Abstract
P2Y1 [P2 (purinergic type-2)-receptor 1] is a G-protein-coupled ADP receptor that regulates platelet activation and ADP-induced Ca2+ signalling. Studies using P2Y1-knockout mice, Gq-deficient mice or P2Y1-selective inhibitors have previously identified a key role for P2Y1 in pathophysiological thrombus formation at high shear stress. We provide evidence that a positively charged juxtamembrane sequence within the cytoplasmic C-terminal tail of P2Y1 can bind directly to the cytosolic regulatory protein calmodulin. Deletion by mutagenesis of the calmodulin-binding domain of P2Y1 inhibits intracellular Ca2+ flux in transfected cells. These results suggest that the interaction of calmodulin with the P2Y1 C-terminal tail may regulate P2Y1-dependent platelet aggregation.
Keywords: calmodulin, G-protein-coupled platelet ADP receptor, maltose-binding protein (MBP), pathophysiological thrombus formation, platelet aggregation, P2 (purinergic type-2)-receptor 1 (P2Y1)
Abbreviations: AR-C69931MX, N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene-ATP; ECL® (Amersham), enhanced chemiluminescence; GP, glycoprotein; GST, glutathione S-transferase; MBP, maltose-binding protein; 2-MeSADP, 2-methylthio-ADP; PKC, protein kinase C; P2Y1, P2 (purinergic type-2)-receptor 1; W-7, N-(6-aminohexyl)-5-chloronaphthalene-1-sulfonamide
INTRODUCTION
P2Y1 [P2 (purinergic type-2)-receptor 1] is a G-protein (Gαq)-linked seven-transmembrane receptor of the purinergic receptor family that activates platelets in response to ADP and regulates Ca2+-dependent signalling events, initiating shape change and reversible αIIbβ3-dependent aggregation [1,2]. P2Y1-dependent activation is reinforced by a second ADP receptor on platelets, P2Y12, which is Gαi-linked and promotes irreversible platelet aggregation [1–3]. The major evidence for a key pathophysiological role for P2Y1 in thrombus formation comes from studies in P2Y1-deficient mice, Gq-deficient mice or normal mice treated with P2Y1 antagonists such as MRS-2179 or MRS-2500 [4–12]. Without P2Y1 (and despite normal P2Y12 expression), platelets show a decreased propensity to form a stable thrombus. Platelets lacking P2Y1 aggregate only at high ADP concentrations (via P2Y12), and do so without shape change or elevation of cytosolic Ca2+ levels. P2Y1-null mice show little or no tendency for spontaneous bleeding, but do show markedly increased resistance to thromboembolism in vivo, when induced by intravenous injection of ADP or collagen plus adrenaline [6,7]. Most recently, it was shown in mice deficient in P2Y1, or treated with the P2Y1-antagonists MRS-2179 or MRS-2500, that arterial thrombosis was significantly less than controls in FeCl3- or laser-induced arterial wall injury models (high-shear conditions); however, venous thrombosis (lower shear) was only slightly inhibited in these models [10,12]. Combined blockade of P2Y1 and P2Y12 is essentially additive when inhibiting thrombus formation under shear stress [8,10,12,13].
Recent studies support the notion that calmodulin plays a central role in the initiation of platelet thrombus formation. In human platelets, aggregation at high shear stress is triggered by the adhesion receptor GPIb (glycoprotein Ib)–IX–V, which binds von Willebrand factor and initiates thrombus involving other receptors, including the collagen receptor, GPVI, and integrins (chiefly αIIbβ3). Cytoplasmic domains of both GPVI and GPIb–IX–V (subunits GPIbβ and GPV) contain discrete juxtamembrane sequences that directly bind calmodulin, interactions which dissociate upon platelet activation [14–19]. Engagement of GPIbα (the major ligand-binding subunit of GPIb–IX–V) or GPVI leads to secretion of ADP that acts via P2Y1 and P2Y12 to increase αIIbβ3-dependent aggregation. It was shown recently [13], that GPIbα-dependent thrombus formation on von Willebrand factor at high shear is specifically impaired by P2Y1 blockade, including elevation of cytosolic Ca2+ associated with platelet arrest, whereas P2Y12 blockade inhibited formation of larger aggregates. Together, these studies indicate a key role for P2Y1 in thrombus formation under conditions of shear stress in flowing blood, and support the promise of P2Y1 as a future antithrombotic target [1,3,9,11]. The goal of the present study was to determine whether the cytoplasmic domain of P2Y1 binds calmodulin in the same manner as other receptors with analogous sequences (Figure 1), and whether the interaction may regulate P2Y1-dependent platelet activation.
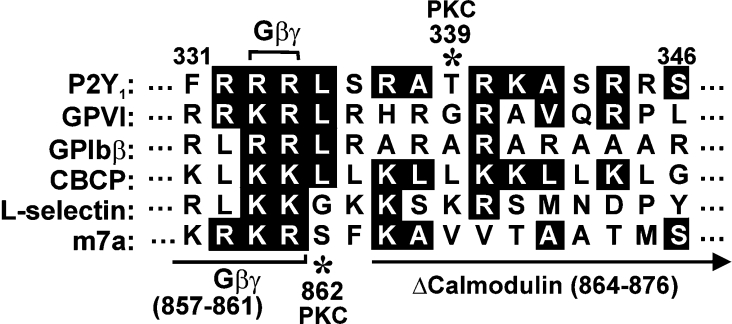
Figure 1. Comparison of calmodulin-binding sequences.
Juxtamembrane calmodulin-binding sequence within the P2Y1 C-terminal cytoplasmic tail, compared with known calmodulin-binding sequences in platelet GPVI [15–17], GPIbβ [14], a non-physiological calmodulin-binding control peptide that forms amphipathic α-helix [20], leucocyte adhesion receptor, L-selectin [21] and the G-protein-coupled glutamate receptor m7a [22]. Identical residues or conservative substitutions are highlighted. The Gβγ-binding region (residues 857–861) and the site of a deletion (residues 864–876) that blocks calmodulin binding are shown on the m7a sequence [22] compared with the G-protein-binding site at Arg333/Arg334 of P2Y1 [23]. Asterisks show known PKC-dependent phosphorylation sites in m7a (Ser862) [24] and P2Y1 (Thr339) [25,26].
MATERIALS AND METHODS
General reagents
Amylose–agarose was purchased from New England Biolabs (Beverly, MA, U.S.A.). A synthetic peptide based on the human P2Y1 C-terminal tail sequence Arg332–Arg345 (R332RRLSRATRKASRR345), purified by reverse-phase HPLC and characterized by MS, was from Mimotopes (Clayton, VIC, Australia). The calmodulin inhibitor W-7 [N-(6-aminohexyl)-5-chloronaphthalene-1-sulfonamide] was obtained from Calbiochem (La Jolla, CA, U.S.A.). The P2Y12 inhibitor AR-C69931MX [N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene-ATP], was the gift of Dr Shaun Jackson (Australian Centre for Blood Diseases, Clayton, Monash University, VIC, Australia).
Gel-shift assay
The capacity of a synthetic peptide based on a P2Y1 C-terminal tail sequence (R332RRLSRATRKASRR345) to form a complex with purified calmodulin was examined using a gel-shift assay previously used to identify calmodulin-binding peptides [13,14,19,21,22]. Briefly, 0.3 nmol of bovine calmodulin (Sigma, St Louis, MO, U.S.A.) was mixed with 0–3.0 nmol of P2Y1 peptide in 0.1 M Tris/HCl and 4 M urea, pH 7.5, in the presence of either 1 mM Ca2+ or 10 mM EGTA. After 30 min at 22 °C, 0.5 vol. of 50% (v/v) glycerol containing two drops of 0.1% Bromophenol Blue was added to each sample prior to resolution on 12.5%-polyacrylamide gels containing 4 M urea, and stained with Coomassie Blue.
Preparation of MBP (maltose-binding protein)–P2Y1 fusion proteins
cDNA corresponding to the P2Y1 C-terminal tail sequence (Phe325–Leu373) was amplified by PCR from full-length cDNA encoding wild-type human P2Y1 in a pCDNA3 vector (Invitrogen, Carlsbad, CA, U.S.A.) [6]. Forward and reverse primers included unique restriction sites (EcoR1 and BamH1 respectively) in non-complementary 5′ ends for subcloning of the amplified fragment into an MBP-fusion vector. The PCR fragment was purified using a QIAquick PCR purification kit (Qiagen, Doncaster, VIC, Australia), digested with EcoR1 and BamH1 and inserted into the pMAL-C2X vector (New England Biolabs) encoding MBP N-terminal to the P2Y1 tail insert. A calmodulin-deletion mutant of MBP–P2Y1 tail lacking residues Arg337–Asn349 was prepared using a blunt-end ligation method as previously described [27]. The correct sequence of the vectors was confirmed by sequencing. MBP alone, or wild-type or mutant MBP–P2Y1 C-terminal tail fusion proteins expressed in Escherichia coli strain BL21 were purified on amylose–agarose and dialysed into TS buffer (0.01 M Tris/HCl and 0.15 M NaCl, pH 7.4) using standard methods [15]. Subcloning of wild-type or calmodulin-deleted P2Y1 tail sequences in GST (glutathione S-transferase) vector (pGEX-4T-1; Amersham Pharmacia), expression and purification of fusion proteins on glutathione–Sepharose was performed using similar methods.
Pull-down assays with P2Y1 fusion proteins
The ability of the full-length P2Y1 C-terminal tail expressed as a MBP-fusion protein to bind calmodulin in human platelet cytosol was assessed using a pull-down assay, as previously described [15]. Briefly, MBP alone, MBP–P2Y1 wild-type C-terminal tail, or MBP–P2Y1 calmodulin-deletion mutant was incubated with cytosol and rocked for 16 h at 4 °C. An equivalent volume of amylose beads (1:1 suspension in TS buffer) was added, and the mixture incubated for a further 4 h. Beads were washed with TS buffer and immunoblotted with anti-calmodulin antibody (Upstate Biotechnology, Lake Placid, NY, U.S.A.) or anti-MBP antibody (New England Biolabs) as previously described [15]. Pull-downs using GST alone, GST–P2Y1 wild-type C-terminal tail or GST–P2Y1 calmodulin-deletion mutant from human platelet lysates [28] with glutathione–Sepharose beads were performed using essentially the same methods, and immunoblotted with anti-Gβ2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). Blots were visualized using ECL® (enhanced chemiluminescence; Amersham, Bucks., U.K.).
Platelet aggregation
Platelet aggregation was performed in a whole-blood Lumi-Aggregometer (Chrono-Log, Havertown, PA, U.SA) using human citrated platelet-rich plasma as previously described [28]. Aggregation was induced by addition of ADP (5 μM). Samples were preincubated with TS buffer or the calmodulin inhibitor W-7 (50–150 μM) for 3 min at 37 °C, prior to adding ADP. Assay mixtures included the P2Y12 inhibitor ARC369931MX (50 nM).
Intracellular Ca2+ measurements
As a functional test of the effect of calmodulin site deletion on ADP responses, intracellular Ca2+ measurements in 1321N1 cells transfected with either P2Y1 wild-type or the calmodulin-deleted mutant lacking residues Arg337–Asn349 [recombinant human P2Y1 in pEGFP-N3 vector (Clontech)] were carried out as described previously [29,30].
RESULTS AND DISCUSSION
In the present study we show that the cytosolic regulatory protein calmodulin directly interacts with a juxtamembrane positively charged sequence within the C-terminal cytoplasmic tail of P2Y1. We recently reported that two platelet-adhesion receptors, GPIb–IX–V and GPVI, which bind von Willebrand factor or collagen respectively to initiate platelet aggregation, bound directly to calmodulin via positively charged juxtamembrane sequences within their cytoplasmic domains [14,15]. In view of the functional role of these receptors, it was noteworthy that the G-protein-coupled ADP receptor P2Y1, which also initiates platelet aggregation [1–11,13], contains an analogous sequence in the juxtamembrane region of its cytoplasmic C-terminal tail (Figure 1). This sequence of P2Y1 contains positively charged and hydrophobic residues spaced in a manner similar to calmodulin-binding sequences in other proteins that form amphipathic helices (reviewed in [31]). The corresponding juxtamembrane region of the other platelet ADP receptor, P2Y12 (F304RNLSLISMLKCPNSAT320) [32], does not contain a consensus calmodulin-binding sequence. Other G-protein-coupled receptors contain a calmodulin-binding site in the same region of the cytoplasmic C-terminal tail as P2Y1, including the glutamate receptor m7a [22,24] (Figure 1).
Calmodulin binds to a P2Y1-based peptide
Using a gel-shift assay, a synthetic peptide based on the juxtamembrane sequence of the P2Y1 C-terminal tail sequence, Arg332–Arg345 (R332RRLSRATRKASRR345), was shown to bind calmodulin. The peptide induced a dose-dependent shift in migration of purified bovine calmodulin on polyacrylamide gels in the presence of Ca2+, generating a single band representing a calmodulin–peptide complex (Figure 2A). In contrast, in the presence of EGTA, there was significantly less calmodulin–peptide complex (Figure 2A), suggesting the calmodulin-P2Y1 peptide interaction under these conditions is Ca2+-dependent.
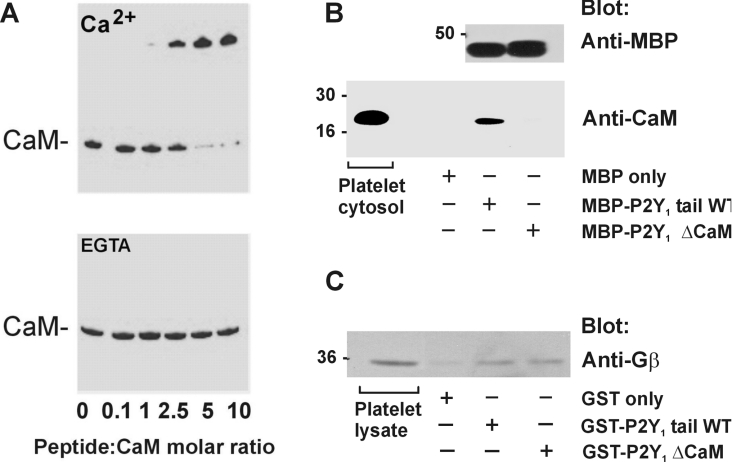
Figure 2. Gel-shift assay (A) and pull-down experiments (B and C).
(A) Non-denaturing gel-shift assay of purified calmodulin (CaM) and increasing concentrations of the P2Y1-related peptide in the presence of Ca2+ (upper panel) or EGTA (lower panel). Proteins were stained with Coomassie Blue. Data are representative of four separate experiments. (B) Pull-down of calmodulin from platelet cytosol by amylose beads and MBP alone, MBP–P2Y1 wild-type C-terminal tail or MBP–P2Y1 ΔCaM mutant. Samples of platelet cytosol or precipitates were analysed by Western blotting with anti-calmodulin antibody (lower panel) or fusion proteins were probed with anti-MBP antibody (upper panel) and visualized using ECL®. (C) Pull-down of Gβ subunits from platelet lysate by glutathione–Sepharose beads and GST alone, GST–P2Y1 wild-type C-terminal tail or GST–P2Y1 ΔCaM mutant. Samples were analysed by Western blotting with anti-Gβ antibody and visualized using ECL®.
Calmodulin binds to MBP–P2Y1 C-terminal tail fusion proteins
We expressed two MBP fusion proteins containing the C-terminal region of P2Y1 to see whether they could bind calmodulin, as did the P2Y1-based synthetic peptide. One of the fusion proteins consisted of MBP–P2Y1, which contained the full-length wild-type cytoplasmic tail of P2Y1 (Phe325–Leu373). A second MBP–P2Y1 fusion protein was a calmodulin-deletion mutant lacking residues Arg337–Asn349 within the calmodulin-binding sequence (MBP–P2Y1 ΔCaM). This deletion was based on a corresponding calmodulin-deletion mutation previously reported to abolish calmodulin binding of the m7a receptor (Figure 1) [24]. The MBP–P2Y1 wild-type tail, but not the MBP–P2Y1 ΔCaM mutant, could specifically pull down calmodulin from platelet cytosol as shown by Western blotting precipitates with an anti-calmodulin antibody (Figure 2B). MBP alone was used as a specificity control. Blotting the MBP–P2Y1 wild-type tail and MBP–P2Y1 ΔCaM pull-down samples with anti-MBP antibody [15] confirmed there was equivalent bait in each lane (Figure 2B). These results suggest there is a single calmodulin-binding site in the P2Y1 C-terminal tail involving residues Arg337–Asn349. The cytoplasmic tail of seven-transmembrane receptors is involved in G-protein binding, and the calmodulin-binding site of P2Y1 is proximal to the residues (Arg333 and Arg334) responsible for G-protein association [23]. We therefore investigated whether deletion of the calmodulin-binding site interferes with binding of G-protein subunits. Probing GST–P2Y1 wild-type tail and GST–P2Y1 ΔCaM pull-down samples with an antibody raised against amino acids 1–300 of the N-terminus of human Gβ2 (Santa Cruz Biotechnology), which recognizes Gβ1–Gβ4 and, to a lesser extent, Gβ5, confirmed that the P2Y1 calmodulin-deleted mutant was still able to bind Gβγ subunits (Figure 2C).
Calmodulin inhibition by W-7 inhibits P2Y1-dependent platelet aggregation
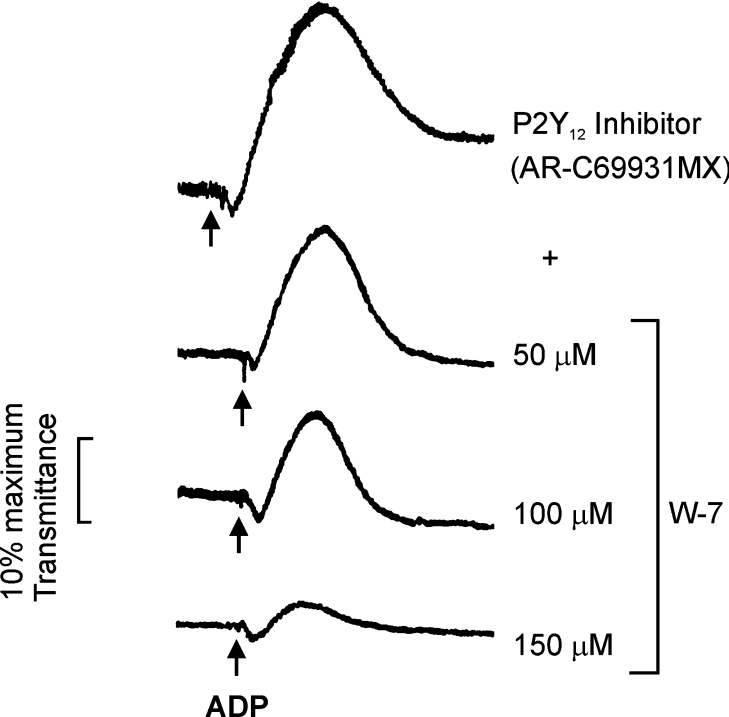
To determine whether there is a functional role for calmodulin in regulating P2Y1 receptor function, we first tested the effect of the calmodulin inhibitor W-7 on P2Y1-dependent platelet aggregation. Assays were carried out under conditions where the other ADP receptor on platelets, P2Y12, was blocked using the P2Y12-selective inhibitor AR-C69931MX. This inhibitor is an ATP analogue which selectively blocks the human ADP-receptor P2Y12, but has no effect on P2Y1 [33]. In AR-C69931MX-treated platelets, ADP acts on P2Y1 and induces reversible aggregation, without P2Y12-dependent reinforcement leading to stable aggregates [33]. Aggregation occurs up to ∼1 min, but is then reversed. P2Y1-dependent platelet aggregation induced by ADP in the presence of AR-C69931MX was inhibited by the calmodulin inhibitor W-7 (50–150 μM), with maximal inhibition at ∼150 μM (Figure 3), concentrations of W-7 previously shown to inhibit other calmodulin-mediated events in platelets or other cells [17,20,34,35]. W-7 has been reported to inhibit Ca2+-dependent platelet shape change and aggregation in response to agonists such as collagen via a myosin light-chain kinase-dependent mechanism [18,34,35]. Accordingly, our results do not mean that W-7 only inhibits P2Y1-associated calmodulin, but are consistent with calmodulin playing a critical role in P2Y1-dependent aggregation.
Figure 3. Inhibition of ADP-induced platelet aggregation by the calmodulin inhibitor W-7.
W-7 (50–150 μM) was preincubated with citrated platelet-rich plasma for 3 min at 37 °C in the presence of the P2Y12 receptor antagonist AR-C69931MX (50 nM) before addition of ADP (5 μM).
Calmodulin-binding site deletion inhibits ADP-induced increases in intracellular Ca2+ in P2Y1-transfected cells
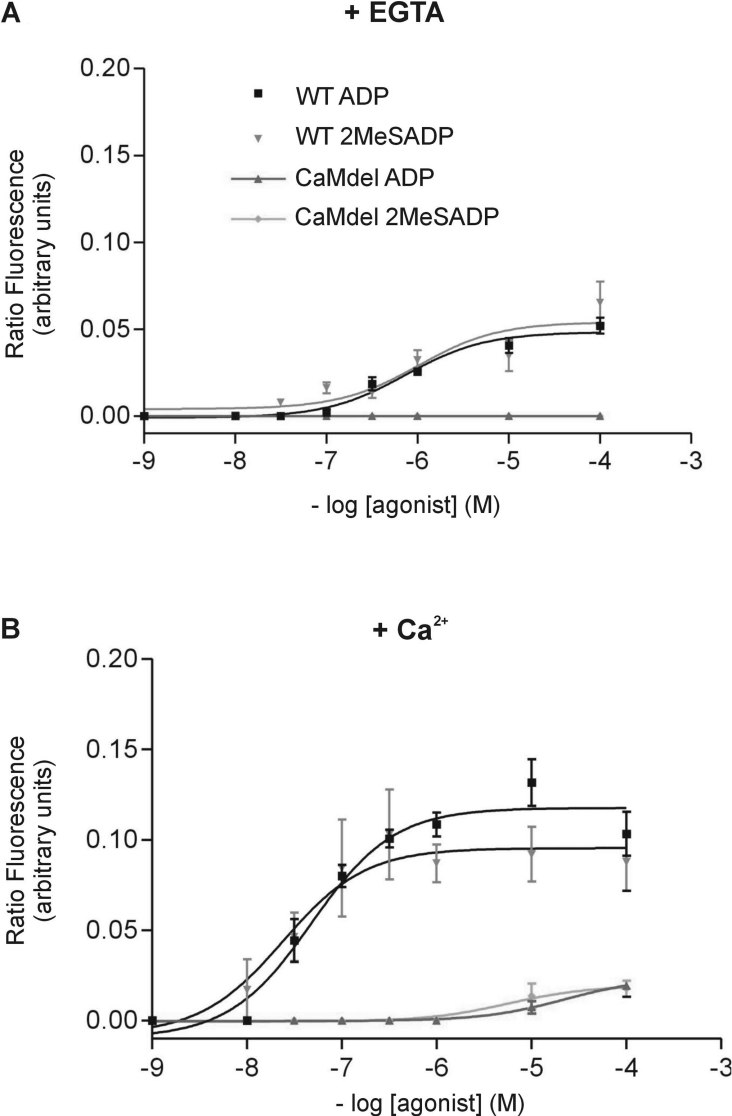
A transient increase in intracellular Ca2+ occurs downstream of P2Y1 ligation following treatment of platelets or P2Y1-transfected cells with ADP [29]. To determine more specifically whether calmodulin binding has a role in P2Y1 function, cells were transfected with either wild-type P2Y1 or calmodulin-disrupted P2Y1. Flow cytometry confirmed that similar levels of either wild-type (9.68±0.51%) or mutant receptor (8.54±0.38%) were expressed relative to untransfected cells (0.10±0.21%). Cells expressing wild-type P2Y1 or calmodulin-deleted P2Y1 were then stimulated with ADP or the highly selective P2Y1 agonist 2MeSADP (2-methylthio-ADP; 10−9–10−4 μM) in the presence or absence of 2 mM Ca2+. In the absence of Ca2+, wild-type P2Y1-transfected cells demonstrated a small increase in intracellular Ca2+ in response to both ADP and 2MeSADP, whereas cells transfected with calmodulin-deleted P2Y1 failed to respond to either agonist (Figure 4A). In the presence of 2 mM extracellular Ca2+, ADP and 2MeSADP elicited a concentration-dependent increase in intracellular Ca2+ in wild-type cells, which was strongly inhibited in the P2Y1 calmodulin-deleted mutants (Figure 4B). These data demonstrate that deletion of the calmodulin-binding region from the cytoplasmic tail of P2Y1 significantly inhibits the increase in intracellular Ca2+ induced by ADP or 2MeSADP, indicating a requirement for calmodulin in P2Y1 function.
Figure 4. Concentration-dependent increases in intracellular Ca2+ concentration in cells transfected with wild-type (WT) or calmodulin-deleted (CAMdel) P2Y1.
[Ca2+]i was induced by ADP or 2MeSADP in either the absence (A) or presence (B) of 2 mM Ca2+. Each curve represents the mean for three independent experiments and the error bars represent the S.E.M.
Calmodulin regulates surface expression of GPVI [17] and GPV of the GPIb–IX–V complex [19], which initiate thrombus formation at high shear [18]. Calmodulin binding to the C-terminal cytoplasmic domain could also regulate surface expression or internalization of P2Y1. Overexpression of P2Y1 on mouse platelets results in reduced bleeding time and increased reactivity to ADP; these results emphasize the potential importance of receptor expression levels in relation to thrombotic states in humans [36]. Activated P2Y1 receptors are internalized through a pathway distinct from that of P2Y12 receptors [30]. Calmodulin dissociation from activated P2Y1 would provide a mechanism for regulating surface expression, as for G-protein-coupled receptors on other cells [22].
Together, these results suggest that the interaction of calmodulin with the P2Y1 C-terminal tail may regulate aspects of P2Y1-dependent platelet aggregation. The calmodulin-binding sequence is proximal to functional sites for G-protein association (Arg333–Arg334) [23] and PKC (protein kinase C)-dependent phosphorylation (Ser339) [25] in the C-terminal tail of P2Y1 (Figure 1). Further studies are warranted in order to unravel the functional role of calmodulin-mediated events at the cytoplasmic face of P2Y1 and their relationship to calmodulin-dependent regulation of other receptors that initiate thrombosis.
Acknowledgments
This work was supported in part by the National Health and Medical Research Council of Australia, the National Heart Foundation of Australia and Monash University. We gratefully acknowledge the excellent technical assistance of Ms Andrea Aprico and Ms Jana Yip.
References
- 1.Gachet C. Regulation of platelet functions by P2 receptors. Annu. Rev. Pharmacol. Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 2.Nurden A. T., Nurden P. Advantages of fast-acting ADP receptor blockade in ischemic heart disease. Arterioscler. Thromb. Vasc. Biol. 2003;23:158–159. doi: 10.1161/01.atv.0000053387.06709.32. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt D. L., Topol E. J. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2003;2:15–28. doi: 10.1038/nrd985. [DOI] [PubMed] [Google Scholar]
- 4.Leon C., Hechler B., Vial C., Leray C., Cazenave J. P., Gachet C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 5.Offermanns S., Toombs C. F., Hu Y. H., Simon M. I. Defective platelet activation in Gαq-deficient mice. Nature (London) 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 6.Leon C., Hechler B., Freund M., Eckly A., Vial C., Ohlmann P., Dierich A., LeMeur M., Cazenave J. P., Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J. Clin. Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabre J. E., Nguyen M., Latour A., Keifer J. A., Audoly L. P., Coffman T. M., Koller B. H. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat. Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 8.Turner N. A., Moake J. L., McIntire L. V. Blockade of adenosine diphosphate receptors P2Y12 and P2Y1 is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98:3340–3345. doi: 10.1182/blood.v98.12.3340. [DOI] [PubMed] [Google Scholar]
- 9.Baurand A., Gachet C. The P2Y1 receptor as a target for new antithrombotic drugs: a review of the P2Y1 antagonist MRS-2179. Cardiovasc. Drug Rev. 2003;21:67–76. doi: 10.1111/j.1527-3466.2003.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 10.Lenain N., Freund M., Leon C., Cazenave J. P., Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J. Thromb. Haemostasis. 2003;1:1144–1149. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson S. P., Schoenwaelder S. M. Antiplatelet therapy: in search of the ‘magic bullet’. Nat. Rev. Drug Discov. 2003;2:775–789. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 12.Hechler B., Nonne C., Roh E. J., Cattaneo M., Cazenave J.-P., Lanza F., Jacobson K. A., Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J. Pharmacol. Exp. Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzucato M., Cozzi M. R., Pradella P., Ruggeri Z. M., De Marco L. Distinct roles of ADP receptors in von Willebrand factor-mediated platelet signaling and activation under high flow. Blood. 2004;104:3221–3227. doi: 10.1182/blood-2004-03-1145. [DOI] [PubMed] [Google Scholar]
- 14.Andrews R. K., Munday A. D., Mitchell C. A., Berndt M. C. Interaction of calmodulin with the cytoplasmic domain of the platelet membrane glycoprotein Ib–IX–V complex. Blood. 2001;98:681–687. doi: 10.1182/blood.v98.3.681. [DOI] [PubMed] [Google Scholar]
- 15.Andrews R. K., Suzuki-Inoue K., Shen Y., Tulasne D., Watson S. P., Berndt M. C. Interaction of calmodulin with the cytoplasmic domain of the platelet membrane glycoprotein VI. Blood. 2002;99:4219–4221. doi: 10.1182/blood-2001-11-0008. [DOI] [PubMed] [Google Scholar]
- 16.Locke D., Liu C., Peng X., Chen H., Kahn M. L. Fc Rγ-independent signaling by the platelet collagen receptor glycoprotein VI. J. Biol. Chem. 2003;278:15441–15448. doi: 10.1074/jbc.M212338200. [DOI] [PubMed] [Google Scholar]
- 17.Gardiner E. E., Arthur J. F., Kahn M. L., Berndt M. C., Andrews R. K. Regulation of platelet membrane levels of glycoprotein VI by a platelet-derived metalloproteinase. Blood. 2004;104:3611–3617. doi: 10.1182/blood-2004-04-1549. [DOI] [PubMed] [Google Scholar]
- 18.Gardiner E., Arthur J., Berndt M., Andrews R. Role of calmodulin in platelet receptor function. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2005;3:283–287. doi: 10.2174/156801605774322283. [DOI] [PubMed] [Google Scholar]
- 19.Rabie T., Strehl A., Ludwig A., Nieswandt B. Evidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein V. J. Biol. Chem. 2005;280:14462–14468. doi: 10.1074/jbc.M500041200. [DOI] [PubMed] [Google Scholar]
- 20.Thomas W. G., Pipolo L., Qian H. Identification of a Ca2+/calmodulin-binding domain within the carboxyl-terminus of the angiotensin II (AT1A) receptor. FEBS Lett. 1999;455:367–371. doi: 10.1016/s0014-5793(99)00904-7. [DOI] [PubMed] [Google Scholar]
- 21.Kahn J., Walcheck B., Migaki G. I., Jutila M. A., Kishimoto T. K. Calmodulin regulates L-selectin adhesion molecule expression and function through a protease-dependent mechanism. Cell. 1998;92:809–818. doi: 10.1016/s0092-8674(00)81408-7. [DOI] [PubMed] [Google Scholar]
- 22.El Far O., Betz H. G-protein-coupled receptors for neurotransmitter amino acids: C-terminal tails, crowded signalosomes. Biochem. J. 2002;365:329–336. doi: 10.1042/BJ20020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Z., Tuluc F., Bandivadekar K. R., Zhang L., Jin J., Kunapuli S. P. Arg333 and Arg334 in the COOH terminus of the human P2Y1 receptor are crucial for Gq coupling. Am. J. Physiol. Cell Physiol. 2005;288:C559–C567. doi: 10.1152/ajpcell.00401.2004. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor V., El Far O., Bofill-Cardona E., Nanoff C., Freissmuth M., Karschin A., Airas J. M., Betz H., Boehm S. Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science. 1999;286:1180–1184. doi: 10.1126/science.286.5442.1180. [DOI] [PubMed] [Google Scholar]
- 25.Fam S. R., Gallagher C. J., Kalia L. V., Salter M. W. Differential frequency dependence of P2Y1- and P2Y2- mediated Ca2+ signaling in astrocytes. J. Neurosci. 2003;23:4437–4444. doi: 10.1523/JNEUROSCI.23-11-04437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy A. R., Jones M. L., Mundell S. J., Poole A. W. Reciprocal cross-talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood. 2004;104:1745–1752. doi: 10.1182/blood-2004-02-0534. [DOI] [PubMed] [Google Scholar]
- 27.Shen Y., Romo G. M., Dong J. F., Schade A., McIntire L. V., Kenny D., Whisstock J. C., Berndt M. C., Lopez J. A., Andrews R. K. Requirement of leucine-rich repeats of glycoprotein (GP) Ibα for shear-dependent and static binding of von Willebrand factor to the platelet membrane GP Ib–IX–V complex. Blood. 2000;95:903–910. [PubMed] [Google Scholar]
- 28.Arthur J. F., Gardiner E. E., Matzaris M., Taylor S. G., Wijeyewickrema L., Ozaki Y., Kahn M. L., Andrews R. K., Berndt M. C. Glycoprotein VI is associated with GPIb-IX-V on the membrane of resting and activated platelets. Thromb. Haemostasis. 2005;93:716–723. doi: 10.1160/TH04-09-0584. [DOI] [PubMed] [Google Scholar]
- 29.Hechler B., Leon C., Vial C., Vigne P., Frelin C., Cazenave J. P., Gachet C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998;92:152–159. [PubMed] [Google Scholar]
- 30.Baurand A., Eckly A., Hechler B., Kauffenstein G., Galzi J. L., Cazenave J. P., Leon C., Gachet C. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol. Pharmacol. 2005;67:721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- 31.Hoeflich K. P., Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 32.Hollopeter G., Jantzen H. M., Vincent D., Li G., England L., Ramakrishnan V., Yang R. B., Nurden P., Nurden A., Julius D., Conley P. B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature (London) 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 33.van Giezen J. J., Humphries R. G. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin. Thromb. Hemostasis. 2005;31:195–204. doi: 10.1055/s-2005-869525. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa M., Hidaka H. Role of calmodulin in platelet aggregation. Structure–activity relationship of calmodulin antagonists. J. Clin. Invest. 1982;69:1348–1355. doi: 10.1172/JCI110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riondino S., Gazzaniga P. P., Pulcinelli F. M. Convulxin induces platelet shape change through myosin light chain kinase and Rho kinase. Eur. J. Biochem. 2002;269:5878–5884. doi: 10.1046/j.1432-1033.2002.03305.x. [DOI] [PubMed] [Google Scholar]
- 36.Hechler B., Zhang Y., Eckly A., Cazenave J. P., Gachet C., Ravid K. Lineage-specific overexpression of the P2Y1 receptor induces platelet hyper-reactivity in transgenic mice. J. Thromb. Haemostasis. 2003;1:155–163. doi: 10.1046/j.1538-7836.2003.00003.x. [DOI] [PubMed] [Google Scholar]