Abstract
The retromer complex is involved in the retrograde transport of the CI-M6PR (cation-independent mannose 6-phosphate receptor) from endosomes to the Golgi. It is a hetero-trimeric complex composed of Vps26 (vacuolar sorting protein 26), Vps29 and Vps35 proteins, which are conserved in eukaryote evolution. Recently, elucidation of the crystal structure of Vps29 revealed that Vps29 contains a metallo-phosphoesterase fold [Wang, Guo, Liang, Fan, Zhu, Zang, Zhu, Li, Teng, Niu et al. (2005) J. Biol. Chem. 280, 22962–22967; Collins, Skinner, Watson, Seaman and Owen (2005) Nat. Struct. Mol. Biol. 12, 594–602]. We demonstrate that recombinant hVps29 (human Vps29) displays in vitro phosphatase activity towards a serine-phosphorylated peptide, containing the acidic-cluster dileucine motif of the cytoplasmatic tail of the CI-M6PR. Efficient dephosphorylation required the additional presence of recombinant hVps26 and hVps35 proteins, which interact with hVps29. Phosphatase activity of hVps29 was greatly decreased by alanine substitutions of active-site residues that are predicted to co-ordinate metal ions. Using inductively coupled plasma MS, we demonstrate that recombinant hVps29 binds zinc. Moreover, hVps29-dependent phosphatase activity is greatly reduced by non-specific and zinc-specific metal ion chelators, which can be completely restored by addition of excess ZnCl2. The binuclear Zn2+ centre and phosphate group were modelled into the hVps29 catalytic site and pKa calculations provided further insight into the molecular mechanisms of Vps29 phosphatase activity. We conclude that the retromer complex displays Vps29-dependent in vitro phosphatase activity towards a serinephosphorylated acidic-cluster dileucine motif that is involved in endosomal trafficking of the CI-M6PR. The potential significance of these findings with respect to regulation of transport of cycling trans-Golgi network proteins is discussed.
Keywords: cargo protein, cation-independent mannose 6-phosphate receptor (CI-M6PR), metallo-phosphoesterase, retromer, trans-Golgi network, transport, vacuolar sorting protein 29 (Vps29)
Abbreviations: CI-M6PR, cation-independent mannose 6-phosphate receptor; CPY, carboxypeptidase Y; GFP, green fluorescent protein; GGA, Golgi-associated γ-adaptin ear homology domain Arf (ADP-ribosylation factor)-interacting protein; GST, glutathione S-transferase; ICP-MS, inductively coupled plasma MS; IPTG, isopropyl β-D-thiogalactoside; MCAC, metal chelation affinity chromatography; Ni-NTA, Ni2+-nitrilotriacetate; PBE, Poisson–Boltzmann equation; ppb, parts per billion; PPP, phosphoprotein phosphatase; SNX, sorting nexin; TBS, Tris-buffered saline; TGN, trans-Golgi network; TPEN, N,N,N′,N′-tetrakis(2-pyridylmethyl-)ethylene-diamine; Vps, vacuolar sorting protein; hVps, human Vps; VHS, Vps27, Hrs and Stam; WipKa, WHAT IF pKa; YFP, yellow fluorescent protein
INTRODUCTION
Eukaryotic cells contain several membrane-enclosed compartments (organelles), each of which has a unique function that is maintained by correct localization and retention of its resident proteins. Eukaryote subcellular organization therefore requires the existence of specific protein sorting and transport pathways to and between various organelles. Genetic screens in Saccharomyces cerevisiae have identified several Vps (vacuolar sorting protein) genes whose products are involved, directly or indirectly, in anterograde or retrograde transport between the Golgi and the lysosome-like vacuole [1–3]. Five of those gene products form the retromer complex in yeast: Vps26, Vps29, Vps35, Vps5 and Vps17, which co-localize on early endosomes [4–6] and are involved in retrograde endosome-to-Golgi transport [7].
In yeast, the Vps35 subunit interacts with the cargo protein Vps10p, which is a type I transmembrane receptor that is involved in the sorting of newly synthesized soluble vacuolar hydrolases, such as CPY (carboxypeptidase Y), from the TGN (trans-Golgi network) to the pre-vacuolar compartment. In the acidic environment, CPY dissociates from Vps10p and is then transported to the vacuole [8–10]. In contrast, Vps10p is recognized by the Vps35 subunit of the retromer complex, which functions in the retrograde transport of Vps10p from the pre-vacuolar compartment back to the TGN [11]. In the TGN, Vps10 can carry out additional rounds of enzyme delivery [9,10].
Orthologues of the yeast retromer proteins appear to be conserved in nearly all eukaryotes, including mammals. Given the role of the retromer complex in yeast, it is likely that the mammalian retromer functions in transport between the endosomes and the TGN. Although yeast protein Vps10p does not have an orthologue in mammals, mannose 6-phosphate receptors function in an essentially similar manner. Indeed, retromer subunits co-localize with the CI-M6PR (cation-independent mannose 6-phosphate receptor) cargo protein [4,6] and hVps35 (human Vps35) directly interacts with the CI-M6PR in the pre-lysosomal compartment [4]. The CI-M6PR is involved in the sorting and transport of newly synthesized lysosomal hydrolases that contain mannose 6-phosphate moieties on their N-glycan chains, which serve as sorting signals for TGN-to-endosome transport [12]. One of the triggers of this anterograde transport is the phosphorylation of Ser2492 in the cytoplasmic tail of the CI-M6PR [13]. This serine residue is part of the acidic-cluster dileucine sequence DDSDEDLLHI, present in the C-terminus of the CI-M6PR. GGA [Golgi-associated γ-adaptin ear homology domain Arf (ADP-ribosylation factor)-interacting protein] proteins, which constitute a family of clathrin coat adaptor proteins [14–18], bind with their so-called VHS (Vps27, Hrs and Stam) domain to these acidic-cluster dileucine sorting signals. GGA proteins transport the CI-M6PR, in clathrin-coated vesicles, from the TGN to an endosomal compartment en route to lysosomes [19–21]. Mutational analysis reveals that Ser2492 itself is likely to play a role in GGA binding [22] and GST (glutathione S-transferase) pull-down assays have demonstrated that phosphorylation of this serine increases the affinity of the CI-M6PR for the VHS domains of GGA1 and GGA3 [23].
In the endosomal compartment, the CI-M6PR is able to bind hVps35 [4]. hVps35 is not only the cargo recognition component, but also the scaffold protein of the retromer complex, interacting directly with hVps26 and hVps29 [5,24]. So far, the role of Vps29 and Vps26 is less clear; Vps29p probably assists Vps35p in cargo binding and Vps26p promotes the interaction between Vps35p and the SNXs (sorting nexins) Vps5p/Vps17p [25]. Vps5p binds with its C-terminal domain to Vps17p to form a dimer [26] that is able to form oligomeric structures, due to the self-assembly activity of Vps5p [7,27]. This dimer is likely to have a structural role in vesicle budding. In mammals, the Vps5p/Vps17p dimer is replaced by its orthologue SNX1 and probably another SNX protein [24]. In the case of the CI-M6PR, the co-operation of all components of the retromer complex results in the retrograde transport of the receptor from the endosome to the TGN, where it can continue the transport of hydrolases [4,6].
Recently, two crystal structures of mammalian Vps29 have been published [5,28], which revealed that Vps29 contains a metallo-phosphoesterase fold. Metallo-phosphoesterases are enzymes involved in the hydrolysis of phosphate esters [29] that contain two metal ions in their active site. In the present study, we analyse the function of Vps29 in more detail. Here, we tested the hypothesis that hVps29 acts as a metallo-phosphoesterase for the CI-M6PR. We demonstrate that hVps29 is a zinc-binding protein that acts in vitro as a phosphatase on a synthetic phosphopeptide sequence, which is based on the serine-phosphorylated C-terminus of the CI-M6PR.
MATERIALS AND METHODS
Constructs
hVps29 catalytic site point mutants were constructed by PCR, using the pCIneo hVps29 mammalian expression vector (kindly provided by C. R. Haft, National Institutes of Health, Bethesda, MD, U.S.A.) as a template. For the hVps29 D8A mutation, the following forward and reverse primers (5′–3′) were used: CCACTTTGCCTTTCTCTCCA and GTTGCACCGGTGTGGGATGTGCAGAGCTCCTAATACCAA. The PCR product was subcloned using EcoRI and AgeI sites. For all other mutations, a two-step PCR strategy was used with the following forward and reverse primers: CCACTTTGCCTTTCTCTCCA and ACTGCATTCTAGTTGTGGTT and, in addition, for hVps29 N39A/N39D, ATTCTCTGCACAGGAG(C/A)TCTTTGCACCAAAGAG and CTCTTTGGTGCAAAGA(G/T)CTCCTGTGCAGAGAAT; for hVps29 D62A/D62N, CATATTGTGAGAGG(CGC/AAA)CTTCGATGAGAATCTG and CAGATTCTCATCGAAG(GCG/CGC)CCTCTCACAATATG; for hVps29 H86A, AAAATTGGTCTGATCGCTGGACATCAAGTTAT and ATAACTTGATGTCCAGCCATCAGACCAATTTT; for hVps29 H117A, ATCTCGGGACACACAGCCAAATTTGAAGCATT and AATGCTTCAAATTTGGCTGTGTGTCCCGAGAT. PCR products were subcloned using EcoRI and NotI sites. All mutations were verified by sequencing.
YFP (yellow fluorescent protein) and GST fusion constructs of the retromer subunits were constructed using the pEYFP-C1 vector (Clontech) or pGEX4T1/3 vector (Amersham Biosciences). For hVps26, the N-terminal Myc tag in the pCIneo Myc hVps26 expression construct was replaced by a unique EcoRI cloning site followed by an initiation codon, and the C-terminal EcoRI site was replaced by a unique NotI restriction site. For hVps29, a unique EcoRI cloning site was introduced at the 5′-end of the pCIneo hVps29 Myc cDNA, while the C-terminal Myc tag was replaced by a stop codon and a unique NotI cloning site. The hVps26 and hVps29 cDNAs were cloned in frame into pEYFP-C1 and pGEX4T3 using EcoRI and NotI/Bsp120I enzymes. For hVps35, the Myc tag was removed from the pCIneo Myc hVps35 construct and the hVps35 cDNA was cloned in frame into the pEYFP-C1 and pGEX4T1 vector using XhoI and NotI/Bsp120I enzymes.
Myc–His fusion constructs of hVps26, hVps29 and hVps35 were constructed by introducing a KpnI restriction site before the GST cDNA of the pGEX vectors and replacing the GST coding sequence by a Myc–His coding sequence using linked primers.
Production of GST fusion proteins
GST fusion proteins were produced in the Escherichia coli XL-2 Blue strain (Stratagene) containing the appropriate pGEX plasmid. Cultures (0.5–5.0 litres) were grown to exponential phase [A600 (absorbance) of 0.6–0.8] and protein expression was induced by addition of IPTG (isopropyl β-D-thiogalactoside) (Invitrogen) to 0.1 mM final concentration. After 1–3 h of induction, cells were harvested, rinsed with TBS (Tris-buffered saline) (pH 7.4) and resuspended in TBS containing 1% Triton X-100, 100 μg/ml lysozyme and 10 μg/ml each of leupeptin, pepstatin and aprotonin. The suspension was frozen at −20 °C for at least 16 h and subsequently thawed at 42 °C followed by sonication. Lysates were cleared by centrifugation for 15 min at 12000 g and then incubated with 0.5–3 ml of a 50% slurry of glutathione–Sepharose beads (Sigma) for 2–4 h at 4 °C with rotation. The glutathione–Sepharose beads were washed four times with TBS (pH 7.4).
Production of Myc–His fusion proteins
Myc–His fusion proteins were produced in the E. coli XL-2 Blue strain containing the appropriate Myc–His plasmid. Cultures of 200 ml were grown to exponential phase (A600 0.6–0.8) and protein expression was induced by addition of IPTG (Invitrogen) to 0.1 mM final concentration. After 4–5 h of induction, cells were frozen at −20 °C for 16 h. Cells were thawed on ice and resuspended in 4 ml of lysis buffer (10 mM imidazole, 150 mM NaCl and 50 mM Tris/HCl, pH 8.0) and lysozyme was added to a final concentration of 1 mM. The lysates were sonicated and cleared by centrifugation for 15 min at 12000 g. Cleared lysates were incubated with 1 ml of Ni-NTA (Ni2+-nitrilotriacetate) beads (Qiagen) for 2–3 h at 4 °C with rotation. The Ni-NTA beads were washed three times (10 mM imidazole, 300 mM NaCl and 50 mM Tris/HCl, pH 8.0) and subsequently eluted with elution buffer (250 mM imidazole, 300 mM NaCl and 50 mM Tris/HCl, pH 8.0) and dialysed against water.
Pull-down assays
NIH3T3 cells were cultured on dishes coated with 0.1% gelatin and grown at 37 °C in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) newborn calf serum. pEYFP-C1 vector, Vps26, Vps29 and Vps35 YFP fusion constructs were transiently transfected into NIH3T3 cells using Lipofectamine™ (Invitrogen) according to the manufacturer's instructions. After 22–24 h of transfection, the cells were lysed in lysis buffer [150 mM NaCl, 25 mM Tris/HCl, pH 7.5, 5 mM EDTA, pH 8.0, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 μg/ml pepstatin] and the lysates were transferred to Eppendorf tubes and centrifuged at 13000 g for 10 min at 4 °C in an Eppendorf centrifuge to remove nuclei and cell debris. The cleared lysates were added to purified GST fusion proteins of hVps26, hVps29 or hVps35 or to GST alone that were bound to GSH–agarose beads and incubated for at least 2 h at 4 °C. Unbound proteins were then removed by washing twice with lysis buffer and once with PBS.
In case of the pull-down experiments with the Myc–His fusion proteins, Myc–His-tagged hVps29 and hVps35 were purified on Ni-NTA beads, eluted and incubated for at least 2 h at 4 °C in the presence of hVps26–GST immobilized on GSH–agarose beads. Unbound proteins were then removed by washing three times with TBS. Samples were then subjected to in vitro phosphatase assays or Western-blot analysis.
For Western-blot analysis, the nitrocellulose membranes were blocked for 1 h with 5% (w/v) BSA, incubated for 1 h at room temperature (20 °C) with anti-GFP (green fluorescent protein) antibody or anti-c-Myc (9E10) antibody (Santa Cruz Biotechnology), incubated for 30 min with appropriate peroxidase-linked secondary antibodies and the immunocomplexes were visualized with ECL® (enhanced chemiluminescence).
In vitro phosphatase assays
For in vitro phosphatase assays on GST fusion proteins alone, GST fusion proteins were separated from their GST tags by incubating the proteins with 10 units of thrombin (Amersham Biosciences) in TBS (pH 7.4) in a total volume of 100 μl for at least 16 h at room temperature. To each sample, 55 μg (1.1 mg/ml) of serine-phosphorylated peptide SFHDDpSDEDLLHI (>95% purity; Sigma-GenoSys) and 100 μl of serine/threonine assay buffer (50 mM Tris/HCl, pH 7.0, and 100 μM CaCl2) (Upstate Biotechnology) were added. The proteins were incubated with the phosphopeptide at 37 °C for 30 min or 16 h. From each sample, 35 μl were added to 100 μl of Biomol Green (Biomol) in a 96-well dish (Nunc). To analyse the amount of free phosphate, A630 was measured with a Wallac Multilabel counter 1420.
In case of the metal ion chelator experiment, GST fusion proteins were incubated with 5 mM EDTA, EGTA or TPEN [N,N,N′,N′-tetrakis(2-pyridylmethyl-)ethylene-diamine] (Molecular Probes) for 15 min at room temperature and washed three times with TBS prior to thrombin cleavage. For the experiments in the presence of zinc and for the experiments using Myc–His constructs, ZnCl2 was added to the serine/threonine assay buffer at a final concentration of 1 mM.
The volume of the remaining supernatant of the samples was reduced by means of a Speed Vac concentrator (type Svc100; Savant), subjected to SDS/PAGE and proteins were visualized using Coomassie Blue staining according to standard procedures.
ICP-MS (inductively coupled plasma MS)
GST alone and hVps29–GST fusion proteins were eluted from glutathione–Sepharose beads by incubation with 5 mM GSH in 50 mM Tris/HCl (pH 8.0). The amount of protein in the supernatant was measured using Bio-Rad Protein Assay Dye Reagent concentrate (Bio-Rad). A small amount of the samples was subjected to SDS/PAGE and proteins were visualized using Coomassie Blue staining to confirm expression of the protein. In order to perform accurate measurements, milligram quantities of protein should be analysed. Series of equal amounts of GST alone and hVps29–GST molecules were prepared by dilution with 5 mM GSH in 50 mM Tris/HCl (pH 8.0). HNO3 was added to a final concentration of 2 M to destroy the proteins. The final volume of the samples was 5 ml. Samples were centrifuged for 30 min at 5000 g and the supernatant was analysed by ICP-MS (X Series ICP-MS; Thermo Electron) controlled with PlasmaLab software v.2.3.0 according to the manufacturer's instructions. The measured amount of Mg, Ca, Cr, Fe, Mn, Ni, Co, Cu and Zn metal ions in the samples was quantified in ppb (parts per billion) using dilution series of CentiPur standard solutions (Merck). The number of measured metal molecules was determined using atomic mass numbers (zinc: 65.37 g/mol) and a ratio between protein molecules and metal molecules was calculated.
Molecular modelling
Molecular modelling of the hVps29 active site was done with YASARA (http://www.yasara.org) starting from the crystal structure with two bound Mn2+ ions (PDB ID 1Z2W, [5]). The highly similar structure of the human serine/threonine protein phosphatase 5 [30] containing two Mn2+ ions plus a bound phosphate was structurally aligned using the SHEBA plugin [31], and subsequently the phosphate co-ordinates were transferred to Vps29. The Mn2+ ions were replaced by Zn2+ ions to match our experimental findings. The phosphate was modelled as a mono-anion with one hydrogen atom on O4 (the leaving group) and one on O2, stabilized by the interaction with Asp62. The bridging water molecule was turned into a hydroxide ion. Finally, the protein side chains were energy minimized with the Yamber2 force field to accommodate the changes [32].
pKa calculations
pKa calculations were carried out using the WIpKa (WHAT IF pKa) calculation package as described earlier [33], with the modification that the protein was described by a single dielectric constant of 8. WIpKa relies on DelPhi II [34] to solve the linear form of the PBE (Poisson–Boltzmann equation). The remaining PBE parameters were set as follows: probe radius, 1.4 Å (1 Å=0.1 nm); solvent dielectric, 80; final grid resolution, 0.25 Å/grid point. Charges and radii were assigned using the OPLS force field [35].
RESULTS
Retromer complex performs phosphoesterase activity on a CI-M6PR-based substrate
Vesicular transport between various membrane-enclosed compartments is a fundamental characteristic of all eukaryotes. Most proteins involved in fundamental vesicular trafficking processes are evolutionarily conserved. Indeed, phylogenetic analysis (see Supplementary Figures S1 and S2 at http://www.BiochemJ.org/bj/398/bj3980399add.htm) demonstrates that Vps29 is found in nearly all eukaryotes, including higher eukaryotes (metazoans, plants and fungi) as well as protozoan groups such as diplomonadida, apicomplexa, entamoebidae and kinetoplastidae. Interestingly, BLAST searches also reveal the similarity of eukaryote Vps29 proteins to hypothetical archaeal proteins with (putative) phosphoesterase activity (e.g. MJ0936), even though archaea do not contain organelles. Vps29 shows extremely high sequence conservation among vertebrates; only 14 out of the 182 amino acids show sequence variations, most of which are conservative substitutions (Supplementary Figure S3 at http://www.BiochemJ.org/bj/398/bj3980399add.htm).
Recent crystallographic studies demonstrate that Vps29 belongs to the PPP (phosphoprotein phosphatase) family of calcineurin-like metallo-phosphatases, although Vps29 enzymatic activity has yet to be established [5,28]. This family is not only found in eukaryotes, but also in archaea and prokaryotes [36]. The crystal structures of members of this family are characterized by a double β-sandwich, surrounded by α-helices (reviewed in [37]). Metallo-phosphoesterases contain two metal ions in their catalytic site, which stabilize a highly reactive hydroxyl ion at physiological pH that is in position for nucleophilic attack of the phosphoester bond. The positively charged metal ions can also serve as an electrophilic catalyst and stabilize developing negative charge(s) of the metal–PO4 transition state. The oxy-anion of the substrate leaving group is usually protonated by a catalytic histidine residue that forms a catalytic dyad with a neighbouring aspartic acid residue, which provides the histidine residue with acidic properties. Efficient regeneration of catalytic hydroxyl ions is required for the active status of the enzyme, which has been proposed to occur through a network of hydrogen-bonded water molecules in the active site, leading to protonation of a surrounding basic histidine residue and release of the proton into bulk solvent [29].
The metal-co-ordinating and catalytically important residues, mostly aspartic acid and histidine residues, are conserved among metallo-phosphoesterase family members and are located within five conserved motifs (I through V) (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/398/bj3980399add.htm) in loops connecting the core α and β secondary structure elements, i.e. DXH(X)∼25 GDXXD(X)∼25 GNH[D/E] (motifs I–III [38,39]) and GH(X)∼50 GHX[H/X] (motifs IV–V [40]). The corresponding motifs of hVps29 start with Asp8, Gly38, Gly61, Gly87 and Gly114 respectively (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/398/bj3980399add.htm). Interestingly, hVps29 shows three unique features that distinguish hVps29 from other metallo-phosphoesterases with a known crystal structure (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/398/bj3980399add.htm). First, an aspartic acid residue is normally found in the position of Asn39 (motif II). Secondly, an asparagine is normally found in the position of Asp62 (motif III). Thirdly, the catalytic histidine residue (motif III), which serves as a proton donor for the leaving group, is replaced by Phe63.
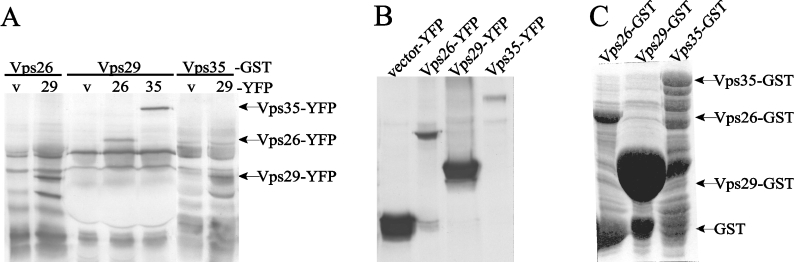
In order to determine whether the retromer complex contains phosphatase activity, we first assessed whether the retromer subunits interact using in vitro affinity precipitations (Figure 1A). Mammalian expressed YFP fusion proteins (Figure 1B) were incubated for 3 h at 4 °C with GST fusion proteins of hVps26, hVps29 and hVps35 (Figure 1C). As seen in Figure 1(A), hVps26–GST and hVps35–GST interact with Vps29–YFP but not with YFP alone, whereas hVps29–GST interacts with hVps26–YFP as well as with hVps35–YFP and not with YFP alone. Thus, consistent with earlier reports [5], interaction between retromer subunits can be demonstrated by in vitro affinity precipitation.
Figure 1. Interaction of hVps29 with hVps26 and hVps35 in vitro.
(A) GST fusion proteins were used as an affinity matrix for hVps26, hVps29 and hVps35 YFP fusion proteins present in the lysates of transiently transfected NIH3T3 cells. Affinity precipitated proteins were visualized by Western-blot analysis with anti-GFP antibodies (v: vector; 26: Vps26; 29: Vps29; and 35: Vps35). (B) Western blot of whole cell lysate samples of NIH3T3 cells transfected with the YFP fusion constructs, detected with anti-GFP antibodies. (C) Coomassie Blue staining of the GST fusion constructs used for the pull-down assay.
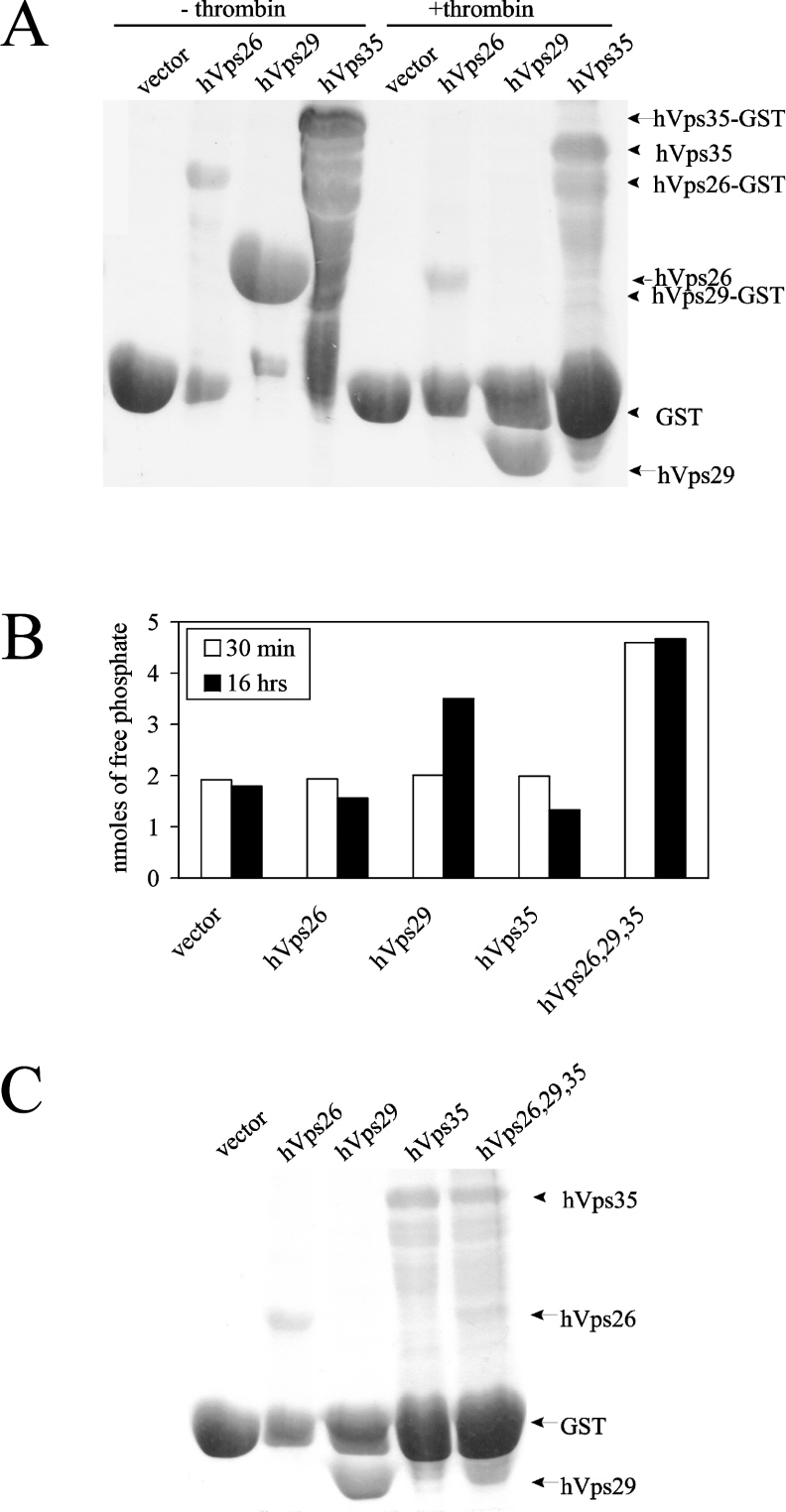
To determine whether hVps29 is an active metallo-phosphoesterase, we assayed recombinant hVps29 for in vitro phosphatase activity. GST fusion proteins of hVps26, hVps29 and hVps35 were separately produced in E. coli and the respective recombinant proteins were separated from their GST tags using the thrombin cleavage site (Figure 2A). Given that (i) the mammalian retromer complex functions in the retrograde transport of cycling TGN proteins, (ii) retromer subunits co-localize with the CI-M6PR, and (iii) mammalian Vps35 directly interacts with the CI-M6PR in the pre-lysosomal compartment [4,6], we used the serine-phosphorylated peptide SFHDDpSDEDLLHI, containing the acidic-cluster dileucine motif of the CI-M6PR C-terminal tail, as an in vitro phosphatase substrate. Recombinant hVps26, hVps29 or hVps35 or a mixture of non-stoichiometric amounts of all three proteins was incubated (30 min and 16 h) with the phosphopeptide at 37 °C. The amount of free phosphate, which correlates with the phosphatase activity, was measured in each sample after 30 min and after 16 h. Figure 2(B) shows that there was no dephosphorylation activity detectable after 30 min for the individual retromer proteins. Interestingly, only hVps29 showed phosphatase activity on its own after 16 h of incubation at 37 °C. The detected amount of free phosphate was considerably higher than the amount of free phosphate measured in the vector control, or measured in hVps26 and hVps35. Importantly, when the three retromer subunits were added simultaneously to the phosphatase reaction, the phosphatase activity was already detectable after 30 min of incubation at 37 °C. Protein expression in the phosphatase reaction was confirmed by Coomassie Brilliant Blue staining of the remaining supernatant of the samples used in the assay (Figure 2C).
Figure 2. Phosphatase activity of hVps29 on a CI-M6PR phosphopeptide.
(A) Coomassie Blue staining of recombinantly expressed GST fusion proteins of vector (26 kDa), hVps26 (36 kDa), hVps29 (20 kDa) and hVps35 (87 kDa) before and after thrombin cleavage. (B) Detected amounts of free phosphate in an in vitro phosphatase assay on the SFHDDpSDEDLLHI phosphopeptide using recombinantly expressed vector, hVps26, hVps29 or hVps35 or a mixture of these three proteins. Levels of free phosphate were determined after 30 min and after 16 h of incubation at 37 °C. (C) Coomassie Blue staining of the remaining supernatant of the protein samples used for the in vitro phosphatase assay.
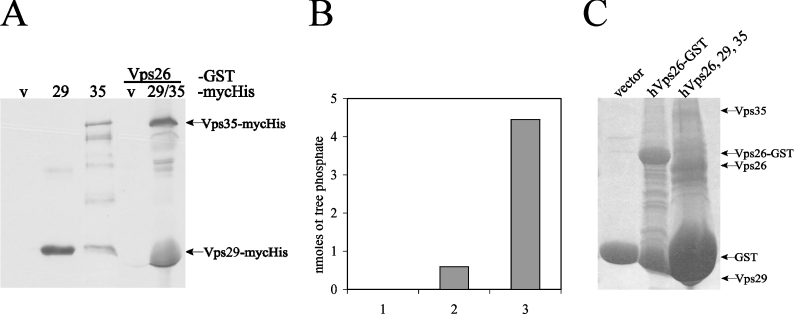
To further confirm phosphatase activity of the retromer subunits as a complex, we used GST–Vps26 or GST alone coupled with GSH–agarose beads as an affinity matrix for recombinant Myc–His-tagged Vps29 and Vps35 proteins that were purified by MCAC (metal chelation affinity chromatography) from bacterial lysates. Consistent with the results shown in Figure 1, both Myc–His-tagged Vps29 and Vps35 were detected by anti-Myc Western blotting in GST–Vps26 affinity precipitates (Figure 3A), indicating that retromer subunits were able to form a complex in vitro. To determine whether these complexes contained phosphatase activity, the GST–Vps26 affinity precipitates containing Myc–His-tagged Vps29 and Vps35 were used for an in vitro phosphatase assay using the serine-phosphorylated SFHDDpSDEDLLHI peptide as a substrate (Figure 3B). Protein expression in the phosphatase assay was confirmed by Coomassie Blue staining of the remaining supernatant of the samples used in the assay (Figure 3C). As shown in Figure 3(B), affinity precipitates of GST–Vps26 but not GST alone contained phosphatase activity towards the CI-M6PR substrate peptide. The amount of phosphatase activity detected in the GST–Vps26 affinity precipitates was consistently much lower than observed when phosphatase activity was assayed on a mixture of GSH–agarose-bound GST–Vps26, GST–Vps29 and GST–Vps35 protein following thrombin cleavage (Figure 3B, third bar). It is likely that the reduced phosphatase activity detected in the GST–Vps26 affinity precipitates is due to the presence of reduced amounts of Myc–His-tagged Vps29 and Vps35 proteins, since we were unable to detect Myc–His-tagged Vps29 and Vps35 in GST–Vps26 affinity precipitates by Coomassie Blue staining (Figure 3C).
Figure 3. Phosphatase assay on affinity-precipitated retromer complex.
(A) Western blot with anti-c-Myc antibody. Lanes 1–3, expression of purified Myc–His constructs following MCAC with Ni-NTA beads. Lanes 4–5, hVps26–GST affinity precipitation following incubation with Myc–His vector or with purified Myc–His hVps29 and hVps35 protein (v: vector; 29: Vps29; and 35: Vps35). (B) Detected amounts of free phosphate in an in vitro phosphatase assay on the SFHDDpSDEDLLHI phosphopeptide using GST vector (bar 1), an affinity precipitation of Vps26–GST containing Vps29 and Vps35 Myc–His fusion proteins (bar 2) or as a positive control, a mixture of hVps26, hVps29 and hVps35 proteins (bar 3), as used in Figure 2. Levels of free phosphate were determined after 30 min of incubation. (C) Coomassie Blue staining of the remaining supernatant of the protein samples used for the in vitro phosphatase assay.
Collectively, the results shown in Figures 1–3 demonstrate that (i) Vps26, Vps29 and Vps35 can co-precipitate in vitro, (ii) Vps29 alone but not Vps26 or Vps35 contains phosphatase activity, (iii) Vps29 phosphatase activity is greatly enhanced by the additional presence of Vps26 and Vps35 retromer subunits, and (iv) GST–Vps26 affinity matrices acquire phosphatase activity following affinity precipitation of – and complex formation with – Myc–His-tagged Vps29 and Vps35 retromer subunits. We conclude that the retromer complex contains in vitro phosphatase activity towards the serine-phosphorylated peptide SFHDDpSDEDLLHI containing the acidic-cluster dileucine motif of the CI-M6PR cytoplasmic tail.
Catalytic-site mutations of hVps29 inhibit its phosphoesterase activity
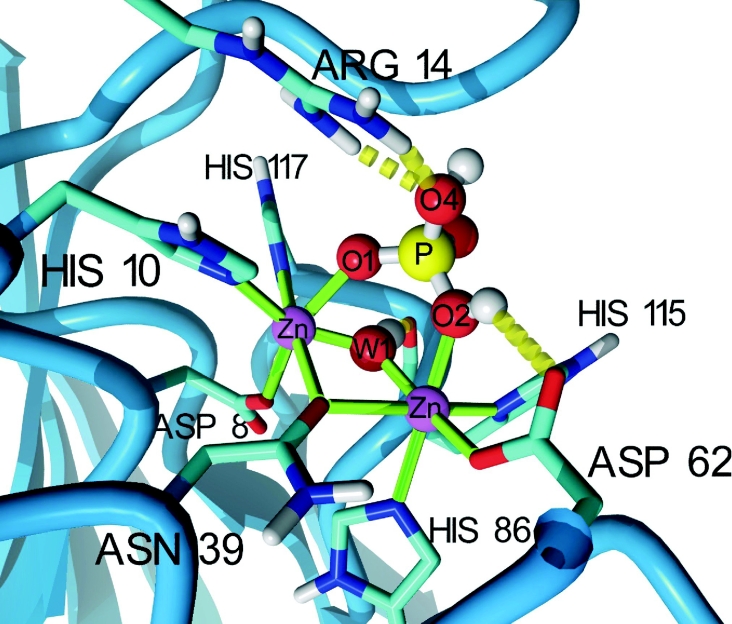
To analyse the phosphoesterase activity of hVps29 in more detail, we generated a model based on the published mVps29 structure [5] of the hVps29 catalytic site containing a binuclear Zn2+ centre (see below) and a bound phosphate (Figure 4). If the substrate phosphopeptide is bound, both metal ions are co-ordinated by six ligands; metal 1 is co-ordinated by Asp8, His10 (motif I), Asn39 (motif II), His117 (motif V), a bridging hydroxide-ion (W1), and O1 of the bound phosphate, whereas metal 2 is co-ordinated by Asn39 (motif II), Asp62 (motif III), His86 (motif IV), His115 (motif V), W1 and O2 of the bound phosphate (Figure 4). In this model, the phosphate group is further positioned by the interaction of the absolutely conserved Arg14 with the leaving group oxygen (O4) of the phosphorylated peptide.
Figure 4. Molecular model of the hVps29 active site.
The catalytic site of hVps29 showing two Zn2+ ions, the co-ordinating amino acid side chains and a bound phosphate. The hydroxide ion bridging the two metal ions is labelled ‘W1’ and is hydrogen-bonded to the backbone oxygen of His115. Only polar hydrogen atoms are shown. The serine residue of the substrate peptide has been omitted, but should be connected to the now protonated phosphate oxygen. The image was created with YASARA (http://www.yasara.org).
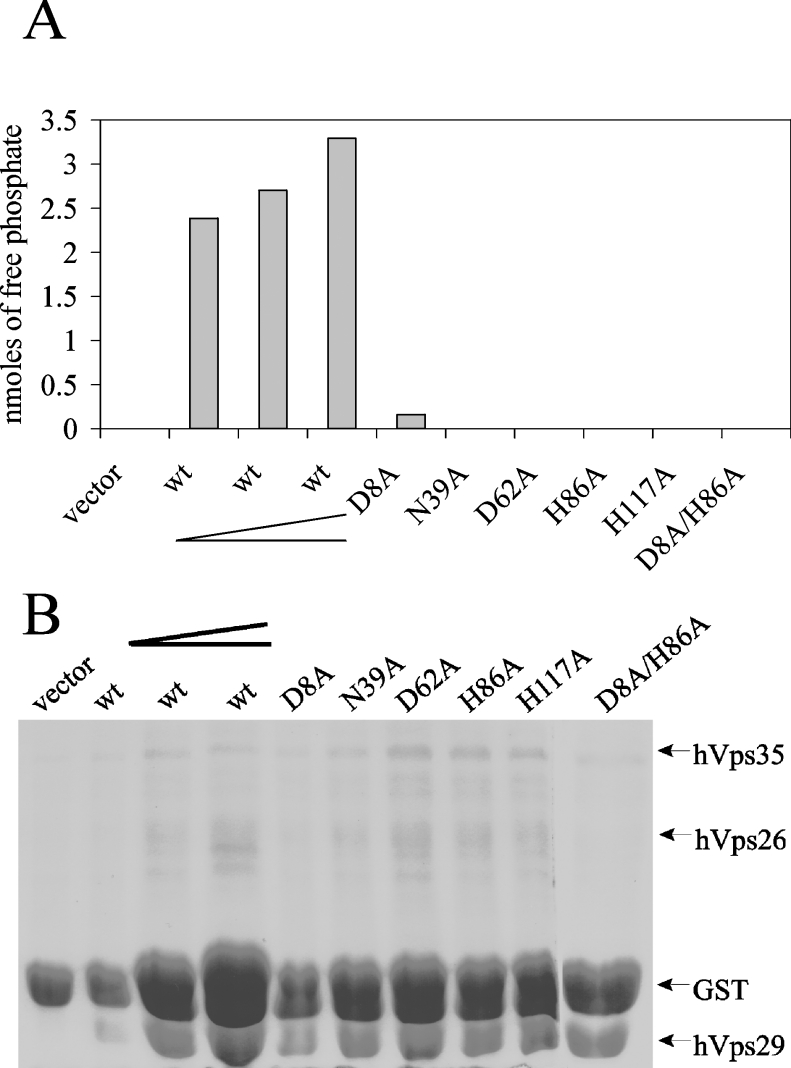
To study the role of these catalytic-site residues in the phosphoesterase reaction, we constructed D8A, N39A, D62A, H86A and H117A mutants as well as a D8A/H86A double mutant of hVps29. GST alone or GST fusion proteins of hVps26, hVps35, wild-type and mutant hVps29 were produced in E. coli and the respective recombinant proteins were separated from their GST tags using thrombin cleavage. Equal amounts of hVps26 and hVps35 proteins were added to samples containing GST alone, wild-type hVps29 or mutant hVps29. Bacterial expression of several catalytic-site mutants was less efficient when compared with the wild-type hVps29 protein, as previously reported by Collins et al. [5]. To compare phosphatase activity of different amounts of mutant hVps29 protein with wild-type hVps29 protein, we used a dilution series of the wild-type protein. Recombinant protein expression of each of the mutant constructs was within the range of the dilution series of the wild-type hVps29 protein (Figure 5B). At all concentrations tested, wild-type hVps29 displayed robust in vitro phosphatase activity towards the phosphorylated SFHDDpSDEDLLHI substrate after 30 min (Figure 5A).
Figure 5. Inhibition of the phosphatase activity of hVps29 by catalytic-site mutants.
(A) Detected amounts of free phosphate in an in vitro phosphatase assay on the SFHDDpSDEDLLHI phosphopeptide using recombinantly expressed hVps26 and hVps35 in combination with vector, wild-type hVps29 in increasing concentrations, as indicated by the elongated chevron, or hVps29 mutants D8A, N39A, D62A, H86A, H117A or D8A/H86A. Levels of free phosphate were determined after 30 min of incubation. (B) Coomassie Blue staining of the remaining supernatant of the protein samples used for the in vitro phosphatase assay.
Each of the catalytic-site mutants showed greatly reduced phosphatase activity towards the CI-M6PR serine-phosphorylated peptide (Figure 5A), demonstrating that phosphatase activity of the retromer complex can be blocked by introduction of mutations in the catalytic site of hVps29. These findings directly demonstrate that hVps29 is a phosphatase. Based on the crystal structure of other metallo-phosphatases, we conclude that mutations in Asp8, Asn39, Asp62, His86 and His117 disrupt binding of one of the metal ions to the catalytic site of hVps29, leading to ablation of enzymatic activity.
Recombinant hVps29 is a zinc-binding protein
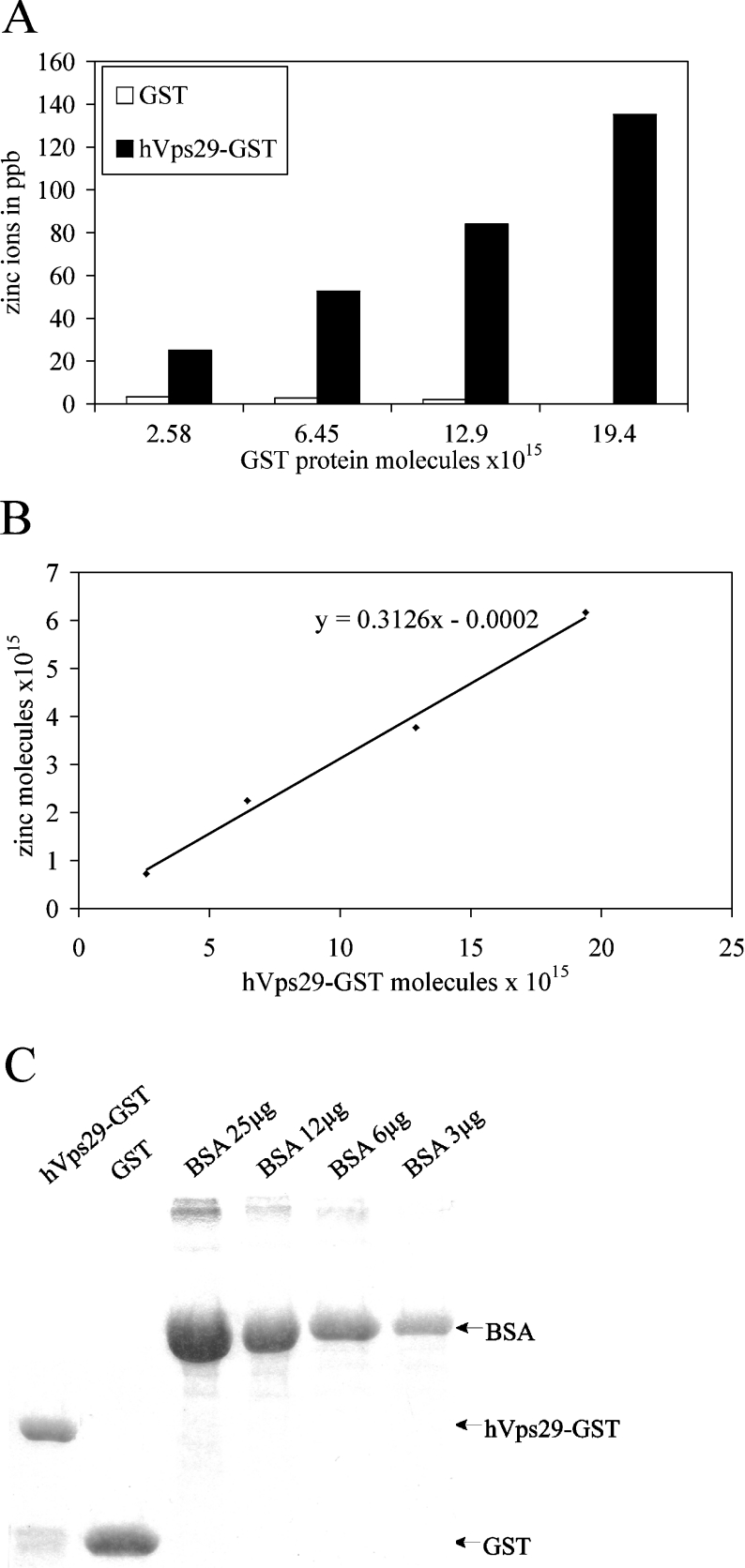
The geometry of the catalytic site of hVps29 best resembles that of the Mn2+/Mn2+ binuclear metal centre of MRE11, a 3′–5′ endonuclease from Pyrococcus furiosus (PDB code 1II7) [41], so we hypothesized that Vps29 bound metal ions, possibly Mn2+, although other metal ions (Cr, Fe, Co, Ni, Cu and Zn) are known to be present in the active site of metallo-phosphatases as well. To identify metal ions bound to native hVps29, we performed ICP-MS on the hVps29–GST fusion protein and on GST alone (see the Materials and methods section). Figure 6(A) shows that the amount of zinc present in samples containing hVps29–GST was much higher than that in the samples containing GST alone. This difference was not detected for Mn, Cr, Fe, Co, Ni or Cu (Table 1) or any other element (results not shown). The amount of zinc in samples containing hVps29–GST increased linearly with the amount of hVps29–GST analysed (Figure 6B), correlating with a relatively constant calculated molar ratio between hVps29–GST and zinc of approx. 1:0.3. Although a molar ratio of 1:2 is expected, there may be an overestimation of the presence of hVps29–GST in the measured samples, due to the presence of a small amount of GST alone and other contaminating proteins (Figure 6C). Moreover, it is possible that a fraction of the hVps29–GST fusion proteins, produced in E. coli, has an incorrect folding or is not able to acquire zinc molecules co- or post-translationally. Nevertheless, we conclude that recombinant hVps29 is a zinc-binding phosphoesterase. Mutant hVps29 proteins could not be analysed by ICP-MS, due to their reduced expression levels.
Figure 6. Detected amounts of zinc ions in hVps29–GST by ICP-MS.
(A) Binding of zinc ions (in ppb) to increasing amounts of recombinantly expressed hVps29–GST fusion protein or GST alone. (B) Linear increase in the amount of zinc induced by increasing amounts of recombinantly expressed hVps29–GST fusion protein corrected for binding to GST alone. The slope of the line indicates the molar ratio of hVps29–GST/zinc. (C) Coomassie Blue staining of a small amount of the recombinantly expressed proteins used in the ICP-MS analysis. The protein amount was measured by photospectrometric analysis (results not shown), and also by comparison with BSA levels.
Table 1. Detected amounts of metal ions in hVps29–GST by ICP-MS.
Samples of recombinantly expressed hVps29–GST fusion protein and GST alone with an increasing amount of molecules were analysed by ICP-MS. Depicted are the amounts in ppb of those metal ions that were the best candidates for binding to hVps29–GST.
| Metal ions in ppb | |||||
|---|---|---|---|---|---|
| Metal ion | 1015×GST protein molecules… | 2.58 | 6.45 | 12.9 | 19.4 |
| Mg | |||||
| GST | 0.757 | 0.058 | 0.436 | 0.681 | |
| Vps29 | 0.978 | 2.372 | 0.034 | 1.125 | |
| Ca | |||||
| GST | 111.7 | 62.8 | 81.7 | 122.1 | |
| Vps29 | 116.2 | 217.4 | 51.1 | 77.5 | |
| Cr | |||||
| GST | 0.337 | 0.212 | 0 | 0.549 | |
| Vps29 | 0 | 0.222 | 0 | 0.164 | |
| Fe | |||||
| GST | 20.56 | 0 | 0 | 102.8 | |
| Vps29 | 0 | 0 | 0 | 0.9 | |
| Mn | |||||
| GST | 0.212 | 0 | 0 | 1.268 | |
| Vps29 | 0 | 0 | 0 | 0.02 | |
| Ni | |||||
| GST | 0.499 | 0.134 | 0 | 1.548 | |
| Vps29 | 0 | 0.183 | 0 | 0.002 | |
| Co | |||||
| GST | 0.036 | 0.006 | 0.006 | 0.021 | |
| Vps29 | 0.006 | 0.013 | 0.025 | 0.036 | |
| Cu | |||||
| GST | 0.515 | 0 | 0 | 0.346 | |
| Vps29 | 0.062 | 1.458 | 0.181 | 0.656 | |
| Zn | |||||
| GST | 3.229 | 2.682 | 1.933 | 0.886 | |
| Vps29 | 25.08 | 52.58 | 84.08 | 135.3 | |
Inhibition of the phosphatase activity by metal ion chelators
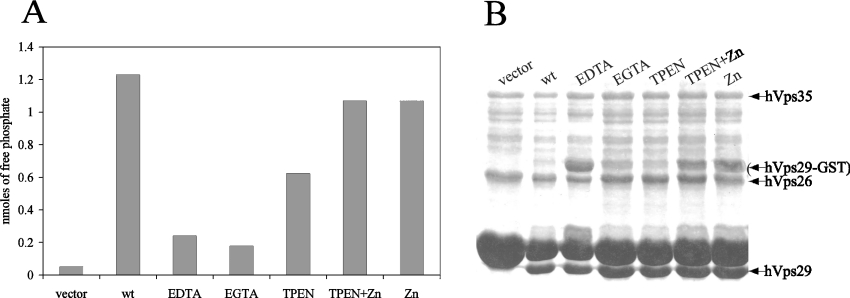
To investigate the role of the presence of metal ions in the catalytic site of hVps29 on its phosphoesterase activity, we performed phosphatase assays after treatment with several metal ion chelators. Treatment of the recombinantly expressed retromer proteins with non-specific metal ion chelators such as EDTA or EGTA prior to thrombin cleavage, resulted in an 80% reduction of the enzymatic activity of the retromer complex on the SFHDDpSDEDLLHI phosphorylated peptide (Figure 7A). More interestingly, pretreatment of the recombinantly expressed retromer proteins with the zinc-specific chelator TPEN led to a reduction of the phosphatase activity by approx. 50% (Figure 7A). Re-addition of ZnCl2 restored the phosphatase activity of the TPEN-treated samples to the level of untreated wild-type hVps29 (Figure 7A). Addition of ZnCl2 without TPEN treatment did not increase the phosphatase activity of the retromer complex on the SFHDDpSDEDLLHI phosphorylated peptide. Protein expression in the phosphatase assay was confirmed by Coomassie Blue staining of the remaining supernatant of the samples used in the assay (Figure 7B). We conclude that hVps29 contains zinc ions that are essential for phosphatase activity of the retromer complex.
Figure 7. Inhibition of the phosphatase activity of hVps29 by metal ion chelators.
(A) Detected amounts of free phosphate in an in vitro phosphatase assay on the SFHDDpSDEDLLHI phosphopeptide using recombinantly expressed hVps26 and hVps35 in combination with vector as a control or wild-type hVps29. Samples were treated with 5 mM of the indicated metal ion chelators prior to thrombin cleavage. When indicated, ZnCl2 was added to the reaction mixture to a final concentration of 1 mM. Levels of free phosphate were determined after 30 min of incubation at 37 °C. (B) Coomassie Blue staining of the remaining supernatant of the protein samples used for the in vitro phosphatase assay.
Functional significance of the Asn39/Asp62 switch in hVps29
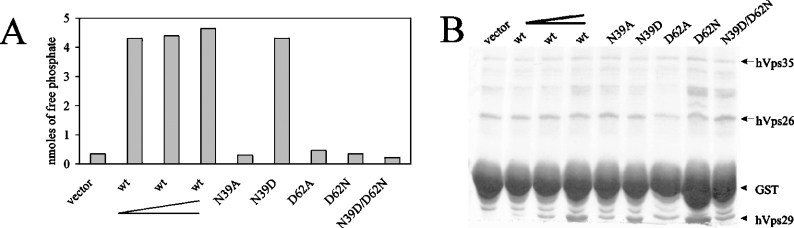
As previously discussed in the subsection ‘Retromer complex performs phosphoesterase activity on a CI-M6PR-based substrate’ in the Results section, Vps29 is unique among PPP-type metallo-phosphatases because of the presence of Asn39 (instead of Asp), Asp62 (instead of Asn) and Phe63 (instead of a catalytic histidine). To determine the functional significance of the Asn39/Asp62 switch for hVps29 phosphatase activity, we introduced the N39D, D62N as well as a N39D/D62N double mutation into pGEX4T3–hVps29 and evaluated the phosphatase activity of these mutants. Recombinant protein expression of each of the mutant constructs was within the range of the dilution series of the wild-type hVps29 protein (Figure 8B). As expected, the N39D mutation had no effect on the phosphatase activity (Figure 8A). Interestingly however, the D62N mutation blocked hVps29 phosphatase activity, which was also seen for the N39D/D62N double mutant. These results indicate that hVps29 Asn39 can be replaced by an aspartic acid residue because both side chains can contribute a metal bridging oxygen at this position. In contrast, Asp62 cannot be replaced by an asparagine residue, indicating that both oxygens in the carboxy group are functionally important in hVps29. This is surprising, as the amino group of asparagine would be ideally suited to bind the phosphate O2 oxygen, an interaction found in all available X-ray structures of ligated metallo-phosphatases.
Figure 8. Effect of the Asn39/Asp62 pair on the phosphatase activity.
(A) Detected amounts of free phosphate in an in vitro phosphatase assay on the SFHDDpSDEDLLHI phosphopeptide using recombinantly expressed hVps26 and hVps35 in combination with vector, wild-type hVps29 in increasing concentrations, as indicated by the elongated chevron, and hVps29 mutants N39A, N39D, D62A, D62N and N39D/D62N. Levels of free phosphate were determined after 30 min of incubation at 37 °C. (B) Coomassie Blue staining of the remaining supernatant of the protein samples used for the in vitro phosphatase assay.
Since Vps29 lacks the catalytic histidine residue in motif III, we considered the possibility that Asp62 might help to replace it. To investigate whether Asp62 could take the role of the general acid and neutralize the oxy-anion of the leaving group, we performed pKa calculations for the active-site residues and found that protonation of Asp62 is highly unlikely; owing to the presence of the metal ions and the strong hydrogen bond between Asn39 and Asp62, the predicted pKa of Asp62 is below zero. The same is true for the histidine residues close by, which are all involved in metal binding. A more plausible explanation for the importance of Asp62 is depicted in Figure 4: Asp62 may help to stabilize a proton on the phosphate O2 atom by forming a hydrogen bond. In the trigonal bipyramidal transition state of the reaction [30], the proton could come sufficiently close to reach the leaving group directly, or alternatively jump via an associated water molecule. Another possible role of Asp62 might involve protein binding, and its importance is best illustrated by the conservation of a charged residue in this position among several plant and fungal species (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/398/bj3980399add.htm). Although the complete function of hVps29 Asp62 remains unclear, its importance for the catalytic activity of hVps29 is clearly demonstrated.
DISCUSSION
hVps29 functions as a phosphoesterase in vitro
In the present study, we have characterized the function of the retromer component hVps29. Recently published crystal structures of Vps29 by Wang et al. [28] and Collins et al. [5] indicate that Vps29 contains a metallo-phosphoesterase fold, but these authors could not experimentally show its phosphoesterase activity. Here, we demonstrate in vitro phosphatase activity for the hVps29-containing retromer complex towards a serine-phosphorylated peptide SFHDDpSDEDLLHI substrate, containing the acidic-cluster dileucine motif of the CI-M6PR tail. There was no phosphatase activity measured towards the threonine-phosphorylated peptide KRpTIRR, which can be dephosphorylated by PP2A (protein phosphatase 2A), demonstrating substrate specificity for the hVps29-containing retromer complex (results not shown).
The discrepancy between the studies with respect to mammalian Vps29 phosphatase activity may be explained by several differences in the experimental conditions. First, we observed that the phosphatase activity of hVps29 alone was only detectable after prolonged overnight incubation. Moreover, we demonstrated by affinity precipitations that hVps29 interacts with the other retromer subunits, consistent with Collins et al. [5], and that the addition of hVps26 and hVps35 to the reaction mixture greatly enhanced the phosphatase activity. These results indicate that the retromer complex functions as a holo-enzyme complex. Indeed, using hVps26–GST as a bait, we were able to affinity precipitate the hVps29 and hVps35 retromer subunits, which resulted in acquisition of phosphatase activity. The requirement for the other retromer subunits may be due to the involvement of Vps26 and/or Vps35 in (i) substrate recognition [4,6,8–10], (ii) contributions of active-site residues (see below), and/or (iii) assembly, folding or stability of the active retromer complex [10,20,25]. Vps35p is very unstable when either Vps29 or Vps26 is mutated or deleted [10,20]. Moreover, anti-Vps26 siRNAs treatment of mammalian cell lines does not only lead to depletion of endogenous Vps26, but also to depletion of Vps29 and Vps35 [4,6], indicating that all retromer subunits must be present to form a stable complex. Secondly, we avoided any influence of the GST protein on the assembly and activity of the retromer complex. We used recombinant proteins only (i) after the recombinant retromer protein was cleaved from the GST moiety by thrombin or (ii) after replacing the GST moiety by the smaller Myc–His tag.
Thirdly, we demonstrated hVps29 phosphatase activity towards the serine-phosphorylated peptide SFHDDpSDEDLLHI substrate. Collins et al. [5] could not detect phosphatase activity of hVps29 towards PNPP (p-nitrophenyl phosphate)-based substrates and several soluble phosphatidylinositol phosphates.
hVps29 is a zinc-binding protein
Apart from the Asn39/Asp62 switch, the catalytic site of hVps29 best resembles that of the MRE11 endonuclease which contains a binuclear Mn2+ centre [41]. Collins et al. [5] and Wang et al. [28] soaked mVps29 crystals in MnSO4 and observed that the mVps29 catalytic site can bind bivalent metal ions. However, these studies did not identify the metal ions bound to native Vps29 or the metal ions required for enzymatic activity. ICP-MS analysis revealed that native hVps29 contains Zn2+. We demonstrated that zinc binding is involved in the enzymatic activity of hVps29 by performing phosphatase assays with the specific zinc metal chelator TPEN, which reduces the phosphatase activity. Moreover, we demonstrated that addition of ZnCl2 after TPEN treatment restores the phosphatase activity.
More than 300 enzymes, covering all six classes of enzymes, have been discovered which require zinc ions for their activity [42]. Moreover, zinc is the most common metal used in metallo-enzymes that catalyse hydrolysis or hydration reactions [29]. Importantly, other PPP-type phosphatases, including purple acid phosphatase (PDB code 4KBP) [43], calcineurin (1AUI) [44] and 5′ nucleotidase (1USH) [45] also contain Zn2+ ions in their catalytic sites.
Implications for the catalytic mechanism
Even though we were able to demonstrate phosphatase activity for the retromer complex, many questions regarding the enzymatic reaction remain. First, the identity of the nucleophile that attacks the phosphate is unclear. The active site of metallo-phosphoesterases contains water molecules, one of which is invariably involved in the co-ordination of both metal ions in a μ-hydroxo bridge (W1) (Figure 4). Frequently, a second water molecule (W2) is detected bound to metal 1. Which of these water molecules acts as the nucleophile during catalysis is a question of debate [46]. In general, the pKa of W1 is sufficiently low to be deprotonated and to become a hydroxide ion. Opposite to the leaving group, W1 is also in an ideal position for in-line attack of the phosphorus atom. However, as W1 binding to both metal ions reduces its nucleophilicity, it has been suggested that metal 1-bound W2 is the nucleophile that attacks the phosphoester bond [40]. However, this mechanism was proposed for purple acid phosphatase co-ordinating two different metal ions, Zn2+ and Fe3+ [43]. Binding of trivalent Fe3+ to W2 can lower the pKa of W2 sufficiently such that W2 exists as a hydroxide ion at physiological pH. In contrast, bivalent Zn2+ ions cannot lower the pKa of terminally ligated water molecule sufficiently to create a hydroxide ion by itself at physiological pH [47]. In that case, a general base may be required to further lower the pKa of W2. Although we cannot exclude that W2 (if existing) is the nucleophilic hydroxide molecule, we favour the hypothesis that the hydroxide ion W1 is the one that is involved in the nucleophilic attack, as discussed in detail by Swingle et al. [30].
The second question that remains concerns the identity of the proton donor that protonates the oxy-anion of the leaving group. Unlike most other eukaryotic PPP-type metallo-phosphatases, Vps29 lacks a catalytic histidine residue in motif III. Building on their negative results with respect to Vps29 phosphatase activity, Collins et al. [5] suggested that another cofactor (such as Vps26 or Vps35) might contribute a catalytic histidine to the Vps29 active site. Based on current (draft) whole genome sequencing programmes, both Vps26 and Vps35 retromer proteins contain histidine residues that are nearly absolutely conserved among all eukaryotes and could be candidates for the delivery of the missing histidine (results not shown). However, the low but measurable in vitro phosphatase activity of hVps29 in the absence of hVps26 and hVps35 seems inconsistent with such a model. In addition, molecular modelling suggests that there is very little space to insert a histidine in the gap that is created by the outward movement of Phe63 that occurs upon metal binding (E. Krieger, unpublished work). It should be noted that we cannot exclude the possibility that conformational changes occur in the Vps29 catalytic site upon assembly of the holo-retromer complex. Importantly, despite lacking a catalytic histidine residue in motif III, the archaeal protein MJ0936 is also an active metallo-phosphoesterase [48], indicating that a catalytic histidine is not absolutely required for phosphoesterase activity. Yet another possibility that needs to be considered is that there is no specific proton donor for the leaving group, and that the leaving group slowly retrieves a proton from bulk water. Finally, it is important to realize that the pKa calculations must be interpreted with caution: they were performed on hVps29 alone, in the presence of the binuclear Zn2+ centre and in the presence or absence of bound tri-anionic phosphate, even though it is clear that the enzymatic activity of hVps29 is greatly enhanced when bound to hVps26 and hVps35. Given that mVps35 binds close to the mVps29 active site [5], it is conceivable that the immediate environment of the Vps29 active site, i.e. the charge distribution, and thus the predicted pKa values, is changed upon incorporation of Vps29 in the holo retromer complex. Crystallization of the holo-retromer complex will probably be necessary to answer some of these questions.
A third question that remains concerns the evolutionary conservation of Vps29 enzymatic function. Although metal-co-ordinating catalytic-site residues of Vps29 are highly conserved in most metazoans, there are multiple substitutions in those residues among pseudo-coelomate nematodes, and various fungal and protozoan species (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/398/bj3980399add.htm). It remains to be demonstrated whether the retromer complex displays phosphatase activity in yeast and other species or whether there may be functional differences in the retromer complex among species.
Trafficking of the CI-M6PR depends on the phosphorylation status of the receptor
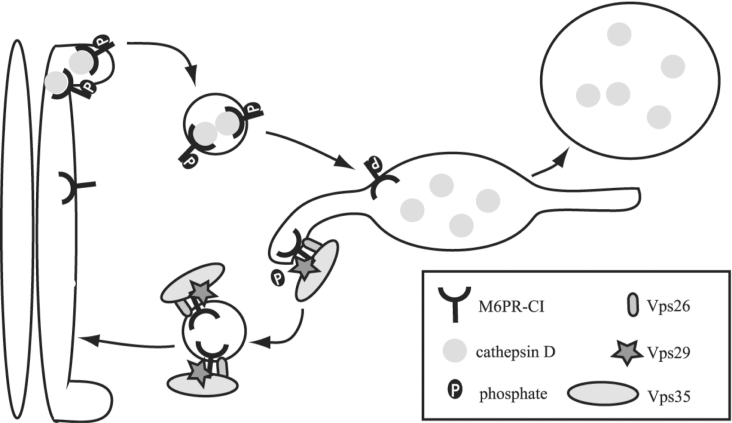
We demonstrate that the retromer component hVps29 interacts with hVps26 and hVps35 retromer subunits and functions in vitro as a phosphoesterase for a phosphopeptide based on the sequence of the CI-M6PR tail. A working model for the in vivo role of hVps29-mediated dephosphorylation of the acidic-cluster dileucine motif of the CI-M6PR is presented in Figure 9. After binding to mannose 6-phosphate-tagged lysosomal hydrolases in the TGN, the phosphorylation of Ser2492 within the acidic-cluster dileucine motif acts as a sorting signal for anterograde transport by binding to GGA1 and GGA3 [23]. The GGA proteins will transport the CI-M6PR by clathrin-coated pits to the endosome. In the endosome the CI-M6PR will dissociate from its ligand, which is subsequently transported to the lysosome. On the endosome, the CI-M6PR co-localizes with the retromer complex [4,6]. It is tempting to speculate that after binding of mammalian Vps35 to the CI-M6PR in the endosome, Vps29 will dephosphorylate the receptor. Dephosphorylation of this trafficking motif might be the trigger for the CI-M6PR to recycle back to the TGN to carry out additional rounds of enzyme delivery. This model remains to be confirmed by in vivo data of dephosphorylation of the CI-M6PR by hVps29. In addition, the exact role of dephosphorylation of the CI-M6PR in the transport of the receptor remains to be investigated. Other proteins that have been implicated in endosome-to-Golgi retrieval of the cycling TGN proteins are TIP47 (47 kDa tail-interacting protein) [49] and PACS-1 (phosphofurin cluster sorting protein-1) [50]. However, neither of these proteins is evolutionarily conserved among eukaryotes and therefore may not be fundamental to the eukaryote retrograde endosome-to-TGN vesicular transport pathway.
Figure 9. Model of phosphate-dependent transport of the CI-M6PR.
The CI-M6PR binds cathepsin D in the TGN. Binding of GGAs to the phosphorylated CI-M6PR causes the transport of the ligand–receptor complex to the endosome. In the endosome, the ligand dissociates from the receptor and after binding to Vps35, Vps29 is able to dephosphorylate the receptor. The retromer recycles the CI-M6PR to the TGN.
In addition, it is interesting to note that Vps29 may function as a phosphoesterase for other substrates as well. Mutational analysis of the serine-phosphorylated peptide SFHDDpSDEDLLHI could reveal a minimal sequence motif required for Vps29-dependent dephosphorylation. In yeast, Vps35 not only binds the Vps10p receptor, but also A-ALP (alkaline phosphatase) [11,51], so its cytosolic tail DPAP A (dipeptidyl aminopeptidase A) is also an interesting candidate for dephosphorylation by Vps29. Since mammalian Vps35 is known to interact with the CI-M6PR [4,6,9], it is tempting to speculate that Vps29 may be involved in dephosphorylation of other acidic-cluster dileucine motifs as well, many of which are regulated by serine phosphorylation like the CD-M6PR (cation-dependent mannose 6-phosphate receptor) and furin [52,53].
Online data
Acknowledgments
We thank Professor E. J. J. van Zoelen, Dr S. M. Jansen (Department of Cell Biology FNWI of the Radboud University Nijmegen) and Dr E. J. Reijerse (Max Planck Institute for Bioinorganic Chemistry, Mulheim an der Ruhr, Germany) for helpful discussions, and E. M. Bilkerdijk (Department of Cell Biology FNWI of the Radboud University Nijmegen) and M. J. C. M. Koopmans (Department of Cell Biology, FNWI of the Radboud University) for construction of several hVps29 mutants. Financial support for this project was provided by research grants from Zon-MW (The Hague, The Netherlands) (908-02-047).
References
- 1.Bankaitis V. A., Johnson L. M., Emr S. D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc. Natl. Acad. Sci. U.S.A. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothman J. H., Stevens T. H. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- 4.Arighi C. N., Hartnell L. M., Aguilar R. C., Haft C. R., Bonifacino J. S. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins B. M., Skinner C. F., Watson P. J., Seaman M. N., Owen D. J. Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat. Struct. Mol. Biol. 2005;12:594–602. doi: 10.1038/nsmb954. [DOI] [PubMed] [Google Scholar]
- 6.Seaman M. N. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaman M. N., McCaffery J. M., Emr S. D. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cereghino J. L., Marcusson E. G., Emr S. D. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell. 1995;6:1089–1102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper A. A., Stevens T. H. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcusson E. G., Horazdovsky B. F., Cereghino J. L., Gharakhanian E., Emr S. D. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 11.Nothwehr S. F., Ha S. A., Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 13.Meresse S., Hoflack B. Phosphorylation of the cation-independent mannose 6-phosphate receptor is closely associated with its exit from the trans-Golgi network. J. Cell Biol. 1993;120:67–75. doi: 10.1083/jcb.120.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boman A. L., Zhang C., Zhu X., Kahn R. A. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell'Angelica E. C., Puertollano R., Mullins C., Aguilar R. C., Vargas J. D., Hartnell L. M., Bonifacino J. S. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirst J., Lui W. W., Bright N. A., Totty N., Seaman M. N., Robinson M. S. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poussu A., Lohi O., Lehto V. P. Vear, a novel Golgi-associated protein with VHS and gamma-adaptin ‘ear’ domains. J. Biol. Chem. 2000;275:7176–7183. doi: 10.1074/jbc.275.10.7176. [DOI] [PubMed] [Google Scholar]
- 18.Takatsu H., Yoshino K., Nakayama K. Adaptor gamma ear homology domain conserved in gamma-adaptin and GGA proteins that interact with gamma-synergin. Biochem. Biophys. Res. Commun. 2000;271:719–725. doi: 10.1006/bbrc.2000.2700. [DOI] [PubMed] [Google Scholar]
- 19.Puertollano R., Randazzo P. A., Presley J. F., Hartnell L. M., Bonifacino J. S. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 20.Takatsu H., Katoh Y., Shiba Y., Nakayama K. Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J. Biol. Chem. 2001;276:28541–28545. doi: 10.1074/jbc.C100218200. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y., Doray B., Poussu A., Lehto V. P., Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
- 22.Doray B., Bruns K., Ghosh P., Kornfeld S. Interaction of the cation-dependent mannose 6-phosphate receptor with GGA proteins. J. Biol. Chem. 2002;277:18477–18482. doi: 10.1074/jbc.M201879200. [DOI] [PubMed] [Google Scholar]
- 23.Kato Y., Misra S., Puertollano R., Hurley J. H., Bonifacino J. S. Phosphoregulation of sorting signal-VHS domain interactions by a direct electrostatic mechanism. Nat. Struct. Biol. 2002;9:532–536. doi: 10.1038/nsb807. [DOI] [PubMed] [Google Scholar]
- 24.Haft C. R., de la Luz Sierra M., Bafford R., Lesniak M. A., Barr V. A., Taylor S. I. Human orthologues of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol. Biol. Cell. 2000;11:4105–4116. doi: 10.1091/mbc.11.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy J. V., Seaman M. N. Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol. Biol. Cell. 2001;12:3242–3256. doi: 10.1091/mbc.12.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horazdovsky B. F., Davies B. A., Seaman M. N., McLaughlin S. A., Yoon S., Emr S. D. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell. 1997;8:1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seaman M. N., Williams H. P. Identification of the functional domains of yeast sorting nexins Vps5p and Vps17p. Mol. Biol. Cell. 2002;13:2826–2840. doi: 10.1091/mbc.02-05-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Guo M., Liang Z., Fan J., Zhu Z., Zang J., Zhu Z., Li X., Teng M., Niu L., et al. Crystal structure of human vacuolar protein sorting protein 29 reveals a phosphodiesterase/nuclease-like fold and two protein–protein interaction sites. J. Biol. Chem. 2005;280:22962–22967. doi: 10.1074/jbc.M500464200. [DOI] [PubMed] [Google Scholar]
- 29.Christianson D. W., Cox J. D. Catalysis by metal-activated hydroxide in zinc and manganese metalloenzymes. Annu. Rev. Biochem. 1999;68:33–57. doi: 10.1146/annurev.biochem.68.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Swingle M. R., Honkanen R. E., Ciszak E. M. Structural basis for the catalytic activity of human serine/threonine protein phosphatase-5. J. Biol. Chem. 2004;279:33992–33999. doi: 10.1074/jbc.M402855200. [DOI] [PubMed] [Google Scholar]
- 31.Jung J., Lee B. Protein structure alignment using environmental profiles. Protein Eng. 2000;13:535–543. doi: 10.1093/protein/13.8.535. [DOI] [PubMed] [Google Scholar]
- 32.Krieger E., Darden T., Nabuurs S. B., Finkelstein A., Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins. 2004;57:678–683. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen J. E., Vriend G. Optimizing the hydrogen-bond network in Poisson–Boltzmann equation-based pK(a) calculations. Proteins. 2001;43:403–412. doi: 10.1002/prot.1053. [DOI] [PubMed] [Google Scholar]
- 34.Nicholls A., Honig B. A rapid finite difference algorithm, utilizing successive over-relaxation to solve the Poisson–Boltzmann equation. J. Comp. Chem. 1991;12:435–445. [Google Scholar]
- 35.Jorgensen W. L., Tirado-Rives J. The OPLS potential functions for proteins: energy minimizations for crystals for cyclic peptides and crambin. J. Am. Chem. Soc. 1988;110:1657–1666. doi: 10.1021/ja00214a001. [DOI] [PubMed] [Google Scholar]
- 36.Kennelly P. J. Archaeal protein kinases and protein phosphatases: insights from genomics and biochemistry. Biochem. J. 2003;370:373–389. doi: 10.1042/BJ20021547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barford D., Das A. K., Egloff M. P. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct. 1998;27:133–164. doi: 10.1146/annurev.biophys.27.1.133. [DOI] [PubMed] [Google Scholar]
- 38.Koonin E. V., Mushegian A. R., Tatusov R. L., Altschul S. F., Bryant S. H., Bork P., Valencia A. Eukaryotic translation elongation factor 1 gamma contains a glutathione transferase domain–study of a diverse, ancient protein superfamily using motif search and structural modeling. Protein Sci. 1994;3:2045–2054. doi: 10.1002/pro.5560031117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuo S., Clemens J. C., Stone R. L., Dixon J. E. Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J. Biol. Chem. 1994;269:26234–26238. [PubMed] [Google Scholar]
- 40.Klabunde T., Strater N., Frohlich R., Witzel H., Krebs B. Mechanism of Fe(III)-Zn(II) purple acid phosphatase based on crystal structures. J. Mol. Biol. 1996;259:737–748. doi: 10.1006/jmbi.1996.0354. [DOI] [PubMed] [Google Scholar]
- 41.Hopfner K. P., Karcher A., Craig L., Woo T. T., Carney J. P., Tainer J. A. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell. 2001;105:473–485. doi: 10.1016/s0092-8674(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 42.McCall K. A., Huang C., Fierke C. A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000;130:1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 43.Strater N., Klabunde T., Tucker P., Witzel H., Krebs B. Crystal structure of a purple acid phosphatase containing a dinuclear Fe(III)-Zn(II) active site. Science. 1995;268:1489–1492. doi: 10.1126/science.7770774. [DOI] [PubMed] [Google Scholar]
- 44.Kissinger C. R., Parge H. E., Knighton D. R., Lewis C. T., Pelletier L. A., Tempczyk A., Kalish V. J., Tucker K. D., Showalter R. E., Moomaw E. W., et al. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature (London) 1995;378:641–644. doi: 10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- 45.Knofel T., Strater N. X-ray structure of the Escherichia coli periplasmic 5′-nucleotidase containing a dimetal catalytic site. Nat. Struct. Biol. 1999;6:448–453. doi: 10.1038/8253. [DOI] [PubMed] [Google Scholar]
- 46.Lindqvist Y., Johansson E., Kaija H., Vihko P., Schneider G. Three-dimensional structure of a mammalian purple acid phosphatase at 2.2 Å resolution with a mu-(hydr)oxo bridged di-iron center. J. Mol. Biol. 1999;291:135–147. doi: 10.1006/jmbi.1999.2962. [DOI] [PubMed] [Google Scholar]
- 47.Baes C. F., Mesmer R. E. New York: Wiley-Interscience; 1976. The Hydrolysis of Cations; pp. 219–237. [Google Scholar]
- 48.Chen S., Yakunin A. F., Kuznetsova E., Busso D., Pufan R., Proudfoot M., Kim R., Kim S. H. Structural and functional characterization of a novel phosphodiesterase from Methanococcus jannaschii. J. Biol. Chem. 2004;279:31854–31862. doi: 10.1074/jbc.M401059200. [DOI] [PubMed] [Google Scholar]
- 49.Diaz E., Pfeffer S. R. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 50.Wan L., Molloy S. S., Thomas L., Liu G., Xiang Y., Rybak S. L., Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 51.Nothwehr S. F., Bruinsma P., Strawn L. A. Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol. Biol. Cell. 1999;10:875–890. doi: 10.1091/mbc.10.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breuer P., Korner C., Boker C., Herzog A., Pohlmann R., Braulke T. Serine phosphorylation site of the 46 kDa mannose 6-phosphate receptor is required for transport to the plasma membrane in Madin–Darby canine kidney and mouse fibroblast cells. Mol. Biol. Cell. 1997;8:567–576. doi: 10.1091/mbc.8.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones B. G., Thomas L., Molloy S. S., Thulin C. D., Fry M. D., Walsh K. A., Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.