection against sharp temperature increases by dissolving in the thylakoid membranes and stabilizing hydrophobic interactions (Sharkey and Singsaas, 1995; Singsaas et al., 1997; Sharkey et al., 2001). However, this effect was not observed in all studies (Logan and Monson, 1999). Isoprene was also suggested to potentially provide a more general protection against stress conditions (Sharkey and Loreto, 1993) and in particular against photooxidative stress (Zeidler et al., 1997) and was recently shown to protect leaves against ozone (Loreto et al., 2001; Loreto and Velikova, 2001). Other alkenes were also found to increase thermotolerance in leaves (Sharkey et al., 2001). The well-known ability of alkenes, such as isoprene, to react with singlet oxygen (1O2), ozone and OH radicals led us to test the hypothesis that isoprene may help to protect leaves against oxidative stress by reacting with 1O2 and other radicals.
Singlet oxygen is produced in leaves by interaction of molecular oxygen with triplet state chlorophyll, which is formed under conditions of excessive excitation. This may occur under high-light intensities or because of environmental stress that limits the use of the absorbed sunlight (Demmig-Adams, 1990). Here, we used Rose Bengal (RB) as a photosensitizer that produces 1O2 upon absorbing green light and the herbicide bromoxynil (BX) that increases the sensitivity of PSII to light, thus, producing 1O2 through photoinhibition (Krieger-Liszkay and Rutherford, 1998).
In this study, we used the Mediterranean shrubs Myrtus communis and Rhamnus alaternus. Both are evergreen shrubs that grow in northern Israel and throughout the Mediterranean region (Heler and Livneh, 1982). M. communis is a high isoprene emitter (Hansen et al., 1997) and emits only small amounts of monoterpenes (Owen et al., 1997). To our knowledge, there is no information of emission from R. alaternus, however, two other Rhamnus spp. are known to emit isoprene (Kesselmeier and Staudt, 1999).
RESULTS
Isoprene Emission from M. communis
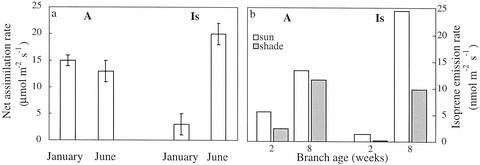
To characterize isoprene emission from M. communis, we examined rates of emission, seasonality, phenology, and CO2 response. Large seasonal variations were observed in isoprene emission rates (Fig. 1a) with maximal rates during the summer and autumn (up to 30 nmol m−2 s−1) and close to detection limit (approximately 0.2 nmol m−2 s−1) in winter. Only small changes in net assimilation rates were observed over the same time (Fig. 1a).
Figure 1.
Seasonal variations (a) and effects of branch age (b) on net assimilation (A) and isoprene emission rates (Is) from a shrub of M. communis. Measurements were performed at leaf temperature of 26°C and light intensity of 1,000 μmol m−2 s−1. The seasonal variations were measured at intercellular CO2 concentration (ci) values between 450 and 500 μL L−1, and the effect of branch age was measured at ambient CO2 concentrations (approximately 350 μL L−1).
Net assimilation and isoprene emission rates were measured in branches that developed from leaves marked at “age zero” (approximately 5 mm2). Both net assimilation and isoprene emission rates increased with leaf age up to 2 months (Fig. 1b). Isoprene emission was first detected, however, only about 1 week later than net CO2 uptake in both sun and shade leaves. The lack of isoprene emission in young leaves was useful in fumigation experiments (see below). Throughout the experiment, both net assimilation and isoprene emission rates were higher in sun leaves than in shade leaves.
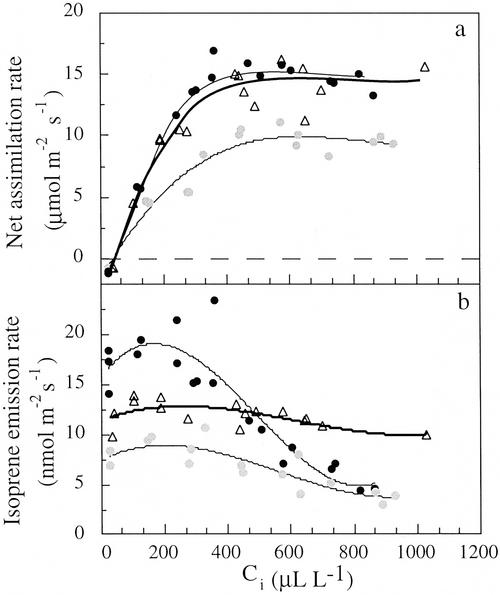
The short-term response of net CO2 assimilation and isoprene emission rates to changes in the intercellular CO2 concentration (ci) was measured at two leaf temperatures (26 ± 0.5°C and 34 ± 0.5°C). Emission rates were relatively constant at ci values between 20 and 300 μL L−1, whereas net assimilation rates increased with ci (Fig. 2). At ci values of approximately 300 to 900 μL L−1, isoprene emission rates decreased by 55% to 75% (at 26°C), whereas net assimilation appeared to be CO2 saturated. The decrease of isoprene emission rates at high ci values was much smaller at 34°C, and isoprene emission rates decreased only by approximately 25% between 300 and 1,000 μL L−1 (Fig. 2b).
Figure 2.
Effects of ci on net assimilation (a) and isoprene emission rates (b) from branches of M. communis measured at 26°C (circles; two different branches) and 34°C (triangles) and light intensity of 1,000 μmol m−2 s−1.
Effects of Singlet Oxygen in M. communis
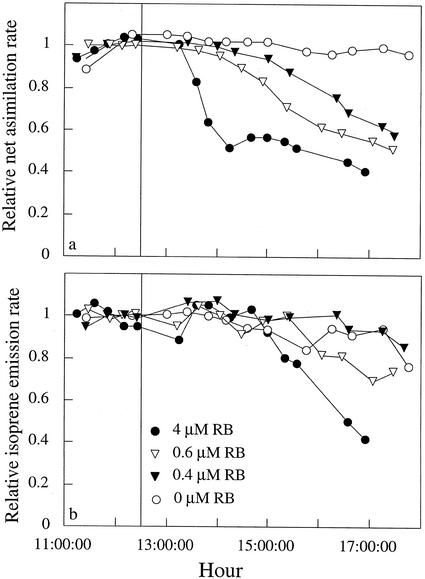
RB was used as a sensitizer to produce 1O2 under light, and its effect on net assimilation and isoprene emission rates was clearly concentration dependent (Fig. 3). Treatments with RB at ambient CO2 concentration resulted in a rather fast decrease in both net assimilation and isoprene emission rates (Figs. 3 and 4a), whereas lesions on the leaves were observed only after 2 d. In control plants (no RB), net assimilation and isoprene emission rates remained relatively constant throughout the measurement day (Fig. 3).
Figure 3.
Effects of different concentration of RB in the feeding solution on net assimilation (a) and isoprene emission rates (b) of M. communis at leaf temperature of 26°C, light intensity of 1,000 μmol m−2 s−1, and ci values of 200 to 250 μL L−1. The vertical line indicates the beginning of RB feeding. Values were normalized to the average before RB feeding.
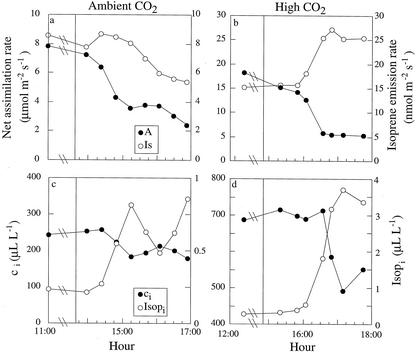
Figure 4.
Effects of singlet oxygen in leaves of M. communis under ambient CO2 concentration (approximately 350 μL L−1; a and b) and high CO2 concentration (approximately 800 μL L−1; c and d) on net assimilation and isoprene emission rates (a and c) and on changes in the ci and isoprene (Isopi; b and d) at leaf temperature of 26°C and light intensity of 1,000 μmol m−2 s−1. The vertical line indicates the beginning of RB feeding.
An important check was made by repeating the treatment and control measurements using light filtered through purple zelofan but maintaining the same total photosynthetically active radiation at the leaf level (the zelofan filter had a broad absorption peak around λmax = 567 nm, as compared with λmax = 547 nm of RB). The filter prevented most of the photochemical formation of 1O2 by RB, and no effect of RB on net assimilation and isoprene emission rates was observed in this case. Net assimilation rates after 2.5 h of RB feeding were 36% of control with no filter (Fig. 4) but 70% of control with the filter. The decrease in net assimilation with filter was similar to that observed in untreated leaves. The results confirmed that the effect of RB was via the photochemical reaction that yields 1O2, and not, for example, by a chemical poisoning.
Comparing the time response of isoprene emission and net assimilation rates showed that, at ambient CO2 concentrations, the decrease in isoprene emission rates was delayed by 1 to 2 h, and was smaller in magnitude, relative to that in net assimilation (Fig. 4a). As a result, the fraction of fixed C allocated to isoprene production increased from 0.5% to 1.1% within 3 h, as did estimated isoprene concentration in the leaf airspaces (Fig. 4c; calculated as by Singsaas et al. [1997]; note that consideration of stomatal patchiness may have small effect on our results). At high CO2 concentrations, net CO2 assimilation rates began to decrease approximately 1.5 h after the beginning of RB feeding, but, in contrast to ambient CO2, isoprene emission rates either did not change or increased (Fig. 4b).
At high CO2 concentrations, the response of isoprene emission to RB treatments was well correlated with changes in ci. In untreated leaves, isoprene emission normally decreased with increasing ci (at 26°C; Fig. 2); whereas in RB-treated leaves, a decrease in ci with time, because of RB effect, led to an increase in isoprene emission rates (comparison of absolute emission rates between Figs. 2 and 4 is difficult because of large differences among the different branches used in the different ci experiments). This was most apparent over the seasonal cycle. During autumn and summer, ci decreased and isoprene emission rates increased in response to RB treatments (Fig. 4, b and d). In contrast, during spring, both ci and isoprene emission rates did not change (data not shown). Furthermore, in an RB treatment in autumn at 34°C and high CO2 concentration, a large decrease in net assimilation and in ci but no change in isoprene emission rates were observed (data not shown). This is consistent with the notion of reduced effect of ci on isoprene emission rate at elevated temperatures (Fig. 2b).
Isoprene Fumigation in M. communis
The possibility that isoprene may provide protection against 1O2 damage was further tested by isoprene fumigation of young, non-emitting branches of M. communis treated by RB or BX. In both isoprene-fumigated and non-fumigated young leaves, RB treatment (0.4 μm) led to a decrease in net assimilation rates. However, in isoprene-fumigated leaves (1–2 μL L−1), the decrease in net assimilation was one-half that of non-fumigated leaves (Table I). Higher isoprene concentration (4–5 μL L−1) did not increase the protection effects, and similar treatments in mature (isoprene-emitting) leaves showed no clear fumigation effects.
Table I.
Effect of isoprene fumigation on net assimilation rates (A) after 4 h of 1O2 treatment in young (non-emitting) leaves of M. communis
| Rose Bengal (0.4 μM)
|
Bromoxynil (50 μM)
|
|||||
|---|---|---|---|---|---|---|
| Fumigation | No fumigation | Ratio | Fumigation | No fumigation | Ratio | |
| 87 | 64 | 1.36 | 66 | 43 | 1.53 | |
| 79 | 49 | 1.61 | 62 | 49 | 1.27 | |
| 109 | 84 | 1.30 | 57 | 54 | 1.53 | |
| Average ± se | 92 ± 9 | 66 ± 10 | 1.42 ± 0.10 | 62 ± 3 | 49 ± 3 | 1.29 ± 0.14 |
Data are presented as the percentage of A left after the treatment. Also presented is fumigation effect on percentage of A, as the ratio between consecutive fumigation and non-fumigation experiments. Light intensity was 1,000 μmol m−2 s−1 and leaf temperature was 26°C. Exogenous isoprene was added to give isoprene concentrations of 1 to 5 and 1 to 15 μL L−1 in the leaf air spaces in the RB and BX experiments, respectively. The fumigation effect was significant at P < 0.005 for RB and P < 0.025 for BX (one tailed paired Student's t test). Data represent results of two shrubs each in the RB and BX experiments.
RB may produce 1O2 at concentrations and locations that do not occur naturally. A more natural cause for 1O2 production in the leaves is photoinhibition, during which the rate of absorption of photons is higher than the rate of utilization of the excitation energy. This leads to formation of triplet state chlorophyll that reacts readily with oxygen, yielding 1O2 (Demmig-Adams, 1990). To better simulate photoinhibition and 1O2 production in the vicinity of PSII, we repeated the isoprene fumigation experiments in leaves treated with the herbicide BX (50 μm). BX increases the sensitivity of PSII to light, leading to production of 1O2 near PSII. In non-fumigated leaves, the treatment resulted in approximately 50% decrease in net assimilation rates within 4 h (beginning 1.5–2 h after feeding). Isoprene-fumigated leaves (1–15 μL L−1) showed approximately 30% smaller decrease in net assimilation due to BX (Table I).
Isoprene Fumigation in R. alaternus
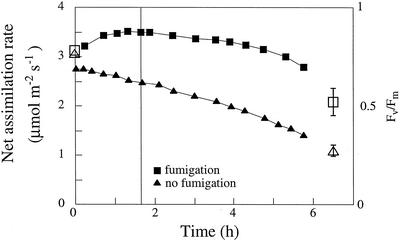
The isoprene 1O2 protection effect was further tested in a different isoprene-emitting species, R. alaternus. Similar fumigation experiments were carried out with young shade leaves treated with RB (0.1 μm). Young leaves of R. alaternus emitted small amounts of isoprene, and isoprene concentrations used in fumigation were up to 20 μL L−1. In non-fumigated leaves, net assimilation decreased significantly and with increasing rates of decrease over time (i.e. increase in the slope of the time response curve) to 0.53 ± 0.02 of control after 4 h (n = 4). In isoprene-fumigated leaves, net assimilation rates decreased to 0.66 ± 0.05 of control (n = 4; Fig. 5; the difference between isoprene-fumigated and non-fumigated leaves was significant at the P < 0.04 level). Interestingly, there was a consistent difference in response between isoprene-fumigated and non-fumigated leaves even before RB feeding, in which non-fumigated leaves of R. alaternus consistently showed a decrease in net assimilation rates from the onset of the experiment, whereas in isoprene-fumigated leaves, net assimilation rates began decreasing only after RB feeding (Fig. 5).
Figure 5.
Effect of RB (0.1 μm) on net assimilation rates (full symbols) and chlorophyll fluorescence yield (Fv/Fm; white symbols) in young shade leaves of R. alaternus at leaf temperature of 26°C, light intensity of 1,000 μmol m−2 s−1. The vertical line indicates the beginning of RB feeding. The fumigation effect was significant at P < 0.04 and P < 0.006 in net assimilation and fluorescence, respectively. The error bars of Fv/Fm before the RB treatment are smaller than the symbols.
Isoprene protection in R. alaternus was also clearly observed in chlorophyll fluorescence measurements (Fv/Fm, an indicator of PSII efficiency, compared before and after each RB treatment experiment). Fv/Fm decreased significantly more in non-fumigated leaves than under isoprene fumigation (from 0.765 ± 0.010 to 0.274 ± 0.027, n = 5; and from 0.778 ± 0.003 to 0.520 ± 0.069, n = 4, in non-fumigated and isoprene-fumigated leaves, respectively; P < 0.006). Slightly better recovery of Fv/Fm after 24 h in low light was observed in isoprene-fumigated leaves (Fv/Fm after 24 h was 0.700 ± 0.020 and 0.639 ± 0.016 in isoprene-fumigated and non-fumigated leaves, respectively; P < 0.03).
DISCUSSION
Characteristics of isoprene emission in M. communis reported here are consistent with reports on other plants (Sharkey et al., 1991; Sharkey and Loreto, 1993; Harley et al., 1994; Monson et al., 1994; Schnitzler et al., 1997). Such characteristics included pronounced seasonality, absence of isoprene emission in young leaves, delayed onset of emission with respect to photosynthetic activity, and higher activity of sun leaves. The effect of ci on rates of net assimilation and isoprene emission at both high and moderate temperatures is similar to that observed in aspen and red oak (Monson and Fall, 1989; Loreto and Sharkey, 1990). This was related to ATP limitation at high ci and moderate temperatures, which is reduced at high temperatures (Loreto and Sharkey, 1990). However, no decrease in isoprene emission rates was observed in M. communis at CO2 free air (ci of approximately 20 μL L−1) for extended time periods, similar to observations in aspen but not in red oak.
The results presented here show for two plant species and using two 1O2 production methods that isoprene can reduce oxidative damage to the photosynthetic apparatus. The beneficial effects are evident in the reduced effect of 1O2 on net assimilation rates and on PSII fluorescence yield (Fv/Fm) in the presence of isoprene.
In discussing the isoprene protection effects observed in this study, it is important to consider the relevance of the experimental results to natural conditions. For this purpose, we consider the concentrations used, the production rates of isoprene as compared with possible production rates of 1O2 in leaves, and other sources for oxidative stress such as O3 and water stress.
A first indirect indication for the usefulness of isoprene in protection against 1O2 is an increase in internal concentration during 1O2 stress. Using RB as a photosensitizer, we observed a decrease in net assimilation and in stomatal conductance. Markedly smaller reduction in isoprene emission than in net assimilation rates (at ambient CO2) or even enhancement (at high CO2) was observed. As a consequence, the plant apparently invested more of the fixed carbon in isoprene formation, and the reduction in stomatal conductance led to an increase in isoprene concentrations in the leaf air spaces during stress. Such increases in intercellular isoprene concentrations may indicate enhancement of isoprene potential to scavenge 1O2.
More directly, isoprene protection against 1O2 was observed in isoprene-fumigated young leaves (little or no endogenous isoprene emission; Table I; Fig. 5). Fumigation experiments are physiologically more relevant if the concentration of isoprene used for fumigation is consistent with concentrations that occur naturally within the leaf air spaces. The concentrations we used for fumigation (1–20 μL L−1) were higher than that occurring regularly in leaves of M. communis but were not unusual in mature stressed leaves (Fig. 4) and in leaves of other plants such as white oak and kudzu (>10 μL L−1; Singsaas et al., 1997). We, therefore, concluded that isoprene concentrations that are expected in the leaf air spaces of isoprene-emitting leaves, are effective in reacting with and scavenging 1O2, as observed under the experimental conditions.
In addition to physiologically relevant concentrations, it also seems that potential rates of 1O2 scavenging by isoprene are physiologically relevant. Although detailed evidence is lacking, results from isolated PSII reaction centers show that approximately 30% of excitation yields 3P680, and most of it is reflected as 1O2 (Durrant et al., 1990; Telfer et al., 1994). In intact leaves, however, about 85% of the excitation of the reaction center leads to photochemistry (Papageorgiou, 1975), and in this case, one can assume that only a few percent of the excitation could yield 1O2. Therefore, the naturally occurring production of 1O2 in the chloroplasts should, at least a priori, be comparable with the production of isoprene that can constitute up to a few percent of the carbon assimilation.
The fumigation experiments reported here seemed to be more efficient with RB than with BX. This, in fact, is consistent with the expected isoprene concentration gradients from a source in the atmosphere to the chloroplasts. RB-produced 1O2 is expected to spread across the leaf, and an external source of isoprene would be more efficient in reacting with it than with 1O2 produced specifically near PSII (i.e. by BX). Under natural conditions, however, a gradient in the opposite direction is likely to exist. In this case, isoprene production is in the chloroplasts, where protection against photoinhibition (simulated here by BX treatment) is expected to be more efficient.
Isoprene protection against 1O2 was not limited to M. communis, and similar effects were observed in young leaves of R. alaternus. Interestingly, non-fumigated leaves of this plant consistently showed a decrease in net assimilation rates even before RB treatment. This was probably due to exposure of shade-adapted leaves to high light intensity during measurements. Our interpretation is supported by the results that fumigation always prevented this effect, apparently protecting the leaves against photoinhibition. We, therefore, speculate that the results show, in fact, two levels of protection before and after RB feeding.
Increased thermotolerance and ozone protection by isoprene were suggested to be achieved through strengthening of the thylakoid membranes (Loreto et al., 2001; Sharkey et al., 2001) and preventing peroxidation of membrane lipids (Loreto and Velikova, 2001). In R. alaternus, the fluorescence yield (Fv/Fm) indicated significantly lower damage to the photosynthetic apparatus in isoprene-fumigated as compared with non-fumigated leaves. This also suggest that isoprene may protect the photosynthetic apparatus embedded in the thylakoid membranes, but, whereas the actual cause for the thermal or ozone damage is not clear, here, it was directly related to 1O2.
Although not the only protection mechanism available, isoprene may have some specific advantages. For example, chloroplast membranes are highly sensitive to photooxidative damage that occurs because of excessive light intensity leading to 1O2 production. The extent of this damage/protection is related to the amount of β-carotene bound to the PSII reaction centers (Telfer et al., 1994). In that sense, isoprene may provide a more dynamic protection mechanism, considering the increase in isoprene emission rates with light intensities (Guenther et al., 1993). This enables the plant to rapidly raise the protection level when needed and before changes in carotenoid synthesis are effective.
The products of reactions between isoprene and 1O2 are likely to be toxic hydroperoxides similar to those produced in reactions with O3 (Mehlhorn and Wellburn, 1987; Hewitt et al., 1990; Salter and Hewitt, 1992). However, we show here that isoprene can protect leaves against 1O2, and isoprene was shown lately to protect leaves against ozone by preventing the collapse of mesophyll cells and chloroplasts membranes (Loreto et al., 2001) and decreasing the amounts of H2O2 and lipid peroxidation (Loreto and Velikova, 2001). The reaction products are apparently less toxic than 1O2 itself or are efficiently scavenged by other agents. Also, the possibility that isoprene could protect against 1O2 by strengthening the membranes, whereas the direct reaction with 1O2 is less significant, cannot be ruled out (isoprene protection against O3 was suggested to be achieved by both mechanisms; Loreto et al., 2001).
Photoinhibition and 1O2 stress are often associated with high light intensities together with low temperatures, as observed in clear winter days. It was consequently shown that in some plants, levels of the carotenoids of the xanthophyll cycle and de-epoxidation state are higher in winter than in summer (e.g. Adams and Demmig-Adams, 1994). However, isoprene emission, proposed here as a protection mechanism, was minimal in winter in M. communis. But note that in Mediterranean plant species, the xanthophyll cycle de-epoxidation state was shown to be higher in summer, well correlated with low water potential (Kyparissis et al., 2000). In the eastern Mediterranean region, photoinhibition and oxidative stress are likely to be more pronounced in summer, when plants are exposed to high light and water stress, whereas winter conditions are optimal for growth. Low stomatal conductance during water stress periods would also enhance intercellular isoprene concentrations (Sharkey and Loreto, 1993; Fang et al., 1996) and the potential protective capacity.
We note in closing that the protective aspects of isoprene discussed above may also be consistent from an evolutionary perspective. Carotenoids of the xanthophyll cycle are known to scavenge both triplet chlorophyll and singlet oxygen (Demmig-Adams, 1990; Havaux and Niyogi, 1999). Zeaxanthin was suggested to decrease membrane fluidity and to increase thermotolerance (Tardy and Havaux, 1997; Havaux, 1998) as was suggested for isoprene (Sharkey et al., 2001; Sharkey and Singsaas, 1995; Singsaas et al., 1997). Isoprene shares a common biochemical production pathway with carotenoids, and it can be speculated that isoprene was a primitive protection mode against 1O2 that evolved into these more dedicated radical scavengers.
MATERIALS AND METHODS
Plant Material
Net assimilation and isoprene emission rates were measured for cut branches of Myrtus communis from plants growing on the campus of the Weizmann Institute of Science (Rehovot, Israel) and for attached branches from plants grown in pots under similar light and temperature conditions. Net CO2 assimilation (6.5 ± 0.5 and 7.5 ± 0.5 μmol m−2 s−1) and isoprene emission rates (6.0 ± 0.5 and 6.5 ± 2.0 nmol m−2 s−1) were similar in attached and cut branches, respectively. Hence, branches were cut under water and were kept with the stem immersed in deionized water for the experiments reported here. Cut branches containing young leaves from a shade growing shrub of Rhamnus alaternus in the Weizmann campus were used in isoprene fumigation experiments.
Gas-Exchange Measurements
The sampling system was centered on a flow-through cuvette (volume of approximately 60 mL) in which the branch was sealed. The cuvette was equipped with a magnet-operated fan for mixing the air. Light (1,000 μmol m−2 s−1 photosynthetically active radiation) was supplied by a halogen lamp (250 W, Quartzline lamp, General Electric Co., Cleveland, OH); the infrared radiation was filtered out using a water bath (3 cm thick). The temperature in the cuvette was controlled by water circulation through a cooling bath (Haake D8-V, Karlsruhe, Germany) and through the bottom of the cuvette and was set to give the desired leaf temperature (normally 26 ± 0.5°C). Air temperature was measured by a shaded thermocouple in the cuvette, and the leaf temperature was measured by a thermocouple touching the abaxial side of the leaf (type T thermocouple digital thermometer, HH82, Omega, Stamford, CT; precision of 0.1°C). Dry air with various CO2 concentrations was supplied by mixing cylinder air with no CO2 and cylinder air having 1% or 2.5% (v/v) CO2 using two mass flow controllers (MKS1179A, Andover, MA; 1,000 mL min−1 and 100 mL min−1 full scale, respectively, used in a relative mode). Total flow rate through the cuvette was 200 mL min−1. Both air flows passed through activated charcoal traps to remove hydrocarbons. The tubing (0.25- and 0.125-inch stainless steel) carrying the air exiting the cuvette was heated to 80°C to avoid condensation of water vapor.
CO2 and H2O concentrations in the air entering and leaving the leaf cuvette were measured by an infrared gas analyzer (Li-6262, LI-COR, Lincoln, NE), at a precision of ±1 μmol mol−1 for CO2 and ±0.1 mmol mol−1 for water vapor. Net assimilation, stomatal conductance, and ci were calculated according to von Caemmerer and Farquhar, (1981) assuming that stomatal patchiness has only small effects (van Kraalingen, 1990). Projected leaf area was estimated at the end of each experiment.
Isoprene Measurements
For hydrocarbons measurements (Greenberg et al., 1994; R.K. Monson, personal communication) an aliquot of the air stream exiting the cuvette was pumped (bypassing the infrared gas analyzer) into a pre-evacuated glass bulb (2 L, 60 mtorr), through a trapping loop (0.125-inch stainless steel, 27 cm long in which the central approximately 9 cm was packed with 212- to 300-μm glass beads [Sigma, St. Louis], with a 2-μm filter [Valco, Houston, TX] placed at the outlet) connected to a six-port valve (Valco). The loop was cooled with either liquid nitrogen or a mixture of ethanol and dry ice (−75°C) for trapping the hydrocarbons in the air. The pressure (pressure transducer, Omega) at the entrance to the glass bulb was used to estimate the amount of air sampled (typical pressure used was 100 torr, namely the sample size was approximately 250 mL). After trapping, the valve was switched to a flow of helium (1.5 mL min−1), the loop was rapidly heated (hot sand, 200°C), and the trapped hydrocarbons passed directly to a gas chromatography (GC) column (HP 5890, Wilmington, DE).
The hydrocarbons were separated using a polar GC column (30 m long, 0.25 mm i.d., 0.25 μm film; Supelcowax 10, Supelco, Bellefonte, PA; temperature program: 35°C for 1 min, temperature increase at 10°C min−1 to 170°C for 2 min). Isoprene was detected using a flame ionization detector kept at 250°C. The area of the peaks obtained was recorded and analyzed by a chromatography software (Borwin, version 1.21.60, JMBS developments, La Fontenil, France).
The GC peak area of isoprene was found to vary linearly with concentration, and to be constant over time. Isoprene gaseous standards were prepared and measured every few months. The precision of isoprene concentration measurements was 4%. Standards were prepared by evaporating isoprene (99%; Aldrich, Milwaukee, WI) into a pre-evacuated 12-L bulb to a pressure of approximately 0.1 torr, on a vacuum line. Nitrogen was added to atmospheric pressure. The bulb was evacuated to approximately 1 torr and refilled with nitrogen to atmospheric pressure, to give 0.1 to 0.2 μL L−1 isoprene.
Singlet Oxygen Treatments
Singlet oxygen was produced in the leaves by RB (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein) or BX (3,5-dibromo-4-hydroxybenzonitrile; Agan chemical manufacturers, Ashdod, Israel) in the light. RB acts as a photosensitizer to singlet oxygen production upon absorbing green light, λmax = 547 nm. BX is a phenolic herbicide that increases the sensitivity of PSII to excess light through formation of P680 triplet (Krieger-Liszkay and Rutherford, 1998). Both chemicals were fed as aqueous solutions through the petiole, and their effect on net assimilation and isoprene emission rates was examined at ambient and high CO2 concentrations, 26°C, and at light intensity of 1,000 μmol m−2 s−1. To use physiologically relevant 1O2 concentrations, RB concentration used in the fumigation experiments (0.4 μm in M. communis and 0.1 μm in R. alaternus) was the lowest to induce decrease in net assimilation rates (Fig. 3), and BX was used as a source for 1O2 near PSII, simulating photoinhibition more specifically than RB.
Isoprene fumigation was done together with the 1O2 treatment in young leaves, by passing the air entering the leaf cuvette through a piece of permeable tubing that was enclosed in an Erlenmeyer containing isoprene and kept in an ice bath. The fumigation dose (1–20 μL L−1) was determined by the permeability of the tubing and the isoprene concentration in the Erlenmeyer.
Chlorophyll Fluorescence
Chlorophyll fluorescence yield (Fv/Fm) was measured in dark-adapted leaves of R. alaternus before and after singlet oxygen experiments, using a portable fluorometer (PAM-2000, Walz, Germany).
Statistical Analysis
The data analysis add-in from Microsoft Excel 1998 (Microsoft Corp., Redmond, WA) was used to calculate Student's t test for determining the significance of difference between fumigation and non-fumigation experiments.
Acknowledgments
We thank Y. Rudich, F. Loreto, R.K. Monson, and two anonymous reviewers for helpful comments and E. Negreanu for technical help. We thank Agan Chemical manufacturers for supplying BX.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010909.
LITERATURE CITED
- Adams WWI, Demmig-Adams B. Carotenoid composition and down regulation of photosystem II in three conifer species during the winter. Physiol Plant. 1994;92:451–458. [Google Scholar]
- Chameides WL, Lindsay RW, Richardson J, Kiang CS. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science. 1988;241:1473–1475. doi: 10.1126/science.3420404. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta. 1990;1020:1–24. [Google Scholar]
- Durrant JR, Giorgi LB, Barber J, Klug DR, Porter G. Characterization of triplet states in isolated photosystem II reaction centers: oxygen quenching as a mechanism for photodamage. Biochim Biophys Acta. 1990;1017:167–175. [Google Scholar]
- Fang C, Monson RK, Cowling EB. Isoprene emission, photosynthesis, and growth in sweetgum (liquidambar styraciflua) seedlings exposed to short- and long-term drying cycles. Tree Physiol. 1996;16:441–446. doi: 10.1093/treephys/16.4.441. [DOI] [PubMed] [Google Scholar]
- Greenberg JP, Lee B, Helmig D, Zimmerman PR. Fully automated gas chromatograph-flame ionization detector system for the in situ determination of atmospheric non-methane hydrocarbons at low parts per trillion concentration. J Chromatogr. 1994;676:389–398. [Google Scholar]
- Guenther A, Zimmerman P, Harley P, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analysis. J Geophys Res. 1993;98:12609–12617. [Google Scholar]
- Hansen U, Van Eijk J, Bertin N, Staudt M, Kotzias D, Seufert G, Fugit JL, Torres L, Cecinato A, Brancaleoni E et al. Biogenic emissions and CO2 gas exchange investigated on four Mediterranean shrubs. Atmos Environ. 1997;31:157–166. [Google Scholar]
- Harley PC, Litvak ME, Sharkey TD, Monson RK. Isoprene emission from velvet bean leaves. Plant Physiol. 1994;105:279–285. doi: 10.1104/pp.105.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Monson RK, Lerdau MT. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia. 1999;118:109–123. doi: 10.1007/s004420050709. [DOI] [PubMed] [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998;3:147–151. [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heler D, Livneh M. Plants and Animals of the Land of Israel. 10A. Tel Aviv: Ministry of Defense Publishing; 1982. Plants with flowers. [Google Scholar]
- Hewitt CN, Kok GL, Fall R. Hydroperoxide in plants exposed to ozone mediate air pollution damage to alkene emitters. Nature. 1990;344:56–58. doi: 10.1038/344056a0. [DOI] [PubMed] [Google Scholar]
- Jacob DJ, Wofsy SC. Photochemistry of biogenic emissions over the Amazon forest. J Geophys Lett. 1988;93:1477–1486. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- Krieger-Liszkay A, Rutherford AW. Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: relevance to photodamage and phytotoxicity. Biochemistry. 1998;37:17339–17344. doi: 10.1021/bi9822628. [DOI] [PubMed] [Google Scholar]
- Kyparissis A, Drilias P, Manetas Y. Seasonal fluctuations in photoprotective (xanthophyll cycle) and photoselective (chlorophylls) capacity in eight Mediterranean plant species belonging to two different growth forms. Aust J Plant Physiol. 2000;27:265–272. [Google Scholar]
- Logan BA, Monson RK. Thermotolerance of leaf discs from four isoprene-emitting species is not enhanced by exposure to exogenous isoprene. Plant Physiol. 1999;120:821–825. doi: 10.1104/pp.120.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 2001;126:993–1000. doi: 10.1104/pp.126.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 1990;182:523–531. doi: 10.1007/BF02341027. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Wellburn AR. Stress ethylene formation determines plant sensitivity to ozone. Nature. 1987;327:417–418. [Google Scholar]
- Monson RK, Fall R. Isoprene emission from Aspen leaves. Plant Physiol. 1989;90:267–274. doi: 10.1104/pp.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak M, Wildermuth M, Guenther AB, Zimmermann PR. Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia. 1994;99:260–270. doi: 10.1007/BF00627738. [DOI] [PubMed] [Google Scholar]
- Owen S, Boissard C, Street RA, Duckham SC, Csiky O, Hewitt CN. The BEMA project: screening of 18 Mediterranean plant species for volatile organic compound emissions. Atmos Environ. 1997;31:101–117. [Google Scholar]
- Papageorgiou G. Govindjee, ed, Bioenergetics of Photosynthesis. New York: Academic Press; 1975. Chlorophyll fluorescence: an intrinsic probe of photosynthesis; pp. 319–371. [Google Scholar]
- Ryerson TB, Trainer M, Holloway JS, Parrish DD, Huey LG, Sueper DT, Frost GJ, Donnelly PD, Schauffler S, Atlas EL et al. Observations of ozone formation in power plant plums and implications for ozone control strategies. Science. 2001;292:719–723. doi: 10.1126/science.1058113. [DOI] [PubMed] [Google Scholar]
- Salter L, Hewitt NC. Ozone-hydrocarbon interactions in plants. Phytochemistry. 1992;31:4045–4050. [Google Scholar]
- Schnitzler JP, Lehning A, Steinbrecher R. Seasonal pattern of isoprene synthase activity in Quercus robur leaves and its significance for modelling isoprene emission rates. Bot Acta. 1997;110:240–243. [Google Scholar]
- Sharkey TD, Chen X, Yeh S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001;125:2001–2006. doi: 10.1104/pp.125.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia. 1993;95:328–333. doi: 10.1007/BF00320984. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF. High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant Cell Environ. 1991;14:333–338. [Google Scholar]
- Sharkey TD, Singsaas EL. Why plants emit isoprene. Nature. 1995;374:769. [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene emitting species. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy F, Havaux M. Thylakoid membrane fluidity and thermostability during the operation of the xanthophyll cycle in higher-plant chloroplasts. Biochim Biophys Acta. 1997;1330:179–193. doi: 10.1016/s0005-2736(97)00168-5. [DOI] [PubMed] [Google Scholar]
- Telfer A, Dhami S, Bishop SM, Phillips D, Barber J. β-Carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry. 1994;33:14469–14474. doi: 10.1021/bi00252a013. [DOI] [PubMed] [Google Scholar]
- Trainer M, Williams EJ, Parish DD, Buhr MP, Allwine EJ, Westberg HH, Fehsenfeld FC, Liu SC. Models and observations of the impact of natural hydrocarbons on rural ozone. Nature. 1987;329:705–707. [Google Scholar]
- van Kraalingen DWG. Implications of non-uniform stomatal closure on gas exchange calculations. Plant Cell Environ. 1990;13:1001–1004. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationship between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Zeidler JG, Lichtenthaler HK, May HU, Lichtenthaler FW. Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z Naturforsch. 1997;52c:15–23. [Google Scholar]