Abstract
Helicases are molecular motor proteins that couple the hydrolysis of NTP to nucleic acid unwinding. The growing number of DNA helicases implicated in human disease suggests that their vital specialized roles in cellular pathways are important for the maintenance of genome stability. In particular, mutations in genes of the RecQ family of DNA helicases result in chromosomal instability diseases of premature aging and/or cancer predisposition. We will discuss the mechanisms of RecQ helicases in pathways of DNA metabolism. A review of RecQ helicases from bacteria to human reveals their importance in genomic stability by their participation with other proteins to resolve DNA replication and recombination intermediates. In the light of their known catalytic activities and protein interactions, proposed models for RecQ function will be summarized with an emphasis on how this distinct class of enzymes functions in chromosomal stability maintenance and prevention of human disease and cancer.
Keywords: aging, cancer, DNA repair, genomic instability, helicase, RecQ
Abbreviations: ALT, alternative lengthening of telomeres; at, Arabidopsis thaliana; ATP[S], adenosine 5′-[γ-thio]triphosphate; BACH1, BRCA1-associated C-terminal helicase; BaP, benzo[a]pyrene; BcPh, benzo[c]phenanthrene; BER, base excision repair; BLAP75, BLM-associated protein 75; BS, Bloom syndrome; DE, diol epoxide; dm, Drosophila melanogaster; DNA-PK, DNA-dependent protein kinase; ds, double-stranded; DSB, double-strand break; ES cells, embryonic stem cells; EXO-1, exonuclease 1; FEN-1, flap endonuclease 1; G4, G-quadruplex; HJ, Holliday junction; HR, homologous recombination; HRDC, helicase and RNaseD C-terminal; NHEJ, non-homologous end-joining; PARP-1, poly(ADP-ribose) polymerase-1; PCNA, proliferating cell nuclear antigen; PML, promyelocytic leukaemia; pol, DNA polymerase; RAPADILINO syndrome, radial hypoplasia/aplasia, patellae hypoplasia/aplasia and cleft or highly arched palate, diarrhoea and dislocated joints, little size and limb malformation, nose slender and normal intelligence; rDNA, ribosomal DNA; RPA, replication protein A; RQC, RecQ C-terminal; RTS, Rothmund–Thomson syndrome; SCE, sister chromatid exchange; SDSA, synthesis-dependent strand annealing; SF, superfamily; ss, single-stranded; SSB, ssDNA-binding protein; t-loop, telomeric loop; Top3, topoisomerase III; TRF, telomeric repeat-binding factor; WEX, Werner-like exonuclease; WS, Werner syndrome
INTRODUCTION
Understanding the molecular mechanisms of RecQ helicases is fundamental to deciphering the roles of these enzymes in cellular DNA metabolism. Since the discovery of Escherichia coli RecQ and its implicated role in genetic recombination, the world of RecQ helicases has become significantly more complex with the identification and characterization of a number of eukaryotic RecQ helicases that are important in the replicational stress response and maintenance of genomic stability.
Classification of RecQ helicases and molecular-genetic analyses of their biochemical and cellular functions gained prominence with the understanding that certain rare genetic disorders [WS (Werner syndrome), BS (Bloom syndrome) and RTS (Rothmund–Thomson syndrome)] are a consequence of mutations in the human RecQ genes WRN, BLM and RECQ4 respectively. WS is characterized by premature aging with an elevated risk of age-associated diseases such as cancer, atherosclerotic cardiovascular disease, diabetes mellitus (Type II) and osteoporosis [1]. Epigenetic inactivation of WRN is detected in a number of human cancers [2]. BS is associated with a very high incidence of different types of cancers, both solid tumours and leukaemia, and also manifested by skin disorders, proportional dwarfism, immunodeficiency and male sterility [1]. People with RTS, also known as poikiloderma congenitale, displays growth deficiency, photosensitivity with poikilodermatous skin changes, early greying and hair loss, juvenile cataracts and a predisposition to malignancy, especially osteogenic sarcomas [1]. Apart from RTS, RECQ4 mutations were detected in Finnish patients with an autosomal recessive disorder RAPADILINO syndrome [radial hypoplasia/aplasia, patellae hypoplasia/aplasia and cleft or highly arched palate, diarrhoea and dislocated joints, little size (height at least 2 S.D. smaller than the average height) and limb malformation, nose slender and normal intelligence] [3]. Although many features of the two genetic disorders overlap, poikiloderma, a hallmark of RTS, has been described as being generally absent from RAPADILINO syndrome. RECQL4 mutations have also been identified in a subgroup of patients with Baller–Gerold syndrome, a rare autosomal recessive condition with radial aplasia/hypoplasia and craniosynostosis [4]. In addition to WRN, BLM and RECQ4, the two other human RecQ helicases are RECQ1 and RECQ5, which are not yet genetically linked to a disease. Conceivably, mutations in RECQ1 or RECQ5 might also lead to a hereditary chromosomal instability disorder or predispose individuals to cancer.
Understanding RecQ deficiency related to genetic pathways of cellular transformation in cancer and other human diseases is an active area of investigation. Results from biochemical studies continue to set the foundation for the proposed models of RecQ cellular functions in DNA transactions. The essence of this review will be to summarize our current knowledge of the mechanisms of RecQ DNA helicases in pathways of cellular DNA metabolism in order to build on previous work and understand better the involvement of RecQ helicases in genome stability maintenance. Although the scope of this review will focus on the biochemical functions of RecQ helicases in DNA replication, repair and recombination, other cellular pathways, such as transcription, may also involve RecQ helicases. Gene expression profiling in WS closely resembles that of normal aging, suggesting that the transcription alterations common to WS and normal aging represent general events in the aging process [5]. Cells from WS and old individuals had similarly aberrant transcriptional responses to IR (ionizing radiation) and UV irradiation, suggesting a role for WRN in stress-induced gene expression [6]. The cellular studies clearly indicate that WS has multiple deficiencies and the WRN gene product is likely to have pleiotropic functions in vivo. The same may hold true for the other RecQ helicases.
SEQUENCE HOMOLOGY AND STRUCTURAL ASPECTS
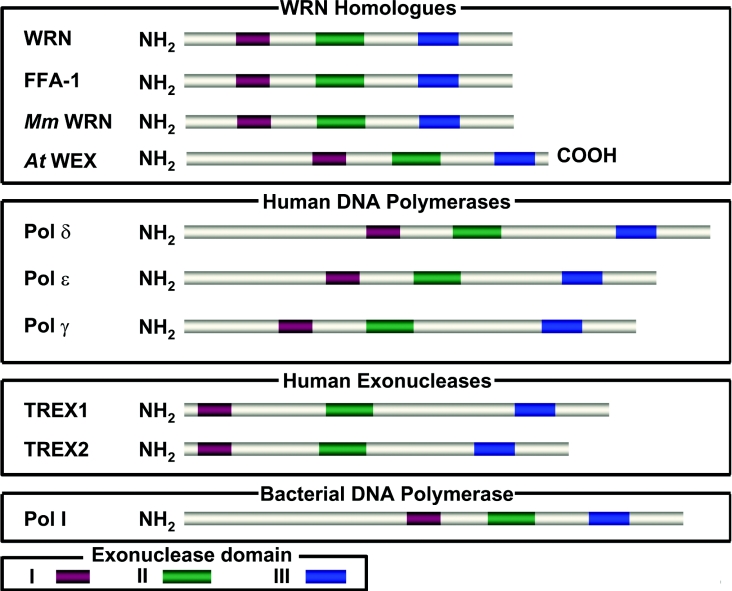
The RecQ helicase domain is highly conserved in evolution from bacteria to humans. A single RecQ family member exists in bacteria and budding or fission yeast, whereas there are generally multiple representatives in higher eukaryotes, including five identified RecQ proteins in humans (Figure 1). Members of the RecQ family can be distinguished from other helicases not only by the sequence homology of the conserved motifs in the ∼450-amino-acid helicase domain, but also by the existence (in most members) of two additional conserved regions: the RQC (RecQ C-terminal) and HRDC (helicase and RNaseD C-terminal) motifs (Figure 1, and discussed below). Recent structural data from RecQ protein domain fragments have ignited an interest in the field to understand RecQ protein architecture, and how the conserved domains are important for biochemical and cellular functions [7].
Figure 1. Schematic representation of RecQ helicase family members.
Human members of the RecQ family of DNA helicases are shown. Proteins are aligned by their conserved RecQ helicase domain. The conserved domains and motifs in each group are shown by different colours as depicted at the bottom.
RecQ signature helicase motifs
Helicases catalytically separate ds (double-stranded) DNA through the binding and hydrolysis of NTP. The unwinding activities of helicases are co-ordinated through a series of seven sequence motifs (I, Ia, II, III, IV, V and VI) that are hallmarks of both SF (superfamily) -1 and -2 helicases. The RecQ family (SF2) contains these seven motifs plus an additional motif (motif 0) that is N-terminal to motif I [8].
An important function of motif 0 is suggested by the observation that a mutation in this region of Sgs1 leads to both a hyper-recombination phenotype and sensitivity to DNA damage [9]. A mutation of the conserved motif 0 glutamine to arginine is sufficient to cause BS [10]. Furthermore, a mutation of the corresponding glutamine in the murine BLM homologue abolishes its ATPase and DNA-unwinding activities [11]. A solution structure of E. coli RecQ provided evidence that motif 0 contributes to the adenine-binding pocket [12]. Motif I in the RecQ family appears to make the canonical phosphate and metal contacts [12]. In support of this, mutations of the invariant phosphate-binding lysine residue in motif I of the human RecQ helicases WRN [13], BLM [14], RECQ1 [15] and RECQ5β [16], and the yeast Sgs1 helicase [17] seriously impair or abolish their ATPase and DNA-unwinding activities, suggesting a functionally conserved role of this motif. Motif II in RecQ proteins represents the canonical Walker B motif [7] and is therefore implicated in NTP hydrolysis.
E. coli RecQ contains a conserved aromatic-rich loop in its helicase domain between motifs II and III that maps to a similar tertiary position as found in the SF1 helicases [18]. The conserved aromatic-rich loop in motif III of SF1 helicases mediates both ATP and ss (single-stranded) DNA binding [19,20]. Mutational analysis of the RecQ aromatic-rich loop provided evidence that this region is critical for coupling ATPase and DNA-binding/unwinding activities [18]. The crystal structure of E. coli RecQ [12] has provided further insight into the functional importance of the conserved helicase motifs (see below).
Conserved RQC, HRDC and other protein domains of RecQ family members
The RQC motif residing just C-terminal to the conserved helicase domain is found in the vast majority of RecQ helicases, with the exception of RECQ4, RECQ5α and RECQ5γ (Figure 1). The RQC region was first determined to contain a Zn2+-binding domain and a winged helix domain from the crystal structure of E. coli RecQ [12]. Mutations in E. coli RecQ within the Zn2+-binding domain severely impaired its DNA binding [21]. Modelling studies suggested a Zn2+-binding domain in the BLM RQC region, and mutational analyses implicate its role in DNA binding and protein conformation [22]. A structure of a recombinant human WRN protein fragment indicated that the WRN RQC region also forms a Zn2+-binding domain and winged helix domain [23]; furthermore the WRN RQC has been shown to bind DNA [24,25] and other proteins (for a review, see [26]).
In addition to the catalytic core, a conserved ∼80-amino-acid motif designated HRDC found in a number of DNA-metabolizing proteins is present in most RecQ family members (Figure 1). Indeed, the HRDC domain of Sgs1 [27] and E. coli RecQ [28] has been shown to bind ssDNA in vitro, suggesting an auxiliary function in substrate recognition. In WRN, the HRDC, RQC and exonuclease motifs confer a DNA substrate-binding specificity [25]. The radioresistant bacterium Deinococcus radiodurans RecQ helicase has three HRDC domains at its C-terminus that are involved in DNA binding and regulatory functions of the helicase [29].
WRN and its homologues contain three motifs near the N-terminus that share identity with the exonuclease domain of E. coli pol (DNA polymerase) I and RNaseD [30]. The conserved exonuclease motifs in WRN are found in three human DNA polymerases that contain proofreading exonuclease domains and two exonucleases that are proposed to have auxiliary proofreading functions (Figure 2).
Figure 2. Conserved nuclease domain of WRN and 3′ to 5′ exonucleases.
The top panel depicts the three conserved exonuclease motifs of human WRN and its homologues in lower eukaryotes. The two middle panels depict human DNA polymerases and exonucleases which share identity with WRN exonuclease. The bottom panel depicts a prokaryotic DNA polymerase with the conserved exonuclease domains. The conserved exonuclease domains are colour-coded as indicated at the bottom. Mm, Mus musculus.
Some of the RecQ homologues have strongly acidic regions primarily in the N-terminal region before the helicase domain (Figure 1). WRN has a highly acidic 27-amino-acid direct repeat located in the N-terminus between the exonuclease and helicase domains; one copy of the same sequence is found in Xenopus WRN (FFA-1). This acidic repeat in WRN has been implicated in transcriptional activation [31] and the physical interaction with the heterotrimeric SSB (ssDNA-binding protein) RPA (replication protein A) [32].
Insights from structures of RecQ protein domains
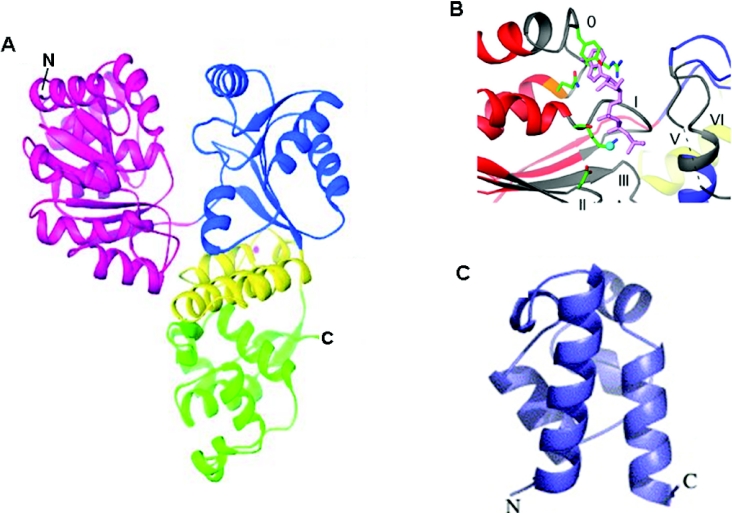
A complete structure for any of the full-length RecQ family helicases has not been obtained to date. However, crystal structures of regions of E. coli RecQ have been obtained. A catalytically active E. coli RecQ domain fragment lacking the C-terminus after the conserved helicase and RQC domains (RecQΔC) was characterized, revealing a tri-lobed modular molecule that has a ‘Y’ shape with an apparent cleft on the surface [12] (Figure 3A). The N-terminal and C-terminal portions constitute each side of the cleft which is lined with the conserved helicase motifs, an arrangement that is similar to other SF2 family helicases. The RecQ helicase crystallized with ATP[S] (adenosine 5′-[γ-thio]triphosphate) indicated that the ATP analogue is packed into a binding site that includes residues from motifs 0, I and II [12] (Figure 3B).
Figure 3. Structures of conserved RecQ protein domains.
(A) X-ray crystal structure of the E. coli RecQ catalytic core [12]. Helicase lobes are coloured red and blue, the Zn2+-binding domain and winged helix domain of the RQC region are in yellow and green respectively. A bound Zn2+ ion is shown as a magenta sphere. (B) Close-up of the ATP-binding site in the structure of the ATP[S]-bound E. coli RecQ catalytic core [7,12]. A bound Mn2+ ion is shown as a cyan sphere. (C) X-ray crystal structure of the E. coli RecQ HRDC domain [28]. Figures are courtesy of Dr James Keck (University of Wisconsin Medical School, Madison, WI, U.S.A.).
The E. coli RecQ crystal structure also revealed a Zn2+-binding site constituted by four α-helices that ligand a Zn2+ ion via four highly conserved cysteine side chains in the RQC (Figure 3A). These cysteine residues have been demonstrated to be important for the cellular roles of RecQ helicases [9,14,33,34]. The RQC motif also contains the structural helix–turn–helix fold which is termed a winged helix domain (Figure 3A). The winged helix domain is structurally similar to a number of DNA-binding domains from other proteins. The recognition helix and wing of the winged helix domain make contacts with dsDNA (see [7] for details). A large cleft found at the intersection of the winged helix and the Zn2+-binding domains in RecQ is proposed to serve as a site for the binding of DNA, possibly dsDNA [12]. A recent solution structure of the human WRN RQC region was also shown to contain a Zn2+-binding domain and a winged helix-like motif [23]. It was proposed that the winged helix of WRN serves as a multi-functional domain that interacts with structure-specific DNA and a variety of DNA-processing proteins.
Bernstein and Keck [28] obtained a high-resolution crystal structure of the HRDC region of E. coli RecQ (Figure 3C). The domain folds independently and forms a globular bundle of six helices that resemble the Saccharomyces cerevisiae Sgs1 HRDC domain [27] and auxiliary DNA-binding domains of Geobacillus stearothermophilus PcrA, E. coli Rep, and rat pol β. On the basis of the structural data and mutagenesis studies, it was suggested that the sequence variations in the HRDC domains in RecQ helicases might serve to target the helicase to different types of DNA structures [28].
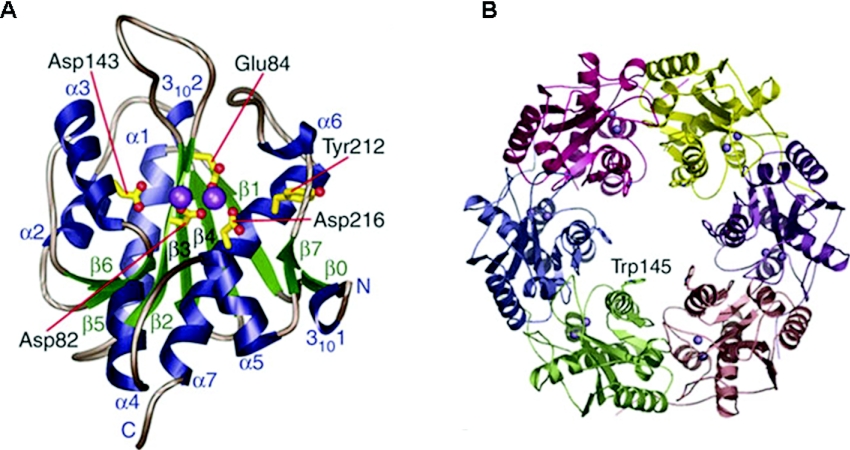
To acquire structural and functional information for the exonuclease domain of WRN protein, crystal structures of complexes containing a catalytically active WRN exonuclease (WRN-exo) domain fragment [WRN-(1–333)] with metal ion were recently solved [35]. The WRN-exo domain has an αβ fold with a central β-sheet surrounded by α-helices and a small two-stranded sheet that shows an overall structural similarity to the DnaQ family of 3′ to 5′ exonucleases (Figure 4A). The metal ion–WRN-exo structural complexes and results from biochemical characterization of WRN nuclease domain site-directed mutant proteins indicated a conserved structure and mechanism with the replicative proofreading exonucleases (Figure 2). Two bivalent cation metal ions (Mg2+ or Mn2+) are accommodated within the active site of the WRN exonuclease domain, suggesting that the WRN exonuclease domain degrades DNA substrates by a two-metal-ion-mediated mechanism, as observed for the Klenow exonuclease [36]. A WRN ring homology model was built based on the structural superimposition of the WRN-exo domain fragment with the Arabidopsis thaliana homologue [35] (Figure 4B), revealing the location of the active site of the exonuclease which faces the centre of the ring. The central cavity of the WRN ring is large enough to accommodate dsDNA, suggesting a possible regulatory mechanism with the Ku heterodimer that also forms rings and stimulates WRN exonuclease activity [26].
Figure 4. WRN exonuclease structure.
(A) X-ray crystal structure of the WRN-exo fold is shown with the conserved active site residues that chelate the two Mn2+ ions (purple) [35]. (B) The WRN-exo hexameric ring model with uniquely coloured WRN-exo subunits was built by superimposition with the A. thaliana homologue [35]. Figures are courtesy of Dr John Tainer (The Scripps Research Institute, La Jolla, CA, U.S.A.).
CATALYTIC ACTIVITIES
Background on helicase mechanism
All helicases unwind nucleic acid with a distinct directional polarity, either 3′ to 5′ or 5′ to 3′ with respect to the strand on which the helicase is presumed to translocate [37]. Helicases utilize energy from the hydrolysis of ATP (or other NTPs), which allows them to translocate and unwind the duplex substrate. Two types of mechanisms have been proposed for duplex unwinding by DNA helicases: passive and active. In passive mechanisms, the helicase itself does not destabilize the duplex, but rather it translocates forward, trapping ssDNA that is released due to thermal fraying of the duplex at the ssDNA/dsDNA junction. On the other hand, in active mechanisms, the helicase dynamically destabilizes the duplex ahead of the fork with one DNA-binding site, while simultaneously holding the unwound ssDNA with the second DNA-binding site.
Several models for the active mechanism have been proposed (for reviews, see [37,38]) (Figure 5). In the inchworm model [20], the functional helicase has two non-identical DNA-binding sites that bind with a defined polarity; the leading site interacts with the duplex region during successive cycles of unwinding, whereas the tail site interacts with the ssDNA. A co-operative inchworm mechanism has been proposed in which multiple helicase molecules line up along the ssDNA lattice [39,40]. The rolling model requires at least two identical DNA-binding sites, both of which can bind to ssDNA and dsDNA, in an alternating fashion. The rolling model requires a dimeric protein, whereas the inchworm model is consistent with any oligomeric state, including a monomer. An alternative multimeric form is the hexameric helicase, which is believed to have a ring structure that encircles one strand of the duplex leaving the other extruded outside the ring. The hexameric helicase may have an advantageous design to prevent premature dissociation of the functional helicase molecule from the DNA substrate. Whether the unwinding mechanism(s) of RecQ helicases could be generally classified into any one of these models or depends on the nature of the DNA substrate to be unwound is of interest for understanding their DNA transactions.
Figure 5. Models depicting active unwinding mechanisms for DNA helicases.
For all of the mechanisms depicted, DNA unwinding is fuelled by the energy of helicase-catalysed NTP hydrolysis. (A) Inchworm mechanism for a monomeric helicase in which the enzyme undergoes energetic and conformational changes to translocate unidirectionally along DNA and destabilize the duplex. (B) Co-operative inchworm mechanism characterized by the alignment of enzyme molecules to promote forward movement and increase helicase activity when multiple helicase monomers co-operate. (C) Rolling model mechanism in which the protomers of a dimeric helicase alternate in binding ssDNA or dsDNA as the enzyme translocates and destabilizes the duplex. (D) Hexameric helicase unwinding mechanism characterized by a ring structure that encircles one strand and displaces the other strand which is extruded outside the ring.
DNA unwinding catalysed by RecQ helicases
Consistent with the presence of the conserved Walker A and B boxes (ATPase motifs I and II), all members of the RecQ helicase family characterized thus far exhibit ATPase activity that is dependent on a bivalent cation (generally Mg2+) and DNA. Except for Sgs1 [41], ATP hydrolysis by RecQ helicases is stimulated to a greater degree by ssDNA compared with dsDNA effector [42–45]. As a general rule, longer ssDNA molecules are significantly more effective in stimulating ATP hydrolysis by RecQ helicases than short oligomers, suggesting that RecQ helicases may have the ability to translocate processively along long stretches of ssDNA without additional binding steps.
All RecQ helicases that have been shown to catalyse dsDNA unwinding do so with a 3′ to 5′ direction with respect to the strand that the enzyme is presumed to bind; however, unidirectional translocation of any RecQ helicase into the duplex has not been shown. In general, the deoxy or ribodeoxy form of ATP is the preferred nucleotide as the energy source for DNA unwinding by RecQ helicases. Of the five human RecQ helicases, only the RTS protein (RECQ4) is reported to be inactive as a helicase, even in the presence of ATP-Mg2+ which can be hydrolysed by the enzyme [44].
E. coli RecQ helicase activity is sensitive to the ratio of Mg2+ to ATP with an optimal ratio of 0. 8 and a free Mg2+ concentration of 50 μM [46]. In addition, E. coli RecQ helicase activity displayed a sigmoidal dependence on ATP concentration [46]. Like E. coli RecQ, human RECQ1 helicase activity is significantly inhibited at Mg2+/ATP ratios greater than 1 [15]. WRN helicase activity was shown to increase with Mg2+/ATP ratios up to 1; however, greater Mg2+/ATP ratios of up to 4 did not significantly increase or decrease WRN unwinding further [47]. Depending on the RecQ helicase, certain bivalent cations can substitute for Mg2+ as a cofactor in the unwinding reaction. For example, Mn2+ and Ni2+ substituted for Mg2+ as a cofactor for WRN helicase, whereas Fe2+ or Cu2+ profoundly inhibited WRN unwinding in the presence of Mg2+ [47]. These results indicate that the unwinding reaction conditions should be optimized for the RecQ helicase under investigation.
RecQ protein assembly state
Helicase assembly state is important for the mechanism of DNA unwinding. Electron and atomic force microscopy studies as well as glycerol gradient and gel filtration analyses have provided evidence for various assembly states of RecQ helicases in solution [48]; however, the active functional state of unwinding for a number of the RecQ helicases remains debatable. E. coli RecQ helicase activity was found to display a sigmoidal dependence on ATP concentration, suggesting multiple interacting ATP-binding sites to mediate duplex DNA unwinding [46]. However, another study reported that RecQ exists as a monomer in solution and that both DNA-unwinding activity and ATPase activity were independent of RecQ concentration [49]. Initial rates of WRN helicase activity displayed a hyperbolic dependence on ATP concentration [47], suggesting that WRN helicase activity was not co-operative with respect to ATP concentration.
An alternative method to address the assembly state of an actively unwinding helicase has been to examine the stoichiometry of the unwinding reaction. Real-time pre-steady-state kinetic analysis of WRN helicase activity demonstrated a 1:1 stoichiometry for the number of WRN protein molecules that unwound each 19 bp forked duplex DNA substrate, indicating that WRN can function as a monomer to unwind the DNA substrate [47]. Quite recently, E. coli RecQ was similarly reported to display a 1:1 ratio between RecQ monomer and duplex DNA substrate unwound, suggesting a monomer [50].
DNA substrate specificity of RecQ helicases
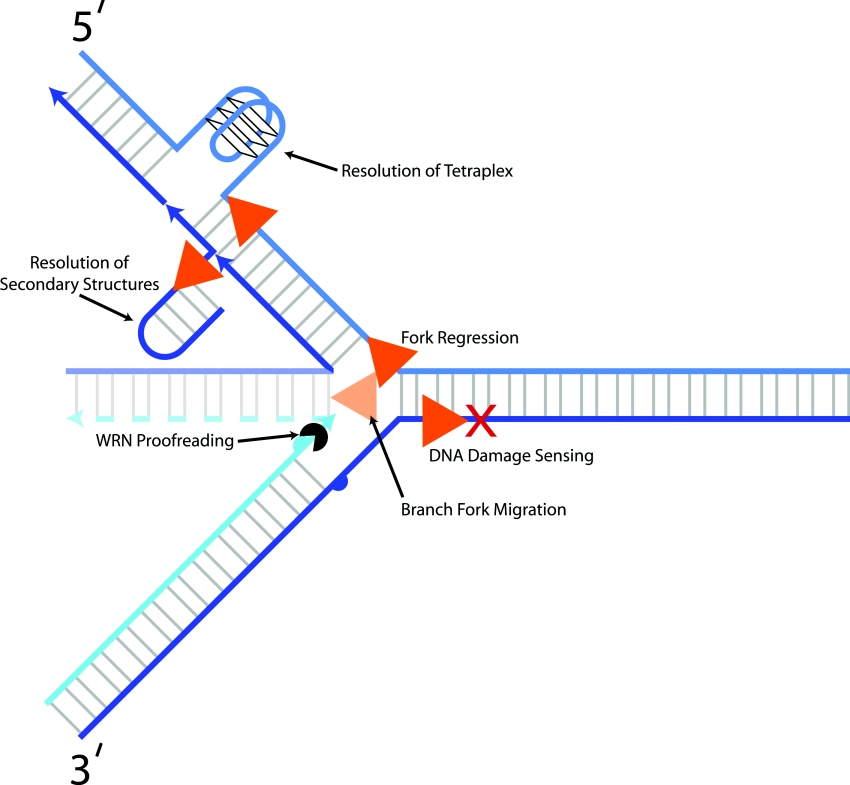
The replication defects and hypersensitivity to DNA-damaging agents of various RecQ mutant cell lines suggest their role in the processes of DNA replication and/or repair. Therefore the catalytic activity of a RecQ helicase may be important at specific structural DNA intermediates that arise at the replication fork (Figure 6) or during DNA repair. Studies using in vitro strand displacement assays have led to insights into the DNA substrate preferences of RecQ helicases. Work in various laboratories has demonstrated that RecQ helicases are fairly promiscuous with respect to the number of DNA substrates that they can unwind, including 3′-tailed or -forked duplexes, HJ (Holliday junction) and other alternative DNA structures. To probe the importance of DNA structural elements for unwinding, WRN helicase activity was tested on dsDNA substrates with specifically positioned biotin–streptavidin complexes [51]. WRN was completely blocked by streptavidin bound to the 3′ ssDNA tail near the ssDNA/dsDNA junction; however, WRN efficiently unwound the forked duplex with streptavidin bound just upstream of the junction, suggesting that WRN recognizes elements of the fork structure to initiate unwinding [51]. WRN also unwinds two important intermediates of DNA replication/repair: a 5′ ssDNA flap substrate that arises during Okazaki fragment processing and DNA repair, and a synthetic replication fork that has fully duplex leading and lagging strand arms [51]. RECQ1 and BLM helicases also unwind the 5′ flap substrate [15,51].
Figure 6. Potential functions of RecQ helicases at replication forks.
Biochemical and cellular studies have suggested that RecQ helicases act upon certain DNA structural intermediates at the replication fork to ensure accurate processing and genome fidelity. RecQ helicases may act upon the replication fork in various capacities (resolution of secondary structures, lagging strand processing, branch fork migration, DNA-damage sensing or bypass, replication fork regression and WRN proofreading function). See the text for details.
Some differences in substrate specificity or directionality of unwinding a synthetic replication fork structure have been observed among the RecQ helicases [51,52], suggesting that these enzymes may have specialized unwinding functions tailored for specific DNA metabolic intermediates (Figure 6). Quite recently, BLM helicase, but not E. coli RecQ, was shown to promote regression of a model replication fork in vitro [53]. It is conceivable that distinct RecQ assembly states may dictate the enzyme's ability to act upon certain DNA structures. For example, a multimeric RecQ helicase may be required to catalyse branch migration of HJ structures.
Action of RecQ helicases on DNA recombination intermediates
Two key intermediates of HR (homologous recombination) are the four-stranded HJ and the three-stranded D-loop. An early important observation was made that BLM and WRN selectively bind HJ structures in vitro and are capable of efficiently promoting ATP-dependent HJ branch migration through greater than 2 kb of DNA [54,55], suggesting they may act upon such four-stranded structures at blocked or collapsed replication forks to allow processing into mature recombinants. Other RecQ helicases that have been shown to efficiently unwind HJ structures include E. coli RecQ, Sgs1, RECQ1 and RECQ5β [15,16,56,57]. The bacterial HJ core recognition protein RuvA inhibits HJ branch migration by BLM, WRN, RECQ1 or RECQ5β [15,16,55,58], suggesting that these RecQ helicases specifically recognize the HJ core where they initiate unwinding.
During HR, Rad51-mediated strand invasion results in the displacement of one strand of the sister chromatid duplex yielding a D-loop intermediate. In vitro evidence that BLM [59], WRN [60,61] and RECQ1 [15] helicases act upon D-loop structures supports a proposed role of RecQ helicases in resolving the recombination intermediate. BLM helicase was shown to preferentially unwind mobile D-loop substrates by releasing the invading strand of the D-loop structure; however, neither the presence nor polarity of the protruding tail were found to be critical in determining the efficiency by which BLM disrupts mobile D-loops [62]. WRN utilizes its dual helicase and exonuclease activities to simultaneously unwind and degrade D-loop structures characterized by an invading 3′ ssDNA tail [60,61]. On the basis of biochemical assays with model t-loop (telomeric loop) substrates, a catalytic role of WRN in telomere metabolism through the ALT (alternative lengthening of telomeres) pathway has been proposed [60] (see the Telomere maintenance section).
Unwinding of alternative DNA structures by RecQ helicases
Oligonucleotides containing runs of guanines readily and spontaneously assemble into four-stranded structures stabilized by G-quartets, planar arrays of four-hydrogen-bonded guanines. Motifs for the formation of G-quadruplex DNA structures are widely dispersed in eukaryotic genomes, and are abundant in regions of biological significance such as telomeres, promoter elements of many important genes and recombination hotspots. Unregulated formation of G4 (G-quadruplex) DNA during replication, recombination, transcription or telomeric DNA elongation may potentially lead to genomic instability [63].
WRN [64,65], BLM [65,66], Sgs1 [67] and E. coli RecQ [68] helicases have been found to efficiently unwind and disrupt G4 DNA. Both Sgs1 and BLM preferentially unwind G4 DNA compared with duplex DNA or HJ substrates [65,67,69]. The substrate preference for G4 DNA binding was mapped to the conserved RQC domain, whereas HJ binding involves both the RQC and HRDC domains [70]. G4 DNA unwinding activity is proposed to contribute to the function of Sgs1 in the maintenance of two G-rich genomic domains, rDNA (ribosomal DNA) [71,72] and telomeres [73–75]. Sgs1 is required for recombination-dependent telomere maintenance in cells lacking telomerase [73–75]. A role of Sgs1 in rDNA metabolism is consistent with the observations that sgs1-deficient strains are characterized by nucleolar fragmentation and production of rDNA circles [76,77]. Like Sgs1, WRN is suspected to have a role in rDNA metabolism on the basis of the observation that a significant fraction of WRN is nucleolar [78]. In vivo evidence suggests that WRN is necessary for efficient replication of G-rich telomeric DNA, maintaining telomeres and genomic stability [79]. The interaction between WRN helicase and pol δ specifically facilitates DNA synthesis through tetraplex and hairpin structures of the d(CGG)n trinucleotide repeat sequence [80], suggesting a role of WRN helicase for enabling replication progression through genomic DNA roadblocks such as secondary structure.
The triple helix is another alternative DNA structure that can be formed by sequences that are widely distributed throughout the human genome [81]. Triplexes are generated when a third strand lies in the major groove of duplex DNA. BLM and WRN helicases were shown to efficiently unwind a DNA triple helix [82]. The helicase reactions were dependent on NTP hydrolysis and required a free 3′ tail attached to the third strand.
Impact of covalent DNA adducts on WRN helicase activity
DNA damage evokes a cellular response by a genome surveillance system that senses DNA structural perturbation at the site of the lesion and elicits an appropriate response that may involve direct repair of the lesion, stabilization of the replication fork or induction of apoptosis. It has been proposed that poor base stacking at the sites of DNA damage may initiate recognition of DNA lesions by a number of repair proteins [83]. Cellular pathways of DNA metabolism are influenced by DNA lesions, and, since DNA helicases are among the first proteins to encounter DNA damage, it is of interest to understand how helicase action is modulated by interaction with chemically modified DNA.
RecQ helicases are likely to be targets for the adverse effects of DNA lesions such as polycyclic aromatic hydrocarbon BcPh (benzo[c]phenanthrene) or BaP (benzo[a]pyrene) DE (diol epoxide) adducts that are mutagenic and carcinogenic. WRN helicase activity is sensitive to the strand, orientation and stereochemistry of intercalated BcPh adducts [84]. WRN or RECQ1 helicase activity was also found to be inhibited in a strand-specific manner by BaP DE–dG adducts [85], which either occupy the minor groove without significant perturbation of DNA structure (trans adducts) or cause base displacement at the adduct site (cis adducts) [86,87]. In contrast, WRN helicase activity is only mildly affected by intercalating BaP DE–dA adducts that locally perturb DNA double-helical structure [85]. Partial unwinding of the duplex at BaP DE–dA adduct sites [88] may make such adducted DNAs more susceptible to the action of WRN helicase than DNA containing the corresponding BcPh DE–dA adducts, which cause little or no destabilization of duplex DNA [89]. The adverse effects of polycyclic aromatic hydrocarbons on WRN or other helicases could contribute to chromosomal damage and carcinogenesis induced by these DEs. Alternatively, RecQ helicases may have the ability to sense particular types of DNA damage and facilitate a mechanism for loading of other DNA repair factors by protein–protein interactions.
Interaction of eukaryotic RecQ helicases with RPA
The length-dependence for duplex DNA unwinding is an important property of DNA helicases [37]. The presence of an auxiliary factor can greatly alter the ability of a helicase to unwind duplex DNA, and in some cases enables a relatively non-processive helicase to unwind very long duplexes. In the presence of the E. coli SSB, the initial rate and extent of unwinding a linear 2.7 kb plasmid DNA substrate by RecQ is increased [46]. In general, eukaryotic RecQ helicases are not very processive and are only able to unwind 100 bp or less. However, the SSB RPA enables RecQ helicases to unwind duplex DNA substantially better. RPA, an essential protein in the cellular processes of DNA replication, repair, recombination and RNA transcription [90], stimulates helicase activity of WRN [13,32,91], BLM [92], RECQ1 [43] and RECQ5β [16] in a specific manner, since heterologous SSBs have only a weak stimulatory effect that is limited to shorter duplex substrates. In fact, WRN and BLM helicases were shown to unwind duplexes up to 850 bp in length when RPA was present [13,32]. However, the RECQ4 helicase failed to unwind a 40 bp partial duplex substrate even in the presence of RPA [44].
Of the human RecQ DNA helicases examined, WRN, BLM and RECQ1 all interact physically with RPA [13,43,92]. A recombinant RPA70 truncated protein stimulated WRN helicase activity to the same extent as the RPA heterotrimer, and the functional interaction is mediated by residues of RPA70 that overlap with the ssDNA-binding domain [93]. Recently, a high-affinity RPA-interaction site was mapped to the two strongly acidic perfect 27-amino-acid direct repeats of WRN and a lower-affinity site in the RQC region [32] (Figure 1). The physical interaction between WRN and RPA was shown to be critically important in the mechanism of RPA-dependent stimulation of WRN helicase activity [32].
RPA was also shown to physically interact with the N-terminal region of Xenopus WRN and stimulate its helicase activity [94]. This region contains the single acidic repeat element found in human WRN that directly binds to RPA [32]. The WRN fragment harbouring the RPA-interaction site exerted a dominant-negative effect on DNA replication in reconstituted Xenopus nuclei, suggesting a biological importance of the WRN–RPA interaction [94].
The biological significance of the WRN/BLM–RPA interaction may be highly relevant to our understanding of the DNA metabolic defects in WS and BS. RPA has been found to co-localize with WRN upon replication arrest [54] and DNA damage [95], suggesting that the two proteins may indeed collaborate to perform certain cellular function(s). BLM co-localizes with RPA at the synaptonemal complex in meiotic prophase nuclei of mammalian spermatocytes [96]. The involvement of WRN and BLM in the recovery of DNA synthesis after replication arrest poses the question of whether these helicases function with RPA in a critical step to resolve a key replication or recombinational intermediate that arises from fork stalling or collapse.
As a component of the replication stress response, RPA may serve to enable eukaryotic RecQ helicases to overcome DNA-blocking base lesions introduced by exogenous DNA-damaging agents or by endogenous biochemical processes such as oxidation. A role for WRN in conferring resistance to the lesions N3-methyladenine and O6-methylguanine was suggested by cellular studies [97]; however, the importance of WRN helicase function remains to be shown. RPA enabled WRN helicase to overcome inhibition by either the trans-R or cis-R BaP DE–dG adduct [85], suggesting that WRN and RPA may function together to unwind duplex DNA harbouring specific covalent adducts that otherwise block WRN helicase acting alone. In addition to base modifications, RPA may also enable WRN to unwind DNA substrates with alterations to the sugar phosphate backbone such as synthetic vinylphosphonate modifications [98]. By their partnerships with RPA, RecQ helicases may be able to overcome inhibition posed by potentially mutagenic lesions and ensure normal DNA transactions.
Strand annealing catalysed by RecQ helicases
Recently, the human RecQ helicases RECQ5β [16], RECQ1 [15], WRN [99], BLM [99,100] and RECQ4 [44], as well as the dmRECQ5β (dm is Drosophila melanogaster) [99], were shown to mediate annealing of complementary ssDNA molecules. Strand annealing catalysed by RecQ helicases does not require ATP or free Mg2+. Analyses of strand annealing utilizing ATP-binding/hydrolysis mutants revealed that nucleotide binding inhibits strand annealing catalysed by either RECQ1 [15] or RECQ5β [16]. Partial proteolysis studies demonstrated that ATP binding induces a conformational change in RECQ1 protein [15], which serves as a molecular switch from a strand-annealing to a DNA-unwinding mode. RPA binding to ssDNA inhibited strand annealing catalysed by human RecQ helicases [15,16,44,100]. This effect of RPA distinguishes the strand-annealing reaction catalysed by human RecQ helicases from that of Rad52-mediated strand annealing which functions co-operatively with RPA [101].
Experiments utilizing deletion mutants indicated that the C-terminal region after the helicase domain of RECQ5β mediates strand annealing [16]. Cheok et al. [100] utilized various truncation mutants of BLM to show that the C-terminal region (amino acids 1267–1417) adjacent to the HRDC domain is important for BLM strand annealing and DNA binding. The C-terminal regions of BLM and RECQ5β show little sequence similarity, suggesting that the annealing function is dictated by the poorly conserved C-terminal regions in these RecQ helicases.
The biological importance of strand annealing catalysed by RecQ helicases remains to be understood. The competition between unwinding and strand annealing by RecQ helicases raises the issue of how they are regulated [102]. Conceivably, strand annealing by RecQ helicases may be important for replication fork regression (formation of a ‘chicken foot’ structure at blocked DNA replication forks), directional migration of a D-loop or HJ structure, or a pathway of HR repair such as SDSA (synthesis-dependent strand annealing) that involves both DNA-unwinding and strand-annealing activities.
WRN exonuclease activity
Biochemical analysis of WRN exonuclease activity indicates that the enzyme degrades DNA in a 3′ to 5′ direction [103–105]. In addition to a DNA duplex with a recessed 3′ end, WRN exonuclease is able to initiate DNA degradation at flush ends of dsDNA substrates that contain alternative structures such as an internal bubble [106]. A 3′-recessed partial duplex substrate with a single 3′ mismatch is acted upon more efficiently by WRN exonuclease compared with the perfectly base-paired substrate [104]. The exonuclease activity of full-length WRN is stimulated by the presence of ATP [106], suggesting that the exonuclease activity may function co-ordinately with ATP binding/hydrolysis. A recombinant N-terminal fragment of WRN that lacks the ATPase/helicase domain retained exonuclease activity [103,107], indicating that exonuclease activity can be uncoupled from helicase activity. Zn2+ strongly stimulated the exonuclease activity of full-length WRN or the WRN N-terminal fragment, suggesting a Zn2+-binding site in the WRN exonuclease domain [47].
Consistent with the idea that WRN exonuclease activity may function by a two-metal-ion mechanism as proposed for pol I [36], the recently solved structure of a WRN exonuclease domain fragment with metal ion indicates two metal ion sites [35]. The 3′ to 5′ exonuclease activity of WRN, like other proofreading exonucleases with which it shares sequence identity (Figure 2), may remove a mismatched nucleotide that was incorporated by a DNA polymerase. In support of this notion, WRN exonuclease was shown in vitro to excise mismatches following misincorporation of nucleotides by pol β [108]. WRN may also participate in DNA replication by providing an extrinsic exonucleolytic proofreading function to ensure DNA replication fidelity. Recent work has demonstrated that extrinsic proofreading can be catalysed by exonucleases that co-operate with DNA polymerases in multiprotein complexes (for a review, see [109]). Replication fork stalling at a DNA lesion may be remedied by translesion synthesis catalysed by an exonuclease-deficient DNA polymerase that is assisted in trans by an auxilliary exonuclease [110], WRN being a candidate.
The association of WRN exonuclease and helicase activities in a single polypeptide clearly distinguishes it from other human RecQ-like helicases and may contribute to the unique phenotypes of WS. An important question is whether the helicase and exonuclease activities co-ordinately function together or separately? D-loop [60,61] substrates are acted upon simultaneously by WRN helicase and exonuclease activities, supporting a co-ordinate function. The heterodimeric factor Ku implicated in NHEJ (non-homologous end-joining) forms a stable complex with WRN, stimulates its exonuclease activity [107,111] and enables the enzyme to digest past the DNA-damage-blocking lesions 8-oxoadenine and 8-oxoguanine [112]. The relative contribution of WRN helicase and exonuclease activities can be modulated by the WRN-interacting proteins RPA and Ku [113]. Biochemical studies with purified recombinant proteins demonstrated that the 3′ to 5′ nucleolytic digestion catalysed by the WRN exonuclease domain fragment, like full-length WRN, can be stimulated by the Ku70/80 heterodimer [35]. These results suggest that the WRN–Ku functional interaction is mediated, at least in part, by the physical interaction of Ku70/80 with the minimal exonuclease domain.
Multiple RecQ-like genes have been identified in plants [114]. Although there is no homologue of this bifunctional WRN protein in the A. thaliana (at) genome, multiple proteins with the conserved RecQ helicase domain and a single protein with similarity to the WRN exonuclease domain, atWEX (Werner-like exonuclease), are found [115]. The atWEX protein was shown to possess 3′ to 5′ exonuclease activity [116] and is stimulated by atKu [117], suggesting the functional interaction between WEXs and Ku has been preserved through evolution of species.
ROLES OF RecQ HELICASES IN GENOME STABILITY MAINTENANCE
The sequence conservation of RecQ helicases throughout evolution has provided the opportunity to exploit different model organisms to study the genetic functions of RecQ helicases. Studies in model systems continue to be useful in identifying and characterizing their unique roles in cellular pathways. Characterization of the protein interactions of RecQ helicases suggest their involvement in macromolecular complexes that function in essential DNA metabolic pathways of replication, recombination or DNA repair that are important for genomic stability (for reviews, see [26,118]). A summary of the protein interactors of the human RecQ helicases is shown in Table 1. In the following section, we have summarized some of the more prominent models proposed for the involvement of RecQ helicases in specific pathways.
Table 1. Protein interactions of human RecQ helicases.
APE-1, apurinic/apyrimidinic endonuclease 1; CAF-1, chromatin assembly factor 1; FA, Fanconi's anaemia; SUMO-1, small ubiquitin-related modifier-1; VCP, valosin-containing protein; WHIP, Werner helicase-interacting protein.
| RecQ helicase | Physical and/or functional protein interactions |
|---|---|
| BLM | BRCA1 [208], CAF-1 [270], FA [268], FEN-1 [15,144,146], MLH1 [204,206], p53 [252,255,257], PML [140,271], Rad51 [140,195], RPA [32,92], Top3α [164–167], TRF2 [217,219], WRN [234] |
| WRN | APE-1 [211], BLM [234], c-Abl [272], DNA-PK [180,181], EXO-1 [149], FEN-1 [58,142,143,145,147,152], Ku70/80 [107,111,112,178,179,273], MRE11 complex [191], p53 [250,251,253–255], PARP-1 [213,214,273], PCNA [274–276], pol β [210], pol δ [125,126], protein kinase A [277], Rad52 [192], RPA [13,32,91,93], SUMO-1 [278], TopI [275,279], TRF2 [60,219,229], VCP [280], WHIP [281] |
| RECQ4 | PML [282], Rad51 [282], UBR1 [283], UBR2 [283] |
| RECQ5β | RPA [16], Top3α [174], Top3β [174] |
| RECQ1 | EXO-1 [148], MLH1 [148], MSH2/6 [148], Qip1/importin α [284], RPA [43,285], Top3α [165,283] |
RecQ helicases as regulators of genetic recombination at stalled replication forks
Mechanistic insight into how Sgs1, the sole RecQ homologue in S. cerevisiae, functions at stalled replication forks is beginning to emerge. DNA polymerase stabilization at stalled replication forks requires the ATM (ataxia telangiectasia mutated)-related kinase Mec1 and Sgs1 [119]. A model was proposed whereby Sgs1 helicase resolves aberrantly paired structures at stalled forks to maintain ssDNA that allows RPA and Mec1 to promote DNA polymerase association. The observation that Sgs1 is present at replication forks and binds RPA suggests an important role for polymerase assembly/stabilization. Further elaboration of the model suggested that Sgs1 stabilizes the replisome at stalled forks by provoking a conformational change in RPA that promotes stable binding of pol α as a primosome [120]. Sgs1 contributes to genomic stability by its non-catalytic function in checkpoint kinase activation through its interaction with Rad53 or its catalytic function in conferring replisome stability and recovery from arrested replication by virtue of the Top3 (topoisomerase III)–Sgs1 resolvase activity [121]. In the absence of the Sgs1–Top3 interaction, the structure-specific endonuclease Slx1–Slx4 cleaves stalled forks to restart replication [122]. Sgs1 maintains genomic stability by co-ordinating replication and recombination during S-phase, but it is not required for processive DNA synthesis [72].
WS and BS cells have a prolonged S-phase, and sensitivity of these cells to DNA damage that stalls or arrests replication forks suggests a role of the RecQ helicases in the stabilization or restart of the replication fork. Xenopus BLM has been implicated in DNA replication [123], and its absence leads to the accumulation of chromosomal breaks during S-phase in the presence or absence of DNA damage [124]. Interaction of WRN and BLM with a number of proteins involved in DNA replication, including pol δ, RPA, PCNA (proliferating cell nuclear antigen), FEN-1 (flap endonuclease-1) and topoisomerases (Table 1), suggests that RecQ helicases are coupled directly to replication fork progression and may be involved in regulating the initiation and/or progression of DNA replication. The direct interaction of WRN with pol δ may help to recruit pol δ to particular sites of DNA synthesis and facilitate DNA copying [125,126]. Xenopus RECQ4 is required for loading of replication factors at origins, an event that is necessary for initiation of DNA replication [127]. A role of RECQ4 in sister chromatid cohesion was suggested from the characterization of mutant RECQ4 mice [128].
Evidence that replication forks of presumably damaged chromosomes stall normally in vivo suggests that cells are likely to have mechanisms to deal with the aberrant DNA structures. One proposed function of RecQ DNA helicases is to prevent aberrant deleterious recombinogenic pathways when replication is perturbed by DNA damage, alternative DNA structure or impaired DNA synthesis. Various RecQ-deficient eukaryotic cells display elevated levels of recombination, suggesting an anti-recombinase role for RecQ helicases. The processing of aberrant DNA structures at the replication fork by RecQ helicases is likely to counter their potential toxicity incurred by recombinogenic pathways. In support of this, recombination-dependent DNA structures were shown to accumulate at damaged replication forks in an sgs1 mutant [129]. Defects in lagging strand replication can be repaired through a pathway dependent on Rad51 and Sgs1 that is parallel to Mus81 endonuclease [130]. Earlier work suggested that HR initiation is normal in WS cells, but a portion of the products of Rad51-dependent HR cannot be successfully resolved in the absence of WRN function [131,132]. Studies in WS cells complemented with either the exonuclease-defective or the helicase-defective WRN suggested that balanced exonuclease and helicase activities of WRN are required for optimal HR [133], whereas either activity was sufficient to promote cell survival after DNA damage in the absence of recombination [134]. The yeast RecQ homologues Sgs1 and Rqh1 were recently shown to function in DSB (double-strand break) repair by HR in a helicase-independent manner [135,136].
Following replication fork stalling, fork reversal can give rise to a chicken foot intermediate resembling a HJ structure that may be processed in non-recombinogenic or recombinogenic mode. Accurate recovery of replication in E. coli was shown to require several proteins in the RecF pathway of recombination, including the RecQ helicase and RecJ 5′ to 3′ exonuclease [137]. It was proposed that RecQ and RecJ act together at blocked replication forks to create a ssDNA gap large enough for RecA to bind and stabilize the fork. RecQ and RecJ co-operate to process a regressed replication fork intermediate that is induced by DNA damage, which can then be maintained by RecA RecFOR [137]. Thus accumulation of HJ structures through fork reversal produces abnormal replication intermediates that can be processed by recombination pathways which lead to genomic instability in certain mutant backgrounds. To counter this, E. coli RecQ processes stalled replication forks and functions to generate an initiating signal that can recruit RecA for SOS induction and recombination at stalled forks, which are required for the cell-cycle checkpoint and resumption of DNA replication [138].
The replication defects in WS or BS cell lines point to an underlying deficiency when fork progression is abnormal. Co-localization of WRN and BLM with arrested replication forks in response to hydroxyurea treatment [54,139] or DNA damage [58,140] is consistent with their role in response to replicational stress. Asymmetry of DNA replication fork progression in WS cells suggest that WRN acts to prevent collapse of replication forks or to resolve DNA junctions at stalled replication forks, and that loss of this capacity may be a contributory factor in premature aging [141]. WRN and FEN-1 form a complex upon replication arrest [58], suggesting that WRN and FEN-1 together might facilitate processing of certain DNA structures that interfere with normal DNA transactions when replication is perturbed. Interactions of RecQ helicases with FEN-1 [58,142–147] or other structure-specific nucleases {e.g. Mus81 and EXO-1 (exonuclease 1) [148–150]} is likely to be important in the process of DNA replication to ensure genome maintenance when the replication fork encounters a block (Figure 7).
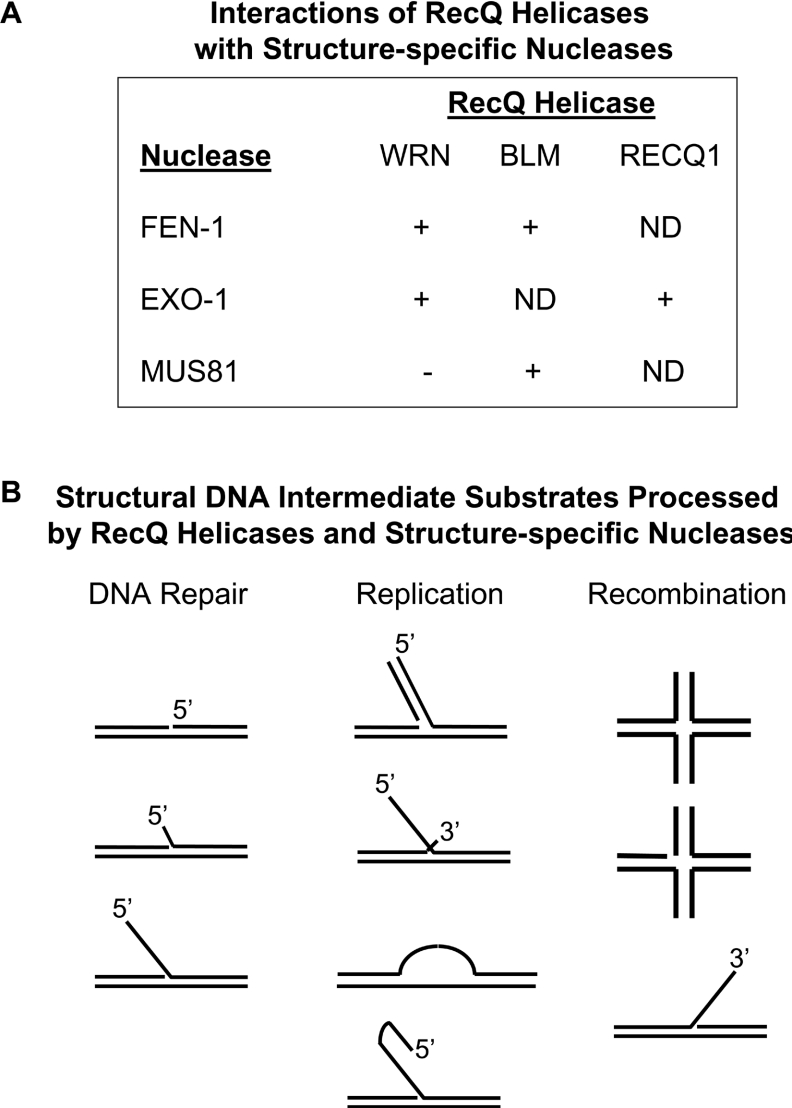
Figure 7. Interactions of RecQ helicases with structure-specific nucleases.
(A) Reported physical and functional RecQ helicase interactions with human FEN-1, EXO-1 and Mus81 nucleases. ND, not determined. (B) Structural DNA intermediates of replication, recombination and repair that are acted upon by the concerted action of a RecQ helicase and a structure-specific nuclease. See the text for details.
A displaced 5′ flap that arises from strand displacement synthesis during lagging strand replication can form hairpins or loops (Figure 7). BLM [146] and WRN [151] have the ability to stimulate FEN-1 cleavage of these structures in vitro, suggesting that the interaction of these RecQ helicases with FEN-1 may serve to resolve secondary structures during Okazaki fragment processing to prevent duplication mutations and repeat expansions. Various types of DNA damage can be repaired by HR through events that involve the processing of DNA structures such as intact or nicked HJ and flap structures (Figure 7). The co-ordinated action of RecQ helicases with structure-specific nucleases on these and related DNA intermediates is likely to be a critical function for the maintenance of genomic stability.
To simulate a condition of replicational stress, WRN function was evaluated in a yeast dna2 mutant background characterized by a replication intermediate processing defect [152]. WRN was found to complement the temperature-sensitive growth defect and G2/M arrest of a dna2-1 mutant. These results indicated that WRN compensates for Dna2 functions during late S-phase to overcome G2/M arrest. Expression of human BLM also complemented the dna2-1 defects [153], suggesting that WRN and BLM may act in the same cellular pathway(s) and that possibly a common domain of the two RecQ helicases may interact with the same DNA substrates and/or protein partners in vivo.
BLM helicase activity is essential for genetic rescue of the dna2-1 mutant [153]; however, a C-terminal non-catalytic WRN domain fragment rescued the dna2-1 phenotypes [152], indicating that a human RecQ helicase can function independently of its catalytic activity in vivo. Genetic complementation of dna2-1 by WRN or BLM is likely to involve the ability of these RecQ helicases to stimulate FEN-1 cleavage of its physiological DNA substrates [58,143,144].
Suppression of elevated SCE (sister chromatid exchange) by the concerted action of BLM and Top3α
Genetic and biochemical results indicate a conserved interaction between RecQ helicases and Top3 in a number of organisms. E. coli RecQ and Top3 together were shown to catalyse the linking and unlinking of covalently closed circular DNA molecules [154,155]. This activity may suppress recombination by either decatenation of newly replicated daughter DNA molecules before cell division or disruption of early recombination intermediates between inappropriately paired DNA molecules. The well-characterized genetic and physical interaction of Sgs1 with Top3 [156–158] is consistent with a model that together they suppress the formation of cross-over products that arise from the resolution of HJ recombination intermediates [159,160]. The fission yeast RecQ helicase Rqh1 works with Top3 in DNA repair during G2 [161]. When Rqh1 is absent, the induction of recombination at a replication fork barrier results in deletion events that correlate with DSBs in the vicinity of the site-specific replication fork barrier [162]. Most recently, rqh1 mutants were shown to be defective in chromosome segregation, with lagging chromosomal DNA that is particularly evident in the rDNA locus [163].
BLM forms a complex in vitro and in vivo with Top3α and the two proteins co-localize to PML (promyelocytic leukaemia) nuclear bodies in exponentially growing human cells [164–166]; moreover, Top3α is mislocalized in BS cells [166]. BLM recruits Top3α to plasmid dsDNA and stimulates Top3α relaxation of negatively supercoiled DNA through its physical association [167]. The N- and C-terminal regions of BLM bind Top3α [166]. The N-terminal region of BLM was found to be essential and sufficient in vivo for the interaction with Top3α and the suppression of SCEs [164].
Recently, a functionally important binding partner of RecQ helicases was identified independently in both yeast and human cells. In yeast, large-scale genetic screens identified RMI1 (NCE4) as a gene that interacts genetically with SGS1 and, when mutated, produces a phenotype very similar to that of top3 strains [168,169]. Sgs1, Top3 and Rmi1 proteins form a complex in yeast cells [168,169]. The human Rmi1 orthologue, named BLAP75 (BLM-associated protein 75), was identified as a protein that co-immunoprecipitates with BLM [170]. Crucially, it was shown that BLAP75 knockdown led to an increase in SCEs, indicating a functional overlap between BLM and BLAP75.
BLM, but not other RecQ helicases, together with Top3α, has the ability to catalyse double HJ dissolution on model DNA substrates in a reaction that requires BLM-mediated ATP hydrolysis and the active-site tyrosine residue of Top3α [171]. BLAP75/RMI promotes this BLM-dependent dissolution of the HR intermediate by recruiting Top3α to the double HJ [172,173]. This reaction gave rise exclusively to non-cross-over products, as predicted from the hemicatenane model, and supports a proposed role of BLM with Top3α as a suppressor of SCEs.
In addition to BLM, RECQ5 and RECQ1 also interact with Top3α [165,174]. RECQ1−/− BLM−/− or RECQ5−/− BLM−/− cells displayed a growth defect, and RECQ5−/− BLM−/− cells had a higher frequency of SCE compared with BLM−/− cells [175]. An independent role of RECQ5 in the suppression of SCEs was demonstrated in ES cells (embryonic stem cells) or differentiated fibroblasts from RECQ5-knockout mice [176]. These studies suggest that RecQ helicases participate in non-redundant pathways to suppress cross-overs during mitosis.
ROLES OF RecQ HELICASES IN DNA REPAIR
The genomic instability and sensitivity to DNA-damaging agents of various organisms with mutations in RecQ helicases indicate a role of these proteins in the DNA-damage response. Interactions of RecQ helicases with DNA repair factors have been identified and characterized, suggesting their participation in pathways of DNA repair (Table 1).
DSB repair
During NHEJ, sites of microsequence homology are joined together by a complex containing both Ku heterodimer and DNA-PK (DNA-dependent protein kinase) [177]. WRN interacts with Ku [107,111,178,179], and its helicase and exonuclease activities are regulated by DNA-PK phosphorylation [180,181]. The interaction of WRN with Ku and DNA-PK may be important to enable the WRN helicase–nuclease to facilitate end-processing before end-joining. The characteristic non-homologous chromosome exchange and formation of large deletions in WS may be due to a defect in end-joining. In support of this, lack of WRN results in extensive deletions of linearized plasmid DNA that bears non-homologous joining ends [182]. BS cells were also found to be defective for end-joining [183–185].
A role for BLM in NHEJ is supported by the observation that dmBLM−/− phenotypes are suppressed by Ku [186]. An interplay of dmBLM with dmKu and role for dmBLM in DSB recognition was suggested based on studies of NHEJ repair of P-element-induced DNA breaks [187]. Genetic studies have also implicated dmBLM in DSB repair through an HR-mediated pathway [188,189]. dmBLM mutants are defective in their ability to execute DNA repair synthesis during SDSA. A model was proposed in which dmBLM acts downstream of strand invasion to unwind a D-loop intermediate to free the nascent strand which can subsequently anneal to its cDNA strand at the site of a DSB. This model helps to explain the role of dmBLM in preventing deletions during DSB repair by promoting HR. The pleiotropic functions of RecQ helicases were shown further by the demonstration that depletion of Xenopus WRN resulted in a significant reduction in the homology-dependent single-strand annealing pathway of DSB repair [190].
The ability of RecQ helicases to interact with proteins implicated in HR repair suggest further their role in this pathway of DSB repair. WRN interacts with the MRE11 complex [191] and the recombination mediator Rad52 [192], and co-localizes with the Rad51 recombinase [95]. Accumulation of WRN at sites of laser-induced DSBs requires its HRDC domain and is independent of PARP-1 [poly(ADP-ribose) polymerase-1], Ku80, DNA-PK, NBS1 and histone H2AX [193]. WRN helicase activity can facilitate the processing of DNA interstrand cross-links in vitro [194], suggesting that WRN may function in early and late steps of DNA cross-link metabolism. Rad51 [195] and Rad51D [196] interact physically with BLM, and Rad51D stimulates BLM-catalysed HJ branch migration activity [196]. It was proposed that BLM functions in multiple downstream repair processes after the ATR/Chk1- and 53BP1-mediated signal from replicational stress is received [197].
Interaction of RecQ helicases with mismatch repair proteins
Mutational analyses suggest that Sgs1 functions to prevent HR [198,199] or act as a sensor for DNA damage [200]. Mutations in SGS1 increased the rate of accumulating gross chromosomal rearrangements, including translocations and deletions containing extended regions of imperfect homology at their breakpoints [198]. Epistasis analysis showed that Sgs1 is redundant with MSH2 for suppressing chromosomal rearrangements and recombination between divergent DNA sequences. Suppression of homoeologous recombination by Sgs1 was suggested to be dependent on mismatch repair [201]. Elevated sister chromatid recombination frequency in sgs1 mutants is decreased by disrupting the RAD52 or MSH2 genes [202]. Genetic and physical analyses of MSH2, MSH6 and SGS1 alleles harbouring mutations that disrupt the biochemical activities of MSH2, MSH6 and Sgs1 support a model in which the MSH2/6 proteins interact with Sgs1 to unwind DNA recombination intermediates that contain mismatches [203]. Physical interactions of Sgs1 with Mlh1 [204] and Rad51 [195] further support a role of Sgs1 as a regulator of recombination.
Although there is no clear supporting evidence that RecQ helicases play a direct role in mismatch repair, a number of the eukaryotic RecQ helicases have been reported to interact with mismatch repair proteins (Table 1). RECQ1 helicase physically binds to the mismatch repair factors MSH2/6, MLH1-PMS2 and EXO-1, and functionally interacts with MSH2/6 and EXO-1 [148]. Physical interactions of BLM with Rad51 [195], MSH6 [205] and MLH1 [204,206] have been shown; however, extracts of BS cells are proficient in mismatch repair [204,206]. Purified recombinant MSH2/6 complex stimulates branch migration activity of BLM on HJ structures [207]. The early identification of BLM in a BASC (BRCA1-associated complex) with proteins such as MSH2/6 and MLH1 that are involved in the recognition and repair of aberrant DNA structures [208] further supports the idea of co-operativity between human RecQ helicases such as BLM and genome surveillance proteins that regulate recombination.
Through their interactions with mismatch repair proteins, RecQ helicases may serve as regulators of homoeologous recombination. A model was proposed that during homoeologous recombination, the mismatch repair recognition complex will stall strand exchange in the presence of mismatches, yielding stabilized branched intermediates [209]. Unwinding of the annealed strands by the RecQ helicase is facilitated by interactions with bound MSH2/6 complex. This permits a subsequent homology search to occur. RecQ helicases may operate in a specialized function in a sub-pathway of HR such as SDSA. Recently, a genetic role for BLM in SDSA was demonstrated [188,189]. In the model presented, BLM is predicted to function downstream of strand invasion to unwind a D-loop intermediate to free the newly synthesized strand. If base-pairing during SDSA is imperfect, a RecQ helicase would be signalled by the MSH complex to disrupt the heteroduplex joint molecule to avoid recombination with the imperfect locus.
BER (base excision repair)
BER is a major pathway for the correction of oxidative DNA damage. WRN interacts with pol β and stimulate pol β strand displacement DNA synthesis via its helicase activity [210]. WRN unwinds several single-strand break BER intermediates in reactions that are regulated by AP endonuclease 1 and pol β [211]. WRN exonuclease and helicase activities were shown to co-operate with pol β on BER intermediates containing a 3′ mismatch [108]. Down-regulation of WRN resulted in increased sensitivity to methylmethanesulfonate, and extracts from these cells displayed reduced long-patch BER [108]. Knockdown of WRN in primary human fibroblasts resulted in increased DNA damage following oxidative stress, particularly in non-dividing cells [212]. A further connection of WRN to BER was evident by the demonstration that the deficiencies of WS cells in the poly(ADP-ribosyl)ation pathway after DNA-damaging treatments is due to the lack of the WRN–PARP-1 interaction [213,214]. Synergistic interaction of WRN and PARP-1 was suggested by the observation that ES cells from PARP-1-null mice with a WRN helicase domain knockout were characterized by a misregulation of genes involved in apoptosis, development and the oxidative stress response; furthermore, the double mutant mice had increased apoptosis, embryonic defects and increased oxidative DNA damage [215].
Telomere maintenance
Mounting evidence indicates a role of RecQ helicases in telomere maintenance. In yeast, Sgs1 participates in a Rad52-dependent recombinational pathway of telomere maintenance in telomerase-negative mutants [216]. The human Sgs1 homologue BLM promotes telomeric DNA synthesis in ALT cells [217] and interacts with the telomeric repeat-binding factors TRF1 and TRF2 [218,219]. BLM helicase activity was shown to be required for the rescue of disrupted telomere lengthening in telomerase-negative sgs1 [220], suggesting that BLM and Sgs1 act catalytically upon similar telomeric structures.
WS fibroblasts display defects associated with telomere dysfunction, including accelerated telomere erosion and premature senescence [221–223]. The forced expression of exogenous telomerase in WS fibroblasts rescued the premature senescent phenotype [224], and the lack of a disease phenotype in WRN-deficient mice with long telomeres [225] implicates telomere attrition in the pathogenesis of WS. Expression of dominant-negative WRN in a human tumour cell line expressing telomerase resulted in an increased telomere loss and chromosome function [226], suggesting a telomerase-independent function of WRN in telomere length maintenance that is consistent with the role of Sgs1 in an ALT pathway for telomere maintenance via HR [73–75]. Members of the RecQ helicase family are known to interact with telomeres [60,222,227,228]. Stochastic loss of telomeres has been reported in cells deficient for WRN protein [222,226]. The WRN and BLM helicases are stimulated by their protein interaction with human TRF2 [217,219,229]. WRN was shown to be present at telomeres along with other DNA repair proteins in cells that employ the ALT pathway; furthermore, WRN promotes unwinding of model telomeric D-loop structures [60], suggesting a helicase-based mechanism to facilitate the ALT pathway. The telomeric SSB POT1 (protection of telomeres-1) stimulates WRN and BLM to unwind telomeric DNA structures [230]. Altogether, the results are consistent with a model (Figure 8) in which telomere binding factors co-operate with mammalian RecQ helicases in resolving telomeric DNA structures in a manner that protects the telomeric 3′ tail as it is exposed during unwinding. Furthermore, WRN is necessary for efficient replication of G-rich telomeric DNA, and defective telomere lagging strand synthesis was reported in cells lacking WRN helicase activity [79].
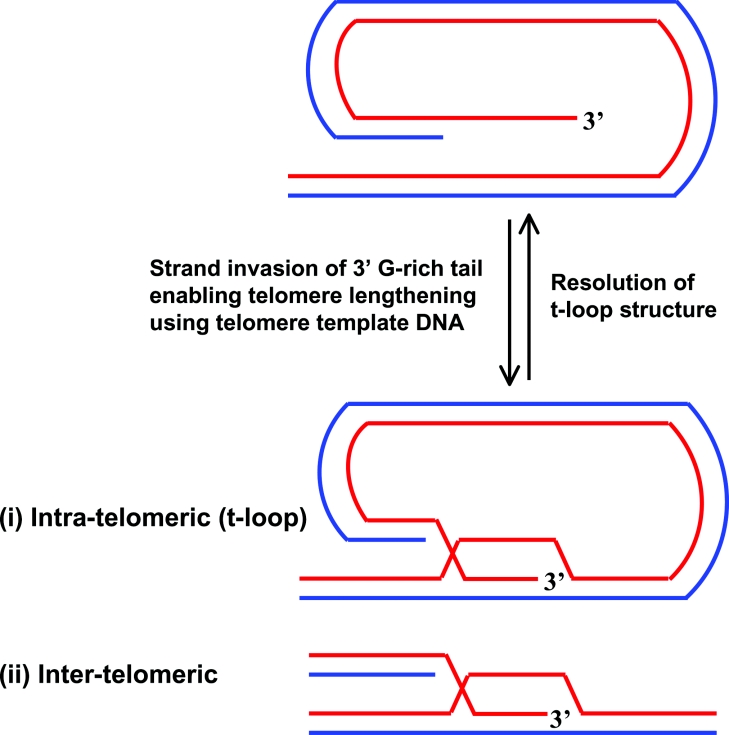
Figure 8. Proposed roles of RecQ helicases in telomere maintenance.
T-loops are created through strand invasion of the 3′ telomeric overhang into the duplex region of the telomere. ALT is a recombination-based pathway that operates in telomerase-deficient cells. This pathway might involve RecQ- and TRF2-mediated strand invasion of the 3′ G-rich tail, enabling the telomere to be lengthened by using telomeric DNA as a template. The telomeric template DNA can originate from two sources: (i) intra-telomeric, where the t-loop is used to prime DNA synthesis, or (ii) inter-telomeric, where the DNA is copied from another telomere or telomeric sequence that exists as extrachromosomal DNA. Additionally, maintenance of telomere length might require a RecQ helicase (e.g. BLM or WRN) to resolve the D-loop structure at the t-loop or unwind a G-quadruplex in the 3′ G-rich tail.
Late-generation telomerase and WRN-deficient mice (mTerc−/− WRN−/−) with dysfunctional telomeres were generated independently by two laboratories [225,231]. The mTerc−/− WRN−/− mice displayed many of the clinical symptoms of premature aging and the types of tumours (osteocarcinomas, soft tissue sarcomas) typically observed in WS patients. Cells from the mTerc−/− WRN−/− mice displayed accelerated telomere shortening, chromosomal instability and replicative senescence. The mTerc−/− WRN−/− cells were also shown to have elevated recombination rates between telomeres of sister chromatids [232]. Restoration of normal telomere SCE was dependent on WRN helicase activity. The WRN-deficient telomere dysfunctional cells escaped senescence by the ALT pathway. These findings suggest that the chromosomal instability and cancer of WS is a consequence of aberrant telomere SCE which activates the ALT pathway (for a review, see [233]).
Consistent with mTerc−/− WRN−/− mice, the pathology of mTerc−/− BLM−/− later-generation mice showed an acceleration of phenotypes characteristic of later-generation Terc mutants [225]. Furthermore, pathology not observed in Terc mutants, but similar to that observed in WS and BS, such as bone loss, was observed. The phenotypes associated with later-generation mice were more severe in mTerc−/− WRN−/− BLM−/− triple mutants than the sum of these defects in each single mutant, suggesting a synergistic interaction of WRN and BLM helicases in telomere biology [225].
These findings and the proposed models are consistent with the observations that the delayed manifestation of the complex pleiotropic phenotypes of WRN or BLM deficiency in mice relates to telomere shortening [231]. The reported interaction of WRN and BLM [234] raises the possibility that these two helicases may act synergistically on sub-telomeric structures to facilitate DNA repair or replication.
RecQ HELICASES AS TARGETS FOR ANTICANCER THERAPY
The pronounced genomic instability of cancer cells and their reliance on fewer DNA repair pathways (owing to mutations in important DNA repair factors) ensures that disrupting a specific DNA repair pathway may have a greater effect on viability of transformed cells. Identifying specific inhibitors of DNA repair factors such as RecQ helicases may lead to development of drugs that target DNA repair in the treatment of human cancers [235]. Small-molecule inhibitors of RecQ helicases that resolve G-tetraplexes may be used to regulate cell-cycle progression by modulating oncogene promoter activation or disrupting telomere maintenance, which are important processes of cellular transformation. The direct link of RecQ helicases (WRN, BLM) to telomere metabolism suggests a molecular target for anticancer therapy that exploits telomere maintenance as a critical process for cell proliferation. Transcription of proto-oncogenes may be regulated by RecQ helicases through their ability to resolve tetraplex structures in promoters. Indeed, c-myc directly stimulates transcription of the WRN gene and experimental evidence suggests that WRN up-regulation by c-myc may promote c-myc-driven tumorigenesis by preventing cellular senescence [236,237].
The design of small molecules which deter G4 resolution may enhance chemotherapy treatment (for a review, see [238]). Compounds such as 2,6-diamido anthraquinones, cationic porphyrins, perylenes or quindolines which stabilize G-quadruplex structures would make ideal candidates for this purpose. The G-quadruplex-binding compound N-methyl mesoporphyrin IX, an effective telomerase inhibitor, is a specific inhibitor of tetraplex unwinding by RecQ helicases [68,69,239]. Trisubstituted acridine ligands were shown to be potent inhibitors of WRN and BLM helicase activity on both G-quadruplex and B-form DNA substrates [240]. Understanding how these and other chemotherapeutic compounds affect the functions of RecQ helicases can provide insight into their potential for combating cancer. In the future, the application of siRNA (small interfering RNA) technology to down-regulate RecQ helicase expression may be coupled to the administration of chemotherapy agents as an approach to aggressively attack cancerous cells which have been rendered susceptible to drugs that induce DNA damage and/or replicational stress.
RecQ POLYMORPHISMS
Population studies of polymorphic variants have begun to address the impact of WRN and other RecQ helicases on age-related disorders and potential as cancer risk factors. A polymorphic WRN C1367R variant residing near the nuclear localization signal was reported to be associated with protection against myocardial infarction [241,242]; however, the C1367R polymorphism had no effect on enzyme function [243,244] or localization [243] and did not influence coronary heart disease for individuals drawn from the Baltimore Longitudinal Study of Aging [243] or Leiden 85-plus Study [245]. Other studies have also begun to investigate the correlations between WRN polymorphisms and age-related illness [246–248]. Most recently, a significant association of the WRN C1367R variant allele C with the tumour suppressor p53 gene in familial breast cancer was reported [249]. Consistent with this, p53 directly interacts with WRN and BLM helicases [250–255], and p53-mediated apoptosis is attenuated in WS and BS cells [254,256,257]. It was suggested that the C1367R polymorphism may have an impact on p53 binding to WRN, leading to an effect on p53-mediated apoptosis [249]. In another study, WRN enzymatic activities were found to be disabled by the WRN R834C polymorphism without a major effect on WRN expression [244]. Genetic instability is elevated significantly in heterozygote carriers [258], suggesting that a WRN dosage effect may possibly modulate the aging process of individuals who are not clinically diagnosed with WS. The possibility also exists that mutations in genes other than WRN might also present symptoms of premature aging closely resembling WS. Lamin A mutations have been identified in a subset of WS patients that do not show mutations at the WRN locus (atypical WS) [259].
Very recently, single nucleotide polymorphisms of RECQ1 were found to be associated with reduced pancreatic cancer survival [260,261]. This raises the possibility that RECQ1 variant alleles can serve as a predictor for cancer survival. Future work characterizing polymorphisms in RecQ helicase genes will probably address the importance of enzymatic activities or protein interactions of RecQ helicases on genomic instability, cancer and aging in the normal population.
CONCLUDING REMARKS AND PERSPECTIVES
Significant progress has been made in appreciating the diverse functions of RecQ helicases; however, a great deal is still unknown about how their activities are dynamically modulated in macromolecular complexes in their tissue or cellular environment. RecQ helicases may act in a concerted action under certain circumstances in DNA metabolic pathways. Although certain functions of RecQ helicases are partially redundant, the distinct clinical and cellular phenotypes of RecQ helicase disorders suggest unique roles of the RecQ helicases that are likely to be carried out by their specialized protein interactions or post-translational modifications. Continued efforts should provide greater insight to understanding the important roles of these DNA-unwinding enzymes in maintaining genomic stability.
In addition to the potentially overlapping roles of RecQ helicases in cellular DNA metabolism, they may function in the same or parallel pathways with other helicases that have not been assigned to the classical RecQ family. A genome-wide synthetic lethality screen of SGS1 identified its interactions with a number of DNA helicases involved in replication, DNA repair and telomere maintenance [262]. DNA helicases that interact with Sgs1 in a helicase network include Rrm3 [263], Srs2 [263] and Dna2 [264]. RecQ helicases may function with a growing list of helicases involved in telomere metabolism [235] to resolve higher-order sub-telomeric DNA structures. For an example in DNA repair, the BACH1 (BRCA1-associated C-terminal helicase) implicated in DSB repair [265] and Fanconi's anaemia [266] displays sequence identity with BLM in the helicase domain [267]. The physical and functional links of BLM with Fanconi's anaemia proteins [268,269] point to their potential co-operation with DNA repair factors such as BACH1. Further studies of ‘RecQ-like’ helicases will lead to greater insights to their cellular roles in pathways of DNA metabolism.
Acknowledgments
We thank Dr Mohammed Hedayati, Dr Wen-Hsing Cheng and Dr Vilhelm Bohr of the Laboratory of Molecular Gerontology for their helpful comments. We wish to thank Dr James Keck (University of Wisconsin Medical School, Madison, WI, U.S.A.) and Dr John Tainer (The Scripps Research Institute, La Jolla, CA, U.S.A.) for kindly providing X-ray crystal structure Figures of RecQ protein domains. This research was supported by the Intramural Research Program of the NIH (National Institutes of Health) National Institute on Aging.
References
- 1.Harrigan J. A., Bohr V. A. Human diseases deficient in RecQ helicases. Biochimie. 2003;85:1185–1193. doi: 10.1016/j.biochi.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Agrelo R., Cheng W. H., Setien F., Ropero S., Espada J., Fraga M. F., Herranz M., Paz M. F., Sanchez-Cespedes M., Artiga M. J., et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siitonen H. A., Kopra O., Kaariainen H., Haravuori H., Winter R. M., Säämänen A. M., Peltonen L., Kestila M. Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Hum. Mol. Genet. 2003;12:2837–2844. doi: 10.1093/hmg/ddg306. [DOI] [PubMed] [Google Scholar]
- 4.Van Maldergem L., Siitonen H. A., Jalkh N., Chouery N., De Roy M., Delague V., Muenke M., Jabs E. W., Cai J., Wang L. L., et al. Revisiting the craniosynostosis-radial ray hypoplasia association: Baller–Gerold syndrome caused by mutations in the RECQL4 gene. J. Med. Genet. 2006;43:148–152. doi: 10.1136/jmg.2005.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyng K. J., May A., Kolvraa S., Bohr V. A. Gene expression profiling in Werner syndrome closely resembles that of normal aging. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12259–12264. doi: 10.1073/pnas.2130723100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyng K. J., May A., Stevnsner T., Becker K. G., Kolvra S., Bohr V. A. Gene expression responses to DNA damage are altered in human aging and in Werner Syndrome. Oncogene. 2005;24:5026–5042. doi: 10.1038/sj.onc.1208692. [DOI] [PubMed] [Google Scholar]
- 7.Bennett R. J., Keck J. L. Structure and function of RecQ DNA helicases. Crit. Rev. Biochem. Mol. Biol. 2004;39:79–97. doi: 10.1080/10409230490460756. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein D. A., Keck J. L. Domain mapping of Escherichia coli RecQ defines the roles of conserved N- and C-terminal regions in the RecQ family. Nucleic Acids Res. 2003;31:2778–2785. doi: 10.1093/nar/gkg376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onoda F., Seki M., Miyajima A., Enomoto T. Elevation of sister chromatid exchange in Saccharomyces cerevisiae sgs1 disruptants and the relevance of the disruptants as a system to evaluate mutations in Bloom's syndrome gene. Mutat. Res. 2000;459:203–209. doi: 10.1016/s0921-8777(99)00071-3. [DOI] [PubMed] [Google Scholar]
- 10.Ellis N. A., German J. Molecular genetics of Bloom's syndrome. Hum. Mol. Genet. 1996;5:1457–1463. doi: 10.1093/hmg/5.supplement_1.1457. [DOI] [PubMed] [Google Scholar]
- 11.Bahr A., De Graeve F., Kedinger C., Chatton B. Point mutations causing Bloom's syndrome abolish ATPase and DNA helicase activities of the BLM protein. Oncogene. 1998;17:2565–2571. doi: 10.1038/sj.onc.1202389. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein D. A., Zittel M. C., Keck J. L. High-resolution structure of the E. coli RecQ helicase catalytic core. EMBO J. 2003;22:4910–4921. doi: 10.1093/emboj/cdg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brosh R. M., Jr, Orren D. K., Nehlin J. O., Ravn P. H., Kenny M. K., Machwe A., Bohr V. A. Functional and physical interaction between WRN helicase and human replication protein A. J. Biol. Chem. 1999;274:18341–18350. doi: 10.1074/jbc.274.26.18341. [DOI] [PubMed] [Google Scholar]
- 14.Neff N. F., Ellis N. A., Ye T. Z., Noonan J., Huang K., Sanz M., Proytcheva M. The DNA helicase activity of BLM is necessary for the correction of the genomic instability of Bloom syndrome cells. Mol. Biol. Cell. 1999;10:665–676. doi: 10.1091/mbc.10.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S., Sommers J. A., Choudhary S., Faulkner J. A., Cui S., Andreoli L., Vindigni A., Brosh R. M., Jr Biochemical analysis of the DNA unwinding and strand annealing activities catalyzed by human RECQ1. J. Biol. Chem. 2005;280:28072–28084. doi: 10.1074/jbc.M500264200. [DOI] [PubMed] [Google Scholar]
- 16.Garcia P. L., Liu Y., Jiricny J., West S. C., Janscak P. Human RECQ5β, a protein with DNA helicase and strand-annealing activities in a single polypeptide. EMBO J. 2004;23:2882–2891. doi: 10.1038/sj.emboj.7600301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J., Mullen J. R., Brill S. J., Kleff S., Romeo A. M., Sternglanz R. Human homologues of yeast helicase. Nature (London) 1996;383:678–679. doi: 10.1038/383678a0. [DOI] [PubMed] [Google Scholar]
- 18.Zittel M. C., Keck J. L. Coupling DNA-binding and ATP hydrolysis in Escherichia coli RecQ: role of a highly conserved aromatic-rich sequence. Nucleic Acids Res. 2005;33:6982–6991. doi: 10.1093/nar/gki999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korolev S., Hsieh J., Gauss G. H., Lohman T. M., Waksman G. Major domain swiveling revealed by the crystal structures of complexes of E. coli Rep helicase bound to single-stranded DNA and ADP. Cell. 1997;90:635–647. doi: 10.1016/s0092-8674(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 20.Velankar S. S., Soultanas P., Dillingham M. S., Subramanya H. S., Wigley D. B. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu J. L., Rigolet P., Dou S. X., Wang P. Y., Xi X. G. The zinc finger motif of Escherichia coli RecQ is implicated in both DNA binding and protein folding. J. Biol. Chem. 2004;279:42794–42802. doi: 10.1074/jbc.M405008200. [DOI] [PubMed] [Google Scholar]
- 22.Guo R. B., Rigolet P., Zargarian L., Fermandjian S., Xi X. G. Structural and functional characterizations reveal the importance of a zinc binding domain in Bloom's syndrome helicase. Nucleic Acids Res. 2005;33:3109–3124. doi: 10.1093/nar/gki619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J. S., Feng H., Zeng W., Lin G.-X., Xi X. G. Solution structure of a multifunctional DNA- and protein-binding motif of human Werner syndrome protein. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18379–18384. doi: 10.1073/pnas.0509380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J. W., Kusumoto R., Doherty K. M., Lin G.-X., Zeng W., Cheng W.-H., von Kobbe C., Brosh R. M., Jr, Bohr V. A. Modulation of Werner syndrome protein function by a single mutation in the conserved RecQ domain. J. Biol. Chem. 2005;280:39627–39636. doi: 10.1074/jbc.M506112200. [DOI] [PubMed] [Google Scholar]
- 25.von Kobbe C., Thoma N. H., Czyzewski B. K., Pavletich N. P., Bohr V. A. Werner syndrome protein contains three structure specific DNA binding domains. J. Biol. Chem. 2003;278:52997–53006. doi: 10.1074/jbc.M308338200. [DOI] [PubMed] [Google Scholar]
- 26.Lee J. W., Harrigan J., Opresko P. L., Bohr V. A. Pathways and functions of the Werner syndrome protein. Mech. Ageing Dev. 2005;126:79–86. doi: 10.1016/j.mad.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z., Macias M. J., Bottomley M. J., Stier G., Linge J. P., Nilges M., Bork P., Sattler M. The three-dimensional structure of the HRDC domain and implications for the Werner and Bloom syndrome proteins. Structure. 1999;7:1557–1566. doi: 10.1016/s0969-2126(00)88346-x. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein D. A., Keck J. L. Conferring substrate specificity to DNA helicases: role of the RecQ HRDC domain. Structure. 2005;13:1173–1182. doi: 10.1016/j.str.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Killoran M. P., Keck J. L. Three HRDC domains differentially modulate Deinococcus radiodurans RecQ DNA helicase biochemical activity. J. Biol. Chem. 2006;281:12849–12857. doi: 10.1074/jbc.M600097200. [DOI] [PubMed] [Google Scholar]
- 30.Moser M. J., Holley W. R., Chatterjee A., Mian I. S. The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res. 1997;25:5110–5118. doi: 10.1093/nar/25.24.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balajee A. S., Machwe A., May A., Gray M. D., Oshima J., Martin G. M., Nehlin J. O., Brosh R. M., Jr, Orren D. K., Bohr V. A. The Werner syndrome protein is involved in RNA polymerase II transcription. Mol. Biol. Cell. 1999;10:2655–2668. doi: 10.1091/mbc.10.8.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty K. M., Sommers J. A., Gray M. D., Lee J. W., von Kobbe C., Thoma N. H., Kureekattil R. P., Kenny M. K., Brosh R. M., Jr Physical and functional mapping of the RPA interaction domain of the Werner and Bloom syndrome helicases. J. Biol. Chem. 2005;280:29494–29505. doi: 10.1074/jbc.M500653200. [DOI] [PubMed] [Google Scholar]
- 33.Ellis N. A., Groden J., Ye T. Z., Straughen J., Lennon D. J., Ciocci S., Proytcheva M., German J. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 34.Janscak P., Garcia P. L., Hamburger F., Makuta Y., Shiraishi K., Imai Y., Ikeda H., Bickle T. A. Characterization and mutational analysis of the RecQ core of the Bloom syndrome protein. J. Mol. Biol. 2003;330:29–42. doi: 10.1016/s0022-2836(03)00534-5. [DOI] [PubMed] [Google Scholar]
- 35.Perry J. J., Yannone S. M., Holden L. G., Hitomi C., Asaithamby A., Han S., Cooper P. K., Chen D. J., Tainer D. J. WRN exonuclease structure and molecular mechanism imply an editing role in DNA end processing. Nat. Struct. Mol. Biol. 2006;13:414–422. doi: 10.1038/nsmb1088. [DOI] [PubMed] [Google Scholar]
- 36.Beese L. S., Steitz T. A. Structural basis for the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. EMBO J. 1991;10:25–33. doi: 10.1002/j.1460-2075.1991.tb07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel S. S., Donmez I. Mechanisms of helicases. J. Biol. Chem. 2006;281:18265–18268. doi: 10.1074/jbc.R600008200. [DOI] [PubMed] [Google Scholar]
- 38.Eoff R. L., Raney K. D. Helicase-catalysed translocation and strand separation. Biochem. Soc. Trans. 2005;33:1474–1478. doi: 10.1042/BST0331474. [DOI] [PubMed] [Google Scholar]
- 39.Byrd A. K., Raney K. D. Protein displacement by an assembly of helicase molecules aligned along single-stranded DNA. Nat. Struct. Mol. Biol. 2004;11:531–538. doi: 10.1038/nsmb774. [DOI] [PubMed] [Google Scholar]
- 40.Byrd A. K., Raney K. D. Increasing the length of the single-stranded overhang enhances unwinding of duplex DNA by bacteriophage T4 Dda helicase. Biochemistry. 2005;44:12990–12997. doi: 10.1021/bi050703z. [DOI] [PubMed] [Google Scholar]
- 41.Bennett R. J., Sharp J. A., Wang J. C. Purification and characterization of the Sgs1 DNA helicase activity of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- 42.Brosh R. M., Jr, Karow J. K., White E. J., Shaw N. D., Hickson I. D., Bohr V. A. Potent inhibition of Werner and Bloom helicases by DNA minor groove binding drugs. Nucleic Acids Res. 2000;28:2420–2430. doi: 10.1093/nar/28.12.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui S., Arosio D., Doherty K. M., Brosh R. M., Jr, Falaschi A., Vindigni A. Analysis of the unwinding activity of the dimeric RECQ1 helicase in the presence of human replication protein A. Nucleic Acids Res. 2004;32:2158–2170. doi: 10.1093/nar/gkh540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macris M. A., Krejci L., Bussen W., Shimamoto A., Sung P. Biochemical characterization of the RECQ4 protein, mutated in Rothmund–Thomson syndrome. DNA Repair. 2006;5:172–180. doi: 10.1016/j.dnarep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Orren D. K., Brosh R. M., Jr, Nehlin J. O., Machwe A., Gray M. D., Bohr V. A. Enzymatic and DNA binding properties of purified WRN protein: high affinity binding to single-stranded DNA but not to DNA damage induced by 4NQO. Nucleic Acids Res. 1999;27:3557–3566. doi: 10.1093/nar/27.17.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harmon F. G., Kowalczykowski S. C. Biochemical characterization of the DNA helicase activity of the Escherichia coli RecQ helicase. J. Biol. Chem. 2001;276:232–243. doi: 10.1074/jbc.M006555200. [DOI] [PubMed] [Google Scholar]
- 47.Choudhary S., Sommers J. A., Brosh R. M., Jr Biochemical and kinetic characterization of the DNA helicase and exonuclease activities of Werner syndrome protein. J. Biol. Chem. 2004;279:34603–34613. doi: 10.1074/jbc.M401901200. [DOI] [PubMed] [Google Scholar]
- 48.Opresko P. L., Cheng W. H., Bohr V. A. At the junction of RecQ helicase biochemistry and human disease. J. Biol. Chem. 2004;279:18099–18102. doi: 10.1074/jbc.R300034200. [DOI] [PubMed] [Google Scholar]
- 49.Xu H. Q., Deprez E., Zhang A. H., Tauc P., Ladjimim M. M., Brochon J. C., Auclaire C., Xi X. G. The Escherichia coli RecQ helicase functions as a monomer. J. Biol. Chem. 2003;278:34925–34933. doi: 10.1074/jbc.M303581200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X. D., Dou S. X., Xie P., Hu J. S., Wang P. Y., Xi X. G. E. coli RecQ is a rapid, efficient and monomeric helicase. J. Biol. Chem. 2006;281:12655–12663. doi: 10.1074/jbc.M513089200. [DOI] [PubMed] [Google Scholar]
- 51.Brosh R. M., Jr, Waheed J., Sommers J. A. Biochemical characterization of the DNA substrate specificity of Werner syndrome helicase. J. Biol. Chem. 2002;277:23236–23245. doi: 10.1074/jbc.M111446200. [DOI] [PubMed] [Google Scholar]
- 52.Ozsoy A. Z., Ragonese H. M., Matson S. W. Analysis of helicase activity and substrate specificity of Drosophila RECQ5. Nucleic Acids Res. 2003;31:1554–1564. doi: 10.1093/nar/gkg243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ralf C., Hickson I. D., Wu L. The Bloom's syndrome helicase can promote the regression of a model replication fork. J. Biol. Chem. 2006 doi: 10.1074/jbc.M604268200. 10.1074/jbc.M604268200. [DOI] [PubMed]
- 54.Constantinou A., Tarsounas M., Karow J. K., Brosh R. M., Jr, Bohr V. A., Hickson I. D., West S. C. Werner's syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 2000;1:80–84. doi: 10.1093/embo-reports/kvd004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karow J. K., Constantinou A., Li J. L., West S. C., Hickson I. D. The Bloom's syndrome gene product promotes branch migration of Holliday junctions. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett R. J., Keck J. L., Wang J. C. Binding specificity determines polarity of DNA unwinding by the Sgs1 protein of S. cerevisiae. J. Mol. Biol. 1999;289:235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 57.Harmon F. G., Kowalczykowski S. C. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 1998;12:1134–1144. doi: 10.1101/gad.12.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma S., Otterlei M., Sommers J. A., Driscoll H. C., Dianov G. L., Kao H. I., Bambara R. A., Brosh R. M., Jr WRN helicase and FEN-1 form a complex upon replication arrest and together process branch-migrating DNA structures associated with the replication fork. Mol. Biol. Cell. 2004;15:734–750. doi: 10.1091/mbc.E03-08-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Brabant A. J., Ye T., Sanz M., German J. L., III, Ellis N. A., Holloman W. K. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- 60.Opresko P. L., Otterlei M., Graakjaer J., Bruheim P., Dawut l., Kolvraa S., May A., Seidman M. M., Bohr V. A. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell. 2004;14:763–774. doi: 10.1016/j.molcel.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Orren D. K., Theodore S., Machwe A. The Werner syndrome helicase/exonuclease (WRN) disrupts and degrades D-loops in vitro. Biochemistry. 2002;41:13483–13488. doi: 10.1021/bi0266986. [DOI] [PubMed] [Google Scholar]
- 62.Bachrati C. Z., Borts R. H., Hickson I. D. Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res. 2006;34:2269–2279. doi: 10.1093/nar/gkl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han H., Hurley L. H. G-quadruplex DNA: a potential target for anti-cancer drug design. Trends Pharmacol. Sci. 2000;21:136–142. doi: 10.1016/s0165-6147(00)01457-7. [DOI] [PubMed] [Google Scholar]
- 64.Fry M., Loeb L. A. Human Werner syndrome DNA helicase unwinds tetrahelical structures of the fragile X syndrome repeat sequence d(CGG)n. J. Biol. Chem. 1999;274:12797–12802. doi: 10.1074/jbc.274.18.12797. [DOI] [PubMed] [Google Scholar]
- 65.Mohaghegh P., Karow J. K., Brosh R. M., Jr, Bohr V. A., Hickson I. D. The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun H., Karow J. K., Hickson I. D., Maizels N. The Bloom's syndrome helicase unwinds G4 DNA. J. Biol. Chem. 1998;273:27587–27592. doi: 10.1074/jbc.273.42.27587. [DOI] [PubMed] [Google Scholar]
- 67.Sun H., Bennett R. J., Maizels N. The Saccharomyces cerevisiae Sgs1 helicase efficiently unwinds G–G paired DNAs. Nucleic Acids Res. 1999;27:1978–1984. doi: 10.1093/nar/27.9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu X., Maizels N. Substrate-specific inhibition of RecQ helicase. Nucleic Acids Res. 2001;29:1765–1771. doi: 10.1093/nar/29.8.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huber M. D., Lee D. C., Maizels N. G4 DNA unwinding by BLM and Sgs1p: substrate specificity and substrate-specific inhibition. Nucleic Acids Res. 2002;30:3954–3961. doi: 10.1093/nar/gkf530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huber M. D., Duquette M. L., Shiels J. C., Maizels N. A conserved G4 DNA binding domain in RecQ family helicases. J. Mol. Biol. 2006;358:1071–1080. doi: 10.1016/j.jmb.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 71.Kaliraman V., Brill S. J. Role of SGS1 and SLX4 in maintaining rDNA structure in Saccharomyces cerevisiae. Curr. Genet. 2002;41:389–400. doi: 10.1007/s00294-002-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Versini G., Comet I., Wu M., Hoopes L., Schwob E., Pasero P. The yeast Sgs1 helicase is differentially required for genomic and ribosomal DNA replication. EMBO J. 2003;22:1939–1949. doi: 10.1093/emboj/cdg180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen H., Sinclair D. A. Recombination-mediated lengthening of terminal telomeric repeats requires the Sgs1 DNA helicase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3174–3179. doi: 10.1073/pnas.061579598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang P., Pryde F. E., Lester D., Maddison R. L., Borts R. H., Hickson I. D., Louis E. J. SGS1 is required for telomere elongation in the absence of telomerase. Curr. Biol. 2001;11:125–129. doi: 10.1016/s0960-9822(01)00021-5. [DOI] [PubMed] [Google Scholar]
- 75.Johnson F. B., Marciniak R. A., McVey M., Stewart S. A., Hahn W. C., Guarente L. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 2001;20:905–913. doi: 10.1093/emboj/20.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sinclair D. A., Guarente L. Extrachromosomal rDNA circles: a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 77.Sinclair D. A., Mills K., Guarente L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science. 1997;277:1313–1316. doi: 10.1126/science.277.5330.1313. [DOI] [PubMed] [Google Scholar]
- 78.Marciniak R. A., Lombard D. B., Johnson F. B., Guarente L. Nucleolar localization of the Werner syndrome protein in human cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6887–6892. doi: 10.1073/pnas.95.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crabbe L., Verdun R. E., Haggblom C. I., Karlseder J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- 80.Kamath-Loeb A. S., Loeb L. A., Johansson E., Burgers P. M., Fry M. Interactions between the Werner syndrome helicase and DNA polymerase δ specifically facilitate copying of tetraplex and hairpin structures of the d(CGG)n trinucleotide repeat sequence. J. Biol. Chem. 2001;276:16439–16446. doi: 10.1074/jbc.M100253200. [DOI] [PubMed] [Google Scholar]
- 81.Manor H., Rao B. S., Martin R. G. Abundance and degree of dispersion of genomic d(GA)n. d(TC)n sequences. J. Mol. Evol. 1988;27:96–101. doi: 10.1007/BF02138367. [DOI] [PubMed] [Google Scholar]
- 82.Brosh R. M., Jr, Majumdar A., Desai S., Hickson I. D., Bohr V. A., Seidman M. M. Unwinding of a DNA triple helix by the Werner and Bloom syndrome helicase. J. Biol. Chem. 2000;276:3024–3030. doi: 10.1074/jbc.M006784200. [DOI] [PubMed] [Google Scholar]
- 83.Yang W. Poor base stacking at DNA lesions may initiate recognition by many repair proteins. DNA Repair. 2006;5:654–666. doi: 10.1016/j.dnarep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Driscoll H. C., Matson S. W., Sayer J. M., Kroth H., Jerina D. M., Brosh R. M., Jr Inhibition of Werner syndrome helicase activity by benzo[c]phenanthrene diol epoxide dA adducts in DNA is both strand-and stereoisomer-dependent. J. Biol. Chem. 2003;278:41126–41135. doi: 10.1074/jbc.M304798200. [DOI] [PubMed] [Google Scholar]
- 85.Choudhary S., Doherty K. M., Handy C. J., Sayer J. M., Kroth H., Jerina D. M., Brosh R. M., Jr Inhibition of Werner syndrome helicase activity by benzo[a]pyrene diol epoxide adducts can be overcome by replication protein A. J. Biol. Chem. 2006;281:6000–6009. doi: 10.1074/jbc.M510122200. [DOI] [PubMed] [Google Scholar]
- 86.Geacintov N. E., Cosman M., Hingerty B. E., Amin S., Broyde S., Patel D. J. NMR solution structures of stereoisometric covalent polycyclic aromatic carcinogen–DNA adduct: principles, patterns, and diversity. Chem. Res. Toxicol. 1997;10:111–146. doi: 10.1021/tx9601418. [DOI] [PubMed] [Google Scholar]
- 87.Volk D. E., Thiviyanathan V., Rice J. S., Luxon B. A., Shah J. H., Yagi H., Sayer J. M., Yeh H. J., Jerina D. M., Gorenstein D. G. Solution structure of a cis-opened (10R)-N6-deoxyadenosine adduct of (9S,10R)-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in a DNA duplex. Biochemistry. 2003;42:1410–1420. doi: 10.1021/bi026745u. [DOI] [PubMed] [Google Scholar]
- 88.Sayer J. M., Shah J. M., Liang C., Xie G., Kroth H., Yagi H., Liu X., Yeh H., Jerina D. M. Effect of absolute configuration on the optical and NMR properties of oligonucleotides containing benzo[a]pyrene adducts at N6 of deoxyadenosine. Polycyclic Aromat. Hydrocarbons. 1999;17:95–104. [Google Scholar]
- 89.Ruan Q., Kolbanovskiy A., Zhuang P., Chen J., Krzeminski J., Amin S., Geacintov N. E. Synthesis and characterization of site-specific and stereoisomeric fjord dibenzo[a,l]pyrene diol epoxide-N6-adenine adducts: unusual thermal stabilization of modified DNA duplexes. Chem. Res. Toxicol. 2002;15:249–261. doi: 10.1021/tx010157k. [DOI] [PubMed] [Google Scholar]
- 90.Wold M. S. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 91.Shen J. C., Gray M. D., Oshima J., Loeb L. A. Characterization of Werner syndrome protein DNA helicase activity: directionality, substrate dependence and stimulation by replication protein A. Nucleic Acids Res. 1998;26:2879–2885. doi: 10.1093/nar/26.12.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brosh R. M., Jr, Li J. L., Kenny M. K., Karrow J. K., Cooper M. P., Kureekattil R. P., Hickson I. D., Bohr V. A. Replication protein A physically interacts with the Bloom's syndrome protein and stimulates its helicase activity. J. Biol. Chem. 2000;275:23500–23508. doi: 10.1074/jbc.M001557200. [DOI] [PubMed] [Google Scholar]
- 93.Shen J. C., Lao Y., Kamath-Loeb A., Wold M. S., Loeb L. A. The N-terminal domain of the large subunit of human replication protein A binds to Werner syndrome protein and stimulates helicase activity. Mech. Ageing Dev. 2003;124:921–930. doi: 10.1016/s0047-6374(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 94.Chen C. Y., Graham J., Yan H. Evidence for a replication function of FFA-1, the Xenopus orthologue of Werner syndrome protein. J. Cell Biol. 2001;152:985–996. doi: 10.1083/jcb.152.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakamoto S., Nishikawa K., Heo S. J., Goto M., Furuichi Y., Shimamoto A. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells. 2001;6:421–430. doi: 10.1046/j.1365-2443.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- 96.Walpita D., Plug A. W., Neff N. F., German J., Ashley T. Bloom's syndrome protein, BLM, colocalizes with replication protein A in meiotic prophase nuclei of mammalian spermatocytes. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5622–5627. doi: 10.1073/pnas.96.10.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blank A., Bobola M. S., Gold B., Varadarajan S., Kolstoe D. D., Meade E. H., Rabinovitch P. S., Loeb L. A., Silber J. R. The Werner syndrome protein confers resistance to the DNA lesions N3-methyladenine and O6-methylguanine: implications for WRN function. DNA Repair. 2004;3:629–638. doi: 10.1016/j.dnarep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Garcia P. L., Bradley G., Hayes C. J., Krintel S., Soultanas P., Janscak P. RPA alleviates the inhibitory effect of vinylphosphonate internucleotide linkages on DNA unwinding by BLM and WRN helicases. Nucleic Acids Res. 2004;32:3771–3778. doi: 10.1093/nar/gkh709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Machwe A., Xiao L., Groden J., Matson S. W., Orren D. K. RecQ family members combine strand pairing and unwinding activities to catalyze strand exchange. J. Biol. Chem. 2005;280:23397–23407. doi: 10.1074/jbc.M414130200. [DOI] [PubMed] [Google Scholar]
- 100.Cheok C. F., Wu L., Garcia P. L., Janscak P., Hickson I. D. The Bloom's syndrome helicase promotes the annealing of complementary single-stranded DNA. Nucleic Acids Res. 2005;33:3932–3941. doi: 10.1093/nar/gki712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sugiyama T., New J. H., Kowalczykowski S. C. DNA annealing by RAD52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6049–6054. doi: 10.1073/pnas.95.11.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Machwe A., Lozada E. M., Xiao L., Orren D. K. Competition between the DNA unwinding and strand pairing activities of the Werner and Bloom syndrome proteins. BMC Mol. Biol. 2006;7:1. doi: 10.1186/1471-2199-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang S., Li B., Gray M. D., Oshima J., Mian I. S., Campisi J. The premature ageing syndrome protein, WRN, is a 3′→5′ exonuclease. Nat. Genet. 1998;20:114–116. doi: 10.1038/2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kamath-Loeb A. S., Shen J. C., Loeb L. A., Fry M. Werner syndrome protein. II. Characterization of the integral 3′→5′ DNA exonuclease. J. Biol. Chem. 1998;273:34145–34150. doi: 10.1074/jbc.273.51.34145. [DOI] [PubMed] [Google Scholar]
- 105.Shen J. C., Gray M. D., Oshima J., Kamath-Loeb A. S., Fry M., Loeb L. A. Werner syndrome protein. I. DNA helicase and DNA exonuclease reside on the same polypeptide. J. Biol. Chem. 1998;273:34139–34144. doi: 10.1074/jbc.273.51.34139. [DOI] [PubMed] [Google Scholar]
- 106.Shen J. C., Loeb L. A. Werner syndrome exonuclease catalyzes structure-dependent degradation of DNA. Nucleic Acids Res. 2000;28:3260–3268. doi: 10.1093/nar/28.17.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cooper M. P., Machwe A., Orren D. K., Brosh R. M., Jr, Ramsden D., Bohr V. A. Ku complex interacts with and stimulates the Werner protein. Genes Dev. 2000;14:907–912. [PMC free article] [PubMed] [Google Scholar]
- 108.Harrigan J. A., Wilson D. M., III, Prasad R., Opresko P. L., Beck G., May A., Wilson S. H., Bohr V. A. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase β. Nucleic Acids Res. 2006;34:745–754. doi: 10.1093/nar/gkj475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Albertson T. M., Preston B. D. DNA replication fidelity: proofreading in trans. Curr. Biol. 2006;16:R209–R211. doi: 10.1016/j.cub.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 110.McElhinny S. A., Pavlov Y. I., Kunkel T. A. Evidence for extrinsic exonucleolytic proofreading. Cell Cycle. 2006;5:958–962. doi: 10.4161/cc.5.9.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li B., Comai L. Functional interaction between Ku and the Werner syndrome protein in DNA end processing. J. Biol. Chem. 2000;275:28349–28352. doi: 10.1074/jbc.C000289200. [DOI] [PubMed] [Google Scholar]
- 112.Orren D. K., Machwe A., Karmakar P., Piotrowski J., Cooper M. P., Bohr V. A. A functional interaction of Ku with Werner exonuclease facilitates digestion of damaged DNA. Nucleic Acids Res. 2001;29:1926–1934. doi: 10.1093/nar/29.9.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Opresko P. L., Laine J. P., Brosh R. M., Jr, Seidman M. M., Bohr V. A. Coordinate action of the helicase and 3′ to 5′ exonuclease of Werner syndrome protein. J. Biol. Chem. 2001;276:44677–22687. doi: 10.1074/jbc.M107548200. [DOI] [PubMed] [Google Scholar]
- 114.Hartung F., Puchta H. The RecQ gene family in plants. J. Plant Physiol. 2006;163:287–296. doi: 10.1016/j.jplph.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 115.Hartung F., Plchova H., Puchta H. Molecular characterisation of RecQ homologues in Arabidopsis thaliana. Nucleic Acids Res. 2000;28:4275–4282. doi: 10.1093/nar/28.21.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plchova H., Hartung F., Puchta H. Biochemical characterization of an exonuclease from Arabidopsis thaliana reveals similarities to the DNA exonuclease of the human Werner syndrome protein. J. Biol. Chem. 2003;278:44128–44138. doi: 10.1074/jbc.M303891200. [DOI] [PubMed] [Google Scholar]
- 117.Li B., Conway N., Navarro S., Comai L., Comai L. A conserved and species-specific functional interaction between the Werner syndrome-like exonuclease atWEX and the Ku heterodimer in Arabidopsis. Nucleic Acids Res. 2005;33:6861–6867. doi: 10.1093/nar/gki984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bachrati C. Z., Hickson I. D. RecQ helicases: suppressors of tumorigenesis and premature ageing. Biochem. J. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cobb J. A., Bjergbaek L., Shimada K., Frei C., Gasser S. M. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–4336. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cobb J. A., Schleker T., Rojas V., Bjergbaek L., Tercero J. A., Gasser S. M. Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev. 2005;19:3055–3069. doi: 10.1101/gad.361805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bjergbaek L., Cobb J. A., Tsai-Pflugfelder M., Gasser S. M. Mechanistically distinct roles for Sgs1p in checkpoint activation and replication fork maintenance. EMBO J. 2005;24:405–417. doi: 10.1038/sj.emboj.7600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fricke W. M., Brill S. J. Slx1–Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1–Top3. Genes Dev. 2003;17:1768–1778. doi: 10.1101/gad.1105203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liao S., Graham J., Yan H. The function of Xenopus Bloom's syndrome protein homolog (xBLM) in DNA replication. Genes Dev. 2000;14:2570–2575. doi: 10.1101/gad.822400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li W., Kim S. M., Lee J., Dunphy W. G. Absence of BLM leads to accumulation of chromosomal DNA breaks during both unperturbed and disrupted S phases. J. Cell Biol. 2004;165:801–812. doi: 10.1083/jcb.200402095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamath-Loeb A. S., Johansson E., Burgers P. M., Loeb L. A. Functional interaction between the Werner syndrome protein and DNA polymerase δ. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4603–4608. doi: 10.1073/pnas.97.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Szekely A. M., Chen Y. H., Zhang C., Oshima J., Weissman S. M. Werner protein recruits DNA polymerase δ to the nucleolus. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11365–11370. doi: 10.1073/pnas.97.21.11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sangrithi M. N., Bernal J. A., Madine M., Philpott A., Lee J., Dunphy W. G., Venkitaraman A. R. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund–Thomson syndrome. Cell. 2005;121:887–898. doi: 10.1016/j.cell.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 128.Mann M. B., Hodges C. A., Barnes E., Vogel H., Hassold T. J., Luo G. Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund–Thomson syndrome. Hum. Mol. Genet. 2005;14:813–825. doi: 10.1093/hmg/ddi075. [DOI] [PubMed] [Google Scholar]
- 129.Liberi G., Maffioletti G., Lucca C., Chiolo I., Baryshnikova A., Cotta-Ramusino C., Lopes M., Pellicioli A., Haber J. E., Foiani M. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 2005;19:339–350. doi: 10.1101/gad.322605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ii M., Brill S. J. Roles of SGS1, MUS81, and RAD51 in the repair of lagging-strand replication defects in Saccharomyces cerevisiae. Curr. Genet. 2005;48:213–225. doi: 10.1007/s00294-005-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prince P. R., Emond M. J., Monnat R. J., Jr Loss of Werner syndrome protein function promotes aberrant mitotic recombination. Genes Dev. 2001;15:933–938. doi: 10.1101/gad.877001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saintigny Y., Makienko K., Swanson C., Emond M. J., Monnat R. J., Jr Homologous recombination resolution defect in Werner syndrome. Mol. Cell. Biol. 2002;22:6971–6978. doi: 10.1128/MCB.22.20.6971-6978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen L., Huang S., Lee L., Davalos A., Schiestl R. H., Campisi J., Oshima J. WRN, the protein deficient in Werner syndrome, plays a critical structural role in optimizing DNA repair. Aging Cell. 2003;2:191–199. doi: 10.1046/j.1474-9728.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 134.Swanson C., Saintigny Y., Emond M. J., Monnat R. J., Jr The Werner syndrome protein has separable recombination and survival functions. DNA Repair. 2004;3:475–482. doi: 10.1016/j.dnarep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 135.Hope J. C., Mense S. M., Jalakas M., Mitsumoto J., Freyer G. A. Rqh1 blocks recombination between sister chromatids during double strand break repair, independent of its helicase activity. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5875–5880. doi: 10.1073/pnas.0601571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lo Y. C., Paffett K. S., Amit O., Clikeman J. A., Sterk R., Brenneman M. A., Nickoloff J. A. Sgs1 regulates gene conversion tract lengths and crossovers independently of its helicase activity. Mol. Cell. Biol. 2006;26:4086–4094. doi: 10.1128/MCB.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Courcelle J., Donaldson J. R., Chow K. H., Courcelle C. T. DNA damage-induced replication fork regression and processing in Escherichia coli. Science. 2003;299:1064–1067. doi: 10.1126/science.1081328. [DOI] [PubMed] [Google Scholar]
- 138.Hishida T., Han Y. W., Shibata T., Kubota Y., Ishino Y., Iwasaki H., Shinagawa H. Role of the Escherichia coli RecQ DNA helicase in SOS signaling and genome stabilization at stalled replication forks. Genes Dev. 2004;18:1886–1897. doi: 10.1101/gad.1223804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davies S. L., North P. S., Dart A., Lakin N. D., Hickson I. D. Phosphorylation of the Bloom's syndrome helicase and its role in recovery from S-phase arrest. Mol. Cell. Biol. 2004;24:1279–1291. doi: 10.1128/MCB.24.3.1279-1291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bischof O., Kim S. H., Irving J., Beresten S., Ellis N. A., Campisi J. Regulation and localization of the Bloom syndrome protein in response to DNA damage. J. Cell Biol. 2001;153:367–380. doi: 10.1083/jcb.153.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodriguez-Lopez A. M., Jackson D. A., Iborra F., Cox L. S. Asymmetry of DNA replication fork progression in Werner's syndrome. Aging Cell. 2002;1:30–39. doi: 10.1046/j.1474-9728.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 142.Brosh R. M., Jr, Driscoll H. C., Dianov G. L., Sommers J. A. Biochemical characterization of the WRN–FEN-1 functional interaction. Biochemistry. 2002;41:12204–12216. doi: 10.1021/bi026031j. [DOI] [PubMed] [Google Scholar]
- 143.Brosh R. M., Jr, von Kobbe C., Sommers J. A., Karmakar P., Opresko P. L., Piotrowski J., Dianova I., Dianov G. L., Bohr V. A. Werner syndrome protein interacts with human Flap endonuclease 1 and stimulates its cleavage activity. EMBO J. 2001;20:5791–5801. doi: 10.1093/emboj/20.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma S., Sommers J. A., Wu L., Bohr V. A., Hickson I. D., Brosh R. M., Jr Stimulation of Flap endonuclease-1 by the Bloom's syndrome protein. J. Biol. Chem. 2004;279:9847–9856. doi: 10.1074/jbc.M309898200. [DOI] [PubMed] [Google Scholar]
- 145.Sharma S., Sommers J. A., Gary R. K., Friedrich-Heineken E., Hubscher U., Brosh R. M., Jr The interaction site of flap endonuclease-1 with WRN helicase suggests a coordination of WRN and PCNA. Nucleic Acids Res. 2005;33:6769–6781. doi: 10.1093/nar/gki1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang W., Bambara R. A. Human Bloom protein stimulates flap endonuclease 1 activity by resolving DNA secondary structure. J. Biol. Chem. 2005;280:5391–5399. doi: 10.1074/jbc.M412359200. [DOI] [PubMed] [Google Scholar]
- 147.Zheng L., Zhou M., Chai Q., Parrish J., Xue D., Patrick S. M., Turchi J. T., Yannone S. M., Chen D., Shen B. Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep. 2005;6:83–89. doi: 10.1038/sj.embor.7400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Doherty K. M., Sharma S., Uzdilla L., Wilson T. M., Cui S., Vindigni A., Brosh R. M., Jr RECQ1 helicase interacts with human mismatch repair factors that regulate genetic recombination. J. Biol. Chem. 2005;280:28085–28094. doi: 10.1074/jbc.M500265200. [DOI] [PubMed] [Google Scholar]
- 149.Sharma S., Sommers J. A., Driscoll H. C., Uzdilla L., Wilson T. M., Brosh R. M., Jr The exonucleolytic and endonucleolytic cleavage activities of human exonuclease 1 are stimulated by an interaction with the carboxyl-terminal region of the Werner syndrome protein. J. Biol. Chem. 2003;278:23487–23496. doi: 10.1074/jbc.M212798200. [DOI] [PubMed] [Google Scholar]
- 150.Zhang R., Sengupta S., Yang Q., Linke S. P., Yanaihara N., Bradsher J., Blais V., McGowan C. H., Harris C. C. BLM helicase facilitates Mus81 endonuclease activity in human cells. Cancer Res. 2005;65:2526–2531. doi: 10.1158/0008-5472.CAN-04-2421. [DOI] [PubMed] [Google Scholar]
- 151.Liu R., Qiu J., Finger L. D., Zheng L., Shen B. The DNA–protein interaction modes of FEN-1 with gap substrates and their implication in preventing duplication mutations. Nucleic Acids Res. 2006;34:1772–1784. doi: 10.1093/nar/gkl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sharma S., Sommers J. A., Brosh R. M., Jr In vivo function of the conserved non-catalytic domain of Werner syndrome helicase in DNA replication. Hum. Mol. Genet. 2004;13:2247–2261. doi: 10.1093/hmg/ddh234. [DOI] [PubMed] [Google Scholar]
- 153.Imamura O., Campbell J. L. The human Bloom syndrome gene suppresses the DNA replication and repair defects of yeast dna2 mutants. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8193–8198. doi: 10.1073/pnas.1431624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harmon F. G., DiGate R. J., Kowalczykowski S. C. RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination. Mol. Cell. 1999;3:611–620. doi: 10.1016/s1097-2765(00)80354-8. [DOI] [PubMed] [Google Scholar]
- 155.Harmon F. G., Brockman J. P., Kowalczykowski S. C. RecQ helicase stimulates both DNA catenation and changes in DNA topology by topoisomerase III. J. Biol. Chem. 2003;278:42668–42678. doi: 10.1074/jbc.M302994200. [DOI] [PubMed] [Google Scholar]
- 156.Bennett R. J., Wang J. C. Association of yeast DNA topoisomerase III and Sgs1 DNA helicase: studies of fusion proteins. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11108–11113. doi: 10.1073/pnas.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fricke W. M., Kaliraman V., Brill S. J. Mapping the DNA topoisomerase III binding domain of the Sgs1 DNA helicase. J. Biol. Chem. 2001;276:8848–8855. doi: 10.1074/jbc.M009719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gangloff S., McDonald J. P., Bendixen C., Arthur L., Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol. Cell. Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ira G., Malkova A., Liberi G., Foiani M., Haber J. E. Srs2 and Sgs1–Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mankouri H. W., Hickson I. D. Understanding the roles of RecQ helicases in the maintenance of genome integrity and suppression of tumorigenesis. Biochem. Soc. Trans. 2004;32:957–958. doi: 10.1042/BST0320957. [DOI] [PubMed] [Google Scholar]
- 161.Laursen L. V., Ampatzidou E., Andersen A. H., Murray J. M. Role for the fission yeast RecQ helicase in DNA repair in G2. Mol. Cell. Biol. 2003;23:3692–3705. doi: 10.1128/MCB.23.10.3692-3705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ahn J. S., Osman F., Whitby M. C. Replication fork blockage by RTS1 at an ectopic site promotes recombination in fission yeast. EMBO J. 2005;24:2011–2023. doi: 10.1038/sj.emboj.7600670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Win T. Z., Mankouri H. W., Hickson I. D., Wang S. W. A role for the fission yeast Rqh1 helicase in chromosome segregation. J. Cell Sci. 2005;118:5777–5784. doi: 10.1242/jcs.02694. [DOI] [PubMed] [Google Scholar]
- 164.Hu P., Beresten S. F., van Brabant A. J., Ye T. Z., Pandolfi P. P., Johnson F. B., Guarente L., Ellis N. A. Evidence for BLM and topoisomerase IIIα interaction in genomic stability. Hum. Mol. Genet. 2001;10:1287–1298. doi: 10.1093/hmg/10.12.1287. [DOI] [PubMed] [Google Scholar]
- 165.Johnson F. B., Lombard D. B., Neff N. F., Mastrangelo M. A., Dewolf W., Ellis N. A., Marciniak R. A., Yin Y., Jaenisch R., Guarente L. Association of the Bloom syndrome protein with topoisomerase IIIα in somatic and meiotic cells. Cancer Res. 2000;60:1162–1167. [PubMed] [Google Scholar]
- 166.Wu L., Davies S. L., North P. S., Goulaouic H., Riou J. F., Turley H., Gatter K. C., Hickson I. D. The Bloom's syndrome gene product interacts with topoisomerase III. J. Biol. Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 167.Wu L., Hickson I. D. The Bloom's syndrome helicase stimulates the activity of human topoisomerase IIIα. Nucleic Acids Res. 2002;30:4823–4829. doi: 10.1093/nar/gkf611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang M., Bellaoui M., Zhang C., Desai R., Morozov P., Delgado-Cruzata L., Rothstein R., Freyer G. A., Boone C., Brown G. W. RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. EMBO J. 2005;24:2024–2033. doi: 10.1038/sj.emboj.7600684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mullen J. R., Nallaseth F. S., Lan Y. Q., Slagle C. E., Brill S. J. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1–Top3 complex. Mol. Cell. Biol. 2005;25:4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yin J., Sobeck A., Xu C., Meetei A. R., Hoatlin M., Li L., Wang W. BLAP75, an essential component of Bloom's syndrome protein complexes that maintain genome integrity. EMBO J. 2005;24:1465–1476. doi: 10.1038/sj.emboj.7600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu L., Hickson I. D. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature (London) 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 172.Raynard S., Bussen W., Sung P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIα, and BLAP75. J. Biol. Chem. 2006;281:13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- 173.Wu L., Bachrati C. Z., Ou J., Xu C., Yin J., Chang M., Wang W., Li L., Brown G. W., Hickson I. D. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shimamoto A., Nishikawa K., Kitao S., Furuichi Y. Human RecQ5β, a large isomer of RecQ5 DNA helicase, localizes in the nucleoplasm and interacts with topoisomerases 3α and 3β. Nucleic Acids Res. 2000;28:1647–1655. doi: 10.1093/nar/28.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang W., Seki M., Narita Y., Nakagawa T., Yoshimura A., Otsuki M., Kawabe Y., Tada S., Yagi H., Ishii Y., Enomoto T. Functional relation among RecQ family helicases RecQL1, RecQL5, and BLM in cell growth and sister chromatid exchange formation. Mol. Cell. Biol. 2003;23:3527–3535. doi: 10.1128/MCB.23.10.3527-3535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu Y., Lu X., Barnes E., Yan M., Lou H., Luo G. Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers. Mol. Cell. Biol. 2005;25:3431–3442. doi: 10.1128/MCB.25.9.3431-3442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cahill D., Connor B., Carney J. P. Mechanisms of eukaryotic DNA double strand break repair. Front. Biosci. 2006;11:1958–1976. doi: 10.2741/1938. [DOI] [PubMed] [Google Scholar]
- 178.Karmakar P., Snowden C. M., Ramsden D. A., Bohr V. A. Ku heterodimer binds to both ends of the Werner protein and functional interaction occurs at the Werner N-terminus. Nucleic Acids Res. 2002;30:3583–3591. doi: 10.1093/nar/gkf482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li B., Comai L. Requirements for the nucleolytic processing of DNA ends by the Werner syndrome protein–Ku70/80 complex. J. Biol. Chem. 2001;276:9896–9902. doi: 10.1074/jbc.M008575200. [DOI] [PubMed] [Google Scholar]
- 180.Karmakar P., Piotrowski J., Brosh R. M., Jr, Sommers J. A., Miller S. P., Cheng W. H., Snowden C. M., Ramsden D. A., Bohr V. A. Werner protein is a target of DNA-dependent protein kinase in vivo and in vitro, and its catalytic activities are regulated by phosphorylation. J. Biol. Chem. 2002;277:18291–18302. doi: 10.1074/jbc.M111523200. [DOI] [PubMed] [Google Scholar]
- 181.Yannone S. M., Roy S., Chan D. W., Murphy M. B., Huang S., Campisi J., Chen D. J. Werner syndrome protein is regulated and phosphorylated by DNA-dependent protein kinase. J. Biol. Chem. 2001;276:38242–38248. doi: 10.1074/jbc.M101913200. [DOI] [PubMed] [Google Scholar]
- 182.Oshima J., Huang S., Pae C., Campisi J., Schiestl R. H. Lack of WRN results in extensive deletion at nonhomologous joining ends. Cancer Res. 2002;62:547–551. [PubMed] [Google Scholar]
- 183.Gaymes T. J., North P. S., Brady N., Hickson I. D., Mufti G. J., Rassool F. V. Increased error-prone non homologous DNA end-joining: a proposed mechanism of chromosomal instability in Bloom's syndrome. Oncogene. 2002;21:2525–2533. doi: 10.1038/sj.onc.1205331. [DOI] [PubMed] [Google Scholar]
- 184.Langland G., Elliott J., Li Y., Creaney J., Dixon K., Groden J. The BLM helicase is necessary for normal DNA double-strand break repair. Cancer Res. 2002;62:2766–2770. [PubMed] [Google Scholar]
- 185.Runger T. M., Kraemer K. H. Joining of linear plasmid DNA is reduced and error-prone in Bloom's syndrome cells. EMBO J. 1989;8:1419–1425. doi: 10.1002/j.1460-2075.1989.tb03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kusano K., Johnson-Schlitz D. M., Engels W. R. Sterility of Drosophila with mutations in the Bloom syndrome gene: complementation by Ku70. Science. 2001;291:2600–2602. doi: 10.1126/science.291.5513.2600. [DOI] [PubMed] [Google Scholar]
- 187.Min B., Weinert B. T., Rio D. C. Interplay between Drosophila Bloom's syndrome helicase and Ku autoantigen during nonhomologous end joining repair of P element-induced DNA breaks. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8906–8911. doi: 10.1073/pnas.0403000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Adams M. D., McVey M., Sekelsky J. J. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299:265–267. doi: 10.1126/science.1077198. [DOI] [PubMed] [Google Scholar]
- 189.McVey M., Adams M., Staeva-Vieira E., Sekelsky J. J. Evidence for multiple cycles of strand invasion during repair of double-strand gaps in Drosophila. Genetics. 2004;167:699–705. doi: 10.1534/genetics.103.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yan H., McCane J., Toczylowski T., Chen C. Analysis of the Xenopus Werner syndrome protein in DNA double-strand break repair. J. Cell Biol. 2005;171:217–227. doi: 10.1083/jcb.200502077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cheng W. H., von Kobbe C., Opresko P. L., Arthur L. M., Komatsu K., Seidman M. M., Carney J. P., Bohr V. A. Linkage between Werner syndrome protein and the Mre11 complex via Nbs1. J. Biol. Chem. 2004;279:21169–21176. doi: 10.1074/jbc.M312770200. [DOI] [PubMed] [Google Scholar]
- 192.Baynton K., Otterlei M., Bjoras M., von Kobbe C., Bohr V. A., Seeberg E. WRN interacts physically and functionally with the recombination mediator protein RAD52. J. Biol. Chem. 2003;278:36476–36486. doi: 10.1074/jbc.M303885200. [DOI] [PubMed] [Google Scholar]
- 193.Lan L., Nakajima S., Komatsu K., Nussenzweig A., Shimamoto A., Oshima J., Yasui A. Accumulation of Werner protein at DNA double-strand breaks in human cells. J. Cell Sci. 2005;118:4153–4162. doi: 10.1242/jcs.02544. [DOI] [PubMed] [Google Scholar]
- 194.Zhang N., Kaur R., Lu X., Shen X., Li L., Legerski R. J. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J. Biol. Chem. 2005;280:40559–40567. doi: 10.1074/jbc.M508453200. [DOI] [PubMed] [Google Scholar]
- 195.Wu L., Davies S. L., Levitt N. C., Hickson I. D. Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51. J. Biol. Chem. 2001;276:19375–19381. doi: 10.1074/jbc.M009471200. [DOI] [PubMed] [Google Scholar]
- 196.Braybrooke J. P., Li J. L., Wu L., Caple F., Benson F. E., Hickson I. D. Functional interaction between the Bloom's syndrome helicase and the RAD51 paralog, RAD51L3 (RAD51D) J. Biol. Chem. 2003;278:48357–48366. doi: 10.1074/jbc.M308838200. [DOI] [PubMed] [Google Scholar]
- 197.Sengupta S., Robles A. I., Linke S. P., Sinogeeva N. I., Zhang R., Pedeux R., Ward I. M., Celeste A., Nussenzweig A., Chen J., et al. Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest. J. Cell Biol. 2004;166:801–813. doi: 10.1083/jcb.200405128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Myung K., Datta A., Chen C., Kolodner R. D. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 2001;27:113–116. doi: 10.1038/83673. [DOI] [PubMed] [Google Scholar]
- 199.Sugawara N., Goldfarb T., Studamire B., Alani E., Haber J. E. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9315–9320. doi: 10.1073/pnas.0305749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cobb J., Bjergbaek L., Gasser S. RecQ helicases: at the heart of genetic stability. FEBS Lett. 2002;529:43–48. doi: 10.1016/s0014-5793(02)03269-6. [DOI] [PubMed] [Google Scholar]
- 201.Spell R. M., Jinks-Robertson S. Examination of the roles of Sgs1 and Srs2 helicases in the enforcement of recombination fidelity in Saccharomyces cerevisiae. Genetics. 2004;168:1855–1865. doi: 10.1534/genetics.104.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Onoda F., Seki M., Wang W., Enomoto T. The hyper unequal sister chromatid recombination in an sgs1 mutant of budding yeast requires MSH2. DNA Repair. 2004;3:1355–1362. doi: 10.1016/j.dnarep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 203.Goldfarb T., Alani E. Distinct roles for the Saccharomyces cerevisiae mismatch repair proteins in heteroduplex rejection, mismatch repair, and non-homologous tail removal. Genetics. 2004;169:563–574. doi: 10.1534/genetics.104.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pedrazzi G., Perrera C., Blaser H., Kuster P., Marra G., Davies S. L., Ryu G.-H., Freire R., Hickson I. D., Jiricny J., Stagljar I. Direct association of Bloom's syndrome gene product with the human mismatch repair protein MLH1. Nucleic Acids Res. 2001;29:4378–4386. doi: 10.1093/nar/29.21.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pedrazzi G., Bachrati C. Z., Selak N., Studer I., Petkovic M., Hickson I. D., Jiricny J., Stagljar I. The Bloom's syndrome helicase interacts directly with the human DNA mismatch repair protein hMSH6. Biol. Chem. 2003;384:1155–1164. doi: 10.1515/BC.2003.128. [DOI] [PubMed] [Google Scholar]
- 206.Langland G., Kordich J., Creaney J., Goss K. H., Lillard-Wetherell K., Bebenek K., Kunkel T. A., Groden J. The Bloom's syndrome protein (BLM) interacts with MLH1 but is not required for DNA mismatch repair. J. Biol. Chem. 2001;276:30031–30035. doi: 10.1074/jbc.M009664200. [DOI] [PubMed] [Google Scholar]
- 207.Yang Q., Zhang R., Wang X. W., Linke S. P., Sengupta S., Hickson I. D., Pedrazzi G., Perrera C., Stagljar I., Littman S. J., et al. The mismatch DNA repair heterodimer, hMSH2/6, regulates BLM helicase. Oncogene. 2004;23:3749–3756. doi: 10.1038/sj.onc.1207462. [DOI] [PubMed] [Google Scholar]
- 208.Wang Y., Cortez D., Yazdi P., Neff N., Elledge S. J., Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 209.Surtees J. A., Argueso J. L., Alani E. Mismatch repair proteins: key regulators of genetic recombination. Cytogenet. Genome Res. 2004;107:146–159. doi: 10.1159/000080593. [DOI] [PubMed] [Google Scholar]
- 210.Harrigan J. A., Opresko P. L., von Kobbe C., Kedar P. S., Prasad R., Wilson S. H., Bohr V. A. The Werner syndrome protein stimulates DNA polymerase β strand displacement synthesis via its helicase activity. J. Biol. Chem. 2003;278:22686–22695. doi: 10.1074/jbc.M213103200. [DOI] [PubMed] [Google Scholar]
- 211.Ahn B., Harrigan J. A., Indig F. E., Wilson D. M., III, Bohr V. A. Regulation of WRN helicase activity in human base excision repair. J. Biol. Chem. 2004;279:53465–53474. doi: 10.1074/jbc.M409624200. [DOI] [PubMed] [Google Scholar]
- 212.Szekely A. M., Bleichert F., Numann A., Van Komen S., Manasanch E., Ben Nasr A., Canaan A., Weissman S. M. Werner protein protects nonproliferating cells from oxidative DNA damage. Mol. Cell. Biol. 2005;25:10492–10506. doi: 10.1128/MCB.25.23.10492-10506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.von Kobbe C., Harrigan J. A., May A., Opresko P. L., Dawut L., Cheng W. H., Bohr V. A. Central role for the Werner syndrome protein/poly(ADP-ribose) polymerase 1 complex in the poly(ADP-ribosyl)ation pathway after DNA damage. Mol. Cell. Biol. 2003;23:8601–8613. doi: 10.1128/MCB.23.23.8601-8613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.von Kobbe C., Harrigan J. A., Schreiber V., Stiegler P., Piotrowski J., Dawut L., Bohr V. A. Poly(ADP-ribose) polymerase 1 regulates both the exonuclease and helicase activities of the Werner syndrome protein. Nucleic Acids Res. 2004;32:4003–4014. doi: 10.1093/nar/gkh721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Deschenes F., Massip L., Garand C., Lebel M. In vivo misregulation of genes involved in apoptosis, development and oxidative stress in mice lacking both functional Werner syndrome protein and poly(ADP-ribose) polymerase-1. Hum. Mol. Genet. 2005;14:3293–3308. doi: 10.1093/hmg/ddi362. [DOI] [PubMed] [Google Scholar]
- 216.Azam M., Lee J. Y., Abraham V., Chanoux R., Schoenly K. A., Johnson F. B. Evidence that the S cerevisiae. Sgs1 protein facilitates recombinational repair of telomeres during senescence. Nucleic Acids Res. 2006;34:506–516. doi: 10.1093/nar/gkj452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stavropoulos D. J., Bradshaw P. S., Li X., Pasic I., Truong K., Ikura M., Ungrin M., Meyn M. S. The Bloom syndrome helicase BLM interacts with TRF2 in ALT cells and promotes telomeric DNA synthesis. Hum. Mol. Genet. 2002;11:3135–3144. doi: 10.1093/hmg/11.25.3135. [DOI] [PubMed] [Google Scholar]
- 218.Lillard-Wetherell K., Machwe A., Langland G. T., Combs K. A., Behbehani G. K., Schonberg S. A., German J., Turchi J. J., Orren D. K., Groden J. Association and regulation of the BLM helicase by the telomere proteins TRF1 and TRF2. Hum. Mol. Genet. 2004;13:1919–1932. doi: 10.1093/hmg/ddh193. [DOI] [PubMed] [Google Scholar]
- 219.Opresko P. L., von Kobbe C., Laine J. P., Harrigan J., Hickson I. D., Bohr V. A. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J. Biol. Chem. 2002;277:41110–41119. doi: 10.1074/jbc.M205396200. [DOI] [PubMed] [Google Scholar]
- 220.Lillard-Wetherell K., Combs K. A., Groden J. BLM helicase complements disrupted type II telomere lengthening in telomerase-negative sgs1 yeast. Cancer Res. 2005;65:5520–5522. doi: 10.1158/0008-5472.CAN-05-0632. [DOI] [PubMed] [Google Scholar]
- 221.Davis T., Singhrao S. K., Wyllie F. S., Haughton M. F., Smith P. J., Wiltshire M., Wynford-Thomas D., Jones C. J., Faragher R. G., Kipling D. Telomere-based proliferative lifespan barriers in Werner-syndrome fibroblasts involve both p53-dependent and p53-independent mechanisms. J. Cell Sci. 2003;116:1349–1357. doi: 10.1242/jcs.00331. [DOI] [PubMed] [Google Scholar]
- 222.Schulz V. P., Zakian V. A., Ogburn C. E., McKay J., Jarzebowicz A. A., Martin G. M., Edland S. D. Accelerated loss of telomeric repeats may not explain accelerated replicative decline of Werner syndrome cells. Hum. Genet. 1996;97:750–754. doi: 10.1007/BF02346184. [DOI] [PubMed] [Google Scholar]
- 223.Tahara H., Tokutake Y., Maeda S., Kataoka H., Watanabe T., Satoh M., Matsumoto T., Sugawara M., Ide T., Goto M., et al. Abnormal telomere dynamics of B-lymphoblastoid cell strains from Werner's syndrome patients transformed by Epstein–Barr virus. Oncogene. 1997;15:1911–1920. doi: 10.1038/sj.onc.1201377. [DOI] [PubMed] [Google Scholar]
- 224.Wyllie F. S., Jones C. J., Skinner J. W., Haughton M. F., Wallis C., Wynford-Thomas D., Faragher R. G., Kipling D. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nat. Genet. 2000;24:16–17. doi: 10.1038/71630. [DOI] [PubMed] [Google Scholar]
- 225.Du X., Shen J., Kugan N., Furth E. E., Lombard D. B., Cheung C., Pak S., Luo G., Pignolo R. J., DePinho R. A., et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol. Cell. Biol. 2004;24:8437–8446. doi: 10.1128/MCB.24.19.8437-8446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bai Y., Murnane J. P. Telomere instability in a human tumor cell line expressing a dominant-negative WRN protein. Hum. Genet. 2003;113:337–347. doi: 10.1007/s00439-003-0972-y. [DOI] [PubMed] [Google Scholar]
- 227.Hickson I. D. RecQ helicases: caretakers of the genome. Nat. Rev. Cancer. 2003;3:169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 228.Schawalder J., Paric E., Neff N. F. Telomere and ribosomal DNA repeats are chromosomal targets of the bloom syndrome DNA helicase. BMC Cell Biol. 2003;4:15. doi: 10.1186/1471-2121-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Machwe A., Xiao L., Orren D. K. TRF2 recruits the Werner syndrome (WRN) exonuclease for processing of telomeric DNA. Oncogene. 2004;23:149–156. doi: 10.1038/sj.onc.1206906. [DOI] [PubMed] [Google Scholar]
- 230.Opresko P. L., Mason P. A., Podell E. R., Lei M., Hickson I. D., Cech T. R., Bohr V. A. POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. J. Biol. Chem. 2005;280:32069–32080. doi: 10.1074/jbc.M505211200. [DOI] [PubMed] [Google Scholar]
- 231.Chang S., Multani A. S., Cabrera N. G., Naylor M. L., Laud P., Lombard D., Pathak S., Guarente L., DePinho R. A. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 232.Laud P. R., Multani A. S., Bailey S. M., Wu L., Ma J., Kingsley C., Lebel M., Pathak S., DePinho R. A., Chang S. Elevated telomere–telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 2005;19:2560–2570. doi: 10.1101/gad.1321305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chang S. A mouse model of Werner Syndrome: what can it tell us about aging and cancer? Int. J. Biochem. Cell Biol. 2005;37:991–999. doi: 10.1016/j.biocel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 234.von Kobbe C., Karmakar P., Dawut L., Opresko P. L., Zeng X., Brosh R. M., Jr, Hickson I. D., Bohr V. A. Colocalization, physical, and functional interaction between Werner and Bloom syndrome proteins. J. Biol. Chem. 2002;277:22035–22044. doi: 10.1074/jbc.M200914200. [DOI] [PubMed] [Google Scholar]
- 235.Sharma S., Doherty K. M., Brosh R. M., Jr DNA helicases as targets for anti-cancer drugs. Curr. Med. Chem. Anticancer Agents. 2005;5:183–199. doi: 10.2174/1568011053765985. [DOI] [PubMed] [Google Scholar]
- 236.Grandori C., Wu K. J., Fernandez P., Ngouenet C., Grim J., Clurman B. E., Moser M. J., Oshima J., Russell D. W., Swisshelm K., et al. Werner syndrome protein limits MYC-induced cellular senescence. Genes Dev. 2003;17:1569–1574. doi: 10.1101/gad.1100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Grandori C., Robinson K. L., Galloway D. A., Swisshelm K. Functional link between Myc and the Werner gene in tumorigenesis. Cell Cycle. 2004;3:22–25. [PubMed] [Google Scholar]
- 238.Doherty K. M., Sharma S., Gupta R., Brosh R. M., Jr Tetraplex binding molecules as anti-cancer agents. Recent Pat. Anti-Cancer Drug Discov. 2006;1:185–200. doi: 10.2174/157489206777442232. [DOI] [PubMed] [Google Scholar]
- 239.Han H., Langley D. R., Rangan A., Hurley L. H. Selective interactions of cationic porphyrins with G-quadruplex structures. J. Am. Chem. Soc. 2001;123:8902–8913. doi: 10.1021/ja002179j. [DOI] [PubMed] [Google Scholar]
- 240.Li J. L., Harrison R. J., Reszk A. P., Brosh R. M., Jr, Bohr V. A., Neidle S., Hickson I. D. Inhibition of the Bloom's and Werner's syndrome helicases by G-quadruplex interacting ligands. Biochemistry. 2001;40:15194–15202. doi: 10.1021/bi011067h. [DOI] [PubMed] [Google Scholar]
- 241.Morita H., Kurihara H., Sugiyama T., Hamada C., Yazaki Y. A polymorphic variant C1367R of the Werner helicase gene and atherosclerotic diseases in the Japanese population. Thromb. Haemostasis. 1999;82:160–161. [PubMed] [Google Scholar]
- 242.Ye L., Miki T., Nakura J., Oshima J., Kamino K., Rakugi H., Ikegami H., Higaki J., Edland S. D., Martin G. M., Ogihara T. Association of a polymorphic variant of the Werner helicase gene with myocardial infarction in a Japanese population. Am. J. Med. Genet. 1997;68:494–498. doi: 10.1002/(sici)1096-8628(19970211)68:4<494::aid-ajmg30>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 243.Bohr V. A., Metter E. J., Harrigan J. A., von Kobbe C., Liu J. L., Gray M. D., Majumdar A., Wilson D. M., III, Seidman M. M. Werner syndrome protein 1367 variants and disposition towards coronary artery disease in Caucasian patients. Mech. Ageing Dev. 2004;125:491–496. doi: 10.1016/j.mad.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 244.Kamath-Loeb A. S., Welcsh P., Waite M., Adman E. T., Loeb L. A. The enzymatic activities of the Werner syndrome protein are disabled by the amino acid polymorphism R834C. J. Biol. Chem. 2004;279:55499–55505. doi: 10.1074/jbc.M407128200. [DOI] [PubMed] [Google Scholar]
- 245.Kuningas M., Slagboom P. E., Westendorp R. G., van Heemst D. Impact of genetic variations in the WRN gene on age related pathologies and mortality. Mech. Ageing Dev. 2006;127:307–313. doi: 10.1016/j.mad.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 246.Bendixen M. H., Nexo B. A., Bohr V. A., Frederiksen H., McGue M., Kolvraa S., Christensen K. A polymorphic marker in the first intron of the Werner gene associates with cognitive function in aged Danish twins. Exp. Gerontol. 2004;39:1101–1107. doi: 10.1016/j.exger.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 247.Payao S. L., de Labio R. W., Gatti L. L., Rigolin V. O., Bertolucci P. H., Smith M. A. Werner helicase polymorphism is not associated with Alzheimer's disease. J. Alzheimers Dis. 2004;6:591–594. doi: 10.3233/jad-2004-6603. [DOI] [PubMed] [Google Scholar]
- 248.Smith M. A., Silva M. D., Araujo L. Q., Ramos L. R., Labio R. W., Burbano R. R., Peres C. A., Andreoli S. B., Payao S. L., Cendoroglo M. S. Frequency of Werner helicase 1367 polymorphism and age-related morbidity in an elderly Brazilian population. Braz. J. Med. Biol. Res. 2005;38:1053–1059. doi: 10.1590/s0100-879x2005000700008. [DOI] [PubMed] [Google Scholar]
- 249.Wirtenberger M., Frank B., Hemminki K., Klaes R., Schmutzler R. K., Wappenschmidt B., Meindl A., Kiechle M., Arnold N., Weber B. H., et al. Interaction of Werner and Bloom syndrome genes with p53 in familial breast cancer. Carcinogenesis. 2006 doi: 10.1093/carcin/bgi374. 10.1093/carcin/bgl067. [DOI] [PubMed]
- 250.Blander G., Kipnis J., Leal J. F., Yu C. E., Schellenberg G. D., Oren M. Physical and functional interaction between p53 and the Werner's syndrome protein. J. Biol. Chem. 1999;274:29463–29469. doi: 10.1074/jbc.274.41.29463. [DOI] [PubMed] [Google Scholar]
- 251.Brosh R. M., Jr, Karmakar P., Sommers J. A., Yang Q., Wang X. W., Spillare E. A., Harris C. C., Bohr V. A. p53 modulates the exonuclease activity of Werner syndrome protein. J. Biol. Chem. 2001;276:35093–35102. doi: 10.1074/jbc.M103332200. [DOI] [PubMed] [Google Scholar]
- 252.Sengupta S., Linke S. P., Pedeux R., Yang Q., Farnsworth J., Garfield S. H., Valerie K., Shay J. W., Ellis N. A., Wasylyk B., Harris C. C. BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J. 2003;22:1210–1222. doi: 10.1093/emboj/cdg114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sommers J. A., Sharma S., Doherty K. M., Karmakar P., Yang Q., Kenny M. K., Harris C. C., Brosh R. M., Jr p53 modulates RPA-dependent and RPA-independent WRN helicase activity. Cancer Res. 2005;65:1223–1233. doi: 10.1158/0008-5472.CAN-03-0231. [DOI] [PubMed] [Google Scholar]
- 254.Spillare E. A., Robles A. I., Wang X. W., Shen J. C., Yu C. E., Schellenberg G. D., Harris C. C. p53-mediated apoptosis is attenuated in Werner syndrome cells. Genes Dev. 1999;13:1355–1360. doi: 10.1101/gad.13.11.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yang Q., Zhang R., Wang X. W., Spillare E. A., Linke S. P., Subramanian D., Griffith J. D., Li J. L., Hickson I. D., Shen J. C., et al. The processing of Holliday junctions by BLM and WRN helicases is regulated by p53. J. Biol. Chem. 2002;277:31980–31987. doi: 10.1074/jbc.M204111200. [DOI] [PubMed] [Google Scholar]
- 256.Spillare E. A., Wang X. W., von Kobbe C., Bohr V. A., Hickson I. D., Harris C. C. Redundancy of DNA helicases in p53-mediated apoptosis. Oncogene. 2005;25:2119–2123. doi: 10.1038/sj.onc.1209242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang X. W., Tseng A., Ellis N. A., Spillare E. A., Linke S. P., Robles A. I., Seker H., Yang Q., Hu P., Beresten S., et al. Functional interaction of p53 and BLM DNA helicase in apoptosis. J. Biol. Chem. 2001;276:32948–32955. doi: 10.1074/jbc.M103298200. [DOI] [PubMed] [Google Scholar]
- 258.Moser M. J., Kamath-Loeb A. S., Jacob J. E., Bennett S. E., Oshima J., Monnat R. J., Jr WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res. 2000;28:648–654. doi: 10.1093/nar/28.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen L., Lee L., Kudlow B. A., Dos Santos H. G., Sletvold O., Shafeghati Y., Botha E. G., Garg A., Hanson N. B., Martin G. M., et al. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- 260.Li D., Frazier M., Evans D. B., Hess K. R., Crane C. H., Jiao L., Abbruzzese J. L. Single nucleotide polymorphisms of RecQ1, RAD54L and ATM genes are associated with reduced survival of pancreatic cancer. J. Clin. Oncol. 2006;24:1720–1728. doi: 10.1200/JCO.2005.04.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li D., Liu H., Jiao L., Chang D. Z., Beinart G., Wolff R. A., Evans D. B., Hassan M. M., Abbruzzese J. L. Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival. Cancer Res. 2006;66:3323–3330. doi: 10.1158/0008-5472.CAN-05-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ooi S. L., Shoemaker D. D., Boeke J. D. DNA helicase gene interaction network defined using synthetic lethality analyzed by microarray. Nat. Genet. 2003;35:277–286. doi: 10.1038/ng1258. [DOI] [PubMed] [Google Scholar]
- 263.Schmidt K. H., Kolodner R. D. Requirement of Rrm3 helicase for repair of spontaneous DNA lesions in cells lacking Srs2 or Sgs1 helicase. Mol. Cell. Biol. 2004;24:3213–3226. doi: 10.1128/MCB.24.8.3213-3226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Budd M. E., Tong A. H., Polaczek P., Peng X., Boone C., Campbell J. L. A network of multi-tasking proteins at the DNA replication fork preserves genome stability. PLoS Genet. 2005;1:e61. doi: 10.1371/journal.pgen.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cantor S. B., Bell D. W., Ganesan S., Kass E. M., Drapkin R., Grossman S., Wahrer D. C., Sgroi D. C., Lane W. S., Haber D. A., Livingston D. M. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–160. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 266.Thompson L. H. Unraveling the Fanconi anemia–DNA repair connection. Nat. Genet. 2005;37:921–922. doi: 10.1038/ng0905-921. [DOI] [PubMed] [Google Scholar]
- 267.Menichini P., Linial M. SUVi and BACH1: a new subfamily of mammalian helicases? Mutat. Res. 2001;487:67–71. doi: 10.1016/s0921-8777(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 268.Meetei A. R., Sechi S., Wallisch M., Yang D., Young M. K., Joenje H., Hoatlin M. E., Wang W. A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol. Cell Biol. 2003;23:3417–3426. doi: 10.1128/MCB.23.10.3417-3426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pichierri P., Franchitto A., Rosselli F. BLM and the FANC proteins collaborate in a common pathway in response to stalled replication forks. EMBO J. 2004;23:3154–3163. doi: 10.1038/sj.emboj.7600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jiao R., Bachrati C. Z., Pedrazzi G., Kuster P., Petkovic M., Li J. L., Egli D., Hickson I. D., Stagljar I. Physical and functional interaction between the Bloom's syndrome gene product and the largest subunit of chromatin assembly factor 1. Mol. Cell. Biol. 2004;24:4710–4719. doi: 10.1128/MCB.24.11.4710-4719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ishov A. M., Sotnikov A. G., Negorev D., Vladimirova O. V., Neff N., Kamitani T., Yeh E. T., Strauss J. F., III, Maul G. G. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cheng W. H., von Kobbe C., Opresko P. L., Fields K. M., Ren J., Kufe D., Bohr V. A. Werner syndrome protein phosphorylation by abl tyrosine kinase regulates its activity and distribution. Mol. Cell. Biol. 2003;23:6385–6395. doi: 10.1128/MCB.23.18.6385-6395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li B., Navarro S., Kasahara N., Comai L. Identification and biochemical characterization of a Werner's syndrome protein complex with Ku70/80 and poly(ADP-ribose) polymerase-1. J. Biol. Chem. 2004;279:13659–13667. doi: 10.1074/jbc.M311606200. [DOI] [PubMed] [Google Scholar]
- 274.Huang S., Beresten S., Li B., Oshima J., Ellis N. A., Campisi J. Characterization of the human and mouse WRN 3′→5′ exonuclease. Nucleic Acids Res. 2000;28:2396–2405. doi: 10.1093/nar/28.12.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lebel M., Spillare E. A., Harris C. C., Leder P. The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J. Biol. Chem. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [DOI] [PubMed] [Google Scholar]
- 276.Rodriguez-Lopez A. M., Jackson D. A., Nehlin J. O., Iborra F., Warren A. V., Cox L. S. Characterisation of the interaction between WRN, the helicase/exonuclease defective in progeroid Werner's syndrome, and an essential replication factor, PCNA. Mech. Ageing Dev. 2003;124:167–174. doi: 10.1016/s0047-6374(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 277.Nguyen D. T., Rovira I. I., Finkel T. Regulation of the Werner helicase through a direct interaction with a subunit of protein kinase A. FEBS Lett. 2002;521:170–174. doi: 10.1016/s0014-5793(02)02868-5. [DOI] [PubMed] [Google Scholar]
- 278.Kawabe Y., Seki M., Seki T., Wang W.-S., Imamura O., Furuichi Y., Saitoh H., Enomoto T. Covalent modification of the Werner's syndrome gene product with the ubiquitin-related protein, SUMO-1. J. Biol. Chem. 2000;275:20963–20966. doi: 10.1074/jbc.C000273200. [DOI] [PubMed] [Google Scholar]
- 279.Laine J. P., Opresko P. L., Indig F. E., Harrigan J. A., von Kobbe C., Bohr V. A. Werner protein stimulates topoisomerase I DNA relaxation activity. Cancer Res. 2003;63:7136–7146. [PubMed] [Google Scholar]
- 280.Partridge J. J., Lopreiato J. O., Jr, Latterich M., Indig F. E. DNA damage modulates nucleolar interaction of the Werner protein with the AAA ATPase p97/VCP. Mol. Biol. Cell. 2003;14:4221–4229. doi: 10.1091/mbc.E03-02-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kawabe Y., Branzei D., Hayashi T., Suzuki H., Masuko T., Onoda F., Heo S. J., Ikeda H., Shimamoto A., Furuichi Y., et al. A novel protein interacts with the Werner's syndrome gene product physically and functionally. J. Biol. Chem. 2001;276:20364–20369. doi: 10.1074/jbc.C100035200. [DOI] [PubMed] [Google Scholar]
- 282.Petkovic M., Dietschy T., Freire R., Jiao R., Stagljar I. The human Rothmund–Thomson syndrome gene product, RECQL4, localizes to distinct nuclear foci that coincide with proteins involved in the maintenance of genome stability. J. Cell Sci. 2005;118:4261–4269. doi: 10.1242/jcs.02556. [DOI] [PubMed] [Google Scholar]
- 283.Yin J., Tae K. Y., Varshavsky A., Wang W. RECQL4, mutated in the Rothmund–Thomson and RAPADILINO syndromes, interacts with ubiquitin ligases UBR1 and UBR2 of the N-end rule pathway. Hum. Mol. Genet. 2004;13:2421–2430. doi: 10.1093/hmg/ddh269. [DOI] [PubMed] [Google Scholar]
- 284.Seki T., Tada S., Katada T., Enomoto T. Cloning of a cDNA encoding a novel importin-α homologue, Qip1: discrimination of Qip1 and Rch1 from hSrp1 by their ability to interact with DNA helicase Q1/RecQL. Biochem. Biophys. Res. Commun. 1997;234:48–53. doi: 10.1006/bbrc.1997.6535. [DOI] [PubMed] [Google Scholar]
- 285.Cui S., Klima R., Ochem A., Arosio D., Falaschi A., Vindigni A. Characterization of the DNA-unwinding activity of human RECQ1, a helicase specifically stimulated by human replication protein A. J. Biol. Chem. 2003;278:1424–1432. doi: 10.1074/jbc.M209407200. [DOI] [PubMed] [Google Scholar]