Abstract
This study examined the effects of a dopamine D1 antagonist, SCH23390, infused into the prelimbic–infralimbic areas on the acquisition of a response and visual-cue discrimination task, as well as a shift from a response to a visual-cue discrimination and vice versa. Each test was carried out in a cross-maze. The response discrimination required learning to always turn in the same direction (right or left) for a cereal reinforcement. The visual-cue discrimination required learning to always enter the arm with the visual cue. In experiment 1, rats were tested on the response discrimination task, followed by the visual-cue discrimination task. In experiment 2, the testing order was reversed. Bilateral infusions of SCH23390 (0.1 or 1 μg/0.5 μL) into the prelimbic–infralimbic areas did not impair acquisition of the response or visual-cue discrimination tasks. SCH23390 injections at 1 μg, but not 0.1 μg impaired performance when shifting from a response to a visual-cue discrimination, and vice versa. Analysis of the errors revealed that the deficit was due to perseveration of the previously learned strategy. These results suggest that activation of dopamine D1 receptors in the prelimbic–infralimbic areas may be critical for the suppression of a previously relevant strategy and/or generating new strategies.
There is accumulating evidence that separate prefrontal cortex regions influence distinct cognitive functions (Kolb et al. 1974; Eichenbaum et al. 1983; Seamans et al. 1995; Delatour and Gisquet-Verrier 1996, 1999, 2000; Goldman-Rakic 1996; Kesner et al. 1996; Petrides 1996; Bussey et al. 1997; DeCoteau et al. 1997; Ragozzino et al. 1998, 1999b; Gisquet-Verrier et al. 2000; Kesner 2000; Ragozzino and Kesner 2001). Experiments in nonhuman primates have shown that different prefrontal cortex areas contribute to separate forms of cognitive flexibility (Dias et al. 1996, 1997). Lesions of the lateral prefrontal cortex produce a selective impairment in extra-dimensional shifts for a visual-cue discrimination task (e.g., learning to make a choice based on shape, then learning to make a choice based on lines). However, lateral prefrontal cortex lesions do not impair reversal learning (e.g., learning to always choose a red object but not a blue object, then learning to choose the opposite colored object). Conversely, lesions of the orbital prefrontal cortex impair reversal learning but not extra-dimensional shifts (Dias et al. 1996, 1997). Taken together, the evidence suggests that the lateral prefrontal cortex and orbital prefrontal cortex regions differentially contribute to cognitive flexibility based on the type of task demands.
The findings from several studies in rodents suggest that the medial prefrontal cortex plays a critical role in behavioral flexibility (deBruin et al. 1994; Aggleton et al. 1995; Granon and Poucet 1995; Bussey et al. 1997; Joel et al. 1997; Ragozzino et al. 1999a,b; Birrell and Brown 2000; Delatour and Gisquet-Verrier 2000; Dias and Aggleton 2000). Recently, a series of experiments found that temporary inactivation or lesions of the prelimbic–infralimbic areas impairs extra-dimensional shifts in discrimination tasks that require the use of different attribute information (Ragozzino et al. 1999a,b; Birrell and Brown 2000). In contrast, prelimbic–infralimbic inactivation or lesions do not impair acquisition or reversal learning of different two-choice discriminations (Ragozzino et al. 1999a,b; Birrell and Brown 2000; Chudasama et al. 2001), suggesting that these prefrontal cortex subregions are selectively involved in behavioral flexibility requiring shifts between different attributes or dimensions.
At present it is unclear what neurochemical mechanisms within the prelimbic–infralimbic subregions enable behavioral flexibility. One neurotransmitter in these prefrontal cortex areas that may contribute to behavioral flexibility is dopamine. The prelimbic–infralimbic areas receive a dopaminergic input from the ventral tegmental area where dopamine neurons synapse on both pyramidal cells and interneurons (Descarries et al. 1987; Van Eden et al. 1987; Carr and Sesack 2000). Voltammetric recordings of dopamine from the prelimbic and infralimbic subregions indicate that dopamine release reliably increases or decreases when reinforcement contingencies are unexpectedly changed in the rat (Richardson and Gratton 1998). In vivo microdialysis studies have revealed that dopamine overflow increases when rats are exposed to novel conditions (Feenstra et al. 1995; Wilkinson et al. 1998). Furthermore, 6-OHDA lesions of the medial prefrontal cortex, including the prelimbic, infralimbic, and medial orbital areas, do not impair acquisition of fear conditioning but do impair extinction of fear conditioning (Morrow et al. 1999). Moreover, dopamine modifies certain forms of synaptic plasticity in the medial prefrontal cortex that may underlie learning and other cognitive functions (i.e., behavioral flexibility; Otani et al. 1998). These studies using a variety of approaches suggest that the dopamine signal may be a key component in modulating activity in the prelimbic–infralimbic areas to facilitate behavioral flexibility.
The dopamine D1 receptor in the prefrontal cortex, in particular, may be critical for mediating dopamine effects on cognitive functioning. Blockade of dopamine D1 receptors in the primate dorsolateral prefrontal cortex, in a dose-dependent manner, may enhance or reduce delay period unit activity during a working-memory task (Williams and Goldman-Rakic 1995). In a comparable fashion, previous investigations have shown that either blockade or superfluous stimulation of dopamine D1 receptors in the prelimbic area impairs working memory (Zahrt et al. 1997; Seamans et al. 1998). This raises the possibility that an optimal level of dopamine D1 receptor activation in prelimbic–infralimbic areas may be important for cognitive functioning (Williams and Goldman-Rakic 1995). To test whether dopamine D1 receptors are critical for behavioral flexibility, our experiments examined the effects of SCH23390, a dopamine D1 antagonist, injected into the prelimbic–infralimbic areas on the acquisition and shift between response and visual-cue discrimination tests.
RESULTS
Histology
Figure 1 illustrates the location of the cannula tips in the prelimbic and infralimbic areas for experiments 1 and 2. Histological examination revealed that the injection tips were located in the anterior–posterior plane at the level of the forceps minor of the corpus callosum. The injection tips were concentrated in the medial portions of the prelimbic area with only a few placements located in the dorsal infralimbic. The dye injections indicated that fluid spread ventrolaterally from the injection site and was concentrated in the prelimbic and infralimbic subregions.
Figure 1.
Placement of cannula tips in the prelimbic and infralimbic areas for rats included in the behavioral analyses for experiments 1 and 2. Cannula tips were concentrated in the prelimbic area 2.7 to 3.7 mm anterior to bregma. Rat brain sections were modified from the atlas of Paxinos and Watson (1996). The number of circles does not match the total number of rats included in the behavioral analyses because certain cannula placements were overlapping to such a large extent that a single circle represented more than one cannula placement.
Experiment 1: The Effects of SCH23390 Infusions into the Prelimbic–Infralimbic areas on Acquisition of a Response Discrimination and Shift to a Visual-Cue Discrimination
The findings on the trials to criterion for response discrimination acquisition are shown in Figure 2A. All groups took ∼70–80 trials to reach criterion. Because there was no difference in trials to criterion on acquisition between the group that received 1 μg of SCH 23390 (mean = 70.7 ± 10.1 sem) and the group that received 0.1 μg of SCH 23390 (mean = 79.3 ± 5.93), their scores were combined into one group for the analysis. The analysis indicated that there was not a significant difference among the groups for initial learning of the response discrimination [F(3,20) = 0.29; P > 0.05]. The difference in probe trials among the groups also was not significant [F(2,20) = 1.09; P > 0.05].
Figure 2.
(A) Mean trials to criterion on acquisition of the response discrimination after bilateral infusions of saline or SCH23390 (0.1 and 1 μg) into the prelimbic–infralimbic areas. Because there was no difference between the effects of SCH23390 at 0.1 μg or 1 μg the data were combined into one group for the analyses. SCH23390 at the 0.1- and 1-μg doses did not impair acquisition compared to that of saline infusions. The treatment received on this test is underlined for each group. (B) Mean trials to criterion on the shift to the visual-cue discrimination after saline or infusions of SCH23390 into the prelimbic–infralimbic areas. SCH23390 at the 1-μg dose, but not the 0.1-μg dose, significantly increased the trials to criterion compared to that of saline injections. The treatment received on this test is underlined for each group. SAL, saline; SCH, SCH23390; *, P < 0.05.
Figure 2B illustrates the results on the trials to criterion for the shift to visual-cue discrimination learning. The two saline groups and the group that received SCH23390 at the 0.1-μg dose reached criterion in ∼60 trials. In contrast, rats that received prelimbic–infralimbic infusions of SCH23390 at a 1-μg dose were impaired in reaching criterion, taking ∼85 trials. The difference in trials to criterion among the groups was significant [F(3,20) = 5.37; P < 0.01]. A post hoc analysis revealed that infusions of SCH23390 at 1 μg significantly increased the trials to criterion compared to those of the saline-treated groups and the SCH23390 0.1-μg group (P < 0.05), but there was not a significant difference in trials to criterion between the saline groups or the SCH23390 0.1-μg group and either saline group (P > 0.05). The difference in the number of probe trials to reach criterion among the groups was not significant [F(3,20) = 0.04; P > 0.05].
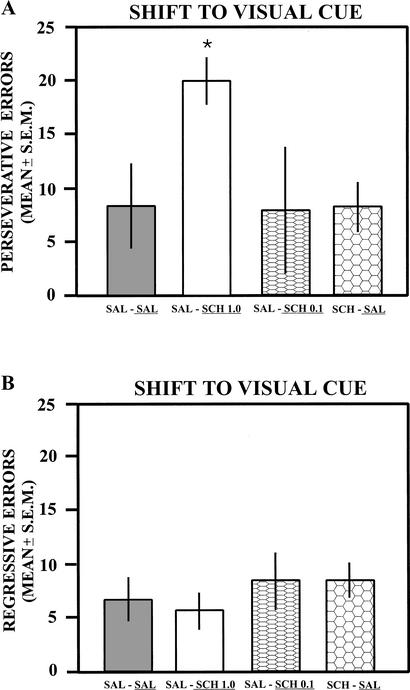
A further analysis of the trials to criterion on the shift to the visual-cue discrimination revealed that the 1-μg dose of SCH23390 impaired learning on the switch due to perseveration (see Fig. 3A). The difference in perseverative errors among the groups was significant [F(3,20) = 5.05; P < 0.01]. Newman-Keuls tests indicated that SCH23390 1-μg-treated rats made significantly more perseverative errors than either of the saline-treated groups or the group that received SCH23390 at the 0.1-μg dose (P < 0.05). The difference in perseverative errors between the saline-treated groups was not significant, as well as between each saline-treated group and the group that received SCH23390 0.1 μg (P > 0.05). In contrast, there was not a significant difference in regressive errors among the groups [F(3,20) = 0.52; P > 0.05; see Fig. 3B).
Figure 3.
(A) Mean number of perseverative errors on the shift to the visual-cue discrimination following saline or injections of SCH23390. Infusions of SCH23390 (1 μg) significantly increased the number of perseverative errors compared to saline infusions or SCH23390 (0.1 μg). The treatment received on this test is underlined for each group. (B) Mean number of regressive errors on the shift to the visual-cue discrimination following saline or SCH23390 injections. Injections of SCH23390 at 0.1 or 1 μg did not significantly increase regressive errors compared to that of saline infusions. The treatment received on this test is underlined for each group. SAL, saline; SCH, SCH23390; *, P < 0.05.
The mean score for the never-reinforced errors in each group was as follows: saline–saline = 5.17 ± 0.79; saline–SCH23390 1 μg = 12.17 ± 2.91; saline–SCH23390 0.1 μg = 4.67 ± 1.09; and SCH23390–saline = 5 ± 1.06. The difference in the errors among the groups was significant [F(3, 20) = 4.58; P < 0.05]. A post hoc analysis revealed that the group infused with SCH23390 at 1 μg made significantly more errors than any of the other three treatment groups (P < 0.05). However, the difference in errors between the saline groups or between the different saline groups and the SCH23390 0.1-μg group was not significant (P > 0.05).
Experiment 2: The Effects of SCH 23390 Injections into the Prelimbic–Infralimbic Areas on Acquisition of a Visual-Cue Discrimination and Shift to a Response Discrimination
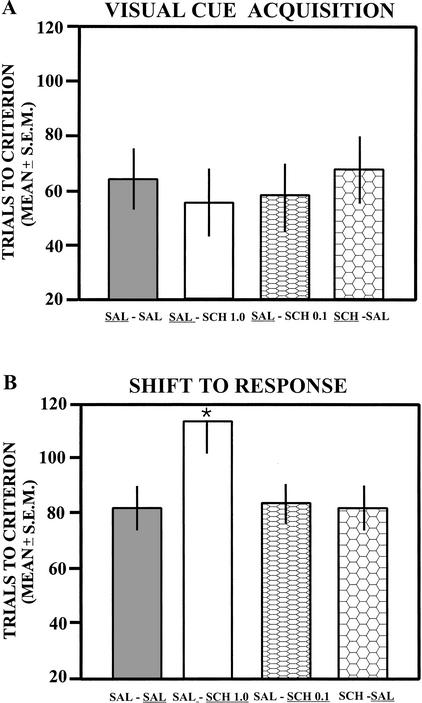
The findings on acquisition of the visual-cue discrimination indicate that rats required ∼55–70 trials to reach criterion (see Fig. 4A). Because there was no difference in trials to criterion between the group that received 1 μg of SCH 23390 (mean = 63 ± 17.6 sem) and the group that received 0.1 μg of SCH 23390 (mean = 74.3 ± 20.7), their scores were combined into one group for the analysis. The difference in trials to criterion among the groups was not significant [F(3,22) = 0.24; P > 0.05]. Furthermore, the difference in the number of probe trials on acquisition was not significant [F(3,22) = 0.19; P > 0.05].
Figure 4.
(A) Mean trials to criterion on acquisition of the visual-cue discrimination after bilateral infusions of saline or SCH23390 (0.1 and 1 μg) into the prelimbic–infralimbic areas. Because there was no difference between the effects of SCH23390 at 0.1 μg or 1 μg the data were combined into one group for the analyses. SCH23390 at the 0.1- and 1-μg doses did not impair acquisition compared to that of saline infusions. The treatment received on this test is underlined for each group. (B) Mean trials to criterion on the shift to the visual-cue discrimination after saline or infusions of SCH23390 into the prelimbic–infralimbic areas. SCH23390 at the 1-μg dose, but not the 0.1-μg dose, significantly increased the trials to criterion compared to that of saline injections. The treatment received on this test is underlined for each group. SAL, saline; SCH, SCH23390; *, P < 0.05.
Infusions of SCH23390 into the prelimbic–infralimbic areas impaired the shift to response discrimination learning in a dose-dependent manner (see Fig. 4B). The saline groups and the group that received SCH23390 0.1 μg reached criterion in ∼80–85 trials. In contrast, the group that received SCH23390 1 μg was slower in reaching criterion, needing ∼115 trials. The difference in trials to criterion was significant among the groups [F(3,22) = 3.21; P < 0.05]. Post hoc tests revealed that the SCH23390 1-μg-treated group took significantly more trials to reach criterion compared to either saline-treated group or the group that received SCH23390 0.1 μg (P < 0.05). The difference in trials to criterion between the saline groups was not significant (P > 0.05). The difference in trials to criterion between the SCH23390 0.1-μg-treated group and either saline-treated group was not significant (P > 0.05). The difference in the number of probe trials to reach criterion among the groups was not significant [F(3,22) = 0.69; P > 0.05].
A further analysis of the trials to criterion on the shift to response discrimination revealed that SCH23390 at 1 μg impaired learning on the switch due to perseveration (see Fig. 5A). The difference in perseverative errors among the groups was significant [F(3,22) = 3.58; P < 0.05]. Post hoc tests indicated that SCH23390 1-μg-treated rats made significantly more perseverative errors then either of the saline-treated groups and the SCH23390 0.1-μg-treated group (P < 0.05). The difference in perseverative errors between the saline-treated groups was not significant (P > 0.05), nor was it significant between either saline-treated group and the SCH23390 0.1-μg-treated group (P > 0.05). There was not a significant difference in regressive errors among the groups [F(3,22) = 0.4; P > 0.05; see Fig. 5B].
Figure 5.
(A) Mean number of perseverative errors on the shift to the response discrimination following saline or injections of SCH23390. Infusions of SCH23390 (1 μg) significantly increased the number of perseverative errors compared to saline infusions or SCH23390 (0.1 μg). The treatment received on this test is underlined for each group. (B) Mean number of regressive errors on the shift to the visual-cue discrimination following saline or SCH23390 injections. Injections of SCH23390 at 0.1 or 1 μg did not significantly increase regressive errors compared to that of saline infusions. The treatment received on this test is underlined for each group. SAL, saline; SCH, SCH23390; *, P < 0.05.
The mean score for the never-reinforced errors in the groups was as follows: saline–saline = 3.67 ± 1.02; saline–SCH23390 1 μg = 8.83 ± 1.56; saline–SCH23390 0.1 μg = 4.33 ± 0.76; and SCH23390–saline = 4.75 ± 1. The difference in the errors among the groups was significant [F(3, 22) = 4.58; P < 0.05]. Post hoc tests revealed that the group infused with SCH23390 at 1 μg made significantly more of these errors than any of the other three treatment groups (P < 0.05). However, the difference in errors between the saline groups or between the saline groups and the SCH23390 0.1-μg group was not significant (P > 0.05).
DISCUSSION
The present findings indicate that blockade of dopamine D1 receptors in the prelimbic–infralimbic areas impairs the shifting of strategies between response and visual-cue discriminations in a dose-dependent manner. This pattern of results is comparable to past experiments indicating that prelimbic–infralimbic inactivation produces deficits in shifts between place and response discriminations, as well as between place and visual-cue discriminations (deBruin et al. 1994; Ragozzino et al. 1999a,b). These findings suggest that the prelimbic–infralimbic areas facilitate behavioral flexibility under conditions in which responding based on one type of attribute information must be inhibited and learning to respond based on a different type of attribute is required. Although prelimbic–infralimbic involvement in behavioral flexibility is not limited to shifts between different types of attribute information, it is also involved when rats must flexibly adapt to using a match-to-sample rule or nonmatch-to-sample rule (Granon et al. 1996; Dias and Aggleton 2000). Taken together, the results suggest that the prelimbic–infralimbic areas may facilitate learning under changing conditions when a new strategy must be learned and a previously relevant strategy inhibited. This contrasts with findings showing that prelimbic–infralimbic inactivation does not impair reversal learning in which the strategy remains the same but the specific choice within that general strategy shifts (e.g., reversal learning; Ragozzino et al. 1999a; Birrell and Brown 2000; Chudasama et al. 2001). Therefore, the prelimbic–infralimbic areas may only be involved in certain types of situationally adaptive responses.
The ability of a dopamine D1 antagonist to impair behavioral flexibility between different types of discrimination tests is comparable to findings indicating that dopamine D1 receptor blockade also impairs working memory when infused into the prelimbic areas (Seamans et al. 1998). In this experiment, rats had to learn a delayed nonmatch-to-sample task in a radial maze. This type of task requires behavioral flexibility in that subjects must inhibit choosing the arms entered on the initial phase and choose the remaining arms on the test phase. Interestingly, high levels of D1 receptor stimulation in the medial prefrontal cortex also causes working memory impairments (Zahrt et al. 1997). The results suggest that there may be an optimal window of dopamine D1 receptor stimulation in the prelimbic–infralimbic areas that enhances behavioral and cognitive functions. In particular, either understimulation or overstimulation of dopamine D1 receptors in this area may lead to behavioral impairments. This idea has also been proposed by Williams and Goldman-Rakic (1995) to explain the role of dopamine D1 receptors within the primate dorsolateral prefrontal cortex in working memory.
The present results are also consistent with findings indicating that dopamine level changes in the medial prefrontal cortex alter the extinction of fear conditioning (Wilkinson et al. 1998; Morrow et al. 1999). More specifically, 6-hydroxydopamine lesions of the medial prefrontal cortex do not impair acquisition of a conditioned-fear response but do impair extinction of the conditioned response (Morrow et al. 1999). In measuring dopamine efflux, an in vivo microdialysis study found that dopamine release increased in the prelimbic–infralimbic areas during the initial sessions of extinction in a fear-conditioning task compared to that in the last acquisition session, suggesting that dopamine release in these areas may be a key signal indicating that the environmental conditions have changed (Wilkinson et al. 1998). Consistent with this idea, measurement of dopamine levels by in vivo voltammetry in the prelimbic and infralimbic subregions indicate that dopamine overflow reliably increases or decreases when reinforcement contingencies are unexpectedly changed in the rat (Richardson and Gratton 1998). These results support the theory that dynamic changes in the activity of dopamine neurons reflect deviations from expected conditions and actual conditions (Schultz 1998; Horvitz 2000). Therefore, changes in dopamine release in the prelimbic–infralimbic areas may be an initial signal indicating environmental demands have changed and a different behavioral response pattern must be employed.
In support of the idea that changes in dopamine release in the prelimbic–infralimbic areas may be an initial change to facilitate the learning of a new strategy, infusions of SCH23390 impaired the initial shift away from the previously relevant strategy but did not increase reversions back to the previously relevant strategy once perseveration ceased. This is comparable to previous studies in which temporary inactivation or neurotoxic lesions of the prelimbic–infralimbic areas impaired behavioral flexibility due to an increase in perseveration (Ragozzino et al. 1999a,b; Birrell and Brown 2000; Dias and Aggleton 2000). This pattern of errors may reflect that the prelimbic–infralimbic areas play a critical role in the inhibition of a previously learned strategy and/or generation of new strategies. However, SCH23390 infusions also increased errors during the shift on trials when the new, relevant choice pattern was congruent with the previously relevant choice pattern. For example, on the shift from the visual cue to the response, a rat that was required to turn right had the previously relevant visual cue in the right arm for half the trials. Blockade of dopamine D1 receptors in the prelimbic–infralimbic areas significantly increased a rat turning into the left arm on these trials. The errors are suggestive of a rat trying a new, irrelevant strategy, such as that required in reversal learning in which a rat must inhibit choosing the visual-cue arm and learn to choose the arm without the visual cue. Thus, dopamine D1 receptor antagonism in the prelimbic–infralimbic areas may not prevent the generation of new strategies.
Although blockade of dopamine D1 receptors in the prelimbic–infralimbic activity may not prevent the generation of new strategies, it may bias the use of a strategy that is appropriate for effective reversal learning. As discussed above, prelimbic–infralimbic inactivation impairs extra-dimensional shifts but not reversal learning (Ragozzino et al. 1999a,b; Birrell and Brown 2000; Chudasama et al. 2001). The findings from several experiments suggest that the orbital prefrontal cortex may be critical for behavioral flexibility, requiring reversal learning but not extra-dimensional shifts (Jones and Mishkin 1972; Kolb et al.1974; Becker and Olton 1980; Dias et al. 1996; Schoenbaum et al. 1998). One possibility is that these prefrontal cortex subregions act in opposition and when activity is altered in one subregion the other subregion may predominate. In this case, alterations in prelimbic–infralimbic activity may alter the balance between the two areas, which biases the use of a reversal-learning strategy that is ineffective when conditions demand an extra-dimensional shift.
In support of the suggestion that different brain areas may compete for the expression of particular behavioral patterns, a previous study showed that the nucleus accumbens and medial prefrontal cortex act in opposition for the expression of locomotor versus stereotyped behavior (Whishaw et al. 1992). Specifically, medial prefrontal cortex lesions increase locomotor activity over stereotyped behavior. However, a nucleus accumbens lesion abolishes this effect and reverses the expression of stereotyped behavior and locomotor activity. Thus, damage to one of these areas may alter the balance between these two systems in the expression of these behavioral patterns.
The higher dose of SCH23390 increasing perseverative errors, but not regressive errors, is unlikely due to the effect of the drug wearing off during the later stages of testing. First, a comparable pattern of results is observed with manipulations of the prelimbic–infralimbic areas when using the local anesthetic bupivacaine, which has been found to last 75–90 min when infused into the brain (Alam and Mallick 1990; M.E. Ragozzino unpubl.). Second, previous studies have shown that a comparable dose of SCH23390 injected into the prelimbic region has behavioral effects that last at least 30 min (Seamans et al. 1998; Granon et al. 2000). Finally, rats that received the high dose of SCH23390 and took the longest to complete behavioral testing (∼60 min) were as likely to make never-reinforced errors near the end of a test session than they were at the beginning. Control rats predominantly made never-reinforced errors during the early to middle stages of testing. This last set of findings suggests that SCH23390 at the 1-μg dose may be effective as long as 60 min. The results are comparable to findings observed following infusions of SCH23390 into the primate dorsolateral prefrontal cortex (Sawaguchi and Goldman-Rakic 1991; Williams and Goldman-Rakic 1995).
Another potential issue with the behavioral effects observed in this study is that SCH23390 has an affinity for 5-HT2 receptors, as well as binding to dopamine D1 receptors (Bischoff et al. 1986, 1988). Therefore, the impairments observed in the present study may be due to SCH23390 acting at 5-HT2 receptors. However, this may not be the case because several studies have shown that direct infusions of selective 5-HT2 agents into the prefrontal cortex or systemic injections do not affect performance on tasks that are sensitive to prefrontal cortex manipulations (Sawaguchi and Goldman-Rakic 1991; Meneses et al. 1997; Ruotsalainen et al. 1997).
In contrast to the results on the shift, blockade of dopamine D1 receptors did not impair acquisition of the response or visual-cue discrimination. Because SCH23390 infusions did not affect acquisition, the deficit observed during the switch are not due to a general impairment in learning. Furthermore, the behavioral flexibility deficit is not caused by motivational or motor side effects. This is similar to experiments showing that prelimbic–infralimbic lesions or inactivation do not impair initial learning of different two-choice discriminations (Ragozzino et al. 1999a,b; Birrell and Brown 2000; Delatour and Gisquet-Verrier 2000; Dias and Aggleton 2000; Chudasama et al. 2001).
In conclusion, activation of dopamine D1 receptors in the prelimbic–infralimbic areas may not be critical for the initial learning of response or visual-cue discriminations. However, activation of these receptors in the prelimbic–infralimbic areas may play a critical role in facilitating behavioral flexibility when conditions demand a switch in strategies, as with extra-dimensional shifts. Furthermore, there may be an optimal level of dopamine D1 receptor stimulation in this region for which to facilitate behavioral flexibility. Because blockade of dopamine D1 receptors in these prefrontal subregions increases perseveration and never-reinforced errors, dopamine D1 receptor activity in the prelimbic–infralimbic areas may enhance inhibition of a previously relevant strategy and possibly learning of a strategy that requires the use of different attribute information.
MATERIALS AND METHODS
Subjects
Male Long-Evans rats (Charles River Laboratories) weighing between 325 and 375 g at the beginning of the experiments served as subjects. Rats were housed individually in plastic cages (30.5 × 55 × 20 cm) located in a temperature-controlled room (22°C) that was maintained at 20%–40% humidity. The rats were kept on a 12-hr light–dark cycle (lights on 7:00 a.m.). All rats were food restricted to maintain their weight at ∼85% of their ad libitum weight but had free access to water throughout the experiment. The experiments were conducted in accordance with the United States government principles for the utilization and care of vertebrate animals used in testing, research, and training.
Apparatus
The cross-maze was a four-arm maze made of 1-cm-thick black plexiglass (see Fig. 6). The maze was placed on a table that was elevated 60 cm above the floor. Each arm was 52 cm long and 9 cm wide; the height of the arm wall was 16.5 cm. Each arm contained a food well (3 cm diameter, 2.5 cm high) that was 3.2 cm from the end wall. Each food-well hole was 2 cm in diameter and 1.25 cm deep. The center platform was 18 × 18 cm.
Figure 6.
Example of a rat tested on the response and visual-cue discrimination tasks. In each task, a rat had a choice to turn to the left or to the right. A white visual cue was randomly placed in one of the choice arms on each trial. In the response version, this rat was started from the south (S), west (W), and east (E) arms and always had to make a 90° turn to the right to receive a cereal reinforcement. In the visual-cue version, the rat was started from the same arms but had to always enter the visual-cue arm that did not depend on always making the same type of turn. The arrows in the maze represent the correct navigation pattern to receive reinforcement.
Surgery
Rats received atropine sulfate (0.2 mL of a 250 μg/mL solution, I.P.) 20 min before administering the general anesthetic (sodium pentobarbital, 50 mg/kg, I.P.). A midsaggital incision was made, and the scalp retracted. Each rat received a bilateral implant of an 8-mm stainless steel guide cannula (22 gauge; Plastics One) aimed toward the prelimbic area. The stereotaxic coordinates were 3.1 mm anterior to bregma, ±1.7 lateral to the midline, and 3 mm ventral to dura. The incisor bar was lowered to 3.3 ± 0.2 below horizontal zero so that the height of bregma and λ were equal. The coordinates were based on the atlas of Paxinos and Watson (1996). Four jeweler's screws were placed in the skull surrounding the cannulae. The cannulae were secured in place with dental acrylic (Plastics One). Stylets were secured in the guide cannulae after the dental acrylic dried. After surgery, rats received ground rat chow mixed in water for 1 d.
Habituation Procedure
Rats were allowed 7–10 d to recover from surgery before the habituation procedure commenced. Two days after surgery, rats were food restricted to 85% of their original ad libitum weight. During food restriction rats were handled for 10 min per day. On the first day of habituation, 3 pieces of Froot Loops cereal (Kelloggs) were placed in each arm, with 2 pieces in the food well. A rat was placed in the maze and allowed to freely navigate and consume cereal pieces for 15 min. If a rat consumed all 12 cereal pieces prior to 15 min, then the rat was placed in a holding cage, the maze was rebaited, and the rat was placed back in the maze. On the second habituation day, the procedure was similar except that after a rat consumed 2 cereal pieces, the rat was picked up and placed in a different arm. This acclimated the rat to being handled in the maze after consuming cereal. On subsequent habituation sessions, the procedure was the same as day 2, except that there were only 2 half pieces of cereal put in each food well. Each time a rat consumed all the cereal pieces after being placed in the maze was considered one trial. This procedure continued until a rat consumed cereal from all food wells for four trials or more in a 15-min session. On the last day of habituation, the turn bias for a rat was determined. The maze was arranged such that a black Plexiglas block (9 × 13 × 1 cm) was placed at the center entrance of one of the arms so that it prevented entry into that arm, giving the maze a T-shape. A rat was started from the stem arm and allowed to turn left or right to obtain a half piece of cereal. In one of the choice arms a white piece of posterboard (8 × 48 × 0.3 cm) was placed on the floor (see Fig. 6). After a rat made a turn and consumed a cereal piece, the rat was picked up, placed in the stem arm, and allowed to make a choice. If the rat chose the same arm as in the initial choice, it was returned to the stem arm until it chose the other arm and consumed the cereal piece. After choosing both arms, the rat was returned to the holding cage, the block and visual cue were moved to different arms, and a new trial was begun. Thus, a trial for the turn-bias procedure consisted of entering both choice arms and consuming the cereal. This procedure continued for seven trials. The turn that a rat made first during the initial choice of a trial was recorded and counted toward its turn bias. Whatever direction (right or left) a rat turned, four or more times during these seven trials was considered its turn bias. During response-discrimination testing, a rat was required to turn in the opposite direction of its turn bias. The habituation procedure ranged from 4 to 8 daily sessions.
After determining the turn bias, a rat's stylets were removed from the guide cannulae and an injection cannula was inserted for 1 min. There was no solution injected at this time. This procedure was performed to prevent clogging of the microinfusion on test days. Behavioral testing was started the next day.
Microinfusion
Bilateral injections into the prelimbic–infralimbic areas were made through an inner cannula (28 gauge) that extended 1 mm below the guide cannula. The inner cannula was attached by a polyethylene tube (PE-20) to a 10-μL Hamilton syringe. The syringe was driven by a microinfusion pump (74900 Series, Cole-Parmer) with solutions infused in a volume of 0.5 μL per side for 2 min. The inner cannula was left in place for 1 min after completion of the infusion to allow for diffusion. Rats received either saline or SCH23390 at either 0.1 or 1 μg dose (Tocris Cookson). SCH23390 was mixed in saline. A new batch of SCH23390 was mixed up for each session the drug was used. During the entire injection procedure, a rat was allowed to freely move in its home cage. Behavioral testing started 5 min after removal of the inner cannula.
Response–Visual-Cue Testing Procedure
The testing procedure was similar to that described in Ragozzino et al. (2002) except that all testing was carried across two consecutive sessions. For each discrimination, three start arms were used. In this experiment, each rat was started on the response version. A rat was started from the arms designated west, south, and east (W, S, and E, respectively; see Fig. 6). The visual cue was placed pseudorandomly in one of the choice arms such that for every consecutive set of 12 trials it occurred an equal number of times in each choice arm. During the acquisition session, a rat always had a choice to make a turn to the left or to the right. A rat had to turn in the opposite direction of its turn bias to receive a half piece of Froot Loops cereal. Figure 1 (top) illustrates an example of the correct navigation patterns for a rat that was required to always make a turn to the right. Between trials a rat was placed back in the holding cage, which sat on a shelf next to the maze. Subsequently, the maze arms were wiped down with a sponge moistened with 1% ammonium chloride solution. The intertrial interval was ∼10 sec. To minimize the use of intramaze cues from the apparatus, every 4 trials the maze was turned 90° clockwise relative to the experimenter. A rat reached criterion when it made 10 correct choices consecutively. There was no limit on the number of trials a rat was allotted to reach this criterion. Once a rat made 10 correct choices consecutively, a probe trial was given. The probe trial consisted of starting the rat from the fourth arm (north, N) that was not used during testing. If a rat correctly turned the same direction as on testing, then the response procedure was completed. If a rat made an incorrect turn, then response testing was continued until a rat made an additional 5 correct choices consecutively, at which time another probe trial was administered. This procedure was continued until a rat made a correct choice on the probe trial. The following measures were taken for each rat: (1) acquisition criterion, defined as the total number of test trials to complete 10 consecutive correct choices in a session; (2) trials to criterion, defined as the total number of test trials completed before a correct choice on the probe trial was made; and (3) probe trials, defined as the total number of probe trials to get one correct. Based on these criteria it was possible that the scores for the acquisition criterion and trials to criterion were the same if a rat made a correct choice on the first probe trial.
The day after reaching criterion on the response version, rats were switched to the visual-cue version. In the visual-cue version a similar procedure was used as in the response version. However, in this test the rat always had to enter the arm with the visual cue. The visual cue was pseudorandomly varied in the left and right arms such that it occurred in each arm an equal amount for every consecutive set of 12 trials. Figure 1 (bottom) shows an example of a rat that learned to always enter the visual-cue arm. The same start arms and criteria to complete the visual-cue version were used as described in the response version. Additional measures were analyzed on the switch to determine whether treatments altered perseveration or reversions back to the previously correct response pattern after perseveration had ceased. Perseveration involved continuing to make the same egocentric response, as required on the response version, when the trial required turning the opposite direction to enter the visual-cue arm. For every consecutive 12 trials in a session, half the trials consisted of these trials. As in a previous experiment (Ragozzino et al. 2002), these trials were separated into consecutive blocks of 4 trials each. Perseveration was defined as entering the incorrect arm in 3 or more trials per block. This is a similar criterion as used in previous experiments measuring perseveration (Hunt and Aggleton 1998; Ragozzino et al. 1999a, 2002; Dias and Aggleton 2000). Once a rat made less than three errors in a block the first time, all subsequent errors were no longer counted as perseverative errors. After a rat stopped perseverating as defined above, the number of errors was counted when a rat reverted back to previously correct response on those same type of trials that required the opposite turn as on the response version. These errors are referred to as regressive errors. During the shift a third type of error could be made if a rat turned in the opposite direction. For the other half of the trials in which a turn in the visual cue arm was the same as egocentric response required in the acquisition phase an error could be made if a rat turned in the other arm. For example, during acquisition a rat might be required to always turn right. During the shift, a rat is then required to always enter the arm with the visual cue. For half of the trials, the visual cue will be in the arm on the right. However, a rat may make an error by turning in the left arm. These errors are referred to as never-reinforced errors and were calculated for each rat. The regressive and never-reinforced measures provide an index of the ability to maintain a new discrimination strategy.
Five minutes before each test session, rats received a microinfusion. Each rat was randomly assigned to one of the five treatment groups. Group assignment was determined by which treatment was administered during each version: (1) response version–saline and visual-cue version–saline (n = 6); (2) response version–saline and visual-cue version–SCH23390 1 μg (n = 6); (3) response version–saline and visual-cue version–SCH23390 0.1 μg (n = 6); (4) response version–SCH23390 1 μg and visual-cue version–saline (n = 3); and (5) response version–SCH23390 0.1 μg and visual-cue version–saline (n = 3). Group 1 served as the control group. Groups 2 and 3 determined whether blockade of dopamine D1 receptors in the prelimbic–infralimbic areas impaired behavioral flexibility when switched to a different discrimination version. Groups 4 and 5 determined whether blockade of dopamine D1 receptors in the prelimbic–infralimbic areas impaired acquisition of response-discrimination learning. Saline infusions were administered to groups 4 and 5 on the switch to determine whether blockade of dopamine D1 receptors on acquisition may have altered neuronal activity that led to a behavioral impairment but was not manifested until rats were switched to a different discrimination.
Visual-Cue–Response Testing Procedure
In this experiment rats were started on the visual-cue version followed by testing on the response version. All other aspects of the testing procedure were as described in experiment 1. On the switch to the response version the same measures were assessed as those during the switch in experiment 1. In this case, perseveration and regressive errors were analyzed from the trials in which a rat was required to turn in the arm opposite to that of the visual cue. Each rat was randomly assigned to one of the following groups: (1) visual-cue version–saline and response version–saline (n = 6); (2) visual-cue version–saline and response version–SCH 23390 1 μg (n = 6); (3) visual-cue version–saline and response version–SCH 23390 0.1 μg (n = 7); (4) visual-cue version–SCH 23390 1 μg and response version–saline (n = 4); and (5) visual-cue version–SCH 23390 0.1 μg and response version–saline (n = 4).
Histology
After completion of behavioral testing, rats received a lethal dose of sodium pentobarbital, followed by a 0.5-μL injection of 2.5% Chicago blue stain through each guide cannula. As in previous experiments (Ragozzino et al. 1999a,b), the stain was used to highlight the approximate spread of the intracranial injections. Rats were perfused intracardially with 0.9% saline, followed by a 4% formaldehyde solution. Brains were removed and stored in a 30% sucrose-formalin solution. The brains were frozen and cut in coronal sections (40 μm) on a cryostat. The sections were mounted on slides and then dried and examined to determine the spread of the stain. The sections were subsequently stained with cresyl violet to identify the location of the cannula tips.
Statistical Analysis
In both experiments there was not a difference between acquisition criterion and trials to criterion. Because of this, only the analysis on the trials to criterion is presented. However, analysis on the number of probe trials is presented. Because there was no difference between the two groups that received SCH 23390 on acquisition of either the response or visual-cue discrimination, as described below, they were combined as a single group for the analyses. A separate one-way analysis of variance (ANOVA) was done on the acquisition version and the switch version for both experiments. One-way ANOVA tests were used to assess differences in perseverative, regressive, and never-reinforced errors among the groups. Newman-Keuls post hoc tests were used to make subsequent comparisons.
Acknowledgments
This research was supported by grant NIH MH 61889.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
Present address: Department of Psychology, University of Illinois at Chicago, 1007 West Harrison St., Chicago, IL 60607, USA.
E-MAIL mrago@uic.edu; FAX (312) 413-4122.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.45802.
REFERENCES
- Aggleton JP, Neave N, Nagle S, Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: Evidence of a double dissociation between frontal and cingulum bundle contributions. J Neurosci. 1995;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Mallick BN. Differential acute influence of medial and lateral preoptic areas on sleep–wakefulness in freely moving rats. Brain Res. 1990;525:242–248. doi: 10.1016/0006-8993(90)90870-h. [DOI] [PubMed] [Google Scholar]
- Becker JT, Olton DS. Discrimination by rats: The role of the frontal and hippocampal systems in retention and reversal. Physiol Behav. 1980;24:33–38. doi: 10.1016/0031-9384(80)90010-4. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Heinrich M, Sonntag JM, Krauss J. The D-1 dopamine receptor antagonist SCH 23390 also interacts with brain serotonin (5-HT2) receptors. Eur J Neurosci. 1986;129:367–370. doi: 10.1016/0014-2999(86)90449-8. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Heinrich M, Krauss J, Sills MA, Williams M, Vassout A. Interaction of the D1 receptor antagonist SCH 23390 with the central 5-HT system: Radioligand binding studies, measurements of biochemical parameters and effects on L-5-HTP syndrome. J Recept Res. 1988;8:107–120. doi: 10.3109/10799898809048981. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial prefrontal cortices on visual discrimination using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Dopamine terminals synapse on callosal projection neurons in the rat prefrontal cortex. J Comp Neurol. 2000;425:275–283. doi: 10.1002/1096-9861(20000918)425:2<275::aid-cne9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- deBruin JPC, Sanchez-Santed F, Heinsbroek RPW, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: Evidence for behavioral flexibility, but not for impaired navigation. Brain Res. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP, Williams JM. Short-term memory for food reward magnitude: The role of the prefrontal cortex. Behav Brain Res. 1997;88:239–249. doi: 10.1016/s0166-4328(97)00044-2. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Prelimbic cortex specific lesions disrupt delayed-variable response tasks in the rat. Behav Neurosci. 1996;110:1282–1298. doi: 10.1037//0735-7044.110.6.1282. [DOI] [PubMed] [Google Scholar]
- ————— Lesions of the prelimbic and infralimbic cortices in rats do not disrupt response selection but induce delay-dependent deficits: Evidence for a role in working memory? Behav Neurosci. 1999;113:941–955. doi: 10.1037//0735-7044.113.5.941. [DOI] [PubMed] [Google Scholar]
- ————— Functional role of the prelimbic–infralimbic cortices in spatial memory: Evidence for its involvement in attention and behavioural flexibility. Behav Brain Res. 2000;109:113–128. doi: 10.1016/s0166-4328(99)00168-0. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience. 1987;21:807–824. doi: 10.1016/0306-4522(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbic–infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- ————— Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sorting Test: Restriction to novel situations and independence from “on-line” information. J Neurosci. 1997;17:9265–9277. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79:434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Feenstra MGP, Botterblom MHA, van Uum JFM. Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: Inhibition by diazepam. Neurosci Lett. 1995;189:81–84. doi: 10.1016/0304-3940(95)11456-7. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Winocur G, Delatour B. Functional dissociation between dorsal and ventral regions of the medial prefrontal cortex in rats. Psychobiology. 2000;28:248–260. [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Granon S, Poucet B. Medial prefrontal lesions in the rat and spatial navigation: Evidence for impaired planning. Behav Neurosci. 1995;108:478–484. doi: 10.1037//0735-7044.109.3.474. [DOI] [PubMed] [Google Scholar]
- Granon S, Etienne S, Buhot MC, Poucet B. Effortful information processing in a spontaneous spatial situation by rats with medial prefrontal lesions. Behav Brain Res. 1996;78:147–154. doi: 10.1016/0166-4328(95)00242-1. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhance and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hunt PR, Aggleton JP. Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed match to place by rats: A deficit in shifting response rules. J Neurosci. 1998;18:10045–10052. doi: 10.1523/JNEUROSCI.18-23-10045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Tarrasch R, Feldon J, Weiner I. Effects of electrolytic lesions of the medial prefrontal cortex or its subfields on 4-arm baited radial maze, two-way active avoidance and conditioned fear tasks in the rat. Brain Res. 1997;765:37–50. doi: 10.1016/s0006-8993(97)00334-x. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28:219–228. [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J Comp Physiol Psych. 1974;87:772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- Meneses A, Terron JA, Hong E. Effects of the 5-HT receptor antagonists GR127935 (5-HT1B/1D) and MDL1000907 (5-HT2A) in the consolidation of learning. Behav Brain Res. 1997;89:217–223. doi: 10.1016/s0166-4328(97)00055-7. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesocortical dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:553–564. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce JM, Crepel F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience. 1998;85:669–676. doi: 10.1016/s0306-4522(97)00677-5. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc. 1996;351:1455–1461. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 3d ed. Sydney, Australia: Academic Press; 1996. In. [Google Scholar]
- Ragozzino ME, Kesner RP. The role of rat dorsomedial prefrontal cortex in working memory for egocentric responses. Neurosci Lett. 2001;308:145–148. doi: 10.1016/s0304-3940(01)02020-1. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic–infralimbic areas of the rodent prefrontal in spatial working memory. Behav Neurosci. 1998;112:233–243. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999a;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999b;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. The role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Gratton A. Changes in medial prefrontal cortical dopamine levels associated with response-contingent food reward: An electrochemical study in rat. J Neurosci. 1998;18:9130–9138. doi: 10.1523/JNEUROSCI.18-21-09130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen S, Sirvio J, Jakala P, Puumala T, MacDonald E, Riekkinen P. Differential effects of three 5-HT receptor antagonists on the performance of rats in attentional and working memory tasks. Eur Neuropsychopharmacol. 1997;7:99–108. doi: 10.1016/s0924-977x(96)00389-6. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal–prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden C, Hoorneman E, Buijs R, Matthijssen M, Geffard M, Uylings H. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopic level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Fiorino D, Mittleman G, Castaneda E. Do forebrain structures compete for behavioral expression? Evidence from amphetamine-induced behavior, microdialysis, and caudate-accumbens lesions in medial frontal cortex damaged rats. Brain Res. 1992;576:1–11. doi: 10.1016/0006-8993(92)90604-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Humby T, Killcross AS, Torres EM, Everitt BJ, Robbins TW. Dissociations in dopamine release in medial prefrontal cortex and ventral striatum during the acquisition and extinction of classical aversive conditioning in the rat. Eur J Neurosci. 1998;10:1019–1026. doi: 10.1046/j.1460-9568.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–574. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]