Abstract
Exposures to uncontrollable stress have been shown to alter ensuing synaptic plasticity in the hippocampus and interfere with hippocampal-dependent spatial memory in rats. The present study examined whether stress, which impairs hippocampal long-term potentiation (LTP), also affects (nonspatial) hippocampal-dependent object-recognition memory, as tested on the visual paired comparison task (VPC) in rats. After undergoing an inescapable restraint–tailshock stress experience, rats exhibited markedly impaired recognition memory at the 3-h (long) familiarization-to-test phase delay but not at the 5-min (short) delay. In contrast, unstressed control animals showed robust recognition memory (i.e., they exhibited reliable preferences for novel over familiar objects) at both short- and long-delay periods. The impairing effect of stress on long-delay recognition memory was transient because 48 h after undergoing stress experience, animals performed normally at the long delay. Similar to stress, microinfusions of DL-2-amino-5-phosphonovaleric acid (APV), a competitive N-methyl-D-aspartate receptor (NMDAR) antagonist that blocks LTP, into the dorsal hippocampus selectively impaired object-recognition memory at the long-delay period. Together, these results suggest that stress and intrahippocampal administration of APV affect recognition memory by influencing synaptic plasticity in the hippocampus.
[The following individuals kindly provided reagents, samples, or unpublished information as indicated in the paper H. Blair.]
The hippocampus is a part of a medial temporal lobe system necessary for the formation of stable declarative memory in humans (Scoville and Milner 1957; Squire and Zola-Morgan 1991; Eichenbaum 2000) and spatial memory in rodents (O'Keefe and Nadel 1978; Morris et al. 1982; Moser et al. 1998). This seahorse-resembling structure has one of the highest concentrations of receptors for corticosteroids (glucocorticoid hormones whose levels elevate in response to stress) in the mammalian brain and participates in the glucocorticoid-mediated negative feedback of the hypothalamus-pituitary-adrenal (HPA) axis (McEwen 1982). In the rat hippocampus, corticosterone has been shown to regulate neuronal metabolism, physiological functions, genomic expression, and alter neuronal morphology (McEwen and Magarinos 1997). Consequently, certain hippocampal functions (such as learning and memory) appear to be susceptible to uncontrollable stress (for reviews, see Sapolsky 1992; Kim and Yoon 1998; de Kloet et al. 1999).
In support of this idea, evidence is emerging that exposure to stress (and corticosteroids) impairs subsequent hippocampal-dependent forms of memory in both humans and animals (Lupien and McEwen 1997). For example, posttraumatic stress disorder patients exhibit deficits in verbal recall tasks (Bremner et al. 1993; Utto et al. 1993), and administration of high doses of cortisol in normal human subjects selectively impairs verbal declarative memory without affecting nonverbal (procedural) memory (Newcomer et al. 1994, 1999). Similarly, stress and stress hormones have been shown to impair succeeding spatial memory tasks in rats (Diamond and Rose 1994; Luine et al. 1994; Bodnoff et al. 1995; de Quervain et al. 1998; Diamond et al. 1999; Nishimura et al. 1999).
Consistent with the behavioral data are in vitro and in vivo electrophysiological studies indicating that stress and glucocorticoids impair hippocampal long-term potentiation (LTP), a putative synaptic mnemonic mechanism in the mammalian brain (Bliss and Lomo 1973; Teyler and DiScenna 1987; Bliss and Collingridge 1993; Martin et al. 2000). For example, hippocampal slices from rats that experienced uncontrollable tailshock stress exhibit LTP impairments in the CA1 and dentate gyrus regions (Foy et al. 1987; Shors et al. 1989; Shors and Dryver 1994; Kim et al. 1996, 2001). Other stressors (e.g., exposures to a brightly lit chamber and a cat predator) also impair LTP and/or primed burst potentiation (a low threshold form of LTP) in awake rats (Diamond and Rose 1994; Xu et al. 1997; Diamond et al. 1999). It is conceivable then that stress affects hippocampal memory via affecting hippocampal plasticity.
In rats, the majority of studies examining stress effects on memory utilize spatial learning tasks (e.g., Diamond and Rose 1994; Luine et al. 1994; Bodnoff et al. 1995; de Quervain et al. 1998; Nishimura et al. 1999). Thus, much less is known about stress effects on nonspatial hippocampal memory. A recent study reported that stress facilitates hippocampal-dependent trace eyeblink conditioning in rats (Beylin and Shors 1998), a finding which suggests that stress exerts differential effects on different forms of hippocampal memory—impairing spatial memory and enhancing nonspatial memory. To test this possibility, the present study examined stress effects on the visual paired comparison task (VPC), a test of nonspatial object recognition memory that has recently been shown to be hippocampal-dependent in rats (Clark et al. 2000). Specifically, we found that exposures to restraint–tailshock stress, which affects LTP in the hippocampus, also influences object-recognition memory in rats. Additionally, intrahippocampal infusions of the N-methyl-D-aspartate receptor (NMDAR) antagonist DL-2-amino-5-phosphonovaleric acid (APV), which pharmacologically mimic stress effects on hippocampal LTP, produce similar effects on recognition memory as stress.
RESULTS
Stress and VPC Task
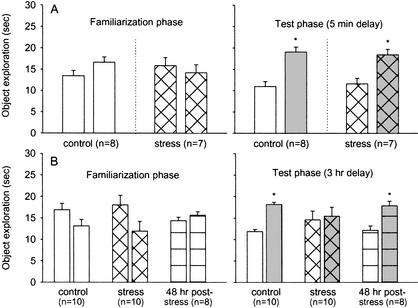
Figure 1 shows that stress did not affect object-recognition memory with a 5-min (short) delay between the familiarization-to-test phase; both control-5 min (t7 = 3.51; P < 0.01) and stress-5 min (t6 = 2.62; P < 0.05) groups spent significantly greater time exploring the novel object than the previously explored (familiar) object. During the familiarization phase, both groups exhibited a comparable amount of time exploring the two identical objects (both P > 0.2).
Figure 1.
(A) Mean time in seconds (+S.E.) control-5 min and stress-5 min animals spent exploring (left panel) two identical objects (unshaded bars) during the familiarization phase, and (right panel) 5 min later one novel object (shaded bars) and one previously explored, familiar (unshaded bars) object during the test phase. (B) Mean time in seconds (+S.E.) control-3 h, stress-3 h, and 48-h poststress-3 hr animals spent exploring (left panel) two identical objects (unshaded bars) during the familiarization phase and (right panel) 3 h later one novel object (shaded bars) and one previously explored (unshaded bars) object during the test phase. Asterisk denotes statistical significance.
With a 3-h (long) delay between the familiarization-to-test phase, animals in the stress-3 h group failed to exhibit preference for the novel object over the familiar object (t9 = 0.22; P > 0.05), whereas the control-3 h animals significantly did (t9 = 6.22; P < 0.01). However, the impairing effect of stress on the 3-hour-delay test was transient; animals that underwent stress experience 48 h prior to testing (the 48-h poststress-3 h group) spent reliably more time exploring the novel object than the familiar object t7 = 2.75; P < 0.05). During the familiarization phase, none of the groups exhibited statistically reliable left–right place bias by spending more time exploring an object placed in one corner over an identical object placed in another corner (all P > 0.2). The fact that familiar and novel objects were subsequently counterbalanced with respect to left and right corners effectively excludes the possibility of spatial bias influencing the results.
Table 1 shows the mean total time in the arena required for animals in control-5 min, stress-5 min, control-3 h, stress-3 h, and 48-h poststress-3 h groups to reach the 30-sec exploration criterion during the familiarization phase (two identical objects) and the test phase (a familiar object and a novel object). Except for the stress-5 min group—animals that required a significantly shorter time to reach the 30-sec exploration criterion during the familiarization phase (F1,13 = 5.3; P < 0.05]—all other groups required comparable amounts of time to reach the 30-sec exploration criterion during both the familiarization and test phases. Note, however, that the stress-5 min animals performed normally during the test phase by exhibiting a reliable preference for the novel object. Importantly, the fact that stress-3 h animals required similar amounts of exploration time during both the familiarization and test phases indicates that the impairment observed in this group is not due to alterations in the exploratory behavior.
Table 1.
Mean Total Time (in Sec ± S.E.) in the Arena to Reach 30-Sec Exploration Criterion
| Group | Familiarization phase | Test phase |
|---|---|---|
| Control (5 min) | 138.0 ± 25.2 | 170.6 ± 24.7 |
| Stress (5 min) | 72.2 ± 9.8 | 117.3 ± 15.8 |
| Control (3 h) | 126.1 ± 18.9 | 186.0 ± 21.1 |
| Stress (3 h) | 120.5 ± 23.3 | 212.0 ± 30.2 |
| 48-h Poststress (3 h) | 122.8 ± 13.0 | 177.5 ± 16.2 |
Stress and Hippocampal LTP
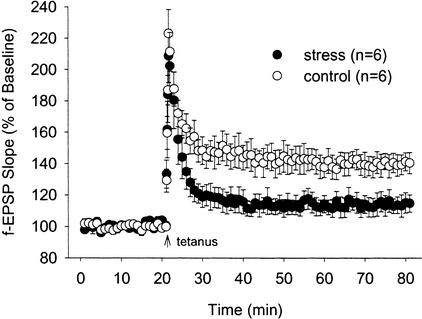
As shown in Figure 2, hippocampal slices prepared from animals that experienced the same stress (as those animals used in the VPC task) exhibited impaired LTP (normalized field excitatory postsynaptic potential [f-EPSP] slopes measured 40–60 min after the tetanus: 113.9 + 6.2%) compared to LTP observed from slices obtained from unstressed control animals (139.8 + 6.6%; F1,10 = 8.4; P < 0.05).
Figure 2.
Effects of restrain–tailshock stress on Schaffer collateral/commissural-CA1 long-term potentiation. Synaptic strength in the CA1 area of the hippocampus from control (open circles; n = 6) and stress (filled circles; n = 6) is expressed as a percentage of the average pretetanus field excitatory postsynaptic potentials (f-EPSP) over time (in minutes).
Intrahippocampal APV and VPC Task
Figure 3 shows a composite of the hippocampal injection sites from the 5-min (left panel) and 3-h (right panel) groups based on a reconstruction of cannulae placements (Paxinos and Watson 1997). As can be seen, all guide cannulae tips were located in or just above the dorsal hippocampus. Because the injection cannulae extended 1 mm beyond the guide cannulae, the drug infusions were reasonably targeted in the dorsal hippocampus.
Figure 3.
Location of injection sites based on a reconstruction of guide cannulae placements in the dorsal hippocampus from 5-min (left panel) and 3-h (right panel) groups of rats.
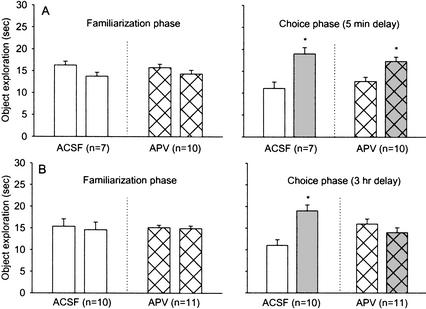
Figure 4 shows that animals in the artificial cerebrospinal fluid-5 min (ACSF) (t6 = 2.72; P < 0.05), APV-5 min (t9 = 2.4; P < 0.05) and ACSF-3 h (t9 = 2.99; P < 0.05) groups spent significantly greater time exploring the novel object than the familiar object. In contrast, the APV-3 h animals spent comparable time exploring novel and familiar objects (t10 = −0.89; P > 0.05), indicating that intrahippocampal APV disrupts recognition memory with long familiarization-to-test delays.
Figure 4.
(A) Mean time (+S.E.) ACSF-5 min and APV-5 min animals spent exploring (left panel) two identical objects during the familiarization phase and (right panel) 5 min later one novel object and one familiar object during the test phase. (B) Mean time (+S.E.) ACSF-3 h and APV-3 h animals spent exploring (left panel) two identical objects during the familiarization phase and (right panel) 3 h later one novel object and one familiar object during the test phase.
During the familiarization phase, none of the groups exhibited statistically reliable place bias of exploration; animals spent similar time exploring an object placed in the left corner and an identical object placed in the right corner (all P > 0.2). No reliable group differences were observed in the mean total arena time required to reach the 30-sec exploration criterion during the familiarization and test phases for the drug infusion groups (data not shown).
DISCUSSION
The present findings indicate that uncontrollable stress, which effectively impairs LTP in the hippocampus, impairs nonspatial recognition memory (as tested on the VPC task). Whereas control (unstressed) animals exhibited significant preferences for a novel object over a familiar object at both short (5 min) and long (3 h) delays, animals subjected to restraint–tailshock stress exhibited a reliable preference for a novel object only at a short delay; with a long delay, stressed animals spent comparable time exploring novel and familiar objects. The impairment observed with the stress-3 h group is not due to stress-induced alterations in motor or motivational factors because the total time in the arena during the familiarization and test phases did not significantly differ between the stress and nonstress groups. The fact that performance in recognition memory was normal with a short delay provides further evidence that stress did not produce changes in extraneous factors that could potentially affect recognition memory performance (such as motor behavior, motivation for exploration, or spontaneous novelty preference). Thus, experiencing stress, which impairs LTP in the hippocampus, selectively impairs long-delay recognition memory in rats.
Beck and Luine (1999) have also reported that chronic restraint stress (6 h per day for 21 d) impairs object-recognition memory when the delay was extended beyond 1 h; however, the effect of chronic restraint stress on hippocampal LTP was not examined. Given that the stress paradigm employed in the present study also impairs LTP in the hippocampus (Foy et al. 1987; Shors et al. 1989; Kim et al. 1996, 2001), it is plausible that stress-induced LTP impairments in the hippocampus might contribute to impairments in recognition memory. Consistent with this notion, the duration of stress effects on recognition memory (which lasted <48 h poststress) corresponds to the duration of stress effects on hippocampal LTP (which also lasts ≤48 h poststress; Garcia et al. 1997; Shors et al. 1997).
Similar to stress, microinfusions of the NMDAR antagonist APV directly into the hippocampus impaired object recognition memory at a 3- h (long) delay but not at a 5-min (short) delay on the VPC task. The concentration of APV used in this study is comparable to those found to effectively block LTP in the hippocampus (Collingridge et al. 1983; Morris 1989) and interfere with hippocampal-dependent spatial learning in the water maze (Davis et al. 1992) and contextual fear conditioning (Young et al. 1994). Importantly, the drug APV did not induce motor or motivational effects that might have indirectly affected the VPC task, as indicated by normal recognition memory at short delay and by comparable total amount of times required to explore the objects (during both familiarization and test phases) as ACSF-treated animals. These results suggest that recognition memory at long delays (but not at short delays) requires the involvement of some forms of NMDAR-dependent synaptic plasticity (e.g., LTP) in the hippocampus.
The possible connection between hippocampal plasticity (i.e., LTP) and recognition memory is also supported by a recent study showing that knockout mice that lack the NMDAR-2 subunit in the CA1 region of the hippocampus exhibit impairments in both hippocampal LTP (in the CA1 region) and performance on the VPC task (Rampon et al. 2000). Similarly, transgenic mice with overexpression of NMDA receptor 2B subunit exhibit enhanced hippocampal LTP and recognition memory (Tang et al. 1999).
Several human, monkey, and rat studies that employed object-recognition tasks indicate that the hippocampus plays an important role in recognition memory. For example, amnestic patients with relatively limited brain damage that includes the hippocampus exhibit impaired recognition memory with long, but not short, delays (McKee and Squire 1993). Another study found an equivalent impairment in spatial and nonspatial object recognition memory in human patients with hippocampal damage (Cave and Squire 1991). Similarly, monkeys with damage confined to the hippocampus (Zola et al. 2000) or with damage also including other medial temporal lobe regions (Bachevalier et al. 1990, 1993; Pascalis and Bachevalier 1999) were found to exhibit recognition memory impairments. Also rats with hippocampal damage do not seem to show the normal preference for investigating novel environments over familiar environments (O'Keefe and Nadel 1978). Recently, Clark et al. (2000) examined rats with either radio-frequency or ibotenic acid lesions of the hippocampus and found that object recognition memory was severely impaired at relatively long delays (10 min, 1 h, and 24 h) but not at short delays (10 sec and 1 min). Additionally, Wood et al. (1993) observed impairments in a nonspatial recognition task in rats with ischemic lesions of the hippocampus, with damage mainly restricted to the CA1 region. In contrast, rats with lesions of the fornix (which connects the hippocampus to the septum, mammillary bodies, and anterior thalamus but leaves hippocampal-cortical connections intact) were not impaired in the VPC (Ennaceur and Aggleton 1994, 1997; Ennaceur et al. 1996; Bussey et al. 2000; Clark et al. 2000). Thus, it appears that lesions of the hippocampus and lesions of the fornix are not functionally equivalent with respect to the VPC task.
As mentioned above, although stress has been found to impair hippocampal-dependent spatial memory (Diamond and Rose 1994; Luine et al. 1994; Bodnoff et al. 1995; de Quervain et al. 1998; Nishimura et al. 1999), stress has also been shown to facilitate cerebellar-dependent eyeblink conditioning in rats (Shors et al. 1992; Beylin and Shors 1998). Although both delay and trace eyeblink conditioning tasks are dependent on an intact cerebellum (for review, see Anderson and Steinmetz 1994), the hippocampus is also necessary for trace (but not delay) eyeblink paradigm (Solomon et al. 1986; Moyer et al. 1990; Kim et al. 1995). It is plausible then that stress acts via the cerebellum in enhancing both delay and trace eyeblink learning. This possibility, however, is not supported by the fact that the cerebellum also plays a role in the spatial water-maze task in rodents (for review, see Lalonde 1994), which is impaired by stress. Because stress also facilitates fear conditioning (Maier 1990; Pugh et al. 1997a,b; Conrad et al. 1999; Rudy et al. 1999), it has also been suggested that stress exerts differential effects on different kinds of learning—enhancing classical conditioning but impairing instrumental learning (Shors 1998). Although the present finding that stress impairs long-delay object-recognition memory is consistent with this view, a recent study reported that the same stress, which impairs hippocampal LTP, also enhances a hippocampal-independent cue-platform learning in a water-maze task, an instrumental task (Kim et al. 2001). Thus, it appears stress is likely to produce a complex effect on different learning systems.
In summary, both stress, which impairs hippocampal LTP, and intrahippocampal infusions of the NMDAR antagonist APV, which also blocks hippocampal LTP, reliably and similarly affected object-recognition memory in rats. Our results highlight the critical involvement of the hippocampus in nonspatial memory and also underscore the importance of understanding the potential of stress effects on normal functions of the hippocampus.
MATERIALS AND METHODS
Subjects
Experimentally naive male Charles River Long-Evans rats (weighing 275–300 g) were individually housed in our Association for Assessment and Accreditation of Laboratory Animal Care (AAALC) accredited animal care facility and maintained on a 12:12-h light:dark cycle (lights on at 7:00 a.m.). They were permitted free access to food and water and gently handled daily to minimize stress. All experiments were conducted during the light phase of the cycle, and were in strict compliance with Yale Animal Resource Center guidelines.
Stress Paradigm
Rats were restrained in a Plexiglas tube and exposed to 60 tailshocks (1-mA intensity; 1-sec duration; 60-sec intershock interval). This stress procedure, adapted from the learned-helplessness paradigm—in which animals undergo an aversive experience under conditions in which they cannot perform any adaptive response (e.g., Seligman and Maier 1967; Maier and Seligman 1976)—effectively impairs hippocampal LTP in rats (Foy et al. 1987; Shors et al. 1989; Kim et al. 1996, 2001).
Stress and VPC Task
The VPC procedure described by Clark et al. (2000) was used in this study. The VPC is an object-recognition task that utilizes the rat's natural tendency to explore novel stimuli (Ennaceur and Delacour 1988), is distinct from other behavioral patterns (e.g., trial-and-error learning; Lorenz 1981), and is analogous to cognition studies with human infants (Cohen et al. 1971). This task is nonspatial in nature and is viewed as a pure object recognition task because there is no rule learning or reward/punishment involved (Clark et al. 2000). A total of 43 rats were used.
Apparatus
Testing took place inside an open-field arena (60 × 60 × 45 cm high; constructed of wood and painted white) placed on a table (80 cm from the floor) with a white-noise source (centered beneath the table) providing constant background noise (72 dB) and illuminated by a fluorescent room light. A CCD camera mounted on the ceiling was connected to a monitor, VCR, and computer (located outside the room) to observe and record the animal's behavior.
Objects
Three identical sets of different objects (made of glass, plastic, metal, or ceramic and varied in shape and texture) were used. Objects were chosen based on weight and size; they were no larger than the rat and were relatively immovable when placed in the open field. All rats were exposed to two different types of objects (to become familiar and novel) simultaneously with the order of object presentations counterbalanced. Also the familiar and novel objects were always placed in the same two corners of the arena in a counterbalanced manner (to preclude possible spatial-location bias).
Habituation
All animals were transported to a waiting room (adjacent to the testing room) and handled for 5 consecutive days. Between 30 and 60 min after transportation, each rat was placed inside the open-field arena (always facing the wall opposite the wall where objects will be placed later) and allowed to explore and become familiar with the arena (context) for 5 min. No object was placed inside the arena during habituation. The open-field was wiped with 5% ammonium hydroxide prior to habituation of the next rat. After 5 min had elapsed, the animals were placed back in the waiting room before being transported back to the vivarium.
Testing
All animals were subjected to a single test trial consisting of a familiarization phase followed by a test phase (Fig. 1).
Familiarization phase
Animals in the stress groups were tested either between 30 and 60 min after experiencing stress or 48 h poststress. Animals in the unstressed control groups were tested in a time-matched manner with the stressed animals. At the start of a trial, each animal was placed in the empty arena (wiped previously with 5% ammonium hydroxide) for 1 min of rehabituation. Afterwards, animals were placed in a holding cage and two identical objects were placed in the arena. Animals were then placed back in the arena and remained there until they had explored the objects for 30 sec (Clark et al., 2000). Upon reaching this criterion, animals were placed back in their home cages, located in the waiting room, for a delay of 5 min or 3 h prior to the test phase.
Test Phase
During the delay period, two objects were placed in the same position as the familiarization phase: one object identical to those in the familiarization phase (but not scent-marked) and the other a novel object. At the end of the delay interval (5 min or 3 h), animals were returned to the arena and remained inside until they again accumulated a total of 30 sec exploration of the two different objects.
In total, there were five groups of animals: (1) control with 5-min delay (control-5 min), (2) stress with 5-min delay (stress-5 min), (3) control with 3-h delay (control-3 h), (4) stress with 3-h delay (stress-3 h), and (5) 48 h after stress with 3-h delay (48-h poststress-3 h).
Scoring
A custom-written computer-assisted scoring program (in QBASIC) was used to score exploratory behavior in the manner similar to Clark et al. (2000). Manual keystrokes on the computer keyboard recorded the duration and the frequency of exploration of objects. Exploration was scored only when the rat's head was (1) within a predefined object boundary outlined on the monitor screen and (2) directed toward the object. Exploration was not scored when the rat climbed on top of the object or if another part of the rat's body touched the object. The computer scoring of behavior was performed by a trained observer who was unaware of the subjects' treatment. Because the times spent exploring the two objects (during familiarization and test phases) are obtained from the same animals, the one-sample t-test (two-tailed significance; with the test value setting of 15 sec denoting no object preference) was employed to analyze the data (Kirk 1984).
Stress and Hippocampal LTP
Twelve naive rats were used in the slice experiment. Between 30 and 60 min after stress, animals were decapitated under halothane anesthesia and hippocampal slices were prepared in a standard manner (Teyler 1980). In brief, transverse hippocampal slices (400 μm) were maintained in an interface-recording chamber (Fine Science Tools), continuously perfused (∼2 mL/min) with 95% O2- and 5% CO2-saturated artificial cerebrospinal fluid (124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO2, 26 mM NaHCO3, 3 mM CaCl2, and 10 mM glucose) at 32°C. After a stabilization period of at least 1 h, a concentric bipolar electrode (25 μm inner-contact diameter) delivering 100 μsec pulses stimulated the Schaffer collateral/commissural fibers. A glass electrode filled with 2 M NaCl (1.5–2.5 MΩ) was placed in the stratum radiatum in CA1 under a microscope to record f-EPSPs. The test stimulus intensity was adjusted to produce a response that was 50% of the maximum evoked responses (without population spike). Baseline synaptic transmission was monitored every 20 sec for 20 min before delivering a tetanus (five trains of 100 Hz, each lasting 200 msec at an intertrain interval of 10 sec). The f-EPSPs (amplified in the band of 0.1–5000 Hz) were monitored up to 1 h after the tetanus. During the tetanus, f-EPSPs evoked by the first pulse in each of the five trains were recorded to assess the development of potentiation. Data were collected and analyzed online using a computer program written in AXOBASIC/QUICKBASIC (Axon Instruments). The initial (negative) slope of the f-EPSPs was used in statistical analyses (Kim et al. 1996). Only those slices that exhibited a stable baseline for 20 min were included in the analysis. The change in f-EPSPs after tetanus was averaged across slices for each rat (two hippocampal slices per rat). The magnitude of LTP was measured between 40 and 60 min after the tetanus and statistical comparisons (one-way ANOVA with repeated measures) were made between the two groups of slices (control and stress).
Intrahippocampal APV and VPC Task
Recently, it has been shown by Clark et al. (2000) that both radio frequency and excitotoxic (ibotenic acid) lesions of the hippocampus impair VPC task, suggesting that the hippocampus is critically involved in recognition memory in rats. To test whether pharmacological blockade of synaptic plasticity (LTP) in the hippocampus affects the VPC task similarly to stress, here we examined the effects of the NMDA receptor antagonist APV infusions into the hippocampus on the VPC task. If stress effects on VPC task (i.e., with a 3-h delay) are due to stress affecting synaptic plasticity in the hippocampus, then intrahippocampal infusions of APV (at concentrations known to block hippocampal LTP) should produce effects on VPC similar to stress.
Surgery
A total of 39 rats were used. Animals were anesthetized via intraperitoneal injection of a ketamine (30 mg/kg)/xylazine (2.5 mg/kg) solution with supplemental injections given as needed. Under aseptic conditions, a stereotaxic instrument (Stoelting) was used to implant 23 gauge guide cannulae bilaterally into the dorsal hippocampus (from bregma: anteroposterior, −2.8 mm; mediolateral, ±2 mm; dorsoventral, −2.8 mm; anteroposterior, −4.1 mm; mediolateral, ±3 mm; and dorsoventral, −2.8 mm; a total of 4 cannulae). Implanted cannulae were cemented to three anchoring screws on the skull. During 7 d of postoperative recovery, the rats were adapted to transportation and handling, and each day the stylet was removed and replaced with a clean one.
Drugs, Injection, and VPC Task
APV (RBI), dissolved in ACSF (pH ∼7.4), was microinfused into the hippocampus (3.75 μg per site; a total of 4 sites) by backloading the drug up a 30-gauge internal cannula into polyethylene (PE 10) tubing connected to 10-μL Hamilton microsyringes (Hamilton Company). The internal cannula extended 1 mm beyond the guide cannula. An injection volume of 0.45 μL (per side) was delivered using a Harvard PHD 2000 (Harvard Apparatus) syringe pump over the course of 3 min (at a rate of 0.15 μL/min). The internal cannula remained in place for at least 30 sec after the infusions before being pulled out. The dosage of APV (3.75 μg/0.45 μL per injection site) used is comparable to the APV concentrations that have been shown to effectively block the induction of LTP in the hippocampus (Morris 1989) and impair hippocampal-dependent spatial learning in the Morris water-maze task (Davis et al. 1992).
Approximately 3 min after APV and ACSF infusions into the hippocampus, animals underwent the VPC task (with familiarization-to-test delay periods of 5 min and 3 h) in the same manner described above. In total, there were four groups of animals: (1) ACSF with 5-min delay (ACSF-5 min), (2) APV with 5-min delay (APV-5 min), (3) ACSF with 3-h delay (ACSF-3 h), and (4) APV with 3-h delay (APV-3 h).
Histology
At the completion of behavioral testing, the rats were overdosed with ketamine HCl and xylazine and perfused intracardially with 0.9% saline, followed by 10% buffered formalin. The brains were removed and stored in 10% formalin for at least 2 wk before slicing. Transverse sections (80 μm) were taken through the extent of the cannulae placement, mounted on gelatinized slides, and stained with cresyl violet dye. An observer unaware of the behavioral data determined the locations of the cannulae tips, and subjects with inaccurate cannulae placements (i.e., one or more cannulae tips misplaced) were excluded from the statistical analysis. One animal from the 3-h APV group was excluded.
Acknowledgments
We thank Dr. Hugh T. Blair for the computer data-scoring program, Dr. Karyn M. Frick for helpful comments on the manuscript, and Toni Mundy and Robin Gardner for their assistance in the experiment. This work was supported by the Whitehall Foundation, Claude D. Pepper Older Americans Independence Center (NIA P60AG10469), and Yale Junior Faculty Fellowship (J.J.K.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jeansok.kim@yale.edu; FAX (203) 432-7172.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.46102.
REFERENCES
- Anderson BJ, Steinmetz JE. Cerebellar and brainstem circuits involved in classical eyeblink conditioning. Rev Neurosci. 1994;5:251–273. doi: 10.1515/revneuro.1994.5.3.251. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C, Mishkin M. Age and sex differences in the effects of selective temporal lobe lesion on the formation of visual discrimination habits in rhesus monkeys (Macaca mulatta) Behav Neurosci. 1990;104:885–899. doi: 10.1037//0735-7044.104.6.885. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav Neurosci. 1998;112:1327–1338. doi: 10.1037//0735-7044.112.6.1327. [DOI] [PubMed] [Google Scholar]
- Bliss TP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Equivalent impairment of spatial and nonspatial memory following damage to the human hippocampus. Hippocampus. 1991;1:329–340. doi: 10.1002/hipo.450010323. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LB, Gelber ER, Lazar MA. Infant habituation and generalization to differing degrees of stimulus novelty. J Exp Child Psychol. 1971;11:379–389. doi: 10.1016/0022-0965(71)90043-9. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann N Y Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: Effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- ————— The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1. Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Garcia R, Musleh W, Tocco G, Thompson RF, Baudry M. Time-dependent blockade of STD and LTP in hippocampal slices following acute stress in mice. Neurosci Letters. 1997;233:41–44. doi: 10.1016/s0304-3940(97)00621-6. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Yoon KS. Stress: Metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han J-S, Packard M. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation (LTP) and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Elementary statistics. 2nd ed. Monterey, CA: Brooks/Cole; 1984. [Google Scholar]
- Lalonde R. Cerebellar contributions to instrumental learning. Neurosci Biobehav Rev. 1994;18:161–170. doi: 10.1016/0149-7634(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Lorenz KZ. The foundations of ethology. New York: Springer-Verlag; 1981. [Google Scholar]
- Luine VN, Villegas M, Martinez C, McEwen BS. Repeated stress causes impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: Theory and evidence. J Exp Psychol. 1976;105:3–46. [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids and hippocampus: Receptors in search of a function. Curr Top Neuroendocrinol. 1982;2:1–22. [Google Scholar]
- McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann NY Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cogn. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: Selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Nishimura J, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neurosci Letters. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997a;111:503–511. [PubMed] [Google Scholar]
- Pugh CR, Fleshner M, Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiol Learn Mem. 1997b;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Kuwagama K, Pugh CR. Isolation reduces contextual but not auditory-cue fear conditioning: A role for endogenous opioids. Behav Neurosci. 1999;113:316–323. doi: 10.1037//0735-7044.113.2.316. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, the aging brain, and the mechanisms of neuron death. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP, Maier SF. Failure to escape traumatic shock. J Exp Psychol Gen. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stress and sex effects on associative learning: For better or for worse. Neuroscientist. 1998;4:353–364. [Google Scholar]
- Shors TJ, Dryver E. Effect of stress and long-term potentiation (LTP) on subsequent LTP and the theta burst response in the dentate gyrus. Brain Res. 1994;666:232–238. doi: 10.1016/0006-8993(94)90777-3. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Gallegos RA, Breindl A. Transient and persistent consequences of acute stress on long-term potentiation (LTP), synaptic efficacy, theta rhythms and bursts in area CA1 of the hippocampus. Synapse. 1997;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Tang Y-P, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Teyler TJ. Brain slice preparation: Hippocampus. Brain Res Bulletin. 1980;5:391–403. doi: 10.1016/s0361-9230(80)80009-8. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- Utto M, Vasterling JJ, Brailey K, Sutker PB. Memory and attention in combat-related posttraumatic-stress-disorder (PTSD) J Psychopath Behav Assess. 1993;15:43–52. [Google Scholar]
- Wood ER, Mumby DG, Pinel JP, Phillips AG. Impaired object recognition memory in rats following ischemia-induced damage to the hippocampus. Behav Neurosci. 1993;10:51–62. doi: 10.1037//0735-7044.107.1.51. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: Immunization against amnesia by context preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired cognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]