Abstract
To better understand the molecular pathogenesis of OPLL (ossification of the posterior longitudinal ligament) of the spine, an ectopic bone formation disease, we performed cDNA microarray analysis on cultured ligament cells from OPLL patients. We found that TSG-6 (tumour necrosis factor α-stimulated gene-6) is down-regulated during osteoblastic differentiation. Adenovirus vector-mediated overexpression of TSG-6 inhibited osteoblastic differentiation of human mesenchymal stem cells induced by BMP (bone morphogenetic protein)-2 or OS (osteogenic differentiation medium). TSG-6 suppressed phosphorylation and nuclear accumulation of Smad 1/5 induced by BMP-2, probably by inhibiting binding of the ligand to the receptor, since interaction between TSG-6 and BMP-2 was observed in vitro. TSG-6 has two functional domains, a Link domain (a hyaluronan binding domain) and a CUB domain implicated in protein interaction. The inhibitory effect on osteoblastic differentiation was completely lost with exogenously added Link domain-truncated TSG-6, while partial inhibition was retained by the CUB domain-truncated protein. In addition, the inhibitory action of TSG-6 and the in vitro interaction of TSG-6 with BMP-2 were abolished by the addition of hyaluronan. Thus, TSG-6, identified as a down-regulated gene during osteoblastic differentiation, suppresses osteoblastic differentiation induced by both BMP-2 and OS and is a plausible target for therapeutic intervention in OPLL.
Keywords: bone morphogenetic protein (BMP), DNA microarray, human mesenchymal stem cell, ossification of the posterior longitudinal ligament (OPLL), osteoblastic differentiation, tumour necrosis factor α-stimulated gene-6 (TSG-6)
Abbreviations: ALP, alkaline phosphatase; BMP, bone morphogenetic protein; CBF, core-binding factor; DMEM, Dulbecco's modified Eagle's medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HA, hyaluronan; hMSC, human mesenchymal stem cell; OA, osteoarthritis; OCN, osteocalcin; OPLL, ossification of the posterior longitudinal ligament; OS, osteogenic differentiation medium; RT, reverse transcriptase; TNFα, tumour necrosis factor α; TSG-6, TNFα-stimulated gene-6
INTRODUCTION
OPLL (ossification of the posterior longitudinal ligament) of the spine is a bone forming disease characterized by ectopic ossification in the spinal ligaments that compresses the spinal canal leading to various neurological symptoms. OPLL is common among elderly populations in East Asia, and is the most frequent disorder of spinal cord function in Japan [1,2]. The incidence of this disorder in Japan is reported to be between 2–4% of the general population over 30 years of age. Despite the late age of onset (average over 50 years), OPLL has a strong genetic background as shown in classical epidemiological studies and by an estimated relative risk to siblings of approximately ten [2]. We previously performed genetic linkage and association studies to identify causalities in OPLL, and successfully identified the collagen 11A2 and collagen 6A1 genes [3–5]. Although determination of the genetic factors of OPLL has important implications, the precise molecular pathogenesis remains unclear, primarily because the small biological effect of the variant is the only observation. To better understand the molecular pathogenesis of OPLL, osteoblastic differentiation was induced in cultured ligament cells from OPLL patients and non-OPLL controls by OS (osteogenic differentiation medium) and the gene expression profiles were monitored by cDNA microarray analysis as described previously [6]. Among the genes up-regulated during OS-induced osteoblastic differentiation, the zinc-finger protein 145 gene (PLZF) was found to promote osteoblastic differentiation of hMSCs (human mesenchymal stem cells) as an upstream transcriptional regulator of CBFA1 (core-binding factor A1) gene. Among the down-regulated genes during OS-induced osteoblastic differentiation, we focused on TSG-6 (tumour necrosis factor α-stimulated gene-6), a novel suppressor of osteoblastic differentiation in hMSCs.
TSG-6 was first identified from a cDNA library prepared from TNFα- (tumour necrosis factor α)-treated human FS-4 fibroblasts [7]. The expression of TSG-6 is tightly regulated by TNFα or interleukin-1 and has a very low constitutive expression in adult tissues. TSG-6 protein, a 35 kDa glycoprotein, is secreted from chondrocytes, fibroblasts, monocytes and vascular smooth muscle cells and plays a role in extracellular matrix remodelling, leucocyte migration and cell proliferation [8]. TSG-6 protein has been detected in synovial fluid of OA (osteoarthritis) and rheumatoid arthritis patients, but is undetectable in the synovial fluid of individuals with no history of joint disease [9,10]. TSG-6 protein has a chondro-protective effect when the recombinant protein is administered into the knee joints of mice with antigeninduced arthritis [11]. TSG-6 was mapped to human chromosome 2q23.3 by radiation hybrid mapping [12,13] where putative genetic linkage to OA was reported [14,15], although the involvement of TSG-6 in genetic susceptibility to OA remains uncertain. Taken together, these results suggest that TSG-6 may play a substantial role in the inhibition of extracellular matrix degradation and joint tissue damage. Thus far, the functional participation of TSG-6 in bone metabolism has not been investigated, although the roles of TNFα, a potent inducer of TSG-6, have been extensively studied.
TNFα is a pluripotent cytokine secreted primarily from cells derived from the immune system such as macrophages, monocytes, neutrophils and T-lymphocytes [16,17]. Despite its role as a host defence cytokine, inflammatory insult occasionally leads to a high level of TNFα secretion that can have various detrimental effects on cellular components of the skeleton. TNFα stimulates proliferation and differentiation of monocytes and macrophages to form resorbing cells, osteoclasts, which leads to the bone loss involved in most inflammatory bone diseases [18]. Although the roles of TNFα in bone resorption and bone osteolysis have been investigated extensively, the molecular mechanism by which the cytokine has an impact on osteoblastic differentiation and bone formation is less well understood. Since treatment of hMSCs with TNFα is sufficient to suppress osteoblastic differentiation [19,20] and concurrently induces TSG-6 in our observations, we investigated the role of TSG-6 in the regulation of osteoblastic differentiation of hMSCs induced by OS or BMP (bone morphogenetic protein)-2.
MATERIALS AND METHODS
Cell culture and osteoblastic induction
hMSCs were purchased from Cambrex Bio Sciences. C2C12 cells were obtained from the American Type Culture Collection. Primary ligament cells from OPLL and non-OPLL controls were provided by Hirosaki University as described previously [6]. The cells were maintained in DMEM (Dulbecco's modified Eagle's medium) containing 10% fetal bovine serum and antibiotic/antimycotic solution (100 units/ml penicillin, 100 μg/ml streptomycin and 250 ng/ml amphotericin B). Osteoblastic differentiation of hMSCs was induced by adding OS (0.1 μM dexamethasone, 0.05 mM ascorbic acid-2-phosphate and 10 mM β-glycerophosphate) or human recombinant BMP-2 (R&D Systems) in serum-free DMEM supplemented with ITS supplements (Sigma), 0.05 mM ascorbic acid-2-phosphate and 10 mM β-glycerophosphate. Osteoblastic differentiation of C2C12 cells was induced by 50 ng/ml recombinant human BMP-2 in DMEM with 5% fetal bovine serum.
RT (reverse transcriptase)-PCR method
Total RNA was purified using TRIzol® reagent, according to the manufacturer's protocol (Invitrogen). cDNA was synthesized using the SuperScript™ III First-Strand Synthesis System (Invitrogen). Real-time PCR was performed using SYBR® Premix Ex Taq™ (Perfect Real Time; TaKaRa Biochemicals) using the ABI Prism® 7900 Sequence detection system (Applied Biosystems). All samples were run in triplicate in each experiment. Quantitative mRNA values were normalized by the amount of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA and results are given as -fold induction. The primer sets for RTPCR were as follows; ALP-F-1228 (5′-ttcttgctggtggaaggaggcagaa-3′) and ALP-R-1521 (5′-cgcccaccaccttgtagccaggccc-3′) for bone/liver/kidney specific ALP (alkaline phosphatase); CBFA1F (5′-acgagctgaacaggaacaacgt-3′) and CBFA1-R (5′-caccagcaagaagaagcctttg-3′) for CBFA1; BMP2-F (5′-agatgaacacagctggtcacaga-3′) and BMP2-R (5′-ggagaggatgcccttttcca-3′) for BMP2: OCN-F (5′-agcaaaggtgcagcctttgt-3′) and OCN-R (5′-gcgcctgggtctcttcact-3′) for OCN (osteocalcin); TSG-6-F (5′-cccattgtgaagccagggcccaactg-3′) and TSG-6-R (5′-ggaagctcatctccacagtatcttccc-3′) for TSG-6; COL1A1-F (5′-gacctcaagatgtgccactctg-3′) and COL1A1-R (5′-aggtgatgttctgggaggcctc-3′) for collagen 1A1 (COL1A1); GAPDH-F (5′-agaacatcatccctgcctctactgg-3′) and GAPDH-R (5′-aaaggtggaggagtgggtgtcgctg-3′) for GAPDH. The primer sequences for mouse Id-1, Id-2 and Id-3 were generously provided by Dr T.-C. He (The University of Chicago Medical Centre, Chicago, IL, U.S.A.). The primer sequences for mouse Cbfa-1, osterix and Gapdh were available at http://www.nature.com/emboj/journal/v23/n3/suppinfo/7600067a.html.
Measurement of ALP activity
ALP activity was assessed by histochemical analysis using the staining kit no. 85L-3R (Sigma). The signal intensities were measured using NIH (National Institutes of Health) Image version 1.62.
Immunoblot analysis
A polyclonal antibody against collagen I was purchased from Calbiochem-Novabiochem. Anti-phospho-Smad 1/5 antibody was purchased from Cell Signaling Technology, Inc. Non-phosphospecific Smad 1 and anti-actin antibodies were purchased from Santa Cruz Biotechnology. Anti-FLAG-M2 monoclonal antibody was from Sigma. Cells were lysed in 50 mM Tris/HCl (pH 8.0), 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P40, 150 mM NaCl and 1 mM APMSF [(p-amino diphenyl)-methanesulfonyl fluoride hydrochloride] (Wako). For identification of phosphorylated Smad protein, cells were lysed in 20 mM Tris/HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 50 mM NaF and 1 mM sodium orthovanadate with a protease inhibitor cocktail tablet (Complete™; Roche Applied Science). The lysate was cleared by centrifugation (12000 g for 15 min) and the supernatant (25 μg) was resolved by SDS/PAGE (6% gel). Proteins in the gel were electrophoretically transferred on to PVDF transfer membrane (Hybond-P; Amersham Biosciences). The membrane was incubated for 1 h at room temperature (24 °C) in TBS-T (Tris-buffered saline and 0.1% Tween 20) containing 3% (w/v) non-fat dried milk powder to block non-specific protein binding. The membrane was then incubated with primary antibody at 4 °C for 8 h. Following four washes with TBS-T, the membrane was incubated with a peroxidase-conjugated secondary antibody. Antibody binding was visualized using the ECL® Western blotting detection system (Amersham Biosciences).
Indirect immunofluorescent cell staining
Cells (1×104) cultured on a cover slip were washed twice with PBS and fixed with 2% paraformaldehyde for 20 min at room temperature. After three washes with PBS, the cells were permeabilized with 0.3% Triton X-100 for 10 min at room temperature. After blocking with 3% (w/v) non-fat dried milk powder in PBS for 30 min, cells were incubated for 1 h with anti-phospho-Smad 1/5 antibody diluted to 1:100 in PBS containing 3% (w/v) non-fat dried milk powder. After three washes with PBS, the cells were incubated for 20 min with sheep anti-mouse IgG–cy3 conjugated antibody diluted to 1:100 in PBS containing 3% (w/v) non-fat dried milk powder. The cells were washed three times with PBS, and immunolocalization was examined by fluorescence microscopy (Olympus).
Recombinant adenovirus vector
A recombinant adenovirus vector carrying human full-length TSG-6 cDNA was constructed using an adenovirus expression vector kit (Takara Biochemicals). Briefly, cDNA encoding the full-length TSG-6 was obtained by PCR amplification from a cDNA pool of hMSCs, using primers; TSG-6-SmaI-F (5′-tcccccggggccgccatgatcatcttaatttacttatttc-3′), TSG-6-SmaI-R (5′-tcccccgggtcacttatcgtcgtcatccttgtaatcccctaagtggctaaatcttccagctaaa-3′). The amplicon was digested with SmaI and subcloned into the SwaI site of a pAxCAwt cassette cosmid. The cosmid and adenovirus DNA-terminal protein complex were co-transfected into the E1 trans-complemental 293 cell line to obtain recombinant adenovirus generated by homologous recombination. The recombinant adenovirus was isolated and the insertion of TSG-6 was verified by direct sequencing. The viruses were grown in FreeStyle 293-F cells to achieve high-titre stock (4.0×108 plaque-forming units/ml) and then purified.
Deletion constructs and transient transfection
The full-length TSG-6 cDNA was subcloned into the pCMV-Tag4 vector (Stratagene) containing FLAG epitope in the C-terminus. cDNA encoding either TSG-6 lacking the CUB domain (TSG-6 ΔCUB) or TSG-6 lacking the HA (hyaluronan) domain (TSG-6 ΔHA) was prepared by PCR amplifications using the following primers; TSG-6 ΔCUB antisense (5′-ccggaattctcacttatcgtcgtcatccttgtaatccccgtagcaataggcatcccatctttca-3′) and TSG-6 ΔHA sense (5′-aacccacacgcaaaggagtgtggtgg-3′) and subcloned into pCMV-Tag4 vector.
Immunoprecipitation
pCMV-Tag4 vector carrying TSG-6, TSG-6 ΔCUB or TSG-6 ΔHA cDNA with FLAG epitope in the C-terminus was used for transfection of FreeStyle 293-F cells (Invitrogen). FreeStyle 293-F cells were grown in serum-free FreeStyle 293 expression medium (Invitrogen) and the conditioned medium was harvested three days later. TSG-6-overexpressed conditioned medium (1 ml) of the FreeStyle 293-F cells was incubated with BMP-2 (1 μg) for 2 h at 4 °C, followed by immunoprecipitation with ANTI-FLAG® M2 affinity gel (Sigma) at 4 °C overnight with gentle mixing. The beads were then washed three times with TBS-T and eluted by competition with the FLAG peptide (Sigma). The supernatant was loaded on SDS/PAGE (4–12% gradient gel) and immunoblotted with monoclonal anti-BMP-2 antibody (Sigma).
Production and purification of recombinant human TSG-6 protein
TSG-6, TSG-6 ΔCUB or TSG-6 ΔHA was overexpressed using FreeStyle 293-F cells. The large-scale conditioned medium was collected three days post-transfection, and then incubated with the ANTI-FLAG® M2 affinity gel (Sigma) at 4 °C overnight with gentle mixing to capture the FLAG-tagged TSG-6 protein. FLAG tagged TSG-6 protein was eluted from the resin by competition with FLAG peptide (Sigma). The mixture was centrifuged (1000 g for 5 min), and the supernatant was loaded on to a HiTrap Q FF column (Amersham Biosciences) equilibrated with 50 mM Hepes (pH 8.0). Bound protein was eluted with step-wise concentrations of NaCl (0.25, 0.4, 0.5 and 0.6 M) in 50 mM Hepes (pH 8.0) and extracted from the 0.4 M NaC1 fraction. The fraction containing the bulk of the TSG-6 protein was concentrated and dialysed against 50 mM Hepes (pH 8.0) and 0.15 M NaCl. This resulted in the recovery of partially purified TSG-6 protein as judged by Coomassie Blue staining of SDS/PAGE gels.
RESULTS
Expression of TSG-6 and ALP activity of ligament cells from OPLL patients and non-OPLL controls treated with OS
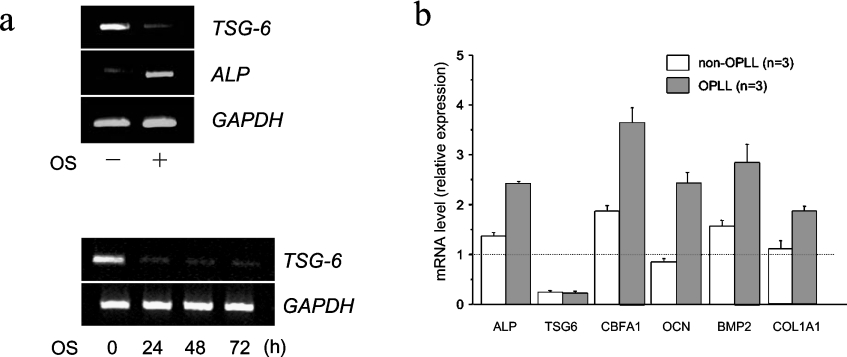
Cultured ligament cells from spinal ligaments of OPLL patients showed enhanced ALP activities after OS induction compared with such cells from non-OPLL patients. cDNA microarray analyses have shown that several genes were down-regulated 48 h post osteoblastic induction in OPLL cells, non-OPLL cells and hMSCs, as listed in Table 2 of [6]. TSG-6 is one of the genes sharply down-regulated during osteoblastic differentiation induced by OS. The decrease in expression was more prominent in hMSCs than in ligament cells. In hMSCs, TSG-6 expression during osteoblastic differentiation decreased 7.7-fold in cDNA microarray analysis and RT-PCR analysis confirmed prolonged down-regulation of TSG-6 for up to three days (Figure 1a).
Figure 1. TSG-6 expression in hMSCs and ligament cells from OPLL and non-OPLL patients.
(a) Expression levels of TSG-6, ALP and GAPDH after OS induction of hMSCs (upper panel) and TSG-6 reduction in hMSCs during prolonged OS treatment (lower panel). hMSCs were treated with OS for five days and total RNA was extracted from the cells. RT-PCR analyses for TSG-6, ALP and GAPDH was performed with each specific primer set. (b) TSG-6 reduction in ligament cells from OPLL patients and non-OPLL controls two days after OS induction. TSG-6 and molecular markers for osteoblastic differentiation (ALP, CBFA1, OCN, BMP-2 and COL1A1) were quantified by real-time RT-PCR as described in the Materials and methods section. Quantitative mRNA values were normalized by the amount of GAPDH mRNA and results are given as -fold induction.
As evaluated by real-time RT-PCR, the expression of TSG-6 was decreased both in OPLL and non-OPLL cells during osteoblastic differentiation induced by OS (Figure 1b). In addition, molecular marker genes for osteoblastic differentiation, ALP, CBFA1, OCN, BMP-2 and COL1A1, were preferentially up-regulated in OPLL cells after OS induction. In the following experiments, hMSCs, well-established pluripotent cells, were used to examine the effects of TSG-6 on osteoblastic differentiation because the ligament cells from OPLL and non-OPLL subjects have not been characterized sufficiently for analysis of the molecular mechanisms underlying ossification.
TNFα inhibits osteoblastic differentiation of hMSCs
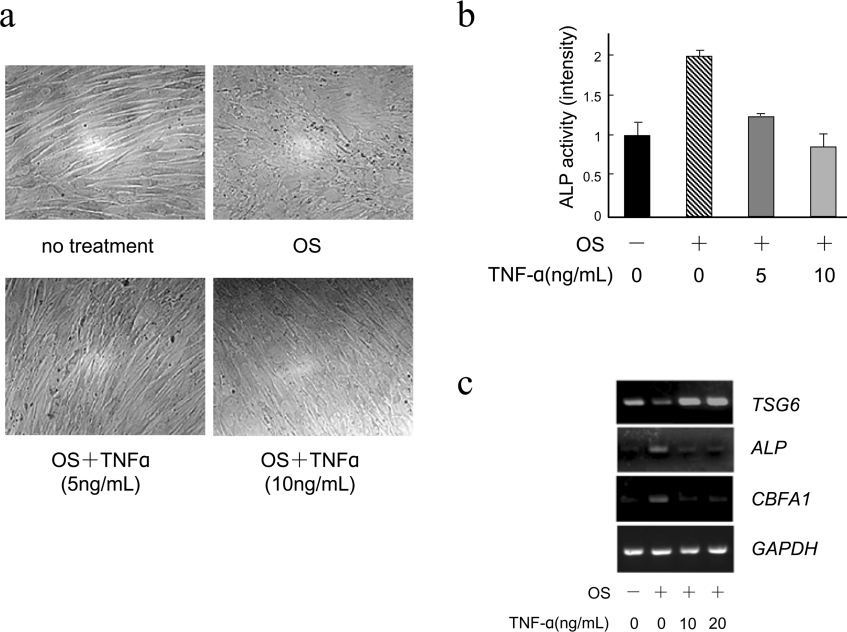
TNFα, a strong inducer of TSG-6, is known to inhibit an early stage of osteoblastic differentiation when added to stromal cell cultures [19,20]. To determine whether TNFα has similar effects on osteoblastic differentiation of hMSCs, the cells were cultured with OS in the presence or absence of TNFα. The addition of TNFα (5 or 10 ng/ml) to the hMSCs suppressed the OS-induced morphological change from spindle to cuboidal shape and also the ALP enhancements in a dose-dependent manner (Figures 2a and 2b). In parallel, OS-induced down-regulation of TSG-6 was completely blocked, even in the presence of the lowest amount of TNFα, together with down-regulation of ALP and CBFA1 (Figure 2c). Thus, TSG-6 may act as a mediator in the suppression of osteoblastic differentiation caused by TNFα.
Figure 2. TNFα inhibits OS-induced osteoblastic differentiation of hMSCs.
(a) Morphologies of hMSCs cultured in control medium or OS in the presence of TNFα (0, 5 or 10 ng/ml). (b) OS-induced ALP activity in the presence of TNFα. ALP activities were determined by ALP staining as described in the Materials and methods section. The intensity of the staining was quantified using NIH Image version 1.62 by measuring four times. The results are expressed as mean±S.D. (c) Expression levels of TSG-6 affected by TNFα. hMSCs were induced by OS in the presence of TNFα (0, 10 or 20 ng/ml) for two days. TSG-6, ALP, CBFA1 and GAPDH mRNA were evaluated by RT-PCR using specific primers.
Overexpression of TSG-6 inhibits osteoblastic differentiation of hMSCs
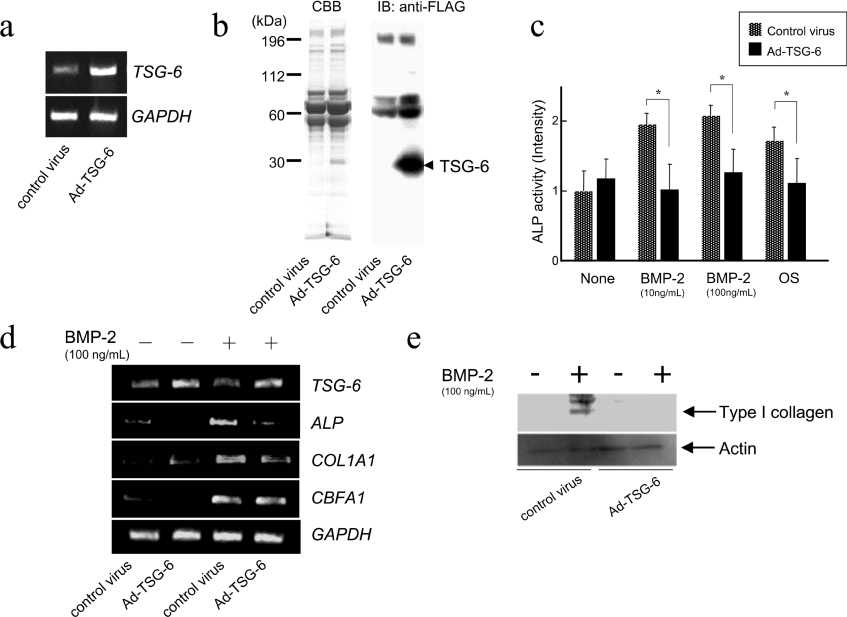
An adenovirus vector carrying full-length TSG-6 cDNA was introduced into hMSCs and the effects of overexpression of TSG-6 on osteoblastic differentiation were investigated. Overexpression of TSG-6 in the cells was confirmed by either RT-PCR (Figure 3a) or protein staining and immunoblotting with anti-FLAG antibody using the adenovirus-infected conditioned media (Figure 3b). Twenty-four hours after adenovirus vector infection, the hMSCs were subjected to osteoblastic induction by BMP-2 or OS and the effect of TSG-6 overexpression on osteoblastic differentiation was examined by ALP staining on day five following induction. The overexpression of TSG-6 resulted in suppression of ALP enhancement induced by BMP-2 as well as that induced by OS (Figure 3c). In addition, overexpression of TSG-6 resulted in reductions in the expression of osteoblast-specific genes such as ALP and COL1A1 in the presence of BMP-2 (100 ng/ml) for three days (Figure 3d). We applied 25 cycles to monitor overexpressed TSG-6, shown in Figure 3(d), making the decrease of TSG-6 in the presence of BMP-2 less evident. In hMSCs, BMP-2-induced expression of CBFA1, a pivotal transcription factor in osteogenesis, was unaffected by TSG-6 overexpression. Overexpression of TSG-6 elicited a marked suppression of type I collagen protein expression induced by BMP-2 (Figure 3e). Taken together, these results strongly suggest that TSG-6 is a suppressive regulator of osteoblastic differentiation of hMSCs.
Figure 3. Adenovirus-mediated overexpression of TSG-6 in hMSCs.
(a) and (b) hMSCs were infected with adenovirus vector carrying full-length TSG-6 cDNA. (a) The overexpression of TSG-6 mRNA was confirmed by RT-PCR. (b) The medium of the adenovirus-infected cell culture (25 μg of total protein) was subjected to SDS/PAGE and FLAG-tagged TSG-6 was detected by Coomassie Blue staining and immunoblot analysis, as described in the Materials and methods section. (c) ALP activities of the adenovirus-infected hMSCs induced by BMP-2 and OS. Statistical significance was evaluated with a simple paired t test (n=3), * denotes P<0.05. (d) The expression of ALP, COL1A1, and CBFA1 transcripts in hMSCs. The adenovirus-infected hMSCs were induced by BMP-2. Three days later, total RNA was extracted and RT-PCR was performed with specific primers. (e) The protein level of type I collagen in hMSCs. Adenovirus-infected hMSCs were induced by BMP-2. Three days later, the cellular protein was extracted and immunoblot analysis was performed. Actin was immunodetected as a loading control.
TSG-6 inhibits BMP-2-signalling by interaction
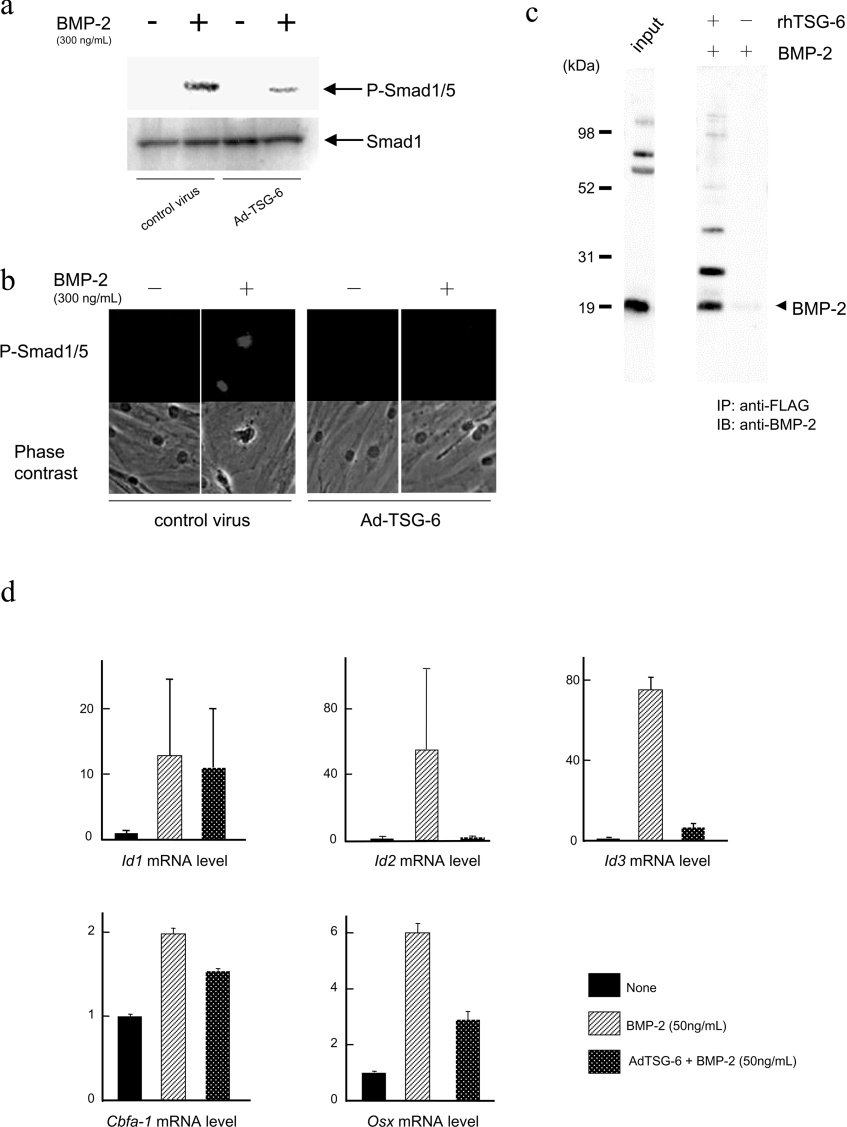
TSG-6, an extracellular glycoprotein, may suppress BMP-2-induced osteoblastic differentiation of hMSCs through inhibition of BMP-2 and the receptor binding. BMPs bind to type I and type II serine/threonine kinase receptors and the activated type I receptor in turn phosphorylates and activates the receptor-associated R-Smads such as Smad 1, 5 and 8 [21,22]. Activated R-Smads are released into the cytoplasm where they form a complex with a co-Smad, Smad 4, and then translocate into the nucleus [23]. Smad 1/5 was phosphorylated and translocated into nuclei after BMP-2 treatment whereas overexpression of TSG-6 inhibited both the phosphorylation and nuclear translocation (Figures 4a and 4b).
Figure 4. TSG-6 exerts inhibitory effects on BMP signalling.
(a) and (b) Effects of TSG-6 on phosphorylation and subcellular localization of Smad 1/5 protein after BMP-2 stimulation. hMSCs were infected with the adenovirus vector carrying TSG-6 cDNA (Ad-TSG-6) or no cDNA (control virus) and incubated in the absence or presence of BMP-2 for 6 h. The total protein was extracted for immunoblot analysis (a) and the cells were subjected to immunohistochemistry (b) with a specific antibody against the phosphorylated form of Smad 1/5. The non-phosphorylated form of Smad 1 was detected with the pan (non-phosphospecific) antibody for an experimental control (a). (c) The in vitro interaction between BMP-2 and TSG-6 proteins. To determine whether TSG-6 protein can interact with BMP-2, the TSG-6-overexpressed conditioned medium of FreeStyle 293-F cells was incubated with recombinant BMP-2 and in vitro binding was examined by immunoprecipitation to detect TSG-6 protein-bound BMP-2 as described in the Materials and methods section. (d) Expression of Id-1, Id-2, Id-3, Cbfa-1 and osterix (Osx) mRNAs was examined by adding BMP-2 to C2C12 cells infected with adenovirus vector carrying TSG-6 cDNA. The C2C12 cells were infected with adenovirus vector carrying TSG-6 cDNA for three days and stimulated with or without BMP-2 (50 ng/ml) for 1 h. The cells were harvested and real-time RT-PCR was performed. Quantitative mRNA values were normalized by the amount of Gapdh mRNA and results are given as the fold induction.
To determine whether TSG-6 protein can interact with BMP-2, FLAG-tagged TSG-6 in a conditioned medium of FreeStyle 293-F cells was incubated with recombinant BMP-2 and in vitro binding was investigated by immunoprecipitation with an anti-FLAG antibody, followed by immunoblot analysis with an anti-BMP-2 antibody to detect TSG-6 protein-bound BMP-2. As shown in Figure 4(c), TSG-6 proteins interacted with BMP-2 in vitro.
Id-1, Id-2 and Id-3 are early-stage targets of BMP signalling and play an important role in regulating BMP-induced bone formation in C2C12 cells [24]. The effects of BMP-2 on expression of these genes together with Cbfa-1 and osterix in TSG-6 overexpressed cells were examined at 1 h after BMP-2-induction using real-time RT-PCR in C2C12 cells (Figure 4d). BMP-2-induced early expressions of Id-2, Id-3 and osterix which were prominently suppressed, remained at the basal level during overexpression of TSG-6, whereas the suppression of Id-1 and Cbfa-1 expression was less evident.
Exogenously applied TSG-6 inhibits BMP-2-induced osteoblastic differentiation
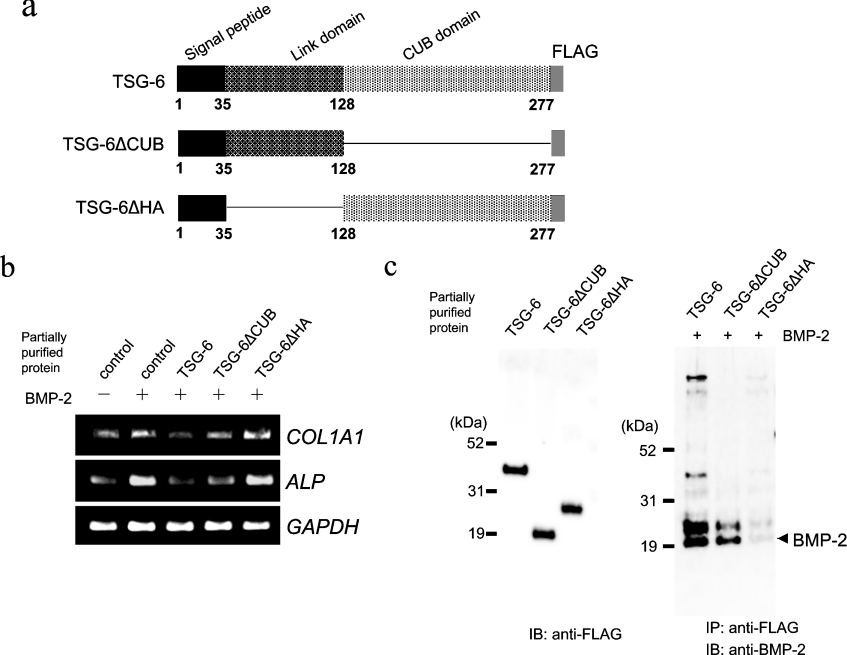
TSG-6 has two functional domains (Figure 5a) [25,26]. The N-terminal Link domain is an HA binding domain that is conserved among HA binding proteins. The C-terminal CUB domain shares sequence homologies with the A chain of the complement component C1s/C1r, uEGF, a protein involved in the development of sea urchin embryos and BMP-1, which has been implicated in protein–protein and protein–carbohydrate interactions [27]. To characterize the functional roles of the Link and CUB domains of TSG-6 in osteoblastic differentiation, TSG-6 cDNA lacking the CUB (TSG-6 ΔCUB) or Link domain (TSG-6 ΔHA) was prepared (Figure 5a). FreeStyle 293-F cells were transfected with a pCMV expression vector bearing either full-length TSG-6, TSG-6 ΔCUB or TSG-6 ΔHA cDNA, and an equivalent amount of partially purified TSG-6 protein as described above was added to the BMP-2-treated hMSCs. Exogenously added full-length TSG-6 protein resulted in suppression of ALP and COL1A1 enhancement induced by BMP-2 (100 ng/ml) for three days, whereas the addition of TSG-6 ΔHA protein resulted in no inhibitory activity and the addition of TSG-6 ΔCUB protein resulted in partial inhibitory activity, strongly suggesting that the HA binding domain is critical in the inhibitory effect of TSG-6 (Figure 5b).
Figure 5. Exogenously added TSG-6 protein inhibits BMP-2-induced osteoblastic differentiation.
(a) The structure of human TSG-6. TSG-6 contains two functional domains, a Link domain and a CUB domain; the deletion constructs lacking the CUB domain (TSG-6 ΔCUB) and the Link domain (TSG-6 ΔHA) are shown. (b) The expression of ALP and COL1A1 mRNA in hMSCs treated with TSG-6, TSG-6 ΔCUB or TSG-6 ΔHA proteins in the presence of BMP-2. Total RNA was extracted at day three and RT-PCR analyses were performed. (c) Partially purified TSG-6 protein and the in vitro interaction between BMP-2 and TSG-6 proteins. The cells were transfected with a pCMV expression vector bearing either TSG-6, TSG-6 ΔCUB or TSG-6 ΔHA cDNA. Seventy-two hours later, the conditioned medium of the cells was collected and concentrated, and the protein was subjected to purification as described in the Materials and methods section. Equal amounts of TSG-6, TSG-6 ΔCUB or TSG-6 ΔHA protein were incubated with BMP-2 and the interaction was examined by immunoprecipitation with an anti-FLAG antibody followed by immunoblot analysis with an anti-BMP-2 antibody.
To examine the roles of the functional domains of TSG-6 protein in interaction with BMP-2, partially purified TSG-6, TSG-6 ΔCUB, or TSG-6 ΔHA protein was prepared in similar amounts (Figure 5c) and incubated with recombinant BMP-2 for in vitro binding. TSG-6 and TSG-6 ΔCUB proteins interacted with BMP-2 whereas TSG-6 ΔHA protein showed a very weak interaction with BMP-2 (Figure 5c).
HA abolishes the inhibitory effect of TSG-6 on BMP-2-induced osteoblastic differentiation and BMP-2–TSG-6 complex formation
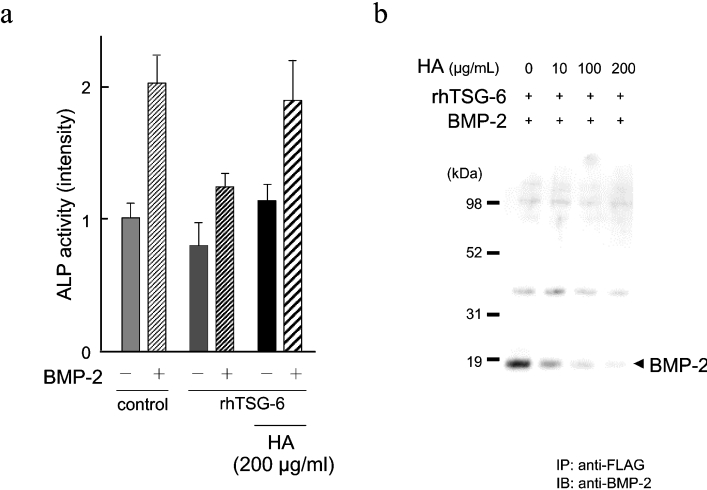
Because TSG-6 ΔHA protein showed no inhibitory effect on BMP-2-induced osteoblastic differentiation, we examined the effects of HA in the regulation of osteoblastic differentiation. The addition of HA completely abolished the inhibitory effect of TSG-6 on BMP-2-induced ALP enhancement in hMSCs (Figure 6a). To examine the effects of HA on the interaction between TSG-6 and BMP-2, the conditioned medium containing overexpressed TSG-6 protein was incubated with BMP-2 in the presence or absence of HA and their interaction in vitro was examined by immunoprecipitation as described above. The interaction of TSG-6 with BMP-2 was diminished by the addition of HA in a dose dependent manner (Figure 6b).
Figure 6. HA reverses the inhibitory effect of TSG-6 on osteoblastic differentiation.
(a) The effect of HA on the suppression of ALP activity by TSG-6. hMSCs were treated with exogenous TSG-6 and induced by BMP-2 in the absence or presence of HA. The ALP activities were evaluated by ALP staining on day five, as described in the Materials and methods section. (b) The effect of HA on the in vitro interaction of TSG-6 and BMP-2. The interaction between TSG-6 and BMP-2 was evaluated by the immunoprecipitation method as described in Figure 4(c) and the effect of the addition of various amounts of HA was examined.
DISCUSSION
OS-treated ligament cells from OPLL patients exhibited enhanced ALP activity compared with those cells from non-OPLL controls, suggesting hyperostosis in OPLL. In previous studies, we identified that the zinc-finger protein 145 gene (PLZF) was up-regulated during OS induction and is a factor that promotes osteoblastic differentiation of hMSCs [6]. In the present study, we focused on TSG-6, a gene down-regulated during osteoblastic differentiation, and examined its suppressive effect on bone formation.
Bone is a dynamic tissue, with bone remodelling involving continuous formation and resorption. OPLL is characterized by ectopic bone formation in the ligament through both endochondral and intramembranous ossification mechanisms and also systemic high bone mass [2]. Imbalance between bone formation and resorption may be a key factor in the aetiology of OPLL, therefore the genes of negative and positive regulators of bone formation both play important roles in ectopic ossification. Previously, negative regulators of bone formation such as Tob (transducer of erbB2), TSH (thyroid-stimulating hormone) and CIZ (Cas-interacting zinc-finger protein) were identified and shown to play important roles in skeletal regulation [28–30]. In the present study, we show that TSG-6 is a suppressive regulator of osteoblastic differentiation of hMSCs (Figures 1 and 3). TSG-6 is tightly regulated by TNFα, a potent pro-inflammatory cytokine ubiquitously distributed throughout the body. TNFα expression is involved in bone resorption through activation of osteoclasts particularly in inflammatory bone diseases. High levels of TNFα have been documented in tissues derived from peri-prosthetic osteolysis and joint arthritis [31]. TNFα exposure of pre-osteoblasts from stromal or calvarial origin strongly inhibits differentiation of the cells into mature matrix-producing osteoblasts [19]. Up-regulation of TSG-6 after TNFα treatment in hMSCs was observed, suggesting that the inhibitory effect of TNFα on OS-induced osteoblastic differentiation may be mediated by stimulated expression of TSG-6 (Figure 2).
Because the signalling pathway in BMP-induced osteoblastic differentiation has been more extensively characterized than that of OS-induced osteoblastic differentiation, we examined the role of TSG-6 in BMP-2-induced osteoblastic differentiation. Adenovirus vector-mediated overexpression of TSG-6 in hMSCs blocked BMP-induced osteoblastic differentiation, shown by decreased expression of COL1A1 and ALP (Figure 3). Thus, the effects of TSG-6 may shift the formation/resorption balance in the skeleton toward resorption underlying, for example, the bone loss in inflammatory arthritis. Exogenously applied TSG-6 also shows a similar suppressive effect on osteoblastic differentiation of hMSCs, indicating that the extracellular space is the site of action (Figure 5). As shown by in vitro immunoprecipitation, TSG-6 interacts with BMP-2 which might well antagonize BMP action, as is the case in inhibition of Smad 1/5 activation (Figure 4). Truncated TSG-6 lacking the Link (HA binding) domain lost both suppressive activity on osteoblastic differentiation and BMP-2 binding activity, whereas the protein lacking the CUB domain only partially lost the suppressive activity (Figure 5). Interestingly HA, which reverses the suppressive effect of TSG-6 on osteoblastic differentiation at the cellular level, inhibits the in vitro interaction between TSG-6 and BMP-2 (Figure 6). Taken together, these results indicate that the HA binding domain is critical for the suppressive effect of TSG-6, presumably through direct binding with BMP-2. BMP signalling is antagonized by secretory cystine knot family members such as noggin, chordin, gremlin, Cerberus, DAN and SOST [32–36]. Especially, noggin is expressed in cells of osteoblastic lineage, and noggin overexpression in mice results in impaired osteoblastic differentiation, reduced bone formation and osteopaenia. Despite the functional similarity to BMP binding with noggin, transgenic mice lacking TSG-6 show no evident abnormality in bone metabolism [37]. The three-dimensional structure of the noggin–BMP-7 complex has been shown to comprise a covalently linked homodimeric noggin bound to BMP-7 that inhibits BMP signalling by blocking the interfaces of the binding sites for the BMP receptors [38]. As there is no sequence homology between TSG-6 and any member of the family, a different mechanism may operate in the case of the TSG-6–BMP-2 complex. The precise mechanism by which TSG-6 exerts its suppressive effects on osteoblastic differentiation and inhibits both OS- and BMP-2-induced osteoblastic differentiation remains uncertain.
TSG-6 protein plays a crucial role in extracellular matrix formation, inflammatory cell migration, cell progression and developmental processes [26]. TSG-6 is capable of binding to serine protease inhibitors, such as inter-α-trypsin inhibitor, together with HA, inhibiting serine protease and subsequent activation of matrix metalloproteinases, therefore protecting the extracellular matrix from protease attack. The extracellular matrix is thought to play a key role in bone formation by providing a scaffold for the migration of osteoblasts and other cells, particularly in ectopic bone formation. Using genetic linkage and association studies we have thus far identified the collagen 11A2 and collagen 6A1 genes, both of which encode extracellular matrix proteins, as representing susceptibility to OPLL. This suggests that the ectopic bone formation in OPLL may result from impaired extracellular matrix formation [3–5]. Interestingly, TSG-6 expression is somewhat increased during chondrogenic differentiation of hMSCs (results not shown) and it is well known that its expression is increased in chondrocyte and other tissues in response to inflammatory stimuli.
In summary, TSG-6, identified as a down-regulated gene during osteoblastic differentiation of ligament cells and hMSCs, suppresses osteoblastic differentiation induced by both OS and BMP-2. TSG-6 may bind to BMP-2 in the extracellular space, thereby inhibiting BMP signalling and bone formation. The novel function of TSG-6 demonstrated in this study suggests the possibility of new anti-ossification therapeutic targets for OPLL patients.
Acknowledgments
This work was supported in part by a Research Grant for Specific Diseases and the Japan Foundation of Aging and Health from the Ministry of Public Health and Welfare, and a Grant-in-Aid for scientific research from the Japanese Ministry of Education, Science, Sports and Culture.
References
- 1.Terayama K. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine. 1989;14:1184–1191. doi: 10.1097/00007632-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Matsunaga S., Sakou T. Ossification of the Posterior Longitudinal Ligament. In: Yonenobu K., Sakou T., Ono K., editors. Tokyo: Springer-Verlag; 1997. pp. 11–17. [Google Scholar]
- 3.Koga H., Sakou T., Taketomi E., Hayashi K., Numasawa T., Harata S., Yone K., Matsunaga S., Otterud B., Inoue I., Leppert M. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am. J. Hum. Genet. 1998;62:1460–1467. doi: 10.1086/301868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda S., Ishidou Y., Koga H., Taketomi E., Ikari K., Komiya S., Takeda J., Sakou T., Inoue I. Functional impact of human collagen α2(XI) gene polymorphism in pathogenesis of ossification of the posterior longitudinal ligament of the spine. J. Bone Miner. Res. 2001;16:948–957. doi: 10.1359/jbmr.2001.16.5.948. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T., Ikari K., Furushima K., Okada A., Tanaka H., Furukawa K., Yoshida K., Ikeda T., Ikegawa S., Hunt S. C., et al. Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am. J. Hum. Genet. 2003;73:812–822. doi: 10.1086/378593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda R., Yoshida K., Tsukahara S., Sakamoto Y., Tanaka H., Furukawa K., Inoue I. The promyelotic leukemia zinc finger promotes osteoblastic differentiation of human mesenchymal stem cells as an upstream regulator of CBFA1. J. Biol. Chem. 2005;280:8523–8530. doi: 10.1074/jbc.M409442200. [DOI] [PubMed] [Google Scholar]
- 7.Lee T. H., Lee G. W., Ziff E. B., Vilcek J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol. Cell. Biol. 1990;10:1982–1988. doi: 10.1128/mcb.10.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milner C. M., Day A. J. TSG-6: a multifunctional protein associated with inflammation. J. Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski H. G., Maier R., Lotz M., Lee S., Klampfer L., Lee T. H., Vilcek J. TSG-6: a TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J. Immunol. 1993;151:6593–6601. [PubMed] [Google Scholar]
- 10.Bayliss M. T., Howat S. L., Dudhia J., Murphy J. M., Barry F. P., Edwards J. C., Day A. J. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarth. Cartil. 2001;9:42–48. doi: 10.1053/joca.2000.0348. [DOI] [PubMed] [Google Scholar]
- 11.Glant T. T., Kamath R. V., Bardos T., Gal I., Szanto S., Murad Y. M., Sandy J. D., Mort J. S., Roughley P. J., Mikecz K. Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor α-stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 2002;46:2207–2218. doi: 10.1002/art.10555. [DOI] [PubMed] [Google Scholar]
- 12.Lee T. H., Klampfer L., Shows T. B., Vilcek J. Transcriptional regulation of TSG6, a tumor necrosis factor- and interleukin-1-inducible primary response gene coding for a secreted hyaluronan-binding protein. J. Biol. Chem. 1993;268:6154–6160. [PubMed] [Google Scholar]
- 13.Nentwich H. A., Mustafa Z., Rugg M. S., Marsden B. D., Cordell M. R., Mahoney D. J., Jenkins S. C., Dowling B., Fries E., Milner C. M., et al. A novel allelic variant of the human TSG-6 gene encoding an amino acid difference in the CUB module. Chromosomal localization, frequency analysis, modeling and expression. J. Biol. Chem. 2002;277:15354–15362. doi: 10.1074/jbc.M110765200. [DOI] [PubMed] [Google Scholar]
- 14.Chapman K., Mustafa Z., Irven C., Carr A. J., Clipsham K., Smith A., Chitnavis J., Sinsheimer J. S., Bloomfield V. A., McCartney M., et al. Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am. J. Hum. Genet. 1999;65:167–174. doi: 10.1086/302465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustafa Z., Chapman K., Irven C., Carr A. J., Clipsham K., Chitnavis J., Sinsheimer J. S., Bloomfield V. A., McCartney M., Cox O., et al. Linkage analysis of candidate genes as susceptibility loci for osteoarthritis-suggestive linkage of COL9A1 to female hip osteoarthritis. Rheumatology (Oxford) 2000;39:299–306. doi: 10.1093/rheumatology/39.3.299. [DOI] [PubMed] [Google Scholar]
- 16.Nanes M. S. Tumor necrosis factor-α: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 17.Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 1991;266:7313–7316. [PubMed] [Google Scholar]
- 18.Kobayashi K., Takahashi N., Jimi E., Udagawa N., Takami M., Kotake S., Nakagawa N., Kinosaki M., Yamaguchi K., Shima N., et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert L., He X., Farmer P., Boden S., Kozlowski M., Rubin J., Nanes M. S. Inhibition of osteoblast differentiation by tumor necrosis factor-α. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 20.Abbas S., Zhang Y. H., Clohisy J. C., Abu-Amer Y. Tumor necrosis factor-α inhibits pre-osteoblast differentiation through its type-1 receptor. Cytokine. 2003;22:33–41. doi: 10.1016/s1043-4666(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 21.Rosenzweig B. L., Imamura T., Okadome T., Cox G. N., Yamashita H., ten Dijke P., Heldin C. H., Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazono K., Kusanagi K., Inoue H. Divergence and convergence of TGF-β/BMP signaling. J. Cell Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 23.Heldin C. H., Miyazono K., ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature (London) 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 24.Peng W. P., Yang Y. C., Kang M. W., Lee Y. T., Chang H. C. Measuring masses of single bacterial whole cells with a quadrupole ion trap. J. Am. Chem. Soc. 2004;126:11766–11767. doi: 10.1021/ja046754l. [DOI] [PubMed] [Google Scholar]
- 25.Lee T. H., Wisniewski H. G., Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 1992;116:545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day A. J., Prestwich G. D. Hyaluronan-binding proteins: tying up the giant. J. Biol. Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- 27.Bork P., Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J. Mol. Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida Y., Tanaka S., Umemori H., Minowa O., Usui M., Ikematsu N., Hosoda E., Imamura T., Kuno J., Yamashita T., et al. Negative regulation of BMP/Smad signaling by Tob in osteoblasts. Cell. 2000;103:1085–1097. doi: 10.1016/s0092-8674(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 29.Abe E., Marians R. C., Yu W., Wu X. B., Ando T., Li Y., Iqbal J., Eldeiry L., Rajendren G., Blair H. C., et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 30.Shen Z. J., Nakamoto T., Tsuji K., Nifuji A., Miyazono K., Komori T., Hirai H., Noda M. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J. Biol. Chem. 2002;277:29840–29846. doi: 10.1074/jbc.M203157200. [DOI] [PubMed] [Google Scholar]
- 31.Merkel K. D., Erdmann J. M., McHugh K. P., Abu-Amer Y., Ross F. P., Teitelbaum S. L. Tumor necrosis factor-α mediates orthopedic implant osteolysis. Am. J. Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren S. M., Brunet L. J., Harland R. M., Economides A. N., Longaker M. T. The BMP antagonist noggin regulates cranial suture fusion. Nature (London) 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D., Ferguson C. M., O'Keefe R. J., Puzas J. E., Rosier R. N., Reynolds P. R. A role for the BMP antagonist chordin in endochondral ossification. J. Bone Miner. Res. 2002;17:293–300. doi: 10.1359/jbmr.2002.17.2.293. [DOI] [PubMed] [Google Scholar]
- 34.Khokha M. K., Hsu D., Brunet L. J., Dionne M. S., Harland R. M. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat. Genet. 2003;34:303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 35.Piccolo S., Agius E., Leyns L., Bhattacharyya S., Grunz H., Bouwmeester T., De Robertis E. M. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature (London) 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dionne M. S., Skarnes W. C., Harland R. M. Mutation and analysis of Dan, the founding member of the Dan family of transforming growth factor β antagonists. Mol. Cell. Biol. 2001;21:636–643. doi: 10.1128/MCB.21.2.636-643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulop C., Szanto S., Mukhopadhyay D., Bardos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- 38.Groppe J., Greenwald J., Wiater E., Rodriguez-Leon J., Economides A. N., Kwiatkowski W., Affolter M., Vale W. W., Belmonte J. C., Choe S. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature (London) 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]