Abstract
Evidence indicates that prostanoids, such as prostaglandins, play a regulatory role in several forms of neural plasticity, including long-term potentiation, a cellular model for certain forms of learning and memory. In these experiments, the significance of the COX isoforms cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) in post-training memory processes was assessed. Adult male Long-Evans rats underwent an eight-trial (30-sec intertrial interval) training session on a hippocampus-dependent (hidden platform) or dorsal striatal–dependent (visible platform) tasks in a water maze. After the completion of training, rats received an intraperitoneal injection of the nonselective COX inhibitor indomethacin, the COX-1–specific inhibitor piroxicam, the COX-2–specific inhibitor N-[2-cyclohexyloxy-4-nitrophenyl]-methanesulfonamide (NS-398), vehicle (45% 2-hydroxypropyl-β-cyclodextrin in distilled water), or saline. On a two-trial retention test session 24 h later, latency to mount the escape platform was used as a measure of memory. In the hidden platform task, the retention test escape latencies of rats administered indomethacin (5 and 10 mg/kg) or NS-398 (2 and 5 mg/kg) were significantly higher than those of vehicle-treated rats, indicating an impairment in retention. Injections of indomethacin or NS-398 that were delayed 2 h post-training had no effect on retention. Post-training indomethacin or NS-398 had no influence on retention of the visible platform version of the water maze at any of the doses administered. Furthermore, selective inhibition of COX-1 via post-training piroxicam administration had no effect on retention of either task. These findings indicate that COX-2 is a required biochemical component mediating the consolidation of hippocampal-dependent memory.
Prostaglandins (PGs) and thromboxanes (TXs), collectively known as prostanoids, are metabolites of arachidonic acid (AA) synthesized and released by most cell types (for review, see Needleman et al. 1986). Although phospholipases initiate prostanoid synthesis by liberating arachidonic acid (5-8-11-14-eicosatetraenoic acid) from membrane fatty acids (for review, see Smith et al. 1991), cyclooxygenase (COX; prostaglandin H synthetase; prostaglandin-endoperoxide synthase; EC 1.14.99.1) enzymes catalyze the first two committed steps in the biosynthesis of prostanoids. These steps include the oxidation of AA to the hydroperoxy endoperoxide PGG2 and its subsequent reduction to the hydroxy endoperoxide PGH2. PGH2 is then transformed by a variety of enzymes and nonenzymatic mechanisms into the primary prostanoids—PGE2, PGD2, PGF2α, PGI2 (prostacyclin)—and thromboxane A2 (Vane et al. 1998).
Two COX isoforms, cyclooxygenase-1 (COX-1; DeWitt and Smith 1988) and cyclooxygenase-2 (COX-2; Kujubu et al. 1991; Xie et al. 1991), have thus far been identified. COX-2 is often referred to as the inducible isoform of COX, as levels of COX-2 increase in response to several forms of stimulation in various types of tissue (for review, see Vane et al. 1998). In contrast, the constitutive form of COX, COX-1, appears to be involved in housekeeping cellular functions (for reviews, see Smith et al. 1991; Herschmann 1996). Although COX-2 is undetectable in most tissues under basal conditions, marked basal expression has been observed in the dendrites and cell bodies of neurons in the central nervous system (Yamagata et al. 1993; Breder et al. 1995; Kaufman et al. 1996; Teather 1998), indicating a role for COX-2 in cell signaling (Kaufman et al. 1996). Both COX isoforms are present in discrete areas of the mammalian brain (Breder et al. 1992, 1995; Teather 1998), often in species- and developmental stage–dependent patterns. The functional significance of these enzymes in the mammalian central nervous system is now being actively explored, particularly in pathological situations.
Several lines of evidence indicate a potential role for COX in the physiological mechanisms underlying memory formation. First, nonspecific COX inhibitors impair passive avoidance memory in chicks and prevent the learning-induced increase in PG release, which occurs 2 h after training (Holscher 1995). Second, COX-2 is expressed in neurons as an immediate early gene (Yamagata et al. 1993) in an NMDA receptor–dependent manner (Yamagata et al. 1993; Lazarewicz and Salinska 1995). This is of particular interest in view of evidence indicating a role for NMDA receptors in memory (Mondadori et al. 1989; Morris et al. 1990; Packard and Teather, 1997a,b). A role for COX in information processing has also been previously indicated based on the expression patterns of COX-2, which is predominantly localized in the amygdala, cortex, and hippocampus (Breder et al. 1992, 1995).
The present study investigated the possible significance of the COX enzymes in two distinct forms of mammalian memory. A distinction between the neural substrates that mediate cognitive memory and those that mediate stimulus-response (S-R) habit formation has been previously proposed (Hirsh 1974; Mishkin and Petri 1984; Packard et al. 1989). Evidence from double dissociation experiments involving lesion and intracerebral post-training drug injections indicates that the hippocampal system and dorsal striatum are parts of independent memory systems that may selectively mediate cognitive memory and S-R habit formation, respectively (Packard et al. 1989; Packard and McGaugh 1992, 1996; McDonald and White 1993, 1994; Packard and Teather 1997a, 1998). To assess the significance of COX-1 and COX-2 in these two types of memory, indomethacin (a nonselective COX inhibitor), N-[2-cyclohexyloxy-4-nitrophenyl&rdqb;-methanesulfonamide, (NS-398; a COX-2 selective inhibitor), and piroxicam (a COX-1 selective inhibitor; Mitchell et al. 1993) were administered after training to rats trained in either a hidden platform or visible platform water maze task, and retention was assessed 24 h later. In the hippocampus-dependent task (Morris et al. 1982), rats learned to swim to a hidden escape platform located in the same location on each trial, and this task is presumed to involve the acquisition of relational spatial information. In the dorsal striatum–dependent task (Packard and McGaugh 1992; McDonald and White 1994), rats learned to swim to a visibly cued platform that is moved to a new spatial location each trial, and this task may involved the acquisition of a S-R (visible platform–approach) habit.
RESULTS
Peripheral Administration of 45% HBC
A 45% distilled water-hydroxypropyl-β-cyclodextrin (HBC) solution was used to aid drug solubilization. HBC is a nontoxic solubilizer with a hydrophilic character that prevents penetration of HBC into the gastrointestinal tract, whereas the complexed drug is readily absorbed (Pitha 1985, 1989). In pilot work, we compared the effects of post-training administration of the water-HBC vehicle and physiological saline, and consistent with our previously published research (Packard and Teather 1997b), we did not observe any behavioral difference between saline-treated rats and the animals administered the 45% HBC–distilled water vehicle. Only the groups injected with HBC-distilled water vehicle were included for further analyses.
Effect of Post-Training Indomethacin on Retention in the Hidden Platform Task
A two-way one-repeated-measure ANOVA computed on the escape latencies on the training day revealed no significant group differences [F(4, 33) = 0.478, P = NS]. A significant trial effect [F(4, 7) = 67.67, P < 0.0001] indicated that the rate of task acquisition was similar in all groups. The performance of all groups over the eight hidden platform training trials reached asymptotic performance of 8 to 14 sec (data not shown). These findings indicate that any differences observed in retention test performance among the treatment groups were not caused by differential rates of task acquisition.
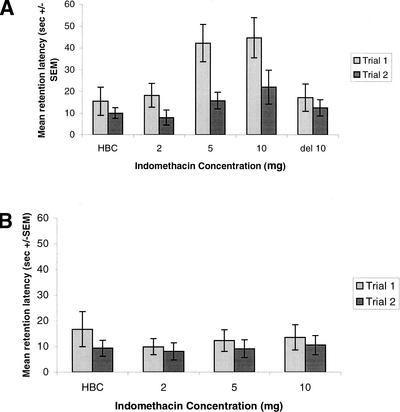
The effect of post-training administration of indomethacin on retention in the hidden platform task is shown in Figure 1A. A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a significant group × trial interaction, [F(3, 1) = 3.1, P < 0.05], a significant group effect [F(3, 27) = 3.17, P < 0.05], and a significant trial effect [F(1, 3) = 13.634, P < 0.001]. Scheffe post-hoc tests showed that the latencies of rats receiving indomethacin at doses of 5 mg/kg (P < 0.05) and 10 mg/kg (P < 0.05) were significantly higher than those of the saline-treated rats on trial 1, indicating an impairment in memory.
Figure 1.
Effect of post-training injections of indomethacin on memory in a hidden (A) and a visible (B) platform water maze task. Retention test escape latencies (seconds ± SEM)
The retention test escape latencies of rats that received injections of indomethacin (10 mg/kg) delayed 2 h post-training were not significantly different than those of vehicle-treated rats (Fig. 1A). A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a nonsignificant group × trial interaction [F(1, 1) = 3.2, P = NS] and a nonsignificant group effect [F(1, 3) = 0.258, P = NS]. A significant trial effect was observed [F(1, 1) = 5.071, P < 0.05], indicating improvement over the two testing trials.
Effect of Post-Training Indomethacin on Retention in the Visible Platform Task
A two-way one-repeated-measure ANOVA computed on the escape latencies on the training day revealed no significant group differences [F(3, 27) = 2.162, P = NS]. A significant trial effect [F(3, 7) = 52.48, P < 0.0001] indicated that the rate of task acquisition was similar in all groups. The performance of all groups over eight training trials in the visible platform task reached asymptotic performance of 9 to 16 sec (data not shown).
The effect of post-training administration of indomethacin on retention in the visible platform task is shown in Figure 1B. A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a nonsignificant group × trial interaction [F(1, 3) = 0.165, P = NS], a nonsignificant group effect [F(3, 28) = 0.331, P = NS], and a nonsignificant trial effect [F(1, 3) = 0.163, P < 0.001], indicating that post-training injections of indomethacin have no influence on retention in the visible platform task.
Effect of Post-Training Piroxicam on Retention in the Hidden Platform Task
A two-way one-repeated-measure ANOVA computed on the escape latencies on the training day revealed no significant group differences [F(3, 27) = 0.647, P = NS]. A significant trial effect [F(3, 7) = 77.86, P < 0.01] indicated that the rate of task acquisition was similar in all groups. The performance of all groups over eight training trials in the hidden platform task reached asymptotic performance of 9 to 18 sec (data not shown).
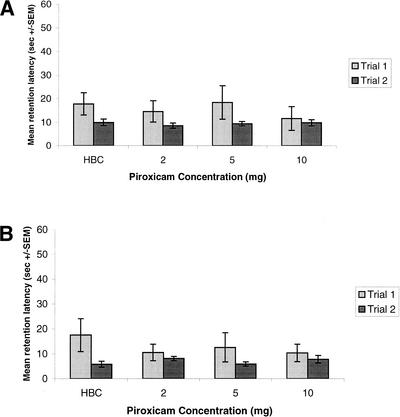
The effect of post-training administration of piroxicam on retention in the hidden platform task is shown in Figure 2A. A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a nonsignificant group × trial interaction [F(1, 3) = 0.426, P = NS] and a nonsignificant group effect [F(3, 27) = 0.131, P = NS], indicating that post-training injections of the COX-1 inhibitor piroxicam have no influence on retention in the hidden platform task.
Figure 2.
Effect of post-training injections of piroxicam on memory in a hidden (A) and a visible (B) platform water maze task. Retention test escape latencies (seconds ± SEM)
Effect of Post-Training Piroxicam on Retention in the Visible Platform Task
A two-way one-repeated-measure ANOVA computed on the escape latencies on the training day revealed no significant group differences [F(3, 28) = 1.03, P = NS]. A significant trial effect [F(3, 7) = 68.51, P < 0.001] indicated that the rate of task acquisition was similar in all groups. The performance of all groups over eight training trials in the visible platform task reached asymptotic performance of 8 to 12 sec (data not shown).
The effect of post-training administration of piroxicam on retention in the visible platform task is shown in Figure 2B. A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a nonsignificant group × trial interaction [F(1, 3) = 1.4, P = NS] and a nonsignificant group effect [F(3, 28) = 0.311, P = NS], indicating that post-training injections of piroxicam have no influence on retention in the visible platform task.
Effect of Post-Training NS-398 on Retention in the Hidden Platform Task
A two-way one-repeated-measure ANOVA computed on the escape latencies on the training day revealed no significant group differences [F(4, 33) = 0.478, P = NS]. A significant trial effect [F(4, 7) = 67.67, P < 0.0001] indicated that the rate of task acquisition was similar in all groups. The performance of all groups over eight training trials in the hidden platform task reached asymptotic performance of 9 to 17 sec (data not shown).
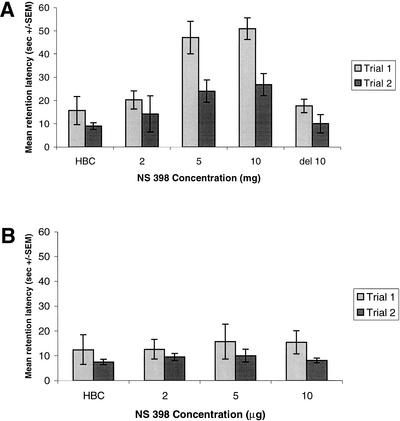
A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a significant group × trial interaction [F(5, 31) = 8.91, P < 0.05], a significant group effect [F(4, 34) = 5.41, P < 0.002], and a significant trial effect [F(1, 4) = 20.172, P < 0.0001]. Tests of simple main effects (group within trial) revealed a significant group effect on retention test trial 1 [F(4, 1) = 2.78, P < 0.001]. Scheffe post-hoc tests showed that the latencies of rats receiving NS-398 at doses of 2 mg/kg (P < 0.05) and 5 mg/kg (P < 0.05) were significantly higher than those of the saline-treated rats on trial 1, indicating an impairment in memory (Fig. 3A).
Figure 3.
Effect of post-training injections of NS-398 on memory in a hidden (A) and a visible (B) platform water maze task. Retention test escape latencies (seconds ± SEM)
The test escape latencies of rats that received injections of NS-398 (5 mg/kg) delayed 2 h post-training were not significantly different than those of vehicle-treated rats (Fig. 3A). A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a nonsignificant group × trial interaction [F(1, 1) = 0.013, P = NS] and a nonsignificant group effect [F(1, 3) = 0.076, P = NS].
Effect of Post-Training NS-398 on Retention in the Visible Platform Task
A two-way one-repeated-measure ANOVA computed on the escape latencies on the training day revealed no significant group differences [F(3, 28) = 0.81, P = NS]. A significant trial effect [F(3, 7) = 64.9, P < 0.001] indicated that the rate of task acquisition was similar in all groups. The performance of all groups over eight training trials in the visible platform task reached asymptotic performance of 7 to 13 sec (data not shown).
The effect of post-training administration of NS-398 on retention in the visible platform task is shown in Figure 3B. A two-way one-repeated-measure ANOVA computed on the escape latencies revealed a nonsignificant group × trial interaction [F(1, 3) = 3.549, P = NS] and a nonsignificant group effect [F(3, 28) = 0.084, P = NS], indicating that post-training injections of NS-398 have no influence on retention in the visible platform task.
DISCUSSION
Effects of Post-Training COX Inhibition on Memory
Post-training peripheral injection of the nonselective COX inhibitor indomethacin and the COX-2–specific inhibitor NS-398, but not the COX-1–specific inhibitor piroxicam, impaired memory in a hidden platform water maze task. Previous findings indicate that COX inhibition attenuates learning-induced induction of COX-2 and impairs memory in chicks trained in a passive avoidance task (Holscher 1995). The present findings confirm a role for COX-2 in mammalian memory formation. In cells and purified enzyme studies, indomethacin has been shown to be a nonselective COX inhibitor (Mitchell et al. 1993), piroxicam has been shown to be selective for COX-1 (Laneuville et al. 1884), and NS-398 has been shown to be selective for COX-2 (Futaki et al. 1994; Masferrer et al. 1994). Although the present findings do not rule out a role for COX-1 in memory processes, the differences observed between the behavioral effects of the various COX inhibitors indicate that these isozymes may not play equivalent roles and that COX-2 is the primary isozyme required for memory consolidation. It should be noted that in the present task, the injections of COX-inhibitors occurred ∼12 to 15 min after the initiation of training (i.e., after the eighth trial in the training session). Therefore, it is conceivable that a role for COX-1 in memory may be present at an earlier time point in the memory formation process. The use of one-trial learning tasks in which the time between initial training and drug administration is shorter (e.g., inhibitory avoidance) may be useful for addressing this possibility.
Injections of indomethacin and NS-398 delayed until 2 h after training did not affect retention, consistent with the hypothesis that the treatments influenced a memory consolidation process (McGaugh 1966, 1973, 1989). Moreover, the time-dependent nature of the treatments indicates that impairing effects of the immediate post-training injections were not caused by a proactive effect of these drugs on nonmnemonic sensory, motivational, attentional, or motor (e.g., swim speed) processes.
In contrast to the impairing effects of COX inhibition in a hidden platform task, post-training administration of indomethacin or NS-398 did not affect memory in a visible platform water maze task. The hidden and visible platform tasks have similar motivational, motoric, and sensory characteristics, indicating that the specific impairment in the hidden platform task is caused by a mnemonic effect of COX-2 inhibition. Acquisition of the hidden platform water maze task is mediated by the hippocampal system (Morris et al. 1982), whereas acquisition of the visible platform task is mediated by a separate memory system that includes the dorsal striatum (Packard and McGaugh 1992; McDonald and White 1994; Packard and Teather 1998; Packard 2001). Therefore, the present findings indicate a selective role for COX function in consolidation of hippocampus-dependent cognitive (Hirsh 1974; Mishkin and Petri 1984) memory. Consistent with this suggestion, COX-2 is expressed at relatively high basal levels in the hippocampus (Yamagata et al. 1993; Breder et al. 1995; Teather 1998). Moreover, COX-2 is constitutively expressed at relatively low levels in the striatum (Yamagata et al. 1993; Breder et al. 1995; Teather 1998), which may in part underlie the present finding that COX-2 inhibition did not affect memory in a striatal-dependent task. A dissociation in COX-2 function in the striatum and hippocampus (and cortex) was also suggested by findings that indicate that peripheral NS-398 attenuated postischemic damage in the cortex and hippocampus, yet had no neuroprotective effect in the striatum (Nogawa et al. 1997).
Post-training administration of the COX-1–specific inhibitor piroxicam (three doses) had no effect on retention of either water maze task, indicating that COX-1 does not play a mnemonic role in either hippocampus-dependent cognitive memory or dorsal striatal–dependent S-R habit formation. As COX-1 appears to perform housekeeping cellular functions rather than activity-dependent or plasticity-related functions (Herschman 1996), the observed lack of effect on memory is perhaps not surprising.
Possible Mechanisms of the Impairing Effect of COX-2 Inhibition on Memory
The present study does not reveal the mechanism(s) by which COX-2 inhibition impairs memory. However, consideration of biochemical and behavioral data from previous studies that examine the roles of various intermediaries in the prostanoid cascade indicates some possibilities. One potential pathway would initially involve the activation of glutamatergic NMDA receptors. An increased level of intracellular calcium after NMDA receptor activation causes a translocation of cPLA2 to the membrane, where it hydrolyzes membrane phospholipids, producing AA (Dumuis et al. 1988; Lazarewicz and Salinska 1995). AA serves as the precursor for prostanoid production, the initial reactions of which are catalyzed by COX. PGH2 is an intermediate formed by the action of COX-2 (or COX-1) and is converted to biologically active prostanoids by specific prostaglandin and thromboxane synthases (for review, see Coleman et al. 1994). Newly formed prostanoids exit the cell via prostaglandin transporters and activate specific G protein–coupled receptors present on the plasma membrane (for review, see Coleman et al. 1994). Prostaglandins are important modulators of adrenergic, noradrenergic (Partington et al. 1980), and glutamatergic (Kimura et al. 1985) neurotransmission.
Neurobehavioral studies support a potential role for various portions of this pathway in memory. For example, extensive evidence indicates a role for NMDA receptor function in memory (Mondadori et al. 1989; Robinson et al. 1989; Packard, and Teather, 1997a,b), and we have previously observed that post-training intrahippocampal infusions of the NMDA receptor antagonist AP5 impairs memory in a hidden platform water maze task (Packard and Teather 1997a). An increase in AA is observed in chick brain slices after training in a passive avoidance task (Holscher et al. 1995), and blockade of PLA2-dependent AA release impairs memory in chicks trained in a passive avoidance task (Holscher and Rose 1994) and in rats trained in a hidden platform water maze task (Holscher et al. 1995). Modulation of adrenergic, noradrenergic, and glutamatergic neurotransmission by prostaglandins could conceivably influence memory processes, as extensive evidence indicates a role for each of these neurotransmitters in memory storage (for review, see McGaugh 1989). In addition to modulating neurotransmission, prostaglandins are involved in the rapid remodeling of actin in the cytoskeleton, and thus can influence the shape of spines and dendrites indicating a potential role for prostaglandins in morphological changes that could conceivably influence synaptic efficiency and information storage.
Finally, in addition to producing AA, cPLA 2 also hydrolyzes membrane phospholipids to produce intracellular platelet activating factor (PAF), a potent bioactive phospholipid (for review, see Bazan et al. 1997). PAF is a putative retrograde messenger in hippocampal long-term potentiation (Clark et al. 1994), and extensive evidence from studies using post-training intracerebral infusions of PAF and PAF receptor antagonists indicates a role for PAF in memory storage processes (Jersusalinsky et al. 1994; Izquierdo et al. 1995; Packard et al. 1996; Teather 1998; Teather et al. 2001). It is of interest to note that PAF has a regulatory effect on the level of COX-2 transcription (Bazan et al. 1994), indicating a possible interaction between intracellular PAF function and prostaglandin pathways in memory. Further research is necessary to reveal the precise mechanism(s) mediating the impairing effects of COX-2 inhibition on memory.
MATERIALS AND METHODS
Subjects
Subjects were 203 male Long-Evans rats (330 to 420 g) from Charles River Breeding Laboratories (Wilmington, MA). Animals were individually housed in a temperature-controlled 12-h light-dark cycle (lights on 7 a.m. to 7 p.m.). Animals were given ad libitum access to food and water.
Apparatus
The water maze was a black circular tank 6 ft (1.83 cm) in diameter and 1.5 ft (0.55 cm) in height. The tank was filled with water (25°C ± 2°C) to a depth of 20 cm and was located in a well-lit room containing several extramaze cues. Four starting positions (north, south, east, west) were spaced around the perimeter of the tank, dividing the pool into four equal quadrants. The rectangular Plexiglas escape platform used for the spatial task (11 × 14 × 19 cm) was submerged at a depth of 1 cm. For the visible platform version of the water maze, a white rubber ball (8 cm in diameter) was attached to the top of the submerged platform and protruded above the water surface. The platform could be used as a step to mount the ball to escape the water.
Drugs
Indomethacin, NS-398, and piroxicam (all purchased from Biomol) were dissolved in 45% HBC in distilled water (Research Biochemicals International.). HBC is a nontoxic solubilizer with a hydrophilic character that prevents penetration of HBC into the gastrointestinal tract, whereas the complexed drug is readily absorbed (Pitha 1989). Intraperitoneal injections had constant injection volumes of 1 mL/kg. All solutions were prepared the day of the injections.
Behavioral Procedures
Hidden Platform Water Maze Task
The behavioral procedures were identical to those previously described (Packard and McGaugh 1994). Rats received one training session consisting of eight trials (i.e., swims). On each trial, the animal was placed into the tank facing the wall at one of four designated start points (north, south, east, and west) and was allowed to escape onto the hidden platform. The submerged platform was located in the same quadrant on every trial. A different starting point was used on each trial such that each starting point was used twice within the eight trials. If an animal did not escape within 60 sec, it was manually guided to the escape platform by the experimenter. After mounting the platform, rats remained on the platform for 20 sec. After each trial, animals were removed from the maze and placed in a holding cage for a 30-sec intertrial interval. The latency to mount the escape platform was recorded and used as a measure of task acquisition. Rats were randomly assigned to treatment groups and were given their respective injections immediately after training (i.e., after the eight trials were completed). Retention was tested 24 h after the completion of training. The submerged escape platform was located in the same quadrant of the maze as it was during training. Latency to mount the escape platform was recorded for two retention test trials and used as a measure of memory for the training session of the previous day.
Additional groups of rats received delayed post-training injections to control for possible nonmnemonic effects of the immediate injections on retention, such as effects on motivational, sensory, or motor processes (McGaugh 1966, 1973, 1989). Doses used for the delayed injections were selected after examining the effectiveness of the immediate post-training injections.
Visible Platform Water Maze Task
The behavioral procedures were identical to those previously described (Packard and McGaugh 1994). Rats received one training session consisting of eight trials (i.e., swims). On each trial, the animal was placed into the tank facing the wall at one of four designated start points (north, south, east, and west) and was allowed to escape onto the visibly cued platform. A different starting point was used on each trial such that each starting point was used twice within the eight trials. If an animal did not escape within 60 sec, it was manually guided to the escape platform by the experimenter. After mounting the platform, rats remained on the platform for 20 sec. After each trial, animals were removed from the maze and placed in a holding cage for a 30-sec intertrial interval. The latency to mount the escape platform was recorded and used as a measure of task acquisition.
The visible escape platform was placed in a different quadrant on each trial such that each of the four quadrants contained the escape platform on two of the eight trials. The locations of the start points were arranged so that the distance to the platform (i.e., proximal or distal) and location of the platform relative to the start point (i.e., left or right) were counterbalanced across the eight trials. Rats were randomly assigned to treatment groups and were given their respective injections immediately post-training (i.e., after the eight trials were completed). Retention was tested 24 h after the completion of training. The visible escape platform was located in a different quadrant of the maze for each test trial. Latency to mount the escape platform was recorded for two retention test trials and used as a measure of memory for the training session of the previous day.
Acknowledgments
Research was supported by National Institutes of Health grants NS 23002 (N.G.B.) and 1R2956973 (M.P.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL lteather@mit.edu; FAX (617) 253-6882.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.43602.
REFERENCES
- Bazan NG, Fletcher BS, Herschman HR, Mukherjee PK. Platelet-activating factor and retinoic acid synergistically activate the inducible prostaglandin synthase gene. Proc Natl Acad Sci. 1994;91:5252–5256. doi: 10.1073/pnas.91.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Packard MG, Teather LA, Allan G. Bioactive lipids in excitatory neurotransmission and neuronal plasticity. Neurochem Int. 1997;30:225–231. doi: 10.1016/s0197-0186(96)00020-4. [DOI] [PubMed] [Google Scholar]
- Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper CB. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol. 1992;322:409–438. doi: 10.1002/cne.903220309. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GD, Happel LT, Zorumski CF, Bazan NG. The role of platelet-activating factor in the release of excitotoxic neurotransmitters. J Lipid Mediators and Cell Signaling. 1994;10:95–97. [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: Properties, distribution and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Dewitt D, Smith WL. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci. 1988;85:1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Haynes L, Pin JP, Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: A theory. Behav Biol. 1974;12:421–442. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Holscher C. Inhibitors of cyclooxygenase produce amnesia for a passive avoidance task in the chick. Eur J Neurosci. 1995;7:1360–1365. doi: 10.1111/j.1460-9568.1995.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Holscher C, Rose SPR. Inhibitors of phospholipase A2 produce amnesia for a passive avoidance task in the chick. Behav Neural Biol. 1994;61:225–232. doi: 10.1016/s0163-1047(05)80005-6. [DOI] [PubMed] [Google Scholar]
- Holscher C, Canevari L, Richter-Levin G. Inhibitors of PLA2 and NO synthase cooperate in producing amnesia of a spatial task. Neuroreport. 1995;6:730–732. doi: 10.1097/00001756-199503270-00006. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Fin C, Schmitz PK, Da Silva R, Jerusalinsky D, Quillfeldt JA, Ferreiara MBC, Medina JH, Bazan NG. Memory enhancement by intrahippocampal, intraamygdala, or intraentorhinal infusion of platelet-activating factor measured in an inhibitory avoidance task. Proc Natl Acad Sci. 1995;92:5047–5051. doi: 10.1073/pnas.92.11.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerusalinsky D, Fin C, Quillfeldt JA, Ferreiara MBC, Schmitz PK, Da Silva R, Walz R, Bazan NG, Medina JH, Izquierdo I. Effect of antagonists of platelet-activating factor receptors on memory of inhibitory avoidance in rats. Behav Neural Biol. 1994;62:1–3. doi: 10.1016/s0163-1047(05)80052-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Okamoto K, Sakai Y. Modulatory effects of prostaglandin D2, E2, and F2α on the postsynaptic actions of inhibitory and excitatory amino acids in cerebellar Purkinje cell dendrites in vitro. Brain Res. 1985;330:235–244. doi: 10.1016/0006-8993(85)90682-1. [DOI] [PubMed] [Google Scholar]
- Kujubu DA, Bradley S, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promotor-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- Lazarewicz R, Salinska E. N-methyl-D-aspartate-evoked release of cyclo-oxygenase products in rabbit hippocampus: An in vivo microdialysis study. J Neuorsci Res. 1995;40:660–666. doi: 10.1002/jnr.490400511. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- ————— Parallel information processing in the water maze: Evidence for independent memory systems involving the dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- ————— Drug facilitation of learning and memory storage. Science. 1973;153:229–240. doi: 10.1146/annurev.pa.13.040173.001305. [DOI] [PubMed] [Google Scholar]
- ————— Dissociating learning and performance: Drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Petri HL. Memories and habits: Some implications for the analysis of learning and retention. In: Squire LR, Butters N, editors. Neuropsychology of memory. New York, NY: Guilford; 1984. pp. 287–296. [Google Scholar]
- Mitchell JA, Akarasereenot P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori C, Weiskrantz L, Buerki H, Petschke F, Fagg GE. NMDA receptor antagonists can enhance or impair learning performance in animals. Exp Brain Res. 1989;75:449–456. doi: 10.1007/BF00249896. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Davis S, Butcher SP. Hippocampal synaptic plasticity and NMDA receptors: A role in information storage? Philos Trans R Soc London. 1990;329:187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Needleman PJ, Turk BA, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. On the neurobiology of multiple memory systems: Tolamn versus Hull, system interactions, and the emotion-memory link. Cogn Process. 2001;1:1–22. [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- ————— Quinpirole and d-amphetamine administration post-training enhances memory on spatial and cued discriminations in a water maze. Psychobiology. 1994;22:54–60. [Google Scholar]
- ————— Inactivation of the hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Double dissociation of hippocampal and dorsal-striatal memory systems by post-training intracerebral injections of 2-amino-5-phosphonopentanoic acid. Behav Neurosci. 1997a;111:543–551. doi: 10.1037//0735-7044.111.3.543. [DOI] [PubMed] [Google Scholar]
- ————— Posttraining injections of MK-801 produce a time-dependent impairment of memory in two water maze tasks. Neurobiol Learn Mem. 1997b;68:42–50. doi: 10.1006/nlme.1996.3762. [DOI] [PubMed] [Google Scholar]
- ————— Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Teather LA, Bazan N G. Effects of intrastriatal injections of platelet-activating factor and the PAF antagonist BN 52021 on memory. Neurobiol Learn Mem. 1996;66:176–182. doi: 10.1006/nlme.1996.0058. [DOI] [PubMed] [Google Scholar]
- Partington CR, Edwards MW, Daly JW. Regulation of cyclic AMP formation in brain tissue by α-adrenergic receptors: Requisite intermediacy of prostaglandins of the E series. Proc Natl Acad Sci. 1980;77:3024–3028. doi: 10.1073/pnas.77.5.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha J. Amorphous water soluble derivatives of cyclodextrins: Non-toxic dissolution enhancing excipients. J Pharmacol Sci. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- ————— Cyclodextrins: Solutions to insolubility. Neurotransmissions. 1989;5:1–4. [Google Scholar]
- Robinson GS, Crooks GB, Shinkman PG, Gallagher M. Behavioral effects of MK-801 mimic deficits associated with hippocampal damage. Psychobiology. 1989;17:156–164. [Google Scholar]
- Smith WL, Marnett LJ, DeWitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Ther. 1991;49:153–179. doi: 10.1016/0163-7258(91)90054-p. [DOI] [PubMed] [Google Scholar]
- Teather LA. New Orleans, LA: Louisiana State University Medical Center; 1998. [Google Scholar]
- Teather LA, Packard MG, Bazan NG. Effects of post-training intra-hippocampal injections of platelet-activating factor and PAF antagonists on memory. Neurobiol Learn Mem. 1998;70:349–363. doi: 10.1006/nlme.1998.3862. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Xie W, Chipman JG, Robertson DK, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: Regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]