Abstract
The eye colour mutant sepia (se1) is defective in PDA {6-acetyl-2-amino-3,7,8,9-tetrahydro-4H-pyrimido[4,5-b]-[1,4]diazepin-4-one or pyrimidodiazepine} synthase involved in the conversion of 6-PTP (2-amino-4-oxo-6-pyruvoyl-5,6,7,8-tetrahydropteridine; also known as 6-pyruvoyltetrahydropterin) into PDA, a key intermediate in drosopterin biosynthesis. However, the identity of the gene encoding this enzyme, as well as its molecular properties, have not yet been established. Here, we identify and characterize the gene encoding PDA synthase and show that it is the structural gene for sepia. Based on previously reported information [Wiederrecht, Paton and Brown (1984) J. Biol. Chem. 259, 2195–2200; Wiederrecht and Brown (1984) J. Biol. Chem. 259, 14121–14127; Andres (1945) Drosoph. Inf. Serv. 19, 45; Ingham, Pinchin, Howard and Ish-Horowicz (1985) Genetics 111, 463–486; Howard, Ingham and Rushlow (1988) Genes Dev. 2, 1037–1046], we isolated five candidate genes predicted to encode GSTs (glutathione S-transferases) from the presumed sepia locus (region 66D5 on chromosome 3L). All cloned and expressed candidates exhibited relatively high thiol transferase and dehydroascorbate reductase activities and low activity towards 1-chloro-2,4-dinitrobenzene, characteristic of Omega class GSTs, whereas only CG6781 catalysed the synthesis of PDA in vitro. The molecular mass of recombinant CG6781 was estimated to be 28 kDa by SDS/PAGE and 56 kDa by gel filtration, indicating that it is a homodimer under native conditions. Sequencing of the genomic region spanning CG6781 revealed that the se1 allele has a frameshift mutation from ‘AAGAA’ to ‘GTG’ at nt 190–194, and that this generates a premature stop codon. Expression of the CG6781 open reading frame in an se1 background rescued the eye colour defect as well as PDA synthase activity and drosopterins content. The extent of rescue was dependent on the dosage of transgenic CG6781. In conclusion, we have discovered a new catalytic activity for an Omega class GST and that CG6781 is the structural gene for sepia which encodes PDA synthase.
Keywords: Drosophila, drosopterin, eye colour mutant, glutathione S-transferase, pyrimidodiazepine synthase, sepia
Abbreviations: CDNB, 1-chloro-2,4-dinitrobenzene; CG, computed gene; DHA, dehydroascorbate; HED, β-hydroxyethylene disulfide; GMR-gal4, ‘glass multiple reporter’ promoter-dependent gal4 construct/transgene; GST, glutathione S-transferase; GSTO1, Omega class GST 1; ORF, open reading frame; PDA, 6-acetyl-2-amino-3,7,8,9-tetrahydro-4H-pyrimido[4,5-b]-[1,4]diazepin-4-one or pyrimidodiazepine; 6-PTP, 2-amino-4-oxo-6-pyruvoyl-5,6,7,8-tetrahydropteridine; H2NTP, 2-amino-4-oxo-6-(D-erythro-1′,2′,3′-trihydroxypropyl)-7,8-dihydropteridine triphosphate; poly(A)+ RNA, polyadenylated RNA; RT, reverse transcriptase; UAS, upstream activation sequence
INTRODUCTION
The eye colour pigments of Drosophila melanogaster have been the subject of many investigations since the discovery of the first eye colour mutant. Two classes of pigments, the brown ‘ommochromes’ and red ‘drosopterins’, are responsible for the typical eye colour of Drosophila. Drosopterins consist of at least five compounds that can be separated by TLC [1] and all are made up of a PDA {6-acetyl-2-amino-3,7,8,9-tetrahydro-4H-pyrimido-[4,5-b]-[1,4]diazepin-4-one or pyrimidodiazepine} portion and a pteridine portion [2–4].
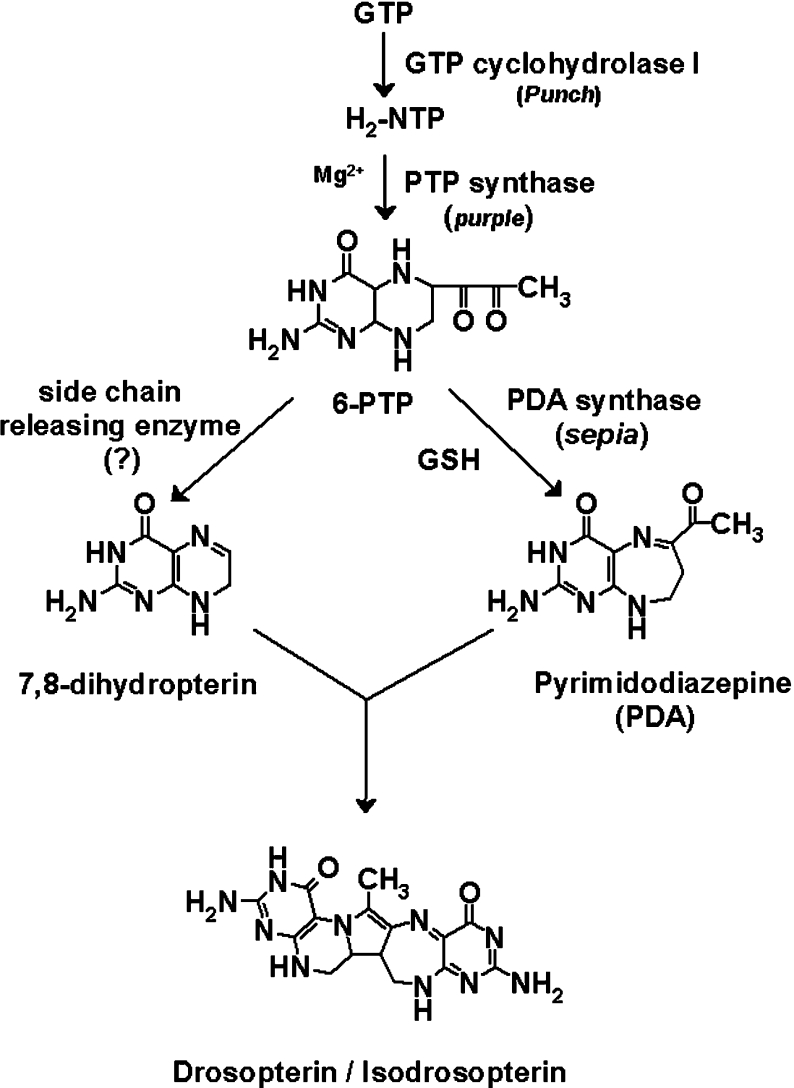
The first step in drosopterin biosynthesis is the formation of H2NTP [2-amino-4-oxo-6-(D-erythro-1′,2′,3′-trihydroxypropyl)-7,8-dihydropteridine triphosphate; also known as 7,8-dihydroneopterin triphosphate] from GTP by GTP cyclohydrolase I, encoded by the Punch gene [5,6]. H2NTP is then converted into 6-PTP (2-amino-4-oxo-6-pyruvoyl-5,6,7,8-tetrahydropteridine; also known as 6-pyruvoyltetrahydropterin) by PTP synthase, the product of the purple gene [7–10]. A better understanding of the biosynthetic pathway was achieved in 1981 with the discovery of a new compound [11] that promotes the incorporation of 14C-labelled H2NTP into drosopterin [12,13]. This compound was shown by Brown and co-workers [11] and Jacobson et al. [14] to be PDA, also known as 6-acetylhomopterin (Scheme 1).
Scheme 1. Proposed pathway of biosynthesis of drosopterins in D. melanogaster.
Wiederrecht and Brown [15] first identified PDA synthase activity and partially purified the enzyme from Drosophila heads. From an analysis of various eye colour mutants, they showed that at least three enzymes are involved in the conversion of H2NTP into PDA [13]: one, called sepiapterin synthase A, present in limited amounts in purple [7,8]; a second, named PDA synthase, that is missing in sepia; and a third deficient in clot. Since addition of partially purified PDA synthase to heated extracts of sepia increased PDA synthesis to the wild-type level, they suggested that sepia+ encodes the structural gene for PDA synthase. While sepia and clot have similar eye colour phenotypes, the ‘leaky’ ability of clot extracts to support the synthesis of PDA led to the suggestion that clot is involved indirectly in the conversion of H2NTP into PDA. The purple gene product, later purified and characterized as 6-PTP synthase [9], is required for the Mg2+-dependent conversion of the H2NTP into 6-PTP [16]. This is converted into PDA by partially purified PDA synthase in an in vitro reaction that requires the presence of GSH at physiological concentrations and an additional thiol compound such as 2-mercaptoethanol. As 2-mercaptoethanol is no longer required under anaerobic conditions, it was proposed that, since PDA has two more electrons than 6-PTP, the reducing power required for the conversion of 6-PTP into PDA is supplied by GSH [13].
Recently, the structural gene for clot was identified in the course of a genetic analysis aimed at recovering P-element-induced mutations in the 25E region of chromosome 2 [17]. The predicted secondary structure, together with protein-domain database searches, indicated that the clot gene encodes a protein related to the glutaredoxin family of the thioredoxin-like enzyme superfamily.
In the present paper, we report identification and characteristics of the structural gene for PDA synthase, based on molecular and biochemical analyses. We have also rescued the phenotype of the se1 mutant by transgenic expression of CG6781, thus confirming that CG6781 is the structural gene for sepia.
EXPERIMENTAL
Drosophila strains
Wild-type D. melanogaster Oregon-R was used to prepare enzyme fractions. Breeding populations of flies were maintained at 25±1 °C on a standard yeast medium with propionic acid added as a mould inhibitor. Adult (0–2 days old) heads were separated from bodies by sieving and stored at −80 °C. se1 flies were obtained from the Bloomington Stock Center (Department of Biology, Indiana University, Bloomington, IN, U.S.A.; BL 1668); the mutant is described in FlyBase (http://flybase.bio.Indiana.edu/).
Cloning and expression of the putative gene
The coding sequences of candidate genes and the clot gene were amplified by RT (reverse transcriptase)–PCR. Total RNA was extracted from 0–2-day-old adult wild-type flies with TRIzol® reagent (Invitrogen) according to the manufacturer's instructions, and PCR was carried out under the following conditions: one cycle of 94 °C for 4 min; 30 cycles of 94 °C for 1 min, annealing for each gene for 1 min, 72 °C for 1 min; and 1 cycle of 72 °C for 7 min using gene-specific primer sets. The sequences and annealing temperatures of primer sets used in the PCR reactions are shown in Table 1. PCR products were subcloned into the multiple cloning site of pET 15b (Novagen) and transformed into BL21 (DE3) cells. To express the proteins, transformants were cultured at 37 °C in 100 ml of LB (Luria–Bertani) medium containing 50 μg/ml ampicillin, and protein expression was induced by adding 0.5 mM isopropyl β-D-thiogalactoside at an absorbance (A) of 0.5. After 3 h, the cells were collected by centrifugation at 5000 g for 10 min and suspended in 4 ml of ice-cold Binding Buffer (pET system manual; Novagen). After sonication, the supernatant containing the soluble enzyme was collected by centrifugation at 10000 g for 20 min at 4 °C, and purified with His·Bind Resin (Novagen) according to the manufacturer's instructions.
Table 1. Primers used for cloning.
Restriction enzymes used were NdeI and BamHI for CG6781, CG6776 and CG6662, and NdeI and XhoI for CG6673A, CG6673B and Clot. Tempa is the annealing temperature used in the corresponding PCR reaction. Underlining indicates the recognition sequences for restriction endonucleases mentioned. The boldface letters indicate start and stop codons in each primer.
| Clone | Forward primer (5′–3′) | Reverse primer (5′–3′) | Tempa (°C) |
|---|---|---|---|
| CG6781 | CATATGAGTAACGGCAGGCATTTGGCAAAAG | GGATCCTCAGGCATCCTTGACCAGCAG | 66 |
| CG6673A | CATATGGCCCTGCCGCAAAAGCACT | CTCGAGCTATGGTGTACCCTTGAAGGCAATGTC | 66 |
| CG6673B | CATATGGCCCTGCCGCAAAAGCACT | CTCGAGTTACAAGGGCTGGAAGGCTACATCGTA | 66 |
| CG6776 | CATATGAGTTCTGGTAAACATTTGGCCAAAGGTTC | GGATCCCTAAGCCAGCAGATCGTAGTTTGC | 65 |
| CG6662 | CATATGAGCAATACTCAGCACTTAACTATTGGCTC | GGATCCCTACCCCAATTTGACACGTTTG | 61 |
| Clot | CATATGGTGGTCACACACAACGTCAAGGGATAC | CTCGAGTTAATCCTCATCCTCGAACATCATCTCG | 63 |
Assay of PDA synthase
PDA synthase activity was assayed spectrophotometrically by separating the reaction product, PDA, using reversed-phase HPLC. The standard reaction mixture (0.1 ml) contained 75 μM H2NTP, 5 mM GSH, 70 mM 2-mercaptoethanol, 10 mM MgCl2, 4 units of PTP synthase, 40 μg of each purified enzyme and 100 mM Pipes buffer (pH 7.5). After incubation at 30 °C for 2 h in the dark, the reaction was stopped by boiling for 5 min and the mixture was then centrifuged to remove precipitated proteins. A portion (0.07 ml) of each mixture was subjected to HPLC with a reversed-phase C18 column (Inertsil ODS-3: 4.6 mm×250 mm; GL Sciences) connected to a photodiode array detector (Waters 996) and a scanning fluorescence detector (Waters 474). The material was eluted isocratically with 85% (v/v) 50 mM ammonium acetate in 15% (v/v) methanol at a flow rate of 1 ml/min. PDA was monitored by the absorbance at 383 nm.
Assay of GST (glutathione S-transferase)
GST activity with CDNB (1-chloro-2,4-dinitrobenzene) as substrate was measured as described by Habig et al. [18]. The standard reaction mixture contained 1 mM GSH, 0.1 M potassium phosphate buffer (pH 6.5) (mixture of 38.1 ml of 1 M K2HPO4 and 61.9 ml of 1 M KH2PO4, which was further diluted to 1000 ml with distilled water) and 100 μg of purified enzyme. The reaction was initiated by addition of 1 mM CDNB at 25 °C, and activity was measured as the increase in absorbance at 340 nm. Thiol transferase activity was measured by the method of Axelsson et al. [19] with HED (β-hydroxyethylene disulfide) as the substrate. The standard reaction mixture contained 0.2 mM NADPH, 1 mM GSH, 50 mM phosphate buffer (pH 7.6), 2 units of glutathione reductase and 30 μg of purified enzyme. The reaction was initiated by the addition of 2 mM HED at 30 °C, and activity was measured as the decrease in NADPH absorbance at 340 nm. Glutathione-dependent DHA (dehydroascorbate) reductase activity was determined by following DHA reduction spectrophotometrically at 265 nm. The standard reaction mixture contained 50 mM potassium phosphate buffer (pH 7.6), 1 mM GSH and 5 μg of purified enzyme, and the reaction was initiated by adding 1 mM DHA after a 1 min pre-incubation at 30 °C [20]. All initial velocities with a range of substrates were measured within the range of 15–20% consumption of each substrate.
Genomic sequencing
To perform PCR analyses, genomic DNA was isolated from adult flies. Thirty flies were homogenized in a glass homogenizer containing 400 μl of Buffer A (100 mM Tris/HCl, pH 7.6, 100 mM EDTA, 100 mM NaCl and 0.5% SDS) and incubated for 30 min at 65 °C. After adding 800 μl of Buffer B (1.5 M potassium acetate and 4.5 M LiCl) to the homogenate, the material was thoroughly mixed, chilled in ice-water and centrifuged for 10 min at 20000 g. The supernatant was retained and the DNA was precipitated with 0.6 vol. of propan-2-ol. CG6781 was amplified with the primer set 5′-CTATCACCACTTGCATCTCTGGACC-3′ and 5′-GGAACCGGTTATGGACTGCATTTAT-3′. PCR was carried out under the following conditions: one cycle of 94 °C for 4 min; 30 cycles of 94 °C for 1 min, 56 °C for 45 s, 72 °C for 1 min 10 s; and 1 cycle of 72 °C for 7 min. The product was gel-purified and cloned into pGEM®-T Easy Vector (Promega). Two or three clones were sequenced using T7 and SP6 primers.
Construction of plasmids for transgenic rescue
A cDNA fragment of the ORF (open reading frame) of CG6781 was generated by RT–PCR. Total RNA was extracted with TRIzol® reagent from 30 pupae of Oregon-R; 2 μg of the total RNA was used as the template for first strand cDNA synthesis with RevertAid M-MuLV RT (MBI Fermentas) according to the manufacturer's instructions, and PCR was carried out with the following pair of primers: Bgl2-5′CG6781(5′-GGAGATCTATGAGTAACGGCAGGCAT-3′) and Kpn1-3′CG678(5′-GGTACCATCAGGCATCCTTGACCA-3′) (the underlining indicates the recognition sequences for restriction endonucleases mentioned). The PCR product was cloned into pGEM®-T-Easy vector and sequenced. A plasmid that had the correct sequence was selected. It was digested with BglII and KpnI, and the resulting fragment was cloned into the BglII/KpnI site of pUAST vector to construct pUAST-CG6781.
P-element-mediated germline transformation and transgenic rescue
Transgenic flies were generated by microinjecting pUAST-CG6781 into w1118 embryos. The microinjection was carried out at Genexel (Daejeon, Korea). Multiple transgenic lines were generated and those with transgenes mapped to the second chromosome were used in the transgenic rescue experiments. For transgenic rescue of sepia phenotypes, UAS [upstream activation sequence (CGGAGTACTGTCCTCC), to which GAL4 binds in order to activate gene transcription] transgenic lines and a GMR-gal4 (‘glass multiple reporter’ promoter-dependent gal4 construct/transgene) driver line were crossed into the se1 background by standard Drosophila genetic techniques using appropriate fly strains. For dosage–effect analysis, y;GMR-gal4 UAS-CG6781/CyO;se was generated by recombining the UAS transgene into the chromosome bearing GMR-gal4. Photographs of Drosophila eyes were taken with an Axiocam (Carl Zeiss) mounted on a Stemi 2000-C (Carl Zeiss).
Quantification of content of PDA and drosopterins
Boiled head extracts (20 heads) prepared in 0.05 ml of 100 mM Pipes buffer (pH 7.5) containing 5 mM GSH, 70 mM 2-mercaptoethanol and 10 mM MgCl2 were analysed for their PDA content by HPLC. Portions (0.03 ml) were injected on to a reversed-phase C18 column (Inertsil ODS-3: 4.6 mm×250 mm; GL Sciences) connected to the HPLC apparatus described above. UV absorbance was monitored at 383 nm and PDA content was determined by integrating the area of the PDA peak. Drosopterin content was assayed spectrophotometrically as described in [21]. Ten fly heads (five from male and five from female 2-day-old adults) split longitudinally into halves were placed in 1 ml of AEA [30% (v/v) ethanol acidified with HCl to pH 2.0] for 30 h with agitation. After centrifugation followed by filtration through glass wool, the absorbance of the supernatant was measured at 482 nm.
Northern-blot analysis
Total RNA was extracted from wild-type D. melanogaster (Oregon-R strain) at various developmental stages using TRIzol® reagent. Poly(A)+ (polyadenylated) mRNA was then purified using an Oligotex® mRNA mini kit (Qiagen). Aliquots (2.5 μg) of mRNA or 90 μg aliquots of total RNA were separated on agarose gels containing 2.2 M formaldehyde, and blotted on to Nytran® N membranes (Schleicher and Schuell) by the conventional capillary transfer method with a TurboBlotter system (Schleicher and Schuell). Blotted membranes were probed with a 32P-labelled cDNA probe covering the full-length ORF of CG6781 using QuikHyb® hybridization solution (Stratagene), and autoradiographed. A probe recognizing the RNA coding for ribosomal protein 49 (rp49) was used as control to ensure that comparable amounts of RNA were loaded in all lanes.
Miscellaneous methods
The molecular mass of native enzyme was estimated with a Sephacryl HR S 200 column (1.5 cm×85 cm) calibrated with standard proteins and Blue Dextran (2000000 Da). The column was developed with 0.1 M potassium phosphate buffer (pH 7.0) at a flow rate of 10 ml/h, and elution of the marker proteins was monitored with a Diode-Array spectrophotometer (Hewlett–Packard, U.S.A.). Protein concentration was determined by the Bradford method [22], with BSA as the standard. SDS/PAGE was performed with SDS/4–20% acrylamide gradient gels. After electrophoresis, proteins on the gel were visualized with Coomassie Brilliant Blue R.
RESULTS
Search for candidate PDA synthase genes at the sepia locus
Previous work [13] has shown that three eye colour mutants, i.e. purple, sepia and clot, are deficient in PDA as well as in the ‘drosopterins’. Purple has a mutation in the structural gene for 6-PTP synthase [7,8] and the structural gene for clot is CG11024, which maps to 25E5 and encodes a protein related to the thioredoxin-like enzyme superfamily [17].
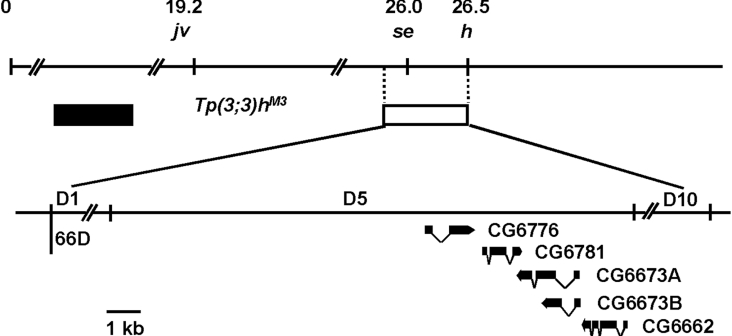
The sepia gene has been mapped by recombination to 3-26.0, very close to the 66D region on the cytological map [23]. In 1985, Ingham et al. [24] generated a new mutant allele, Tp(3;3)hM3, in the course of a genetic analysis of the hairy locus. Tp(3;3)hM3 is associated with transposition to a more distal location on chromosome 3L of material extending distally from the hairy locus and including a region whose breakpoints are at 61E, 66D1–3 and 66D10 on the cytological map (Figure 1). The 66D10 break is located at −10.8 to −9.35 kb on the DNA map of h, which means that the sepia gene is in the 66D1 to 66D10 region [25]. It is also known that PDA synthase requires the presence of GSH, which is thought to supply electrons for the conversion of 6-PTP into PDA [13], and that it appears to be composed of two identical polypeptide chains of 24 kDa [15].
Figure 1. Genetic and cytological map of chromosome 3L showing sepia and its environs.
The fact that sepia is included in the deletion in Tp(3;3)hM3 predicts that the sepia gene is located in the interval 66D1 to 66D10 on the cytological map. The upper and lower solid lines indicate the genetic and cytological locations of the five candidate CGs of PDA synthase found in the 66D5 region. The open box represents the deletion that includes the sepia gene, and the filled box the material transposed in Tp(3;3)hM3. The dotted vertical lines indicate the limits of the chromosomal deletion, while the solid oblique lines indicate their limits on the cytogenetic map. jv (javelin), se (sepia) and h (hairy) have been mapped by recombination to 3–19.2, 3–26.0 and 3–26.5 respectively. The breakpoints of Tp(3;3)hM3 include 61E, 66D1−3 and 66D10 on the cytological map, and its 66D10 break is located at −10.8 to −9.35 kb on the DNA map of h.
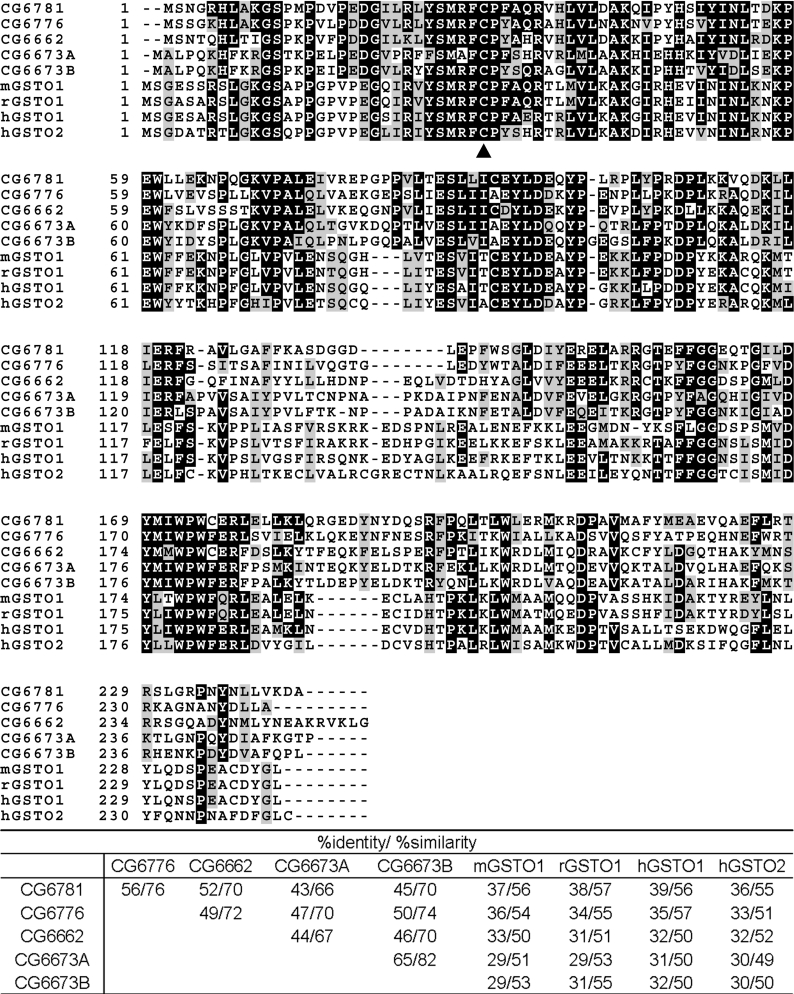
Based on this information, we began to search for the gene encoding PDA synthase. First, we analysed the biochemical functions of all the uncharacterized CGs (computed genes) in the putative sepia locus from 66D1 to 66D10, using the InterPro program (http://www.ebi.ac.uk/InterProScan/). Two main criteria were used: (i) high probability of interaction with glutathione and (ii) calculated molecular mass around 24 kDa. As a result, we identified five candidates, the products of CG6776, CG6781, CG6673A, CG6673B and CG6662 (Figure 1). All five products possess the functional domains of GSTs and are highly similar to the Omega class of GSTs, such as human GSTO1 (Omega class GST 1) [Entrez Protein database (National Center for Biotechnology Information, U.S. National Library of Medicine) accession no. NP_004823], human GSTO2 (Entrez Protein database accession no. NP_899062), mouse GSTO1 (Entrez Protein database accession no. NP_034492) and rat GSTO1 (Entrez Protein database accession no. NP_001007603) (Figure 2). Members of this class have a unique N-terminal extension, and a cysteine residue at the active site, rather than the tyrosine and serine residues found in the active sites of other eukaryotic GSTs [26,27]. In addition to sequence homology, the calculated molecular masses of the products of these candidate CGs, approx. 28 kDa, were similar to the expected molecular size of PDA synthase.
Figure 2. Multiple sequence alignment.
The five candidates for PDA synthase were predicted to be Omega class GSTs using the InterPro program. Multiple sequence alignment of the candidates at 66D5 on the cytological map was performed with human GSTO1 (hGSTO1), human GSTO2 (hGSTO2), mouse GSTO1 (mGSTO1) and rat GSTO1 (rGSTO1) using the ClustalW program. Identical and semi-conserved amino acids are highlighted in black and grey respectively. The arrowhead indicates the putative binding site for the substrate GSH which is characteristic of Omega class GSTs. A % identity/% similarity matrix is shown at the bottom.
Identification and characteristics of the gene encoding PDA synthase
We cloned the ORFs of the five candidate CGs into the pET 15b vector, expressed the proteins in Escherichia coli and purified them by Ni+-affinity column. The conserved active site cysteine residue of Omega class GSTs is able to form a disulfide bond with glutathione and these enzymes have glutathione-dependent thiol transferase and DHA reductase activity, reminiscent of thioredoxin and glutaredoxin enzymes [26,27]. Although there were some differences among the five recombinant proteins, all had relatively high thiol transferase and DHA reductase activities and low activity towards CDNB, a general GST substrate (Table 2). These properties are characteristic of Omega class GSTs.
Table 2. Enzyme activity of recombinant candidate CG products with various substrates.
The activity for each substrate was determined under standard conditions as described in the Experimental section. All values are the means for three determinations. ND, not detectable.
| Specific activity (units/mg) | |||||
|---|---|---|---|---|---|
| CG6781 | CG6673A | CG6673B | CG6776 | CG6662 | |
| PDA synthase* | 124 | ND | ND | ND | ND |
| GST activity for CDNB† | ND | 0.01 | 0.08 | ND | ND |
| Thiol transferase‡ | 5.62 | 2.83 | 54.26 | 1.06 | 0.55 |
| DHA reductase§ | 3.00 | 74.34 | 133.29 | 0.71 | 0.26 |
*One unit of enzymatic activity is defined as the amount of enzyme needed for the formation of 1 nmol of PDA from H2NTP in 2 h at 30 °C in the presence of excess PTP synthase.
†One unit of enzymatic activity is defined as the amount of enzyme catalysing the displacement of 1 μmol of chloride per min at 25 °C.
‡One unit of enzymatic activity is defined as the amount of enzyme catalysing the formation of 1 μmol of GSSG per min at 30 °C.
§One unit of enzymatic activity is defined as the amount of enzyme catalysing the formation of 1 μmol of DHA per min at 30 °C.
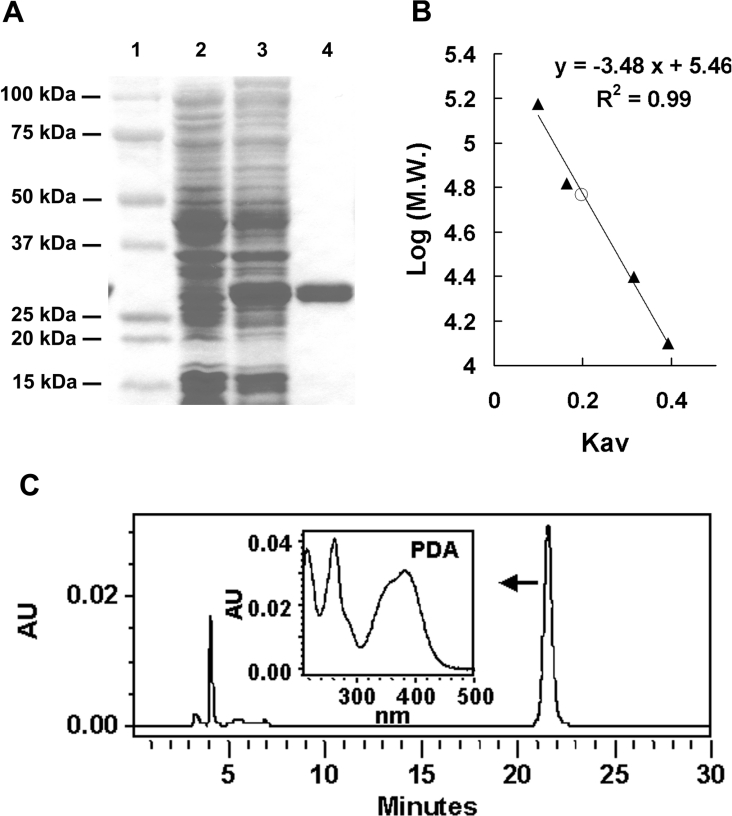
We assayed each recombinant protein for PDA-synthesizing activity as described in the Experimental section. Interestingly, only the product of CG6781 had this activity (Table 2 and Figure 3C), despite the high level of homology between the primary sequences of the five enzymes. The reaction product had a UV absorption spectrum identical with that of authentic PDA [14]. The molecular mass of the CG6781 recombinant protein was estimated to be 28 kDa by SDS/PAGE analysis (Figure 3A). Size-exclusion chromatography on a calibrated Sephacryl HR S 200 column gave its mass as 56 kDa (Figure 3B), indicating that the protein forms homodimer under native conditions.
Figure 3. Molecular mass and PDA synthase activity of the CG6781 product.
(A) Recombinant CG6781, purified with His·Bind resin and subjected to electrophoresis on an SDS/4–20% acrylamide gradient gel, has a molecular mass of approx. 28 kDa. Molecular masses of standards are indicated. 1, standard markers; 2, crude extract before induction; 3, crude extract after induction; 4, purified enzyme. (B) The native molecular mass of the enzyme (○) was estimated to be 56 kDa by size-exclusion chromatography on a calibrated Sephacryl HR S 200 column (1.5 cm×85 cm), as described in the Experimental section. Standard proteins (▲) used for calibration were: alcohol dehydrogenase (150000 Da), BSA (67000 Da), chymotrypsinogen A (25000 Da) and cytochrome c (12500 Da), and a calibration curve of log molecular masses of protein markers versus partition coefficients, Kav, was constructed. (C) PDA synthase activity of CG6781 was assayed as described in the Experimental section. The UV–visible spectrum of the reaction product eluted between 21 and 22 min was identical with that of PDA. The characteristic UV–visible spectrum of PDA determined with photodiode array detector (Waters 996) is shown.
se1 has a frameshift mutation in the ORF of CG6781
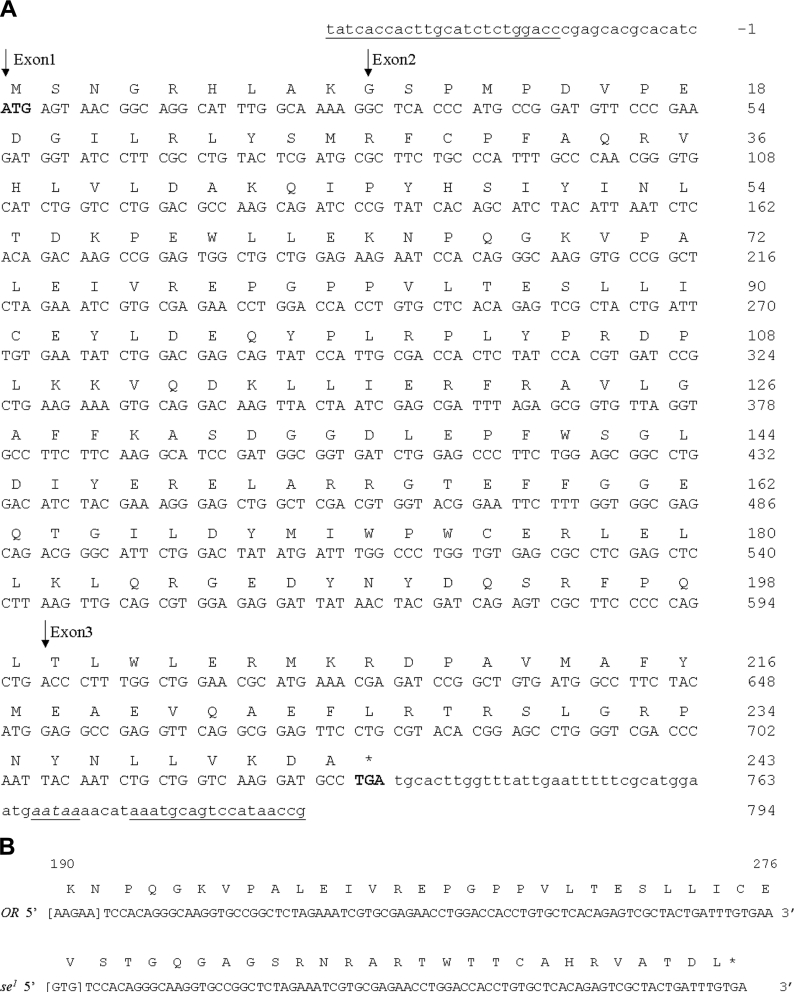
Having identified CG6781 as the PDA synthase gene, we tested whether it is the locus of sepia. As a first step, we investigated the nature of the mutation in se1 by comparing the sequence of CG6781 in wild-type and se1 flies. We generated primers targeted to the untranslated regions at the 5′- and 3′-ends of CG6781 (Figure 4A) based on sequence information from the Drosophila Genome Annotation (release 4.1), and amplified and sequenced the genomic region of CG6781 as described in the Experimental section. This revealed the presence in the se1 mutant of a change of nt 190–194 (numbered from the ATG translation initiation codon), from AAGAA to GTG, leading to a frameshift. This frameshift creates a stop codon (TGA) at nt 273–275 in the second exon (Figure 4B). We also sequenced approx. 1 kb of the upstream region of CG6781, but detected no other mutations.
Figure 4. Nucleotide and amino acid sequence of the se1 allele of CG6781.
(A) Nucleotide and deduced amino acid sequence of CG6781. The sequence of PDA synthase (CG6781) is based on the Drosophila Genome Annotation (release 4.1). CG6781 is composed of three exons, two introns and short untranslated regions at each end. The deduced amino acid sequence is shown above the nucleotide sequence. Lower-case letters represent the untranslated region of the gene. Start and stop codons are shown in boldface, and a putative polyadenylation signal is underlined and in italics. The right-hand side of the sequence gives both nucleotide and amino acid numbering. The beginning of each exon is indicated by downward-pointing arrows. The pair of primers used for genomic sequencing is underlined. (B) The mutation in the coding region of CG6781 in the se1 allele. The cDNA sequence of the coding region of the second exon from 190 to 276 (numbered from the ATG translation initiation codon) is shown. Single-letter amino acid abbreviations are shown above the nucleotide sequence. In se1, nt 190–194, AAGAA, are changed to GTG, which leads to a frameshift mutation. This frameshift mutation produces a stop codon (TGA, underlined) at nt 273–275 in the second exon.
Transgenic expression of CG6781 in the eye rescues the phenotype of se1
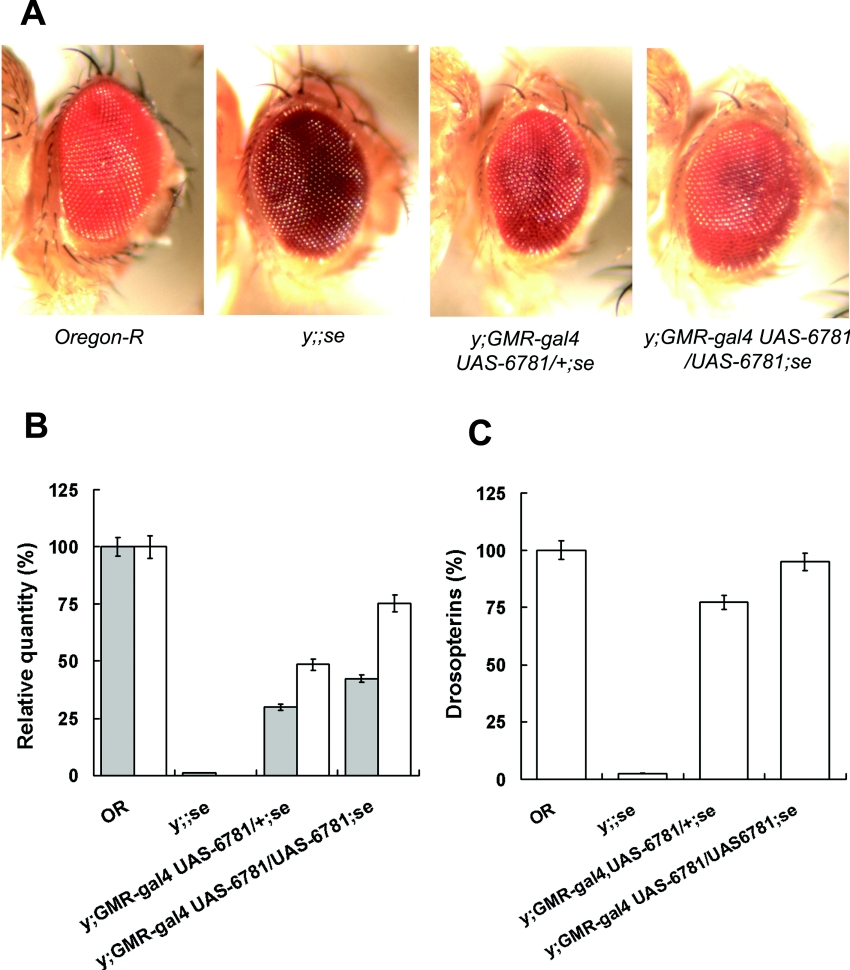
We next enquired whether expression of CG6781 in vivo is sufficient to rescue the mutant phenotype of se1, utilizing the Gal4-UAS binary expression system [28]. The full ORF of CG6781 used in the in vitro enzyme assays was subcloned into pUAST, and transgenic se1 flies harbouring this construct, UAS-CG6781, were generated. In order to drive overexpression in the eyes of these flies, we introduced the eye-specific GMR-gal4 driver into one such transgenic line by recombination and obtained y;GMR-gal4 UAS-CG6781/+;se flies. The photographs in Figure 5(A) demonstrate that in vivo expression of CG6781 rescues the sepia eye colour phenotype. We also measured the amount of PDA, PDA synthase activity and red pigments in these flies. The results in Figures 5(B) and 5(C) reveal that the levels of PDA, PDA synthase activity and drosopterin pigments increased to 30, 49 and 77% respectively of wild-type levels. To determine whether this partial rescue was due to an insufficient level of transgene expression, the dosage of the transgene was increased to two copies by an additional cross yielding y;GMR-gal4 UAS-CG6781/UAS-CG6781;se flies, as described in the Experimental section. This resulted in 43, 75 and 95% rescue of PDA, PDA synthase activity and drosopterins levels respectively (Figures 5B and 5C).
Figure 5. Phenotypic rescue of se1 by transgenic expression of CG6781 in vivo.
The coding region of CG6781 was cloned into pUAST, which was then used to generate UAS-CG6781 transgenic flies by microinjection. (A) Eye colour phenotypes of wild-type (Oregon-R), sepia mutant (y;;se1), flies with one copy each of GMR-gal4 and UAS-CG6781 in the se1 background (y;GMR-gal4 UAS-CG6781/+;se1), and flies with one copy of GMR-gal4 and two copies of UAS-CG6781 in the se1 background (y;GMR-gal4 UAS-CG6781/UAS-CG6781;se1). (B) PDA content (grey bars) and PDA synthase activity (white bars) of adult fly heads of each genotype were determined by HPLC and enzyme assays respectively. (C) The drosopterin content of adult fly heads of each genotype was determined spectrophotometrically as described in the Experimental section. The extent of rescue defined by the content of PDA and PDA synthase activity (B) or of drosopterin (C) is dependent on the dosage of UAS-CG6781. Levels are calculated relative to wild-type flies. The vertical bars represent means±S.E.M.
Expression of CG6781 during development
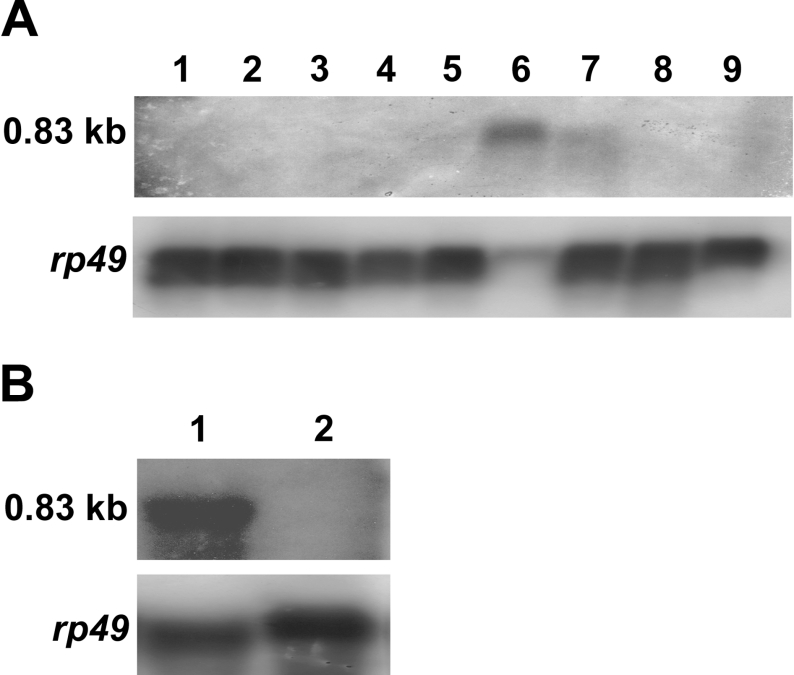
We carried out a Northern-blot analysis to examine the expression profile of CG6781 during development and in adult flies. Blots were probed with a sequence covering the full-length CG6781 ORF. A single species of mRNA (0.83 kb) was detected (Figure 6). This mRNA only appeared at the late pupal stage when it was present at a high level; it was also evident in the early adult (0–2 days old) at a substantially lower level (Figure 6A). Northern blots prepared separately from adult heads and bodies showed that CG6781 is expressed exclusively in the adult head (Figure 6B). It should be noted that GTP cyclohydrolase I [29] and 6-PTP synthase [30], which are involved in the biosynthesis of drosopterins, generally appear at their highest level in the late pupa and the newly emerged adult. Moreover, the developmental profile and head-specific expression of CG6781 agree well with the fact that the formation of red eye pigments starts during the late pupal stage and continues over several days in the eclosed fly [31].
Figure 6. Expression of PDA synthase.
(A) Poly(A)+ RNA (2.5 μg) extracted from wild-type Drosophila (Oregon-R strain) at different developmental stages was analysed by Northern blotting with a 32P-labelled cDNA probe covering the full-length CG6781 ORF. Developmental stages were: 1, early embryos (0–9 h); 2, late embryos (9–18 h), 3, second instar larvae; 4, third instar larvae; 5, white pupae; 6, brown pupae; 7, 0–2-day-old adults; 8, 3–4-day old adults; 9, 5–6-day-old adults. (B) Total RNA (90 μg) extracted from 0–2-day-old adult fly heads (lane 1), and decapitated bodies (lane 2), was analysed by Northern blotting. The lower panel in (A, B) shows the results of hybridization of the same blot with a 32P-labelled rp49 cDNA probe.
DISCUSSION
Drosopterins are one of the major groups of pigments present in the eyes of Drosophila. They consist of a pentacyclic ring system in which two pteridine structures are joined by a pyrrole ring [2–4,32] and are produced non-enzymatically by one-to-one condensation of 7,8-dihydrolumazine or 7,8-dihydropterin with PDA [4,33]. PDA was first discovered as the ‘quench spot’ in fly heads because, unlike other pteridines, it quenches UV light at room temperature (25 °C), but fluoresces an intense bright green at very low temperature [34].
Wiederrecht and Brown [15], using partially purified fly head extracts, identified the enzyme activity responsible for conversion of 6-PTP into PDA, and demonstrated a requirement for glutathione for this reaction. They also suggested that the eye colour mutation sepia was in the structural gene for PDA synthase based on comparison of PDA synthase activity in relevant eye colour mutants. In the present study, we have for the first time identified and characterized the gene encoding PDA synthase, and provided strong biochemical and in vivo evidence that this gene, CG6781, is the structural gene mutated in sepia.
Based on previously reported information that PDA synthase requires glutathione and is composed of two identical subunits of 24 kDa [13,15], five candidate genes of PDA synthase were selected in the presumed sepia locus. These five candidates had already been classified as encoding Omega class GSTs based on amino acid sequence comparisons and phylogenetic analysis of GSTs [35]. We showed that all five products had thiol transferase and DHA reductase activity, reminiscent of thioredoxins and glutaredoxins and characteristic of Omega class GSTs [26,27]. Currently, insect cytosolic GSTs are divided into six classes, Delta (class I), Sigma (class II), Epsilon (class III), Zeta, Theta, Omega and unclassified [36]. Enzymes of the Omega, Zeta and Theta classes are relatively poorly characterized compared with the Delta, Epsilon [37,38] and Sigma [39] types. Our work establishes a new catalytic activity for the Omega class GSTs that CG6781 of Drosophila is involved in pteridine metabolism, in particular in the biosynthesis of red eye pigments. It is likely that the role of CG6781 is restricted to drosopterin biosynthesis, since (i) it is expressed only in the head in adults, (ii) it is expressed in the late pupa and newly emerged adult, and (iii) sepia has no other detectable phenotype than dark-brown eye colour. It is interesting to note that CG6673B has remarkably high DHA reductase activity (Table 2), and its active site motif (CPYS) is identical with that of human GSTO2 (Figure 2), whose DHA reductase activity is also 70–100-fold higher than that of human GSTO1 [40,41]. The biological functions of other Omega class GSTs of Drosophila including CG6673B will be the subject of further studies.
Interestingly, in spite of the strong homology between the five candidates, only the CG6781 protein had PDA synthase activity. Recombinant CG6781 was shown to be a homodimer of 28 kDa subunits. It is well known that all soluble GSTs form dimers and that each subunit contains two independent binding sites. The first is specific for glutathione or a closely related homologue (the G site) and is formed from a conserved group of N-terminal amino acids. The other is the site that binds the hydrophobic substrate (the H site); it is much more variable structurally and is formed from residues in the C-terminal domain [36]. Since these features are present in all five candidates, structural differences in the C-terminal domain may be responsible for the fact that only CG6781 has PDA synthase activity.
Since the first discovery of the naturally occurring sepia allele (se1) in 1913 [42], the nature of this mutation has remained unknown. By sequencing CG6781, we found that nt 190–194 are changed from AAGAA to GTG in se1 and that this leads to a frameshift mutation causing a premature stop codon (TGA) at nt 273–275 in the second exon. Although more rare than spontaneous mutations caused by one or two base changes, this type of complex mutation, involving either insertions/deletions coupled with base substitutions, or insertions coupled with deletions, has been reported in a number of spontaneous frameshift mutations [43–45]. The mechanism involved is obscure. One possibility, suggested by Ripley [46], is that it is caused by quasi-palindromic sequences that could form imperfect hairpin loop structures in which the misaligned bases provide templates for either deletions (by endonucleolytic base excision) or insertions (by gap repair). However, there is no detectable quasi-palindromic sequence in the neighbourhood of the sepia mutation.
Drosopterins are produced non-enzymatically by the condensation of PDA with 7,8-dihydropterin under acidic condition [33]. Due to the lower level of PDA synthase activity in the transgenic flies, PDA would be rate-limiting for the condensation reaction. Therefore drosopterins could accumulate in ommatidial structures as they are synthesized and attain nearly wild-type levels, whereas PDA would not only be in short of supply but would be continuously consumed, so that its steady-state concentration would be less than that of drosopterin (Figures 5B and 5C). Several eye colour gene products are involved in drosopterin biosynthesis. Punch is in the structural gene for the first enzyme, GTP cyclohydrolase I [5,6], and the second step in the pathway is catalysed by PTP synthase encoded by the purple gene [7,8,10]. The mutations sepia and clot affect the production of PDA from 6-PTP, and in the present study we have provided in vitro as well as in vivo evidence that the sepia gene is the structural gene for PDA synthase. The ‘side-chain releasing enzyme’ present in fly head extracts may participate in the conversion of 6-PTP into 7,8-dihydropterin, a component of drosopterin [47]. clot and sepia have similar phenotypes in that they contain neither drosopterins nor PDA, and both produce large amounts of sepiapterin [13]. It has been suggested that clot affects the conversion of H2NTP into PDA indirectly because of the ‘leaky’ nature of its PDA synthase defect. Recently, the clot structural gene was shown to encode a protein related to the glutaredoxin class of the thioredoxin-like enzyme superfamily [17]. Glutaredoxin is a general GSH-disulfide oxidoreductase, catalysing thiol–disulfide exchange reactions. We have cloned the clot ORF and purified the recombinant protein. This did not exhibit PDA synthase activity on its own, nor did it have any effect on the production of PDA when combined with PDA synthase in vitro (results not shown). Whether other factors are needed for Clot to function, or whether its role is simply to balance redox potential for PDA synthesis, is not clear.
Acknowledgments
This work was supported by a grant (M10418000001-05N1800-00110) from the Korea Science and Engineering Foundation under the Specific Research and Development Program funded by the Ministry of Science and Technology, Republic of Korea.
References
- 1.Schwinck I. Epigenetic alternations of the drosopterin pattern in various mutants of Drosophila melanogaster. Genetics. 1971;68:s59–s60. [Google Scholar]
- 2.Theobald N., Pfleiderer W. Ein neuer strukturvorschlag fur die augenpigmente droso-und isodrosopterin aus Drosophila melanogaster. Tetrahedron Lett. 1977;10:841–844. [Google Scholar]
- 3.Theobald N., Pfleiderer W. Ein neuer strukturvorschlag fur die roten augenpigmente drosopterin und isodrosopterin aus Drosophila melanogaster. Chem. Ber. 1978;111:3385–3402. [Google Scholar]
- 4.Yim J., Kim S. J., Walcher G., Pfleiderer W. Structure and nonenzymatic synthesis of aurodrosopterin. Helv. Chim. Acta. 1993;76:1970–1979. [Google Scholar]
- 5.Mackay W. J., O'Donnell J. M. A genetic analysis of the pteridine biosynthetic enzyme, guanosine triphosphate cyclohydrolase, in Drosophila melanogaster. Genetics. 1983;105:35–53. doi: 10.1093/genetics/105.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLean J. R., Krishnakumar S., O'Donnell J. M. Multiple mRNAs from the Punch locus of Drosophila melanogaster encode isoforms of GTP cyclohydrolase I with distinct N-terminal domains. J. Biol. Chem. 1993;268:27191–27197. [PubMed] [Google Scholar]
- 7.Yim J., Grell E. H., Jacobson K. B. Mechanism of suppression in Drosophila: control of sepiapterin synthase at the purple locus. Science. 1977;198:1168–1170. doi: 10.1126/science.412253. [DOI] [PubMed] [Google Scholar]
- 8.Krivi G., Brown G. M. Purification and properties of the enzymes from Drosophila melanogaster that catalyze the synthesis of sepiapterin from dihydroneopterin triphosphate. Biochem. Genet. 1979;17:371–389. doi: 10.1007/BF00498976. [DOI] [PubMed] [Google Scholar]
- 9.Switchenko A. C., Brown G. M. The enzymatic conversion of dihydroneopterin triphosphate to tripolyphosphate and 6-pyruvoyl-tetrahydropterin, an intermediate in the biosynthesis of other pterins in Drosophila melanogaster. J. Biol. Chem. 1985;260:2945–2951. [PubMed] [Google Scholar]
- 10.Park Y. S., Kim J. H., Jacobson K. B., Yim J. Purification and characterization of 6-pyruvoyl-tetrahydropterin synthase from Drosophila melanogaster. Biochim. Biophys. Acta. 1990;1038:186–194. doi: 10.1016/0167-4838(90)90203-r. [DOI] [PubMed] [Google Scholar]
- 11.Wiederrecht G. J., Paton D. R., Brown G. M. The isolation and identification of an intermediate involved in the biosynthesis of drosopterin in Drosophila melanogaster. J. Biol. Chem. 1981;256:10399–10402. [PubMed] [Google Scholar]
- 12.Dorsett D., Yim J., Jacobson K. B. Biosynthesis of ‘drosopterins’ by an enzyme system from Drosophila melanogaster. Biochemistry. 1979;12:2596–2600. doi: 10.1021/bi00579a025. [DOI] [PubMed] [Google Scholar]
- 13.Wiederrecht G. J., Paton D. R., Brown G. M. Enzymatic conversion of dihydroneopterin triphosphate to the pyrimidodiazepine intermediate involved in the biosynthesis of the drosopterins in Drosophila melanogaster. J. Biol. Chem. 1984;259:2195–2200. [PubMed] [Google Scholar]
- 14.Jacobson K. B., Dorsett D., Pfleiderer W., McCloskey J. A., Sethi S. K., Buchanan M. V., Rubin I. B. A naturally occurring pyrimidodiazepine in Drosophila: chemical and spectral properties and relationship to drosopterin. Biochemistry. 1982;21:5700–5706. doi: 10.1021/bi00265a048. [DOI] [PubMed] [Google Scholar]
- 15.Wiederrecht G. J., Brown G. M. Purification and properties of the enzymes from Drosophila melanogaster that catalyze the conversion of dihydroneopterin triphosphate to the pyrimidodiazepine precursor of the drosopterins. J. Biol. Chem. 1984;259:14121–14127. [PubMed] [Google Scholar]
- 16.Switchenko A. C., Primus J. P., Brown G. M. Intermediates in the enzymic synthesis of tetrahydrobiopterin in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 1984;120:754–760. doi: 10.1016/s0006-291x(84)80171-0. [DOI] [PubMed] [Google Scholar]
- 17.Giordano E., Peluso I., Rendina R., Digilio A., Furia M. The clot gene of Drosophila melanogaster encodes a conserved member of the thioredoxin-like protein superfamily. Mol. Genet. Genomics. 2003;268:692–697. doi: 10.1007/s00438-002-0792-0. [DOI] [PubMed] [Google Scholar]
- 18.Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 19.Axelsson K., Eriksson S., Mannervik B. Purification and characterization of cytoplasmic thioltransferase (glutathione:disulfide oxidoreductase) from rat liver. Biochemistry. 1978;17:2978–2984. doi: 10.1021/bi00608a006. [DOI] [PubMed] [Google Scholar]
- 20.Wells W. W., Xu D. P., Washburn M. P. Glutathione: dehydroascorbate oxidoreductases. Methods Enzymol. 1995;252:30–38. doi: 10.1016/0076-6879(95)52006-6. [DOI] [PubMed] [Google Scholar]
- 21.Real M. D., Ferre J., Mensua J. L. Methods for the quantitative estimation of the red and brown pigments of Drosophila melanogaster. Drosoph. Inf. Serv. 1985;61:198–199. [Google Scholar]
- 22.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Andres J. M. New mutants report. Drosoph. Inf. Serv. 1945;19:45. [Google Scholar]
- 24.Ingham P. W., Pinchin S. M., Howard K. R., Ish-Horowicz D. Genetic analysis of the hairy locus in Drosophila melanogaster. Genetics. 1985;111:463–486. doi: 10.1093/genetics/111.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard K., Ingham P., Rushlow C. Region-specific alleles of the Drosophila segmentation gene hairy. Genes Dev. 1988;2:1037–1046. doi: 10.1101/gad.2.8.1037. [DOI] [PubMed] [Google Scholar]
- 26.Board P. G., Coggan M., Chelvanayagam G., Easteal S., Jermiin L. S., Schulte G. K., Danley D. E., Hoth L. R., Griffor M. C., Kamath A. V., et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000;275:24798–24806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 27.Girardini J., Amirante A., Zemzoumi K., Serra E. Characterization of an omega-class glutathione S-transferase from Schistosoma mansoni with glutaredoxin-like dehydroascorbate reductase and thiol transferase activities. Eur. J. Biochem. 2002;269:5512–5521. doi: 10.1046/j.1432-1033.2002.03254.x. [DOI] [PubMed] [Google Scholar]
- 28.Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Fan C. L., Hall L. M., Skrinska A. J., Brown G. M. Correlation of guanosine triphosphate cyclohydrolase activity and the synthesis of pterins in Drosophila melanogaster. Biochem. Genet. 1976;14:271–280. doi: 10.1007/BF00484766. [DOI] [PubMed] [Google Scholar]
- 30.Kim N. S., Kim J. S., Park D. K., Rosen C., Dorsett D., Yim J. Structure and expression of wild-type and suppressible alleles of the Drosophila purple gene. Genetics. 1996;42:1157–1168. doi: 10.1093/genetics/142.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwinck I., Mancini M. The drosopterin pattern in various eye color mutants of the fruitfly, Drosophila melanogaster. Arch. Genet. 1973;46:41–52. [PubMed] [Google Scholar]
- 32.Rokos K., Pfleiderer W. Isolation and properties of neodrosopterin and aurodrosopterin. Chem. Ber. 1975;108:2728–2736. [Google Scholar]
- 33.Paton D. R., Brown G. M. The nonenzymatic synthesis of ‘drosopterins’ from dihydropterin and 2-amino-4-oxo-6-acetyl-3H, 9H-7,8-dihydropyrimido[4,5b][1,4]diazepine. In: Cooper B. A., Whitehead V. M., editors. Chemistry and Biology of Pteridines. Berlin: Walter de Gruyter; 1986. pp. 295–298. [Google Scholar]
- 34.Wilson T. G., Jacobson K. B. Isolation and characterization of pteridines from heads of Drosophila melanogaster by a modified thin-layer chromatography procedure. Biochem. Genet. 1977;15:307–319. doi: 10.1007/BF00484462. [DOI] [PubMed] [Google Scholar]
- 35.Ranson H., Rossiter L., Ortelli F., Jensen B., Wang X., Roth C. W., Collins F. H., Hemingway J. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem. J. 2001;359:295–304. doi: 10.1042/0264-6021:3590295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enayati A. A., Ranson H., Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005;14:3–8. doi: 10.1111/j.1365-2583.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 37.Hemingway J. The molecular basis of two contrasting metabolic mechanisms of insecticide resistance. Insect Biochem. Mol. Biol. 2000;30:1009–1015. doi: 10.1016/s0965-1748(00)00079-5. [DOI] [PubMed] [Google Scholar]
- 38.Prapanthadara L., Promtet N., Koottathep S., Somboon P., Ketterman A. J. Isoenzymes of glutathione S-transferase from the mosquito Anopheles dirus species B: the purification, partial characterization and interaction with various insecticides. Insect Biochem. Mol. Biol. 2000;30:395–403. doi: 10.1016/s0965-1748(00)00013-8. [DOI] [PubMed] [Google Scholar]
- 39.Agianian B., Tucker P. A., Schouten A., Leonard K., Bullard B., Gros P. Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J. Mol. Biol. 2003;326:151–165. doi: 10.1016/s0022-2836(02)01327-x. [DOI] [PubMed] [Google Scholar]
- 40.Whitbread A. K., Tetlow N., Eyre H. J., Sutherland G. R., Board P. G. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics. 2003;13:131–144. doi: 10.1097/00008571-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Schmuck E. M., Board P. G., Whitbread A. K., Tetlow N., Cavanaugh J. A., Blackburn A. C., Masoumi A. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: implications for arsenic metabolism and the age-at-onset of Alzheimer's and Parkinson's diseases. Pharmacogenet. Genomics. 2005;15:493–501. doi: 10.1097/01.fpc.0000165725.81559.e3. [DOI] [PubMed] [Google Scholar]
- 42.Lindsley D. L., Zimm G. G. San Diego, CA: Academic Press; 1992. The Genome of Drosophila melanogaster; pp. 626–627. [Google Scholar]
- 43.Kalinowski D. P., Larimer F. W., Plewa M. J. Analysis of spontaneous frameshift mutations in REV1 and rev1-1 strains of Saccharomyces cerevisiae. Mutat. Res. 1995;331:149–159. doi: 10.1016/0027-5107(95)00064-p. [DOI] [PubMed] [Google Scholar]
- 44.DeMarini D. M., Shelton M. L., Abu-Shakra A., Szakmary A., Levine J. G. Spectra of spontaneous frameshift mutations at the hisD3052 allele of Salmonella typhimurium in four DNA repair backgrounds. Genetics. 1998;149:17–36. doi: 10.1093/genetics/149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellard S., Bulman M. P., Frayling T. M., Shepherd M., Hattersley A. T. Proposed mechanism for a novel insertion/deletion frameshift mutation (I414G415ATCG→CCA) in the hepatocyte nuclear factor 1 alpha (HNF-1 alpha) gene which causes maturity-onset diabetes of the young (MODY) Hum. Mutat. 2000;16:273. doi: 10.1002/1098-1004(200009)16:3<273::AID-HUMU18>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 46.Ripley L. S. Model for the participation of quasi-palindromic DNA sequences in frameshift mutation. Proc. Natl. Acad. Sci. U.S.A. 1982;79:4128–4132. doi: 10.1073/pnas.79.13.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yim J., Jacobson K. B., Crummett D. C. Detection and some properties of an enzyme from Drosophila melanogaster that releases the side chain from dihydroneopterin triphosphate. Insect Biochem. 1981;11:363–370. [Google Scholar]