Abstract
Hyperosmotic stress is known to significantly enhance net uptake of inorganic ions into plant cells. Direct evidence for cell turgor recovery via such a mechanism, however, is still lacking. In the present study, we performed concurrent measurements of net ion fluxes (with the noninvasive microelectrode ion flux estimation technique) and cell turgor changes (with the pressure-probe technique) to provide direct evidence that inorganic ion uptake regulates turgor in osmotically stressed Arabidopsis epidermal root cells. Immediately after onset of hyperosmotic stress (100/100 mm mannitol/sorbitol treatment), the cell turgor dropped from 0.65 to about 0.25 MPa. Turgor recovery started within 2 to 10 min after the treatment and was accompanied by a significant (30–80 nmol m−2 s−1) increase in uptake of K+, Cl−, and Na+ by root cells. In most cells, almost complete (>90% of initial values) recovery of the cell turgor was observed within 40 to 50 min after stress onset. In another set of experiments, we combined the voltage-clamp and the microelectrode ion flux estimation techniques to show that this process is, in part, mediated by voltage-gated K+ transporters at the cell plasma membrane. The possible physiological significance of these findings is discussed.
Improving crop resistance to drought stresses is a long-standing challenge for generations of plant physiologists and agricultural biotechnologists. In the last 15 years, major efforts have been focused on molecular engineering of transgenic species that overexpress genes responsible for biosynthesis of various compatible solutes (Bohnert et al., 1995; Bray, 1997). This approach has been extensively reviewed (Smirnoff, 1998; Bajaj et al., 1999; Bohnert and Shen, 1999; Nuccio et al., 1999; Serrano et al., 1999b; Cushman and Bohnert, 2000). Among the genes targeted were those responsible for biosynthesis of amino acids (Pro, ectoine, and Gly betaine), sugars (Suc, trehalose, and fructan), polyols (mannitol and sorbitol) and quaternary amines (Winicov, 1998; Bajaj et al., 1999; Cushman and Bohnert, 2000; and refs. therein).
The practical outcomes of these extensive studies surprisingly are only marginal (Bajaj et al., 1999; Bohnert and Shen, 1999). To our knowledge, there are no reports of any significant improvements in drought tolerance in transgenic crop species in field trials. This is probably due to the complexity of whole-plant responses to water stress. But at the cellular level, are we on the right track in our attempts to improve the plant's ability to withstand water stress?
It was traditionally believed that the major function of compatible solutes is osmoregulation (Wyn Jones and Pritchard, 1989; Delauney and Verma, 1993; Bajaj et al., 1999). However, it recently became evident that the functions of compatible solutes are not likely to be as straightforward as initially believed. More and more papers question whether compatible solutes are directly involved in regulation of cell turgor, suggesting instead that their possible regulatory role is to adjust metabolic pathways to altered environmental conditions (Bray, 1997; Bohnert and Sheveleva, 1998; Serrano et al., 1999b). For example, although the expression of the yeast TPS1 gene, which encodes trehalose-6-phosphate synthase in tobacco (Nicotiana tabacum) plants, produced significant improvements in drought and salt tolerance (Romero et al., 1997; Serrano et al., 1999a), the measured concentration of trehalose in the transgenic plants was too low (<0.5 mm) for a conventional osmoprotectant effect. It was suggested that trehalose may play a more complex role via regulating numerous metabolic and hormonal pathways rather than directly contributing to osmotic adjustment (Serrano et al., 1999a). Bohnert and Sheveleva (1998) provided strong arguments that, contrary to previous suggestions, the true role of Pro (one of the major compatible solutes believed to be operating in plants; Delauney and Verma, 1993) in osmotic stress protection is still to be determined. It is more likely that the main function of compatible solutes may be stabilization of protein complexes or membranes rather than direct involvement in osmotic adjustment (Bohnert and Shen, 1999).
Meanwhile, practically every plant organism can be affected by osmotic stress of varying severity. If compatible solutes are not directly involved in cell osmotic adjustment, the only way for a plant cell to maintain normal turgor pressure is via uptake of inorganic ions. This option has long been considered a viable alternative to biosynthesis of osmoprotectants (Wyn Jones and Pritchard, 1989; Bohnert et al., 1995).
In previous research, we showed that bean mesophyll cells responded to hyperosmotic stress by increased uptake of K+ and Cl− (Shabala et al., 2000). Our model calculations estimated that up to 85% of the changes in the cell turgor may be compensated by uptake of these two inorganic ions within 1 h after stress onset. However, these calculations have to be supported by direct measurements of the recovery in cell turgor pressure. In the present study, we use concurrent measurements of net ion fluxes (the noninvasive microelectrode ion flux estimation [MIFE] technique) and cell turgor changes (the pressure-probe technique) to provide direct evidence for the role of inorganic ion uptake in turgor regulation in osmotically stressed Arabidopsis epidermal root cells. In another set of experiments, we provide the evidence that this process is at least partially mediated by voltage-gated K+ transporters at the cell plasma membrane.
RESULTS
Altogether, about 40 attempts to measure the kinetics of cell turgor recovery were made. In about 30% of the measurements, the pressure probe became plugged, and measurements were stopped; in about 50% of the measurements, the probe was dislodged as a result of tissue movement upon treatment with the hyperosmotic solution. However, for eight cells, we were able to continuously monitor both the initial turgor and the kinetics of turgor recovery for prolonged intervals, up to 30 to 50 min each. All but one cell showed clear evidence of fast turgor recovery within this time frame.
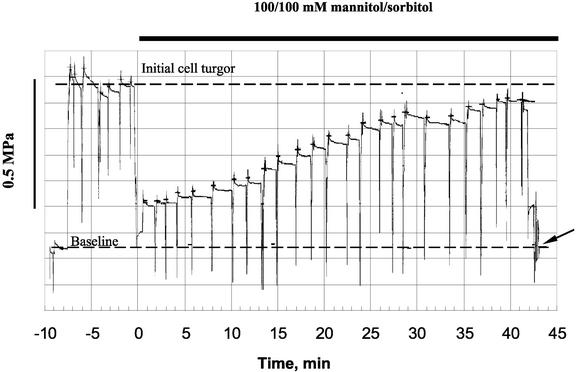
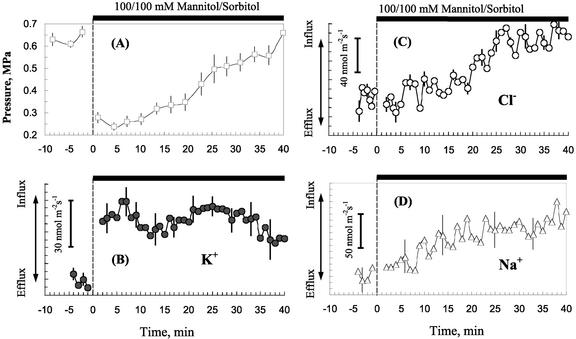
Initial turgor values for epidermal root cells of Arabidopsis incubated in APW were 0.63 ± 0.02 MPa (n = 11 individual plants analyzed). When roots were exposed to hyperosmotic treatment (100/100 mm mannitol/sorbitol), the turgor pressure dropped by about 0.4 MPa in all measured cells. Then a gradual recovery of the cell turgor occurred. This is illustrated in Figures 1 and 2A. The rate of recovery varied between different cells. Some of them had a lag of about 5 to 15 min; in others, the process started almost immediately. In most cells, almost complete (>90% of initial values) recovery of the cell turgor occurred within 40 to 50 min after stress onset (Fig. 2A).
Figure 1.
Example of turgor measurements from an Arabidopsis root epidermal cell. The dotted baseline is the pressure required to offset capillary action to bring the APW/oil interface to the tip. This is the “zero” pressure used in measurements of cell turgor pressure. The micropipette was impaled into the cell, and the position of the meniscus was adjusted to the tip by applying additional pressure to the piston. To make sure that the pipette tip did not become plugged, the meniscus was periodically (every 1.5–2 min) brought out of the cell to clear the tip and then immediately returned to the tip. Due to restricted flow through the small aperture of the micropipette, the pressures recorded during meniscus movement are overshoots and do not reflect the pressure in the cell. For this reason, cell turgor pressure was measured after the rapid transient peak, when the meniscus was adjusted to the tip, but before the slower movement of the meniscus back from the tip because of expansion of the teflon tubing. Hyperosmotic treatment (100/100 mm mannitol/sorbitol) was given at time zero. Cell turgor recovery started approximately 5 min after stress onset. At the end of experiment, the probe was taken out of the cell, and the meniscus position was adjusted in APW (indicated by an arrow) to assure the baseline pressure had not change during the experiment.
Figure 2.
Kinetics of osmotically induced changes in cell turgor (A) and net fluxes of K+ (B), Cl− (C), and Na+ (D) in Arabidopsis root cells. Data are mean ± se (n = 7 individual plants for data presented in A and n = 5 for ion flux data shown in B–D). Hyperosmotic treatment was given at time zero. Almost complete (>90% of initial value) turgor recovery was observed within 40 min after stress onset.
The quickness of turgor recovery implies that this process may be mediated by mechanisms different from biosynthesis of compatible solutes. Our working hypothesis was that the rapid recovery could be mediated by increased uptake mechanisms of major osmotica (inorganic ions) present in solution. Figure 2, B through D, shows average data for K+, Cl−, and Na+ fluxes for five individual plants. Hyperosmotic stress caused increased net uptake of all of these ions into the cell.
The average flux (nanomoles per meter per second) for 4 to 5 min before osmotic treatment was: K+, −80 ± 4; Cl−, −60 ± 12; and Na+, 0 ±, 19 (n = 5). The ion fluxes reflect a complex equilibrium state, most ion uptake is from the gellan gum, resulting in efflux from the side of the root exposed to the low-salt APW at equilibrium. Osmotic treatment decreased or reversed the efflux. To reflect the shift in equilibrium, the fluxes are shown as net flux changes.
In most experiments, the change in K+ uptake was rapid. K+ flux normally stabilized at a new steady level within several minutes after mannitol treatment and then exhibited a pronounced fluctuation around a new baseline (Fig. 2B). Chloride and sodium influx were usually more delayed (up to 20–30 min; Fig. 2, C and D).
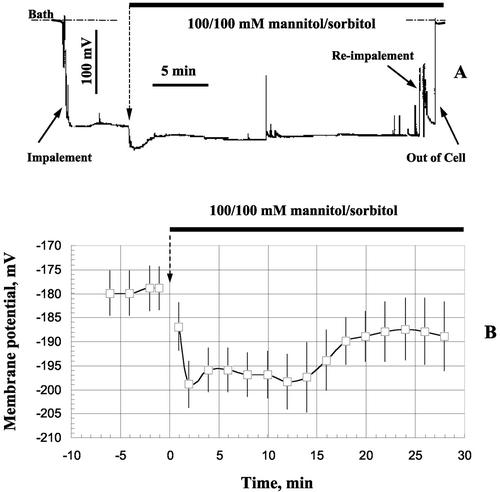
Another pronounced effect of hyperosmotic stress was significant membrane hyperpolarization by about 20 mV (Fig. 3). A typical example of a long-term record of the membrane potential (MP) of an epidermal root cell in response to hyperosmotic treatment is shown in Figure 3A. Average data from seven individual cells are shown in Figure 3B. Hyperosmotic treatment caused immediate and prolonged hyperpolarization in Arabidopsis root cells. This is consistent with previous observations in Arabidopsis (Lew, 1996) and in other species (Kinraide and Wyse, 1986; Reuveni et al., 1987; Kitamura et al., 1997; Zingarelli et al., 1999).
Figure 3.
Osmotically induced changes in plasma MP in Arabidopsis root cells. A, Example of a long-term record from one typical cell. B, Average data for seven plants (error bars are se). Immediate and prolonged manniol-induced hyperpolarization was observed in all cells.
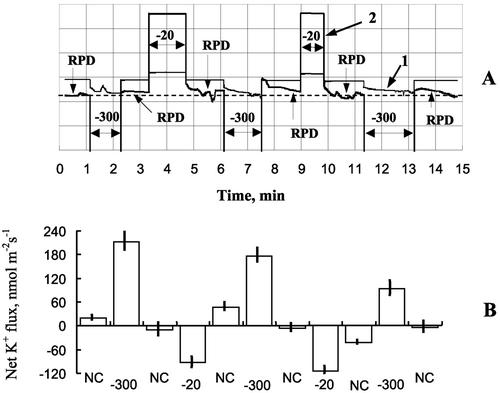
It is known that many membrane transporters, including those for K+ and Cl−, are voltage-dependent (Maathuis and Amtmann, 1999; Tyerman and Skerrett, 1999). To explain the observed increase in net K+ uptake in response to hyperosmotic treatment (Fig. 2B), it was postulated that osmotically induced plasma membrane hyperpolarization may affect K+ uptake via direct control of voltage-gated K+ channels in Arabidopsis root cells (Babourina et al., 2001). Therefore, we performed a series of experiments measuring net K+ flux from the root hair cells clamped at various values above and below the resting potential.
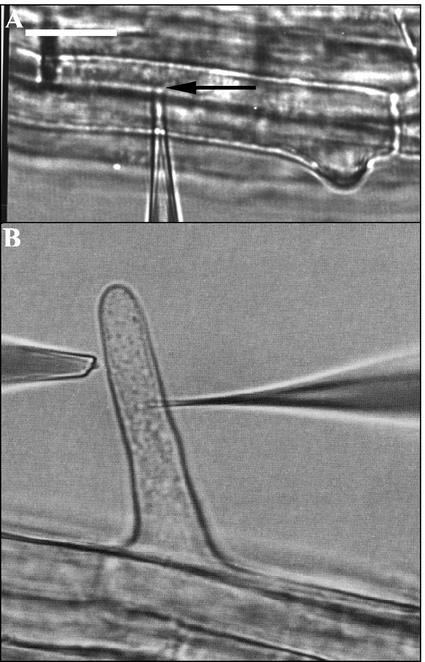
Figure 7A shows a typical root hair cell and two microelectrodes, one (impaled and double-barreled) for voltage clamping and current measurements and another one (near the root hair surface) for net K+ flux measurements. The clamping protocol is illustrated in Figure 4A, and the resultant K+ flux measurements are illustrated in Figure 4B. Clamping the cell to potentials more negative than MP caused a significant uptake of K+ (net K+ influx). When the cell was clamped at a depolarized potential of −20 mV, net K+ flux shifted toward significant net efflux. Thus, direct evidence for voltage-gated control of net K+ fluxes at the tissue level is shown.
Figure 7.
Microphotgraphic examples of pressure probe (A) and ion-flux/voltage-clamp measurements (B). The micropipette tip is indicated by an arrow in A. It is located in the vacuole. In B, the double-barreled microelectrode is impaled into the cytoplasm (Lew, 2000). The ion fluxes from the root hair were measured parallel to the root surface. Bar = 20 μm.
Figure 4.
Control of net K+ fluxes by voltage clamping of Arabidopsis root hair cell. A typical root hair impaled with a double-barreled microelectrode for voltage-clamp and current measurements and a K+-selective MIFE microelectrode for K+ flux measurements is shown in Figure 7B. A, Voltage-clamping protocol for one typical experiment. A bipolar staircase voltage clamp was given, above (−300 mV) and below (−20 mV) the resting potential difference (RPD). Current (1; scale, 20 nA/division) and voltage (2; 50 mV/division) traces are shown. The dotted line indicates the initial level of MP (−175 mV). B, Net K+ fluxes (inward positive) measured in voltage-clamp experiments. The cell clamping at potentials more negative than RPD caused a significant increase in uptake of K+, indicating a direct control of applied voltage over K+ transporters at the plasma membrane (Babourina et al., 2001). Error bars are se (n = 8–12).
DISCUSSION
Elongation growth is crucially dependent on the cell turgor (Cosgrove, 2000). It is not surprising, therefore, that osmotic stress immediately inhibits root cell elongation in higher plants (Hsiao, 2000). However, it is widely reported that this inhibition is only transient, and normal growth usually resumes within 10 to 60 min (Kuzmanoff and Evans, 1981; Itoh et al., 1987; Frensch and Hsiao, 1994; Kitamura et al., 1997). In whole-root measurements of maize (Zea mays), root pressure can recover within this time frame (Azaizeh et al., 1992), although the underlying mechanisms causing recovery were not examined. Itoh et al. (1987) reported that in osmotically stressed mung bean (Vigna radiata) roots, turgor recovery took at least 6 h to be completed. Both initial turgor pressure values and the magnitude of the mannitol-induced drop in the cell turgor were very similar to our data. During long-term (24 h) recovery in maize, hexoses (and the mannitol used in the osmotic treatment) accounted for most of the osmotic adjustment (Pritchard et al., 1996). During this long time, root growth would confound the mechanism causing rapid cell turgor recovery. In the present study, seven of the eight cells measured showed almost complete (>90% of initial value) turgor recovery within 40 min after stress onset (Fig. 2A), much faster than the slow time to recover reported by Itoh et al. (1987). We believe the most important factor accounting for this discrepancy in recovery time was the ionic composition of the nutrient solution.
Our data provide direct evidence that osmotically induced uptake of inorganic ions is an important (and apparently predominant) component of fast turgor recovery in Arabidopsis root cells (Fig. 2A). The model calculations suggested that for a typical Arabidopsis root cell of 12.5 × 80 μm (Lew, 1996) and an average rate of K+, Cl−, and Na+ uptake over a 40-min interval of 40, 35, and 65 nmol m−2 s−1, respectively (Fig. 2, B–D), the total amount of accumulated ions will be 1.31 × 10−12 mol (assuming a uniform flux over the cell surface). According to van't Hoff's law this will change the cell osmotic potential by about 0.28 MPa (see Shabala et al. [2000] for details on calculations). Direct measurements with the pressure-probe suggest that between 0.3 and 0.35 MPa are recovered within 40 min after stress onset (Fig. 2A). Therefore, increased uptake of these three ions measured is responsible for 80% to 90% of quick turgor recovery in osmotically stressed Arabidopsis root cells. The remaining 10% could be probably attributed to increased net uptake of other ions (Mg2+ or Ca2+; Shabala and Newman, 1998), which were not measured. It is important to emphasize that these calculations are for epidermal cells and do not take into account the complexity of root architecture, in which osmotic and turgor regulation may vary by cell type and location, nor can they account for the complexity of whole-plant responses (Kramer and Boyer, 1995). The calculations do implicate strongly inorganic ion uptake in turgor recovery.
Among the ions measured, net uptake of Na+ was the largest one, contributing to up to 0.13 MPa (about 40%) of the cell turgor recovery. The Na+ concentration in the bath solution was five times higher than [K+] (0.5 and 0.1 mm, respectively), and Na+ uptake via low-affinity transport systems is possible (Tyerman and Skerrett, 1999). This may account for the faster turgor recovery we observed compared with Itoh et al. (1987), who used a low nutrient solution and no Na+ and observed much slower recovery of cell turgor, which took several hours to complete. Supporting our statement, Kitamura et al. (1997) have shown that the rate of cell growth recovery was increased when the level of absorbable solutes (KCl in particular) was high.
There are at least two major advantages to using inorganic ions for cell osmotic adjustment. One of them is the rapidity of turgor recovery, the other is the low energetic cost. Almost complete recovery of cell turgor, directly attributable to net ion uptake was achieved within 40 min after onset of hyperosmotic stress (Fig. 2). By comparison, all known reports on de novo synthesis of compatible solutes refer to time scale of several hours (Wyn Jones and Pritchard, 1989; Verbruggen et al., 1996) to days (Kishor et al., 1995). On the other hand, osmotically induced changes in the transcript level of some genes may occur relatively quickly. For example, an Arabidopsis MEKK kinase, ATMEKK1, which complements the osmotic stress-induced HOG pathway in yeast and causes accumulation of the osmoprotectant glycerol is rapidly (within 5 min) induced after salt stress in Arabidopsis (Covic et al., 1999). However, it will take at least another hour, or even longer, before the required amount of compatible solutes is synthesized. This is a crucial time for cell metabolism, especially if the stress is acute. Although synthesis of compatible solutes may have physiological significance for a slowly developing stress, uptake of inorganic ions is the only way to provide fast and efficient osmotic adjustment.
The other important issue to be considered is the energetics of osmotic adjustment. Generating enough organic solutes to achieve full osmotic adjustment under hyperosmotic conditions can be a costly exercise. According to Raven (1985), the difference in the ATP cost between active uptake and compartmentation of inorganic ions and synthesis of compatible solutes is approximately a factor of 10. It is reasonable to suggest, therefore, that the cheapest option (ion uptake) should be given first preference.
Both our experiments (Fig. 3) and literature data (Reid et al., 1984; Kinraide and Wyse, 1986; Reuveni et al., 1987; Kitamura et al., 1997; Teodoro et al., 1998; Zingarelli et al., 1999) suggest that osmotic stress causes rapid, significant, and prolonged hyperpolarization of plasma MP. Because the major source for generating the MP in higher plant cells is the activity of the electrogenic ATP-dependent H+ pump (Spanswick, 1981), it is not surprising that such a pump has long been considered as a potential target of osmotic stress (Rubinstein, 1982; Reinhold et al., 1984; Reuveni et al., 1987). Supporting evidence includes reports of significant osmotic-induced acidification of the bathing medium (Kinraide and Wyse, 1986; Reuveni et al., 1987; Zingarelli et al., 1999) and direct measurements of net H+ extrusion (Lew, 1998; Shabala et al., 2000). The central role of plasma membrane H+ pump in cell osmotic adjustment is also supported by experiments with specific inhibitors of ATPase activity (Oren-Shamir et al., 1990).
Regardless of whether the H+ pump might be acting as a detector or the effector (or both) in turgor maintenance (Reuveni et al., 1987), the physiological consequences of its up-regulation may be increased uptake of nutrients. Reid et al. (1984) reported 7-fold stimulation of the influxes of Cl−, K+, and Na+ caused by hyperosmotic stress in Lamprothamnium sp. Similar observations have been reported elsewhere (Reinhold et al., 1984; Srivastava et al., 1989; Kitamura et al., 1997; Teodoro et al., 1998; Zingarelli et al., 1999). The ionic mechanisms of this process, however, remain to be revealed.
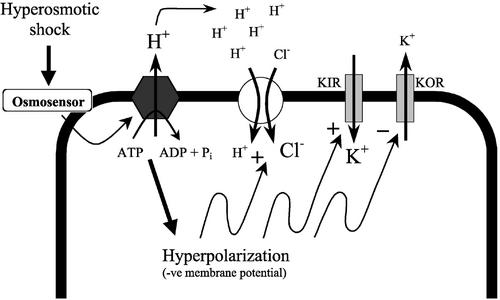
At least two possibilities should be considered. First, in this study, we provide a direct evidence that voltage clamp of the plasma membrane directly affect net K+ fluxes into and out of the cell (Fig. 4). Therefore, the significant (up to 20 mV) membrane hyperpolarization measured in our experiments (Fig. 3) and in Arabidopsis root hairs (Lew, 1996) may affect net K+ uptake via voltage-gated K+ channels (Lew, 1991; Maathuis and Amtmann, 1999). Assuming cytosolic K+ concentration in Arabidopsis root cell to be about 100 mm (Maathuis and Sanders, 1993), Ek = −175 mV for 0.1 mm KCl in the bath, the membrane hyperpolarization from −180 to −200 mV observed in our experiments (Fig. 3) may facilitate K+ influx via inward K+ channels. As an alternative, outward K+ channels may be partially shut down, reducing K+ efflux, consistent with the decreased conductance after hyperosmotic treatment reported by Lew (1996). This is further supported by experiments with radiotracers by Zingarelli et al. (1999), who reported that reduction of K+ efflux rather than stimulation of K+ influx took place in cultured Arabidopsis cells. Both these options are incorporated in our model (Fig. 5).
Figure 5.
A model illustrating pathways of fast turgor adjustment in Arabidopsis root cells. Hyperosmotic shock, sensed via an osmosensor, activates the H+-ATPase. The hyperpolarization (Lew, 1996) increases net K+ uptake thorough an inward K+ channel and concomitantly decreases K+ efflux through an outward K+ channel. Both the hyperpolarized potential and extracellular acidification increase uptake of Cl− through a H+/Cl− symporter.
Another possibility is that activation of H+ pump and resulting extrusion of H+ ions may enhance nutrient uptake via cotransport mechanism. Both H+/K+ and H+/Cl− symporters are known to be present at the plasma membrane (Felle, 1994; Maathuis and Amtmann, 1999). The increase in extracellular [H+] is expected to increase the activity of these secondary active transport mechanisms. This option is also incorporated in our model (Fig. 5).
An important feature of this model is a feedback control of MP by accumulation of ions in the cytosol. Regardless the mechanisms, when K+ uptake increases, MP will be slightly depolarized. Both membrane depolarization and increased cytosolic K+ concentration are expected to reduce further K+ uptake (a negative feedback loop). Increased uptake of Cl− (via H+/Cl− symporter), on the contrary, will lead to a further hyperpolarization due to a positive feedback loop.
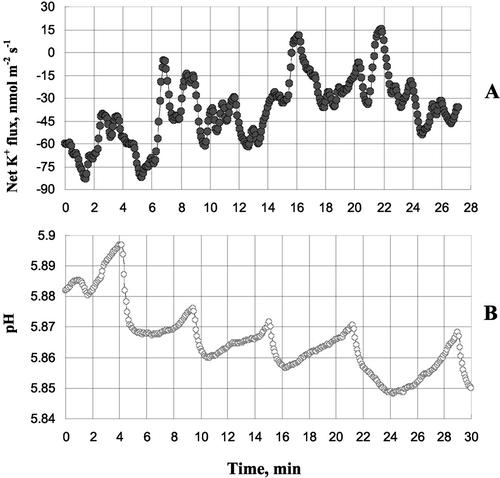
The presence of feedback loops in cell osmotic adjustment implies that that fluxes of ions should exhibit oscillatory behavior. Experimental evidence for that is given in Figure 6, where oscillations in net K+ flux (A) and external pH (due to changes in net H+ flux; B) measured 20 min after onset of hyperosmotic stress are shown. This is consistent with our previous observations from leaf mesophyll cells (Shabala et al., 2000) and some literature reports (Gradmann and Boyd, 1995). Such oscillations are expected to provide a “fine tuning” of cell osmotic potential and, therefore, contribute to the efficacy of cell osmotic adjustment.
Figure 6.
Oscillations in net K+ flux (A) and external pH (B) measured, 20 min after onset of hyperosmotic stress. Two typical examples from two individual plants are shown. Such oscillations are expected to arise from the presence of feedback loops in mechanism of cell osmotic adjustment as suggested by our model (Fig. 5).
It is still unclear how osmotic stress modulates the activity of H+ pump. A decrease in membrane tension caused by decreased turgor may directly activate the plasma membrane H+-ATPase, because the activity of this enzyme is strictly dependent on the lipid environment (Palmgren, 1991). Some authors have ruled out a direct effect of osmoticum on H+ pump activity, suggesting instead that the primary targets in the osmosensory mechanism are stretch-activated Cl− channels inactivated by hyperosmotic stress (Teodoro et al., 1998; Zingarelli et al., 1999). However, in direct experiments with oil injection into the cell, Lew (1996) found no evidence for a turgor-sensing mechanism in Arabidopsis roots. Instead, the existence of osmosensor was postulated. Unraveling the sensor/transduction pathway will require thorough and extensive study. For now, we have been able to establish the central role of ion transport during rapid turgor recovery in root calls.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Columbia wild type) seeds were surface-sterilized with commercial bleach and sown on small (35 mm diameter) petri dishes containing 5.5 mL of 1% gellan gum (ICN Biochemicals, Aurora, OH) made up in modified artificial pond water 5 (0.1 mm KCl, 2.0 mm CaCl2, 1.0 mm MgCl2 1 mm MES, and 0.2 mm Na2SO4; pH adjusted to 5.0 with NaOH). Seedlings were grown at 24°C under constant fluorescent lighting (350–400 lux). The petri dish was oriented in an upright position of about 85°, so roots grew along the surface of the gellan gum essentially without penetrating it. At the same time, roots were firmly anchored in the gum by root hairs embedded in the gum matrix. That provided an opportunity for measurements of both net fluxes from the root surface and normal access for micropipette impalement without having to disturb the roots. Five- to 7-d-old roots were used for measurements.
Ion Flux Measurements
Net fluxes of K+, Cl−, and Na+ were measured noninvasively using ion-selective vibrating microelectrodes (the MIFE technique; University of Tasmania, Hobart, Australia) as described previously (Shabala et al., 1997; Shabala and Newman, 1999). Micropipettes were pulled from borosilicate tubing and then silanized. The tips were broken to an o.d. of about 3 to 5 μm. Commercially available ionophore cocktails (Fluka catalog nos. 60031 for potassium, 24902 for chloride, and 71178 for sodium) were used to fill the tips after backfilling with 0.2 m KCl (K+ selective) or 0.5 m NaCl (Na+ and Cl− selective). The electrodes were calibrated in sets of standards before and after use. Electrodes with a response of less than 50 mV per decade were discarded. The probe excursion distance was either 15 or 25 μm, the movement frequency was 0.1 Hz, and the sampling rate was 15 Hz.
The petri dish containing 5- to 7-d-old Arabidopsis seedlings was filled with APW solution (0.1 mm KCl, 0.1 mm CaCl2, 0.1 mm MgCl2, and 0.5 mm NaCl; unbuffered pH approximately 5.6) and the seedlings were conditioned for 2 to 3 h. The solution was periodically replaced (every 20–30 min) by fresh solution to prevent formation of depletion zones and provide aeration for roots. Twenty minutes before measurements, the petri dish was transferred onto the microscope stage, and electrodes were positioned 20 μm above the root surface, with their tips separated by 2 to 3 μm and aligned parallel to the surface. All measurements were performed in the mature fully elongated zone 6 to 10 mm from the root apex.
Ion fluxes were measured in the steady state for 5 to 10 min and then the hyperosmotic treatment (100/100 mm mannitol/sorbitol made up in APW) was given. About 30 mL of solution was replaced (eight to nine times of the chamber volume), and net ion fluxes were measured for another 50 to 60 min. The time required for solution replacement and establishment of a diffusion gradient (unstirred layer) was about 3 min. This interval was later discarded from the analysis and appears as a gap in most figures.
Voltage-Clamp Experiments
Double-barreled microelectrodes were prepared using a double-pull protocol with intermediate twist as described by Lew (1991). The electrodes were backfilled with 200 mm KCl and connected by AgCl electrodes to IE-251 electrometers (input impedance 1011 Ω; Warner Instruments, Hampden, CT). Before electrodes were impaled in the root hair, the absence of cross talk was confirmed by injecting 1 nA of current through one electrode and checking for significant voltage deflections in the other electrode.
Current-voltage measurements were performed using an operational amplifier controlled by a data acquisition board (Scientific Solutions, Cleveland) via a compiled C program using the current injection capability of the electrometer. The current injected through one of electrodes was measured via the electrometer and sampled by the data acquisition board after filtering at 200 Hz with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA). Both current and voltage traces were displayed on an oscilloscope (TDS430A, Tektronix, Wilsonville, OR) and printed as a hard copy.
The experimental protocol during voltage-clamp measurements involved regular (every 2–3 min) clamping of the MP at different values (−300 to 0 mV range) for 40 to 50 s. In most cases, a bipolar staircase of voltage clamp (alternative clamps above and below the resting potential) was used, each clamp followed by 1 to 2 min of no clamping.
In voltage-clamp experiments, net ion fluxes were measured from the surface of a young root hair cell (as shown in Fig. 7B). The ion-selective K+ vibrating microelectrode was located at a distance of 4 to 6 μm from the root hair surface. The double-barreled microelectrode was impaled from the opposite side of the root hair, and the voltage (resting potential) was monitored for about 1 min. Once the reading was stable, flux measurements were commenced. Net K+ fluxes and clamping currents were averaged for each voltage-clamp treatment.
Cell Turgor Measurements
Pressure-probe measurements were performed as described by Lew (1996). The micropipette was fabricated using a double-pull method from borosilicate tubing with an internal filament. A subminiature pressure transducer (XT-140–300G, Kulite Semiconductor Products, Leonia, NJ) was housed adjacent to the pressure-probe micropipette in a small brass holder connected to a micrometer-driven piston by thick-walled teflon tubing (1.59-mm o.d. × 0.254-mm i.d.; Chromatographic Specialities, Brockville, Ontario, Canada). The piston, tubing, holder, and micropipette were filled with silicon oil (polydimethylsiloxane, Dow Corning, Midland, MN). Franks et al. (1998) used a similar system for small plant cells. It is a modification of the classic pressure probe technique (Husken et al., 1978). The flexible tubing minimizes mechanical vibration, which can damage the cell, especially during long-term measurements. As described previously (Lew, 1996), the micropipette was fabricated using a double-pull protocol to achieve a final diameter of about 1.5 μm. We used this small diameter to avoid cellular damage during impalement and, thus, improve our chances of observing turgor regulation during long-term monitoring (40–50 min) of cell turgor pressure in a single epidermal cell. In fact, after impalement with the small aperture pressure probe, cells maintain normal cytoplasmic streaming (R.R. Lew, unpublished data) and exhibit normal electrical properties (Lew, 1996). However, the small aperture (and relatively large silicone oil reservoir) slows fluid movement through the micropipette tip. The restricted flow means that pressure readings must be made after the meniscus has stopped moving (requiring about 5–10 s) to ensure that hydraulic equilibrium is achieved. Once filled, the micropipette was introduced into the APW bath solution. Capillary action caused the bath solution to enter the tip of the micropipette. This was offset by applying pressure (0.31 ± 0.06 MPa, n = 17) to bring the oil/APW meniscus to the tip of micropipette. The baseline offset was monitored for a minute or so to ensure there was no significant drift both before and after cell turgor measurements. Turgor pressure measurements are referenced to the baseline offset, that is, the baseline offset was used as “zero” pressure in cell turgor measurements (Fig. 1A). Pressure was monitored on a digital oscilloscope as voltage output from the transducer using standard electronics.
The root cells are vacuolate, and, in keeping with previous measurements in root hairs (Lew, 1996) and fluorescent dye tracers used to identify the location of micropipette tips (Lew, 2000), the micropipette tip will be localized in the vacuole upon impalement. Immediately after impalement, the oil/vacuolar sap interface moved back into the micropipette due to the cell turgor. Pressure was applied to bring the meniscus to the pipette tip. The root cell turgor pressure was then calculated from the voltage readings based on calibrations performed with pressurized air and corresponds to the cell turgor pressure.
In addition to restricted hydraulic flow, there were two additional methodological challenges: (a) the small tip makes the probe susceptible to plugging; and (b) the thick-walled teflon tubing used to minimize vibration-induced damage to the cell enlarged very slightly over time, causing the meniscus to move back from the micropipette tip. To minimize tip plugging, every 1.5 to 2 min, the meniscus was brought back from the micropipette tip (estimated as about, 20–40 μm into the micropipette) to clear the tip opening, and then pressure was reapplied immediately to reposition the meniscus at the micropipette tip (Fig. 1). The pressure changes measured by the probe during meniscus adjustment are overshoots and do not reflect pressure in the cell because of the restricted flow at the tip. During readjustment of the meniscus to the micropipette tip, there was a transient pressure spike. Relaxation was at least biphasic. A number of factors could explain multiphasic relaxation, such as restricted flow at the tip and water efflux from the cell in response to the applied pressure (Zimmerman and Steudle, 1980). The latter effect is expected to be rapid in small cells. The T1/2 for water exchange (measured with much larger aperture micropipettes, 4–8 μm) is reported to be about 2 to 9 s in corn root cells (Azaizeh et al., 1992). The slower relaxation of pressure observable in the measurements shown in Figure 1 are, at least in part, due to a slight expansion of the teflon tubing, based on the fact that they occurred concomitantly with meniscus movement back from the tip. The “true” hydrostatic pressure of the cell will fall within the pressure range bounded by the pressure peak and slow relaxation. We used the base of the rapid relaxation to measure the cell turgor pressure for the sake of consistency and reproducibility.
One additional experimental challenge of minimal impact was the inability to visually place the meniscus precisely at the micropipette tip and avoid introducing oil into the cell. The periodic meniscus adjustment during the experiments allowed us to track the meniscus location to confirm its placement near the micropipette tip. Because the meniscus could be placed within 1 to 2 μm of the tip, the effect on steady-state turgor pressure readings was very small because the volume error is extremely small and was ignored in our calculations.
Although the use of a small aperture micropipette has obvious disadvantages, we were able to consistently measure turgor pressures in long-term measurements in a single cell. Electrical properties and cytoplasmic “health” as measured by observations of cytoplasmic streaming were certainly unaffected. We did not observe injury events, such as cytoplasmic leakage from the impalement site, nor did we observe cytoplasmic debris in the miropipette bore. Steady state turgor pressure showed little variability, and the cells were not adversely affected by the periodic and rapid readjustment of the meniscus to the tip, based upon consistently stable turgor pressure readings before and turgor recovery after treatment with osmotica.
Footnotes
This work was supported by the Australian Research Council (grant to S.N.S.) and by the Canadian Natural Sciences and Engineering Research Council (grant to R.R.L.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.020005.
LITERATURE CITED
- Azaizeh H, Gunne B, Steudle E. Effects of NaCl and CaCl2 on water transport across root cells of maize (Zea mays L.) seedlings. Plant Physiol. 1992;99:886–894. doi: 10.1104/pp.99.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babourina O, Hawkins B, Lew RR, Newman I, Shabala S. K+ transport by Arabidopsis root hairs at low pH. Aust J Plant Physiol. 2001;28:635–641. [Google Scholar]
- Bajaj S, Targolli J, Liu LF, Ho THD, Wu R. Transgenic approaches to increase dehydration-stress tolerance in plants. Mol Breed. 1999;5:493–503. [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG. Adaptation to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Shen B. Transformation and compatible solutes. Sci Hortic. 1999;78:237–260. [Google Scholar]
- Bohnert HJ, Sheveleva E. Plant stress adaptation: making metabolism move. Curr Opin Plant Biol. 1998;1:267–274. doi: 10.1016/s1369-5266(98)80115-5. [DOI] [PubMed] [Google Scholar]
- Bray EA. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- Cosgrove DJ. Expansive growth of plant cells. Plant Physiol Biochem. 2000;38:109–124. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Covic L, Silva NF, Lew RR. Functional characterization of ARAKIN (ATMEKK1): a possible mediator in an osmotic stress response pathway in higher plants. Biochim Biophys Acta. 1999;1451:242–254. doi: 10.1016/s0167-4889(99)00096-8. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Bohnert HJ. Genomic approaches to plant stress tolerance. Curr Opin Plant Biol. 2000;3:117–124. doi: 10.1016/s1369-5266(99)00052-7. [DOI] [PubMed] [Google Scholar]
- Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;4:215–223. [Google Scholar]
- Felle HH. The H+/Cl− symporter in root-hair cells of Synapis alba. Plant Physiol. 1994;106:1131–1136. doi: 10.1104/pp.106.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Cowan IR, Tyerman SD, Cleary AL, Lloyd J, Farquhar GD. Guard cell pressure/aperture characteristics measured with the pressure probe. Plant Cell Environ. 1998;18:795–800. [Google Scholar]
- Frensch J, Hsiao TC. Transient responses of cell turgor and growth of maize roots as affected by changes in water potential. Plant Physiol. 1994;104:247–254. doi: 10.1104/pp.104.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradmann D, Boyd CM. Membrane voltage of marine phytoplankton, measured in the diatom Coscinodiscus radiatus. Mar Biol. 1995;123:645–650. [Google Scholar]
- Hsiao TC. Leaf and root growth in relation to water status. HortScience. 2000;35:1051–1058. [Google Scholar]
- Husken D, Steudle E, Zimmerman U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978;61:158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Nakamura Y, Kawata H, Yanada T, Ohta E, Sakata M. Effect of osmotic stress on turgor pressure in mung bean root cells. Plant Cell Physiol. 1987;28:987–994. [Google Scholar]
- Kinraide TB, Wyse RE. Electrical evidence for turgor regulation of proton extrusion in sugar beet taproot. Plant Physiol. 1986;82:1148–1150. doi: 10.1104/pp.82.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor PBK, Hong Z, Miao G-H, Hu C-AA, Verma DPS. Overexpression of delta'-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Mizuno A, Katou K. Adaptive growth responses to osmotic stress of hypocotyl sections of Vigna unguiculata: roles of the xylem proton pump and IAA. Plant Cell Physiol. 1997;38:44–50. [Google Scholar]
- Kramer PJ, Boyer JS. Water Relations in Plants. San Diego: Academic Press; 1995. [Google Scholar]
- Kuzmanoff KM, Evans ML. Kinetics of adaptation to osmotic stress in lentil (Lens culinaris Med.) roots. Plant Physiol. 1981;68:244–247. doi: 10.1104/pp.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RR. Electrogenic transport properties of growing Arabidopsis root hairs. Plant Physiol. 1991;97:1527–1534. doi: 10.1104/pp.97.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RR. Pressure regulation of the electrical properties of growing Arabidopsis thaliana L. root hairs. Plant Physiol. 1996;112:1089–1100. doi: 10.1104/pp.112.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RR. Immediate and steady state extracellular ionic fluxes of growing Arabidopsis thaliana root hairs under hyperosmotic and hypoosmotic conditions. Physiol Plant. 1998;104:397–404. [Google Scholar]
- Lew RR. Electrobiology of root hairs. In: Ridge RW, Emons AMC, editors. Root Hairs. Cell and Molecular Biology. Tokyo: Springer; 2000. pp. 115–139. [Google Scholar]
- Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot. 1999;84:123–133. [Google Scholar]
- Maathuis FJM, Sanders D. Energization of potassium uptake in Arabidopsis thaliana. Planta. 1993;191:302–307. [Google Scholar]
- Nuccio ML, Rhodes D, McNeil SD, Hanson AD. Metabolic engineering of plants for osmotic stress resistance. Curr Opin Plant Biol. 1999;2:128–134. doi: 10.1016/s1369-5266(99)80026-0. [DOI] [PubMed] [Google Scholar]
- Oren-Shamir M, Pick U, Avron M. Plasma membrane potential of the alga Dunaliella, and its relation to osmoregulation. Plant Physiol. 1990;93:403–408. doi: 10.1104/pp.93.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG. Regulation of plant plasma membrane H+-ATPase activity. Physiol Plant. 1991;83:314–323. [Google Scholar]
- Pritchard J, Fricke W, Tomos D. Turgor-regulation during extension growth and osmotic stress of maize roots: an example of single-cell mapping. Plant Soil. 1996;187:11–21. [Google Scholar]
- Raven JA. Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 1985;101:25–77. doi: 10.1111/j.1469-8137.1985.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Reid RJ, Jefferies RL, Pitman MG. Lamprothamnium, a euryhaline charophyte. IV. Membrane potential, ionic fluxes and metabolic activity during turgor adjustment. J Exp Bot. 1984;35:925–937. [Google Scholar]
- Reinhold L, Seiden A, Volokita M. Is modulation of the rate of proton pumping a key event in osmoregulation? Plant Physiol. 1984;75:846–849. doi: 10.1104/pp.75.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M, Colombo R, Lerner HR, Pradet A, Poljakoff-Mayber A. Osmotically induced proton extrusion from carrot cells in suspension culture. Plant Physiol. 1987;85:383–388. doi: 10.1104/pp.85.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C, Bollés JM, Vayá JL, Serrano R, Culiañez-Maciá FA. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta. 1997;201:293–297. doi: 10.1007/s004250050069. [DOI] [PubMed] [Google Scholar]
- Rubinstein B. Regulation of H+ excretion: effect of osmotic shock. Plant Physiol. 1982;69:939–944. doi: 10.1104/pp.69.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R, Culiañez-Maciá FA, Moreno V. Genetic engineering of salt and drought tolerance with yeast regulatory genes. Sci Hortic. 1999a;78:261–269. [Google Scholar]
- Serrano R, Mulet JM, Rios G, Marquez JA, de Larrinoa I, Leube MP, Mendizabal I, Pascual-Ahuir A, Proft M, Ros R et al. A glimpse of the mechanisms of ion homeostasis during salt stress. J Exp Bot. 1999b;50:1023–1036. [Google Scholar]
- Shabala S, Babourina O, Newman I. Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot. 2000;51:1243–1253. [PubMed] [Google Scholar]
- Shabala SN, Newman IA. Osmotic sensitivity of Ca2+ and H+ transporters in corn roots: effect on fluxes and their oscillations in the elongation region. J Membr Biol. 1998;161:45–54. doi: 10.1007/s002329900313. [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA. Light-induced changes in hydrogen, calcium, potassium, and chloride fluxes and concentrations from the mesophyll and epidermal tissues of bean leaves: understanding the ionic basis of light-induced bioelectrogenesis. Plant Physiol. 1999;119:1115–1124. doi: 10.1104/pp.119.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. Plant resistance to environmental stress. Curr Opin Biotechnol. 1998;9:214–219. doi: 10.1016/s0958-1669(98)80118-3. [DOI] [PubMed] [Google Scholar]
- Spanswick RM. Electrogenic ion pumps. Annu Rev Plant Physiol. 1981;32:267–289. [Google Scholar]
- Srivastava A, Pines M, Jacoy B. Enhanced potassium uptake and phosphatidylinositol-phosphate turnover by hypertonic mannitol shock. Physiol Plant. 1989;77:320–325. [Google Scholar]
- Teodoro AE, Zingarelli L, Lado P. Early changes in Cl− efflux and H+ extrusion induced by osmotic stress in Arabidopsis thaliana cells. Physiol Plant. 1998;102:29–37. doi: 10.1034/j.1399-3054.1998.1020105.x. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Skerrett IM. Root ion channels and salinity. Sci Hortic. 1999;78:175–235. [Google Scholar]
- Verbruggen N, Hua X-J, May M, Van Montagu M. Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proc Natl Acad Sci USA. 1996;93:8787–8791. doi: 10.1073/pnas.93.16.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicov I. New molecular approaches to improving salt tolerance in crop plants. Ann Bot. 1998;82:703–710. [Google Scholar]
- Wyn Jones RG, Pritchard J. Stresses, membranes and cell walls. In: Jones HG, Flowers TJ, Jones MB, editors. Plants under Stress: Biochemistry, Physiology, and Ecology and Their Application to Plant Improvement. Cambridge, UK: Cambridge University Press; 1989. , 95–114. [Google Scholar]
- Zimmerman U, Steudle E. Fundamental water relations parameters. In: Spanswick RM, Lucas WJ, Danty J, editors. Plant Membrane Transport: Current Conceptual Issues. Amsterdam: Elsevier/North-Holland Biomedical Press; 1980. pp. 113–127. [Google Scholar]
- Zingarelli L, Marrè MT, Massardi F, Lado P. Effect of hyper-osmotic stress on K+ fluxes, H+ extrusion, transmembrane electric potential difference and comparison with the effects of fusicoccin. Physiol Plant. 1999;106:287–295. [Google Scholar]