Abstract
In addition to its well-documented role in integration of the viral genome, the HIV-1 enzyme IN (integrase) is thought to be involved in the preceding step of importing the viral cDNA into the nucleus. The ability of HIV to transport its cDNA through an intact nuclear envelope allows HIV-1 to infect non-dividing cells, which is thought to be crucial for the persistent nature of HIV/AIDS. Despite this, the mechanism utilized by HIV-1 to import its cDNA into the nucleus, and the viral proteins involved, remains ill-defined. In the present study we utilize in vitro techniques to assess the nuclear import properties of the IN protein, and show that IN interacts with members of the Imp (Importin) family of nuclear transport proteins with high affinity and exhibits rapid nuclear accumulation within an in vitro assay, indicating that IN possesses potent nucleophilic potential. IN nuclear import appears to be dependent on the Imp α/β heterodimer and Ran GTP (Ran in its GTP-bound state), but does not require ATP. Importantly, we show that IN is capable of binding DNA and facilitating its import into the nucleus of semi-intact cells via a process that involves basic residues within amino acids 186–188 of IN. These results confirm IN as an efficient mediator of DNA nuclear import in vitro and imply the potential for IN to fulfil such a role in vivo. These results may not only aid in highlighting potential therapeutic targets for impeding the progression of HIV/AIDS, but may also be relevant for non-viral gene delivery.
Keywords: DNA delivery, HIV-1, Importin, in vitro reconstituted transport system, integrase, nuclear import
Abbreviations: CLSM, confocal laser scanning microscopy; DTAF, 5-[4,6-dichlorotriazinyl]aminofluorescein; Fn/c, nuclear to cytoplasmic fluorescence ratio; β-gal, β-galactosidase; GFP, green fluorescent protein; GST, glutathione S-transferase; GTP[S], guanosine 5′-[γ-thio]triphosphate; HTC cell line, hepatoma tissue culture cell line; Imp, Importin; IN, integrase; LEDGF, lens epithelium-derived growth factor; NLS, nuclear localization sequence; NPC, nuclear pore complex; PIC, pre-integration complex; SV40, simian virus 40; Tag, tumour antigen; Vpr, viral protein R; WT, wild-type
INTRODUCTION
The unique ability of lentiviruses such as HIV-1 to productively infect non-dividing cell types gives them an advantage over their retroviral counterparts in that they can infect terminally differentiated and enduring cells such as macrophages [1], which provides a reservoir for the virus and is thought to contribute to the long-term pathogenicity of HIV/AIDS. As HIV must integrate its cDNA into the host cell genome, infection of non-dividing cells requires the virus to be translocated through an intact nuclear envelope, with the source of this karyophilic potential being attributed to one or more components of the PIC (pre-integration complex).
Classically, nuclear import requires the cargo protein to contain an NLS (nuclear localization sequence) which is typically a single or bipartite cluster of basic amino acids. The NLS is recognized and bound by members of the Imp (Importin) superfamily of import proteins, either directly by Imp β (or a homologue thereof) or indirectly through the Imp α component of the Imp α/β heterodimer, as is the case for the SV40 (simian virus 40) large Tag (tumour antigen) NLS. Imp β mediates the passage of the import cargo through the NPC (nuclear pore complex) and into the nucleus where binding of Ran in its GTP-bound state (Ran GTP) to Imp β mediates dissociation of the complex and release of the cargo protein (for reviews, see [2,3]).
Formed following the entry and uncoating of HIV-1 within the infected cell, the PIC is a nucleic acid/protein complex primarily comprising the viral genome, reverse transcriptase, IN (integrase), matrix and Vpr (viral protein R) [4,5]. Owing to the unique method of HIV reverse transcription, the cDNA within the PIC also contains a short section of triple-stranded DNA known as the cDNA flap. Although there has been a suggestion that this region may be involved in nuclear import of the PIC [6], this has been disputed [7], and Vpr, matrix and IN are generally accepted as the most likely effectors of PIC nuclear import (reviewed in [8]). Whereas both matrix [9,10] and Vpr [11] have been shown to possess nucleophilic potential, the ability of viruses lacking both the proposed matrix NLS and a functional Vpr gene to productively infect non-dividing cells [12–14] indicates that neither is essential for cDNA nuclear import and implies the existence of a third, more fundamental mediator of this process.
IN is a 288-amino-acid protein consisting of three functional domains; an N-terminal Zn2+-binding domain, a central core domain containing the catalytic DDX35E motif, and a C-terminal domain that has been shown to bind DNA non-specifically (for reviews, see [15,16]). IN is responsible for mediating the enzymatic integration of the HIV-1 cDNA into the genome of the host cell via a well-understood process [16]. Aside from this fundamental role, mutations within IN have been shown to affect numerous other steps during infection, including reverse transcriptase activity/cDNA synthesis [17,18], assembly of viral particles [19,20] and polyprotein processing [20], and, as mentioned above, IN is also purported to be involved in the nuclear import of HIV cDNA (see [8]).
Studies into the cellular localization of transfected IN constructs have largely agreed that IN localizes to the nucleus of cells, although IN constructs with large fusion tags such as β-gal (β-galactosidase) [21] or GFP (green fluorescent protein)-pyruvate kinase [22] fail to enter the nucleus, implying that IN contains only a weak NLS. In vitro nuclear import studies have supported the nucleophilic potential of IN [23–25], although highly atypical import mechanisms requiring ATP but occurring either independently of Imp β [24] or of both Imp α and β [23] have been proposed. An alternative mechanism involving the Imp 7/Imp β heterodimer has also been suggested [25]; however, all three theories remain unsupported. Recently, the transcription factor LEDGF (lens epithelium-derived growth factor) has been implicated in IN nuclear import, and although it appears that IN interacts with LEDGF [26] and overexpression of LEDGF may increase IN nuclear accumulation [27], it is unclear whether LEDGF actively facilitates IN import or merely contributes to nuclear accumulation by enhancing the binding of IN to DNA/chromosomes.
The precise region of IN responsible for mediating nuclear import remains unclear, as ambiguous results have been obtained from in vivo mutagenesis analyses. The original identification of a putative bipartite NLS involving amino acids 185–211 [13] has been questioned [23], as this region has been shown to be important for cDNA integration and IN dimerization [28]. A region of IN within amino acids 161–173 (with critical residues at 165/166) has also been nominated as a potential NLS [24,29], although this too is disputed [22,30] as these regions are believed to be critical for cDNA integration. The multifunctional nature of IN makes in vivo analysis of its nuclear import problematic, especially within the context of an infected cell system. Investigating IN nuclear import within an in vitro system is therefore useful, although these experiments can potentially be adulterated by the inherent ability of IN to bind DNA, which can lead to a false indication of nuclear import potential. Needless to say, an in vitro analysis of the nucleophilic properties of IN using specific nuclear import assays that are capable of determining the contribution that DNA binding makes to overall nuclear accumulation would clearly be useful.
Here, we report that IN exhibits a high-affinity interaction with Imps, particularly Imp α and the Imp α/β heterodimer. We also report the nuclear localization of IN in an in vitro transport assay and show that IN nuclear import is dependent on the Imp α/β heterodimer, is inhibited by Ran GTP[S] (guanosine 5′-[γ-thio]triphosphate) and does not require ATP. Further, we show that the IN protein alone is able to bind DNA and import bound DNA into the nucleus of cells via an Imp α/β-dependent process that involves the K186RK region of IN. These results highlight the ability of IN to transport DNA into the nucleus in vitro and imply the potential for IN to fulfil such a role not only in the context of HIV infection, but also within gene delivery applications.
METHODS
Cell culture
The HTC (hepatoma tissue culture) rat hepatoma cell line was maintained in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) foetal calf serum, L-glutamine, penicillin and streptomycin in a humidified 37 °C incubator with 5% CO2. For in vitro transport assay experiments, HTC cells were trypsinized and seeded on to glass coverslips 2 days prior to use to achieve a confluency of 70% at the time of experimentation.
Mutagenesis of IN NLS
Site-directed mutagenesis was performed on the pINSD.His.Sol plasmid [AIDS Research and Reference Reagent Program no. 2958; Division of AIDS, NIAID (National Institutes of Allergy and Infectious Diseases), NIH (National Institutes of Health), Bethesda, MD, U.S.A.] using a QuikChange® mutagenesis kit (Stratagene) according to the manufacturer's instructions to create a K186RK to AAA mutation within a proposed NLS region of the IN coding gene. DNA sequencing was subsequently used to confirm the integrity of the IN NLS mutant.
Protein expression and purification
His6-tagged IN protein was expressed from the pINSD.His.Sol plasmid in BL21 (DE3) bacteria as previously described [31]. Bacterial pellets were resuspended in native buffer (50 mM NaH2PO4, 1 M NaCl and 5 mM 2-mercaptoethanol, pH 8.0) containing 10 mM imidazole, and lysed with 3 mg/ml lysozyme on ice for 30 min in the presence of 1 unit/ml DNase and Complete™ EDTA-free protease inhibitors (Roche). Insoluble material was pelleted at 11000 g for 1 h at 4 °C and the supernatant was incubated with 4 ml of pre-equilibrated Ni-NTA (Ni2+-nitrilotriacetate) bead slurry (Qiagen) for 1 h at 4 °C. Beads were washed and protein was subsequently eluted in the above buffer containing 40 and 500 mM imidazole respectively. Imidazole was removed via dialysis against native buffer and protein was concentrated in molecular-mass cutoff 30 kDa VivaSpin® concentrators (Millipore). The final protein concentration was determined via a dye binding assay (Bio-Rad) and was typically approx. 1 mg/ml.
GST (glutathione S-transferase)-tagged mouse Imp proteins [32] and GFP [33] and β-gal (Tag NLS-β-gal [34])-tagged SV40 Tag NLS fusion proteins were expressed, purified and labelled (where required) as previously described.
Labelling of IN protein
IN protein was fluorescently labelled with the fluorescent dye DTAF {5-[4,6-dichlorotriazinyl]aminofluorescein; Molecular Probes}. IN protein (500 μg) was incubated with 0.4 mg/ml DTAF dissolved in 250 mM bicine [N,N-bis(2-hydroxyethyl)-glycine] buffer (pH 9.5) for 90 min at room temperature (25 °C). Excess dye was removed via a PD-10 buffer exchange column (Amersham) and dialysis against native buffer. For use in in vitro experiments, the salt concentration was reduced by diluting the labelled protein 1:15 in native buffer containing 150 mM NaCl and re-concentrating the protein as above.
In vitro nuclear transport assay
Nuclear import of fluorescently labelled IN was investigated in vitro using mechanically perforated HTC cells as previously described [35]. Briefly, perforation was used to remove the plasma membrane of cells, but leave the nuclear membrane intact, and the perforated cells were then inverted on to a microscope slide over a chamber of artificial ‘cytoplasm’ containing reticulocyte lysate, an ATP regenerating system (0.125 mg/ml creatine kinase, 30 mM creatine phosphate and 2 mM ATP), 70 kDa Texas Red-conjugated dextran (to assess nuclear integrity), 2 μM DTAF-labelled IN protein and IB buffer (110 mM KCl, 5 mM NaHCO3, 5 mM MgCl2, 1 mM EGTA, 0.1 mM CaCl2, 20 mM Hepes and 1 mM dithiothreitol, pH 7.4) in a final volume of 5 μl. The involvement of individual Imps in IN nuclear import was determined by pre-incubating the reticulocyte lysate for 15 min at room temperature with inhibitory monoclonal antibodies to Imps α1/Rch1 or β1 (BD Biosciences) at 45 μg/ml. The requirement for ATP was tested by pretreatment with apyrase to remove ATP from both the reticulocyte lysate (800 units/ml for 10 min at room temperature) and the unperforated HTC cells (0.2 unit/ml for 15 min at 37 °C) and omitting ATP regenerator from the sample. In some experiments, 5 μM Ran preloaded with the non-hydrolysable GTP analogue, GTP[S], was pre-incubated with reticulocyte lysate for 10 min at room temperature. Where required, 0.025% CHAPS was added to estimate the extent to which IN bound to nuclear components. To assess the ability of IN, both WT (wild-type) and the NLS mutant, to import DNA into the nucleus, a plasmid containing the HIV-1 cDNA (pNL4-3; NIH AIDS Research and Reference Reagent Program no. 114) was fluorescently labelled with YOYO dye (Molecular Probes) according to the manufacturer's instructions and pre-incubated with unlabelled IN protein for 15 min at room temperature prior to use in the transport assay.
ALPHAScreen assay
The interaction of IN with Imp proteins was determined using an established ALPHAScreen assay (PerkinElmer) [36]. His6-tagged IN (60 nM) was bound to Ni2+ chelate acceptor beads and incubated with increasing concentrations of biotinylated GST-tagged Imps (or GST alone) bound to streptavidin-coated donor beads. To detect binding to the Imp α/β complex, biotinylated Imp α was first predimerized to non-biotinylated Imp β at 13.6 μM for 15 min at room temperature in IB. Binding interactions were then detected using a FUSIONα (PerkinElmer) plate reader. To assess the ability of IN to interact with Imps when complexed with DNA, IN protein was pre-incubated with plasmid DNA at a molar ratio of 1:0.005 at room temperature for 15 min prior to the addition of the acceptor beads.
DNA gel-shift assay
The interaction of IN (both WT and mutant) with DNA was assessed via a DNA-binding gel-shift assay. Plasmid DNA (500 ng) was pre-incubated with various concentrations of unlabelled IN protein for 10 min at room temperature. Complexes were resolved on a 0.8% agarose gel run at 4 °C and DNA was visualized via ethidium bromide staining.
RESULTS
IN interacts strongly with Imps
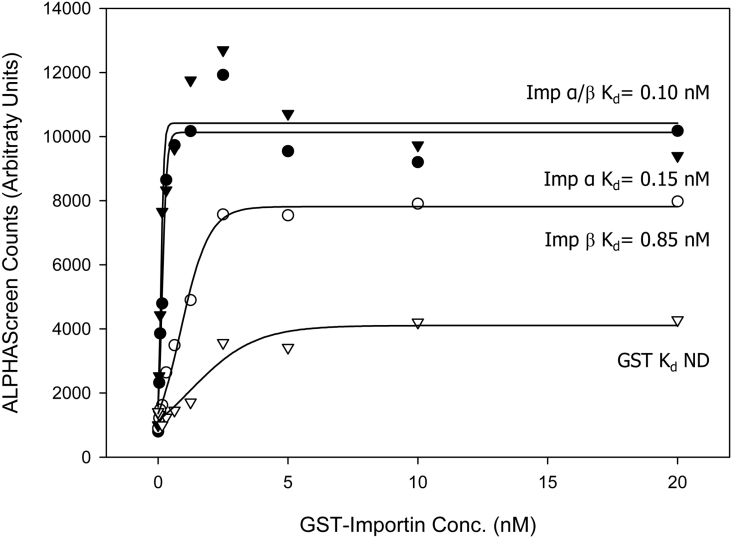
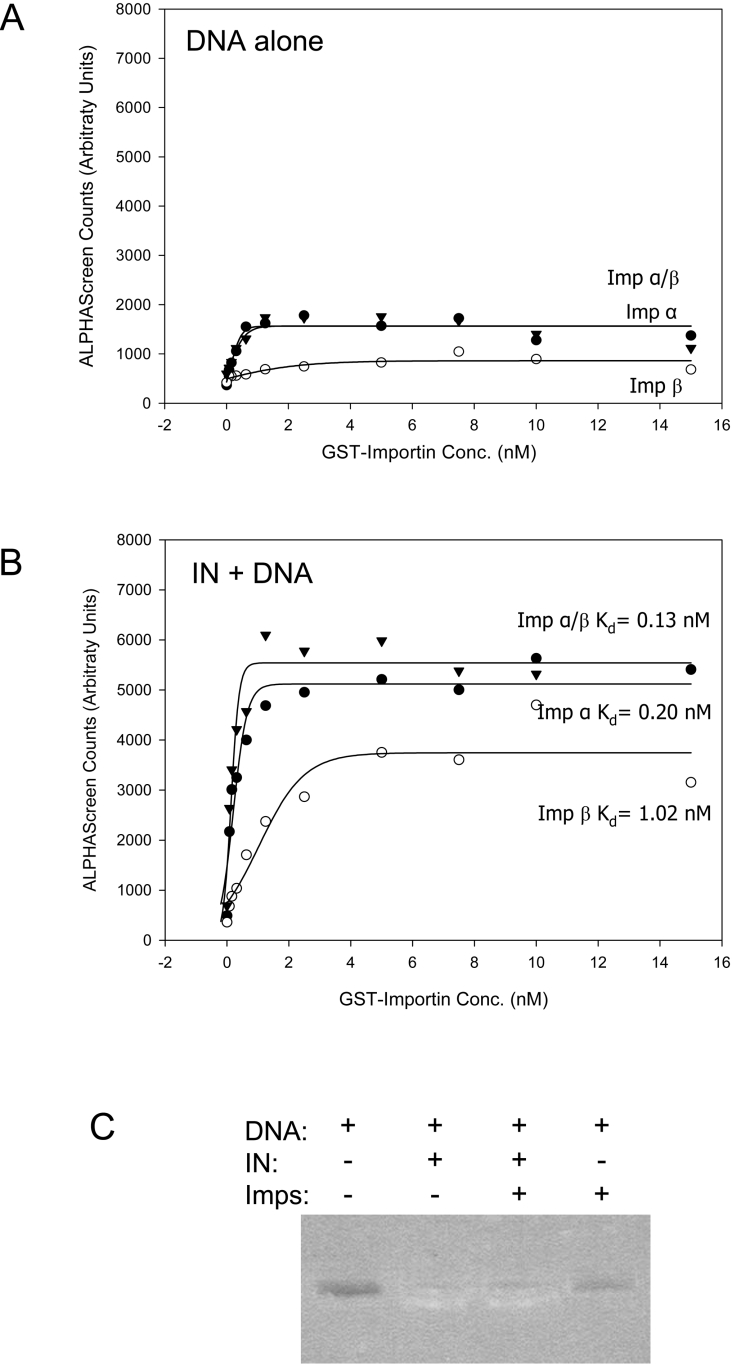
As a first step in assessing the nuclear import potential of IN, we tested its ability to interact with Imps using an ALPHAScreen assay, which has been used previously for Imp-interacting proteins such as SV40 Tag [36]. Figure 1 shows that His–IN exhibits a strong interaction with both Imp α and Imp β proteins alone as well as with the Imp α/β heterodimer with average Kd values of 0.20, 1.44 and 0.18 nM respectively (see Table 1). IN binds both Imp α and the Imp α/β heterodimer with the highest affinity, consistent with the concept that IN interacts with the Imp α portion of the Imp α/β heterodimer and is likely to be imported into the nucleus via the Imp α/β-dependent pathway.
Figure 1. IN interacts with Imps with high affinity as determined using an ALPHAScreen assay.
His6–IN was incubated with increasing concentrations of biotinylated GST–mouse Imp α2 (Rch1), GST–mouse Imp β1, predimerized GST–Imp α/β or GST alone and an ALPHAScreen assay was performed to determine the binding affinity as described in the Methods section. Sigmoidal curves were fitted using the SigmaPlot software to determine the apparent dissociation constants (Kd) as indicated. Each point represents the average of triplicate results from a single representative experiment. ND, not determined.
Table 1. Average Imp binding affinities (Kd).
The Kd values were determined by ALPHAScreen assay (see the Methods section). Kd values shown are the means±S.E.M. for four individual experiments.
| Kd (nM) | |||
|---|---|---|---|
| Imp α | Imp β | Imp α/β | |
| IN | 0.20±0.03 | 1.44±0.36 | 0.18±0.01 |
| IN+DNA | 0.28±0.10 | 2.08±0.40 | 0.23±0.06 |
IN exhibits rapid nuclear accumulation and binds to nuclear components
The nuclear import mechanism of IN was characterized in vitro using a mechanically perforated HTC cell system where CLSM (confocal laser scanning microscopy) images were taken of unfixed cells and the nuclear accumulation of fluorescent protein was monitored over time. By reconstituting nuclear import in vitro, the dependence on factors conventionally required for nuclear import, such as cytosolic components and an ATP regenerating system, can be tested. Such a system is preferable to other such in vitro assays as it enables a quantitative analysis of the kinetics of protein import, rather than a simple qualitative, end-point result provided by similar assays [37].
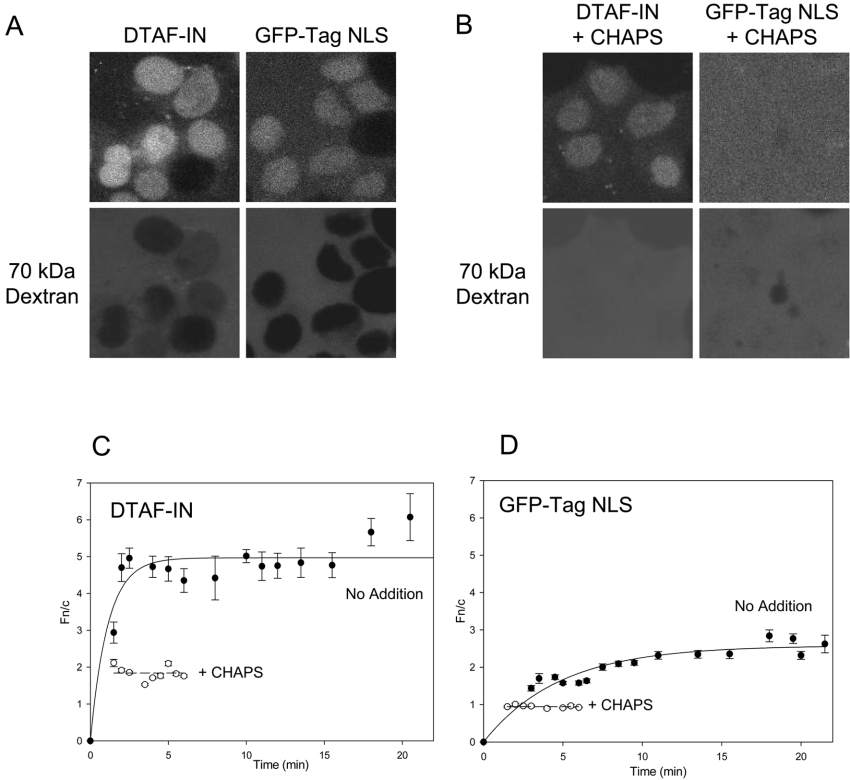
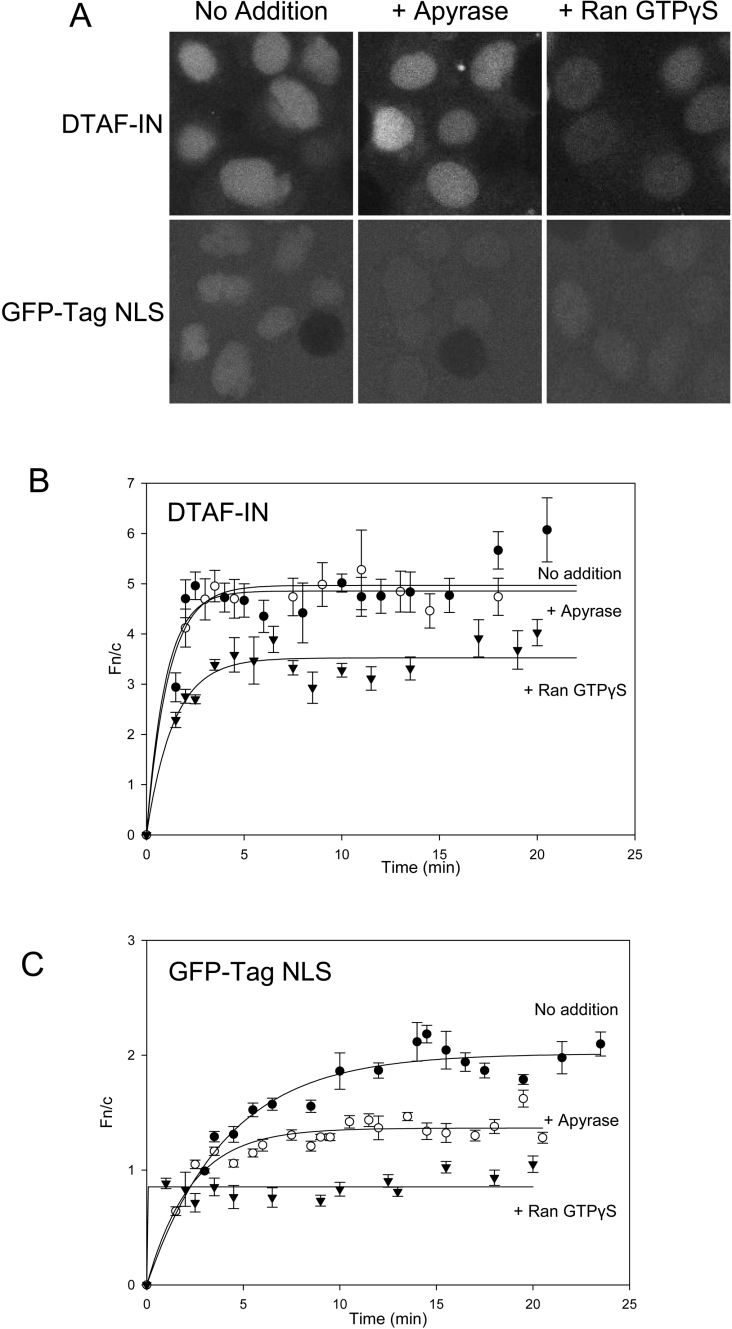
Fluorescently labelled IN (DTAF–IN) exhibited rapid nuclear accumulation when added to the in vitro transport assay, reaching a maximal level of nuclear fluorescence four to five times that of the cytoplasm (Figure 2). The extent of DTAF–IN nuclear import was actually much greater than that observed for the GFP–Tag NLS protein (Figure 2; compare Figures 2C and 2D), used as a positive control in these experiments, indicating that IN is imported into the nucleus via a highly efficient mechanism.
Figure 2. DTAF–IN exhibits nuclear accumulation in an in vitro assay.
Nuclear import of DTAF-labelled IN (DTAF–IN) was reconstituted in vitro in mechanically perforated HTC cells in the presence of exogenous cytosol and an ATP regeneration system as described in the Methods section. (A) CLSM images were acquired periodically for accumulation of DTAF–IN (upper left panel) and the control protein GFP–Tag NLS (upper right panel) into intact nuclei. Nuclear integrity was confirmed by the exclusion of a Texas Red-labelled 70 kDa dextran (lower panels). (B) CLSM images of accumulation of DTAF–IN (upper left panel) and GFP–Tag NLS (upper right panel) in the absence of an intact nuclear membrane (indicated by the lack of exclusion of a 70 kDa dextran; lower panels), induced by the addition of 0.025% CHAPS. (C, D) Image analysis was performed on the CLSM images such as those in (A, B) using ImageJ (NIH) software. All values used were contained within the linear fluorescence range. The nuclear to cytoplasmic fluorescence ratio (Fn/c) was calculated using the equation Fn/c=(Fn−Fb)/(Fc−Fb), where Fn, Fb and Fc represent the nuclear, background and cytoplasmic fluorescence values respectively. Nuclear import kinetics were plotted using SigmaPlot software and exponential curves were fitted for DTAF–IN (C) and GFP–Tag NLS (D) in the absence (solid lines) and presence (dashed lines) of CHAPS as indicated. Each point represents the mean±S.E.M. Note that in the absence of an intact nuclear membrane, steady state is reached within minutes. Therefore the data for CHAPS-treated samples are only presented for the first 5–6 min.
IN possesses inherent DNA binding ability [38] which, combined with its small size (32 kDa), may allow nuclear accumulation to occur via passive diffusion and binding to DNA or other nuclear components. To estimate the extent to which this contributes to the overall level of accumulation, the nuclear membrane was permeabilized with CHAPS and the degree of nuclear accumulation was assessed (Figure 2B). For proteins such as GFP–Tag NLS which do not exhibit binding to nuclear components, the absence of an intact nuclear envelope results in an even distribution of the protein between the nucleus and the cytoplasm, as indicated by an Fn/c (nuclear to cytoplasmic fluorescence ratio) of approx. 1 (Figure 2D). In contrast, DTAF–IN was found to accumulate in the nucleus of CHAPS-treated cells to an Fn/c value of approx. 2 (Figure 2C), which can be attributed to the binding of IN to nuclear components and indicates the basal level of nuclear accumulation displayed by IN in this system. The maximum accumulation in the absence of CHAPS (Figure 2C; Fn/c=5) was much greater than in its presence, suggesting that the binding of IN to nuclear components makes a relatively minor contribution to nuclear accumulation. Although we cannot exclude the possibility that CHAPS may disrupt nuclear-bound proteins which may help to anchor IN within the nucleus, we believe the most likely explanation for the observed differences in accumulation is that facilitated import of IN occurs in the presence of an intact nuclear envelope.
IN nuclear import involves Imp α and Imp β
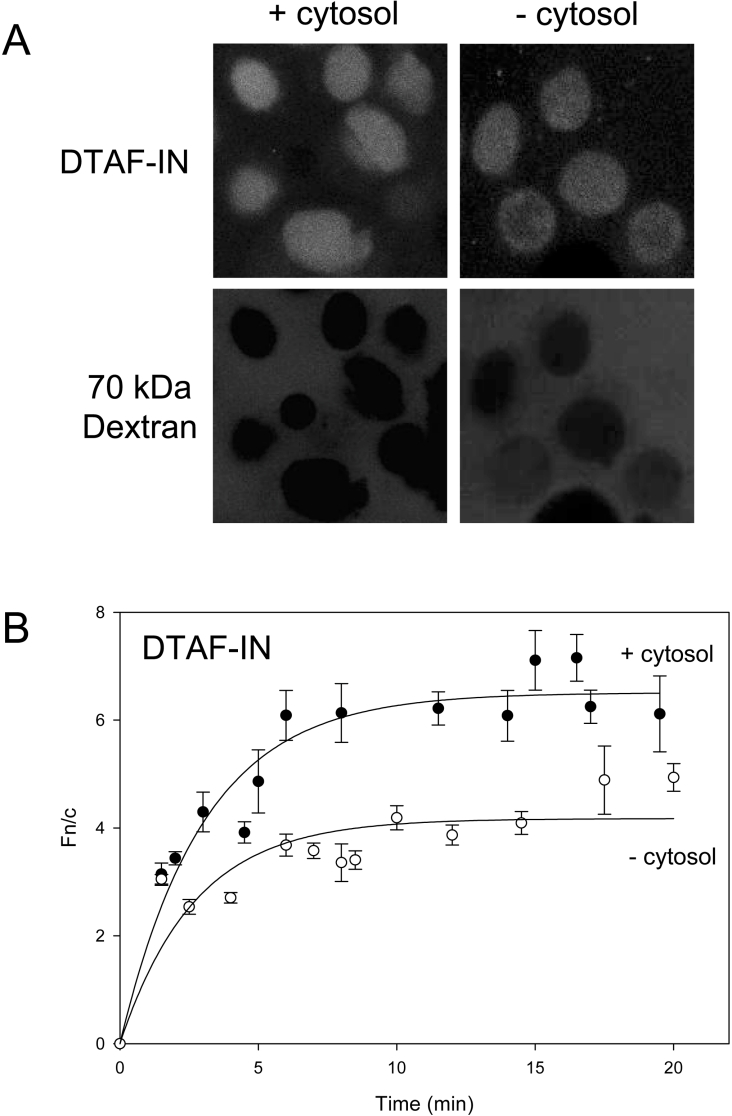
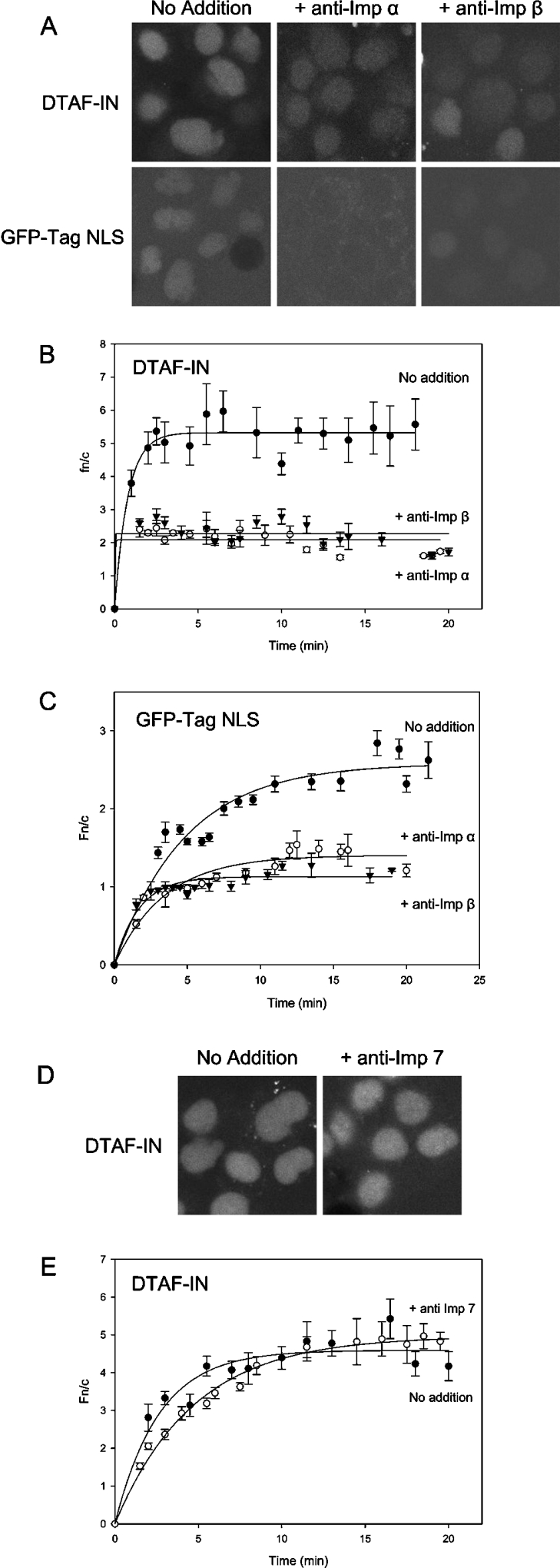
To investigate the dependence of IN nuclear import on cytosolic factors, the assay was carried out in the absence of exogenous cytosol. This was found to significantly decrease the level of nuclear accumulation (Figure 3), indicating that cytosolic components are required for maximal accumulation of IN. The slight degree of facilitated import observed (Fn/c=3 versus Fn/c=2 for CHAPS-treated cells) is attributable to residual endogenous cytosolic factors contained within the perforated cells themselves. To identify which cytosolic factor(s) may be required for IN import, antibodies capable of binding and inhibiting the action of specific Imps were added to the reaction. Antibodies to both Imp α and Imp β dramatically reduced the level of accumulation of both GFP–Tag NLS and DTAF–IN (Figure 4). The Fn/c values for both proteins in the presence of Imp α and β antibodies were similar to those observed in the CHAPS-treated experiments, indicating that facilitated import was almost completely inhibited. The addition of antibodies to Imp 7 did not affect the nuclear accumulation of IN (Figure 4), indicating a lack of requirement for Imp 7 in IN import and verifying the specificity of action of the Imp antibodies. These results indicate that IN nuclear import involves both Imp α and Imp β, most probably in the form of the Imp α/β heterodimer, consistent with the results obtained in the Imp binding assay (see Figure 1).
Figure 3. Nuclear accumulation of IN requires cytosolic factors.
DTAF–IN nuclear import was reconstituted in an in vitro system as per Figure 2 in the presence or absence of exogenous cytosol. (A) CLSM images of DTAF–IN nuclear accumulation in the presence (left panel) or absence (right panel) of exogenous cytosol. (B) Images such as those in (A) were analysed and nuclear import kinetics were determined for DTAF–IN in the presence or absence of exogenous cytosol as per the legend to Figure 2.
Figure 4. Nuclear import of DTAF–IN involves Imp α and Imp β.
Nuclear import of DTAF–IN and GFP–Tag NLS was reconstituted in vitro as per Figure 2 in the presence of antibodies to Imp α, Imp β or Imp 7 at 45 μg/ml. (A) Typical CLSM images of DTAF–IN (upper panels) and GFP–Tag NLS (lower panels) in the absence (left panel) or presence of antibodies to Imp α (middle panel) or Imp β (right panel). (B, C) Nuclear import kinetics of DTAF–IN (B) and GFP–Tag NLS (C) in the absence or presence of antibodies to Imp α or Imp β were determined as described in the legend to Figure 2. (D, E) Typical CLSM images (D) and nuclear import kinetics (E) of DTAF–IN in the presence of antibodies to Imp 7 as described in the legend to Figure 2.
IN nuclear import does not require ATP but can be inhibited by Ran GTP[S]
Previous in vitro characterization of IN nuclear import suggested that ATP was required for this process [23]. To assess the dependence of IN nuclear import on ATP in our system, the ATP-regenerating mixture was omitted from the assay and both the cells and the exogenous cytosol were pretreated with apyrase to remove all traces of ATP. The depletion of the reaction mixture of ATP inhibited the accumulation of GFP–Tag NLS (Figures 5A and 5C), as previously described [39], due to the fact that phosphorylation of regions proximal to the Tag NLS is required to achieve maximal accumulation [34]. In contrast, ATP depletion had no discernible effect on either the maximal level of DTAF–IN accumulation or the rate of nuclear import (Figures 5A and 5B), thus indicating that ATP is not essential for IN nuclear import.
Figure 5. Nuclear import of DTAF–IN does not require ATP but is dependent on Ran GTP.
Nuclear import of DTAF–IN and GFP–Tag NLS was reconstituted in vitro, as described in the legend to Figure 2, following either pretreatment of both the exogenous cytosol and unperforated cells with apyrase (800 units/ml and 0.2 unit/ml respectively) to remove ATP from the system or pre-incubation of exogenous cytosol with 5 μM Ran conjugated with the non-hydrolysable GTP analogue GTP[S] (GTPγS). Accumulation of DTAF–IN and GFP–Tag NLS in untreated cells was also analysed for comparison. (A) Representative CLSM images of DTAF–IN (upper panel) and GFP–Tag NLS (lower panel) nuclear import in untreated cells (left panel) or following pretreatment with apyrase (middle panel) or Ran GTP[S] (right panel). (B, C) CLSM images such as those in (A) were analysed and import kinetics were determined as described in the legend to Figure 2 for the nuclear import of DTAF–IN (B) and GFP–Tag NLS (C) in untreated cells or following treatment with apyrase or Ran GTP[S] as indicated.
The Ran GTP/Ran GDP gradient which exists across the nuclear membrane provides directionality to nuclear transport (for a review, see [40]). To confirm that nuclear accumulation of IN is dependent on a low cytosolic concentration of Ran GTP, import was analysed in the presence of Ran conjugated with the non-hydrolysable GTP analogue GTP[S], which locks Imp β in the Ran GTP bound form and thus prevents its interaction with import cargo. Figure 5 shows that in a similar fashion to GFP–Tag NLS, DTAF–IN nuclear import was significantly inhibited by Ran GTP[S], consistent with an Imp-dependent nuclear import mechanism for IN. In contrast, addition of Ran conjugated with GDP, which does not affect the Imp β–cargo interaction, did not significantly affect IN nuclear accumulation (results not shown).
IN retains the ability to interact with Imps when complexed with DNA
The results presented here reveal IN to be a potent nucleophile. This, combined with the known ability of IN to bind DNA, implies that IN may be able to mediate nuclear import of DNA. To determine whether IN retained the ability to interact with Imps when bound to DNA, an ALPHAScreen assay was again employed to assess the Imp-binding potential of an IN–DNA complex. DNA alone exhibits minimal interaction with Imps (Figure 6A), whereas IN retained a strong interaction with Imps when bound to DNA (Figure 6B), indicating that the DNA and Imp binding regions of IN are discrete. The addition of DNA resulted in only a very minimal reduction in the affinity of the IN–Imp interaction, as indicated by the average Kd values of 0.28, 2.08 and 0.23 nM for Imp α, β and a/β respectively (Table 1), confirming the ability of IN to interact efficiently with DNA and Imps simultaneously.
Figure 6. IN retains the ability to interact with Imps when complexed with DNA.
An ALPHAScreen assay was used to determine the binding affinity of plasmid DNA (A) or His–IN prebound to plasmid DNA (B) with GST–Imp α, GST–Imp β and GST–Imp α/β as per Figure 1. Each point represents the average of triplicate results from a single representative experiment with Kd values as indicated. (C) To confirm that the DNA remained complexed to IN throughout the experiment, a number of control reactions were performed with and without IN, DNA and Imps as indicated, the reactions were run on a 0.8% agarose gel and the DNA was visualized. In the presence of IN (lanes 2 and 3), DNA is attached to the bead network (which prevents it from entering the gel) and DNA is retained in the wells. In the absence of IN (lanes 1 and 4), the DNA does not associate with the beads and is thus absent from the wells.
IN can mediate the import of HIV-1 cDNA into the nucleus via an Imp α/β-dependent process that involves the K186RK region of IN
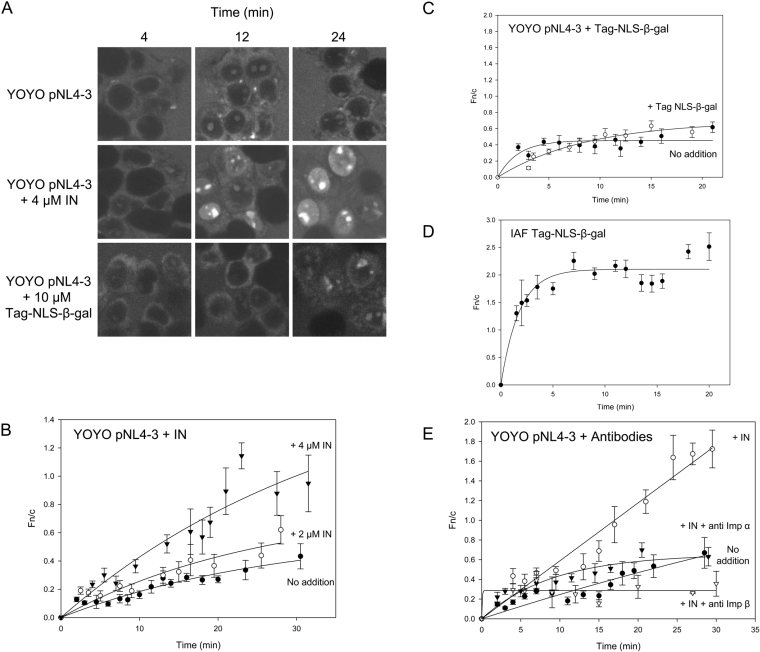
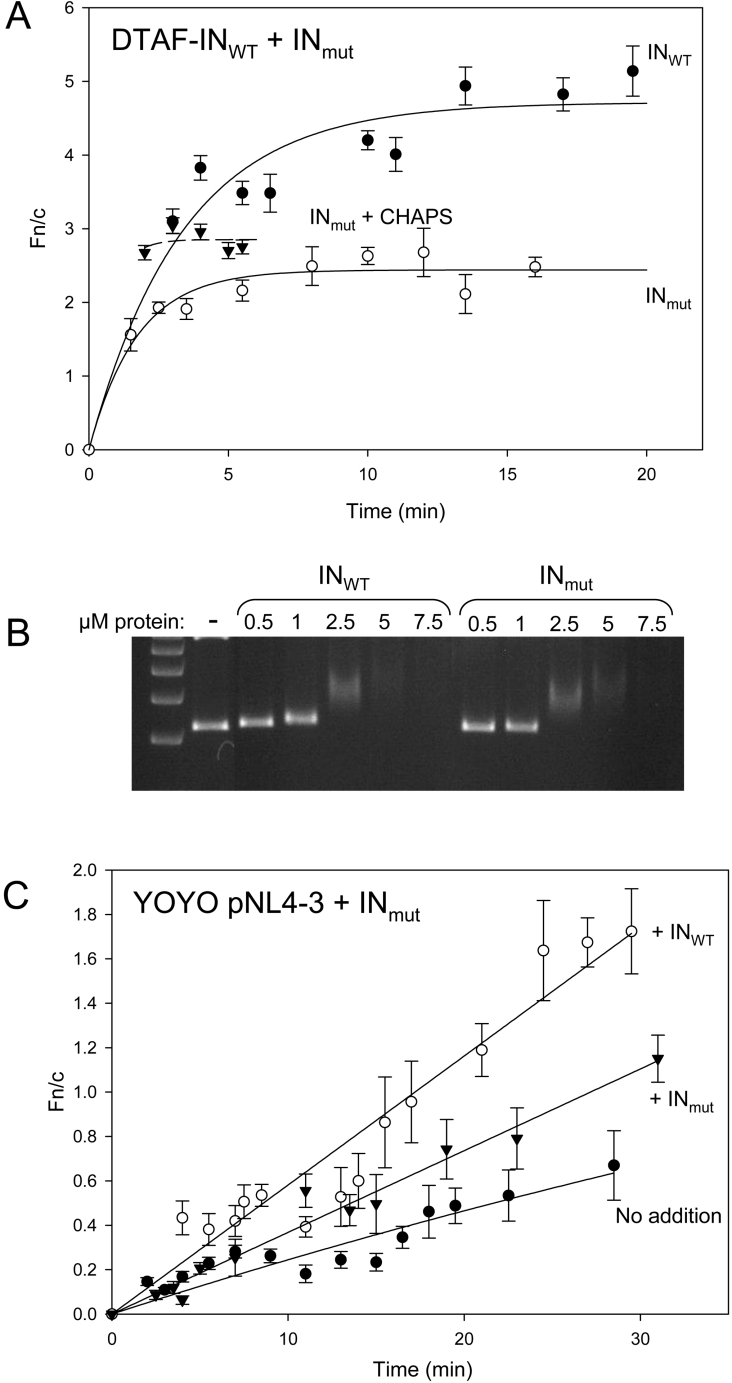
As a first step in assessing the ability of IN to import DNA into the nucleus in vitro, a plasmid containing the HIV-1 cDNA (pNL4-3) was fluorescently labelled with the high-affinity intercalating dye YOYO and its nuclear import was assessed in an in vitro nuclear transport system. Following the addition of labelled DNA, fluorescence was often observed in the nucleolus of intact nuclei (Figure 7A), attributable to the diffusion of unbound YOYO dye molecules into the nucleus and binding to a nucleolar component. Consistent with previous reports [41], YOYO-labelled DNA alone exhibited a small degree of nuclear accumulation (Figure 7B). However, prebinding of the pNL4-3 DNA to increasing concentrations of unlabelled IN was found to increase the rate of import of the DNA into the nucleoplasm in a dose-dependent manner (Figure 7B). A similar enhancement of DNA nuclear delivery by IN was observed when an unrelated DNA plasmid was labelled as above and assayed under similar conditions (results not shown), implying that this effect is not limited to HIV-specific DNA. In contrast, the addition of an excess amount (10 μM) of unlabelled Tag NLS-β-gal protein, which is a potent nuclear targeting protein (Figure 7D), had no effect on the uptake of DNA (Figure 7C), confirming that this enhancement is specific to IN. Similarly, the addition of antibodies to both Imp α and Imp β abolished this effect (Figure 7E), confirming that the enhanced uptake occurs via an Imp α/β-dependent mechanism. Finally, we validated this result by assessing the ability of an IN NLS mutant to import DNA into the nucleus. We created an IN construct containing mutations within the basic K186RK region (previously implicated in IN nuclear import [13,28]) and found it to be specifically impaired in nuclear import (Figure 8A; maximum Fn/c is lower than that for INWT and does not exceed the level of accumulation observed in the presence of CHAPS), while its ability to bind DNA was unaffected (Figure 8B). As expected, the IN NLS mutant showed a reduced ability to import DNA into the nucleus as compared with INWT (Figure 8C). These results indicate the potential of IN to mediate nuclear import of not only HIV cDNA, but DNA in general.
Figure 7. IN is capable of mediating nuclear import of YOYO-labelled DNA in vitro.
The HIV-1 cDNA containing plasmid pNL4-3 was labelled with YOYO as described in the Methods section and nuclear accumulation of the DNA was determined in the absence and presence of unlabelled IN or Tag NLS-β-gal using an in vitro nuclear transport assay (as per Figure 2). (A) CLSM was used to visualize the nuclear import of YOYO-labelled pNL4-3 at the indicated time points either alone (upper panel) or following pre-incubation with 2 μM (results not shown) or 4 μM (middle panel) unlabelled IN protein or 10 μM unlabelled Tag-β-gal protein (lower panel). (B, C) Images such as those in (A) were subjected to image analysis and import kinetics were determined as per Figure 2 for DNA alone or in the presence of 2 or 4 μM unlabelled IN protein (B) or 10 μM Tag-β-gal protein (C) as indicated. (D) The nuclear import of 4 μM 5-iodoacetamidofluorescein (IAF)-labelled Tag-β-gal protein was visualized in vitro and import kinetics were determined as per Figure 2. (E) The nuclear accumulation of YOYO-labelled pNL4-3 DNA was analysed in the presence of 4 μM unlabelled IN protein in the absence or presence of antibodies to Imp α or Imp β as indicated and import kinetics were determined as per Figure 2.
Figure 8. IN K186RK to AAA mutant is defective for nuclear import in vitro, retains its DNA binding ability and shows a reduced ability to import YOYO-labelled DNA into the nucleus.
A K186RK to AAA mutation was introduced into pINSD.His.Sol and mutant (INmut) protein was expressed, purified and DTAF-labelled as described in the Methods section. The nuclear import of INmut was reconstituted in vitro as described in Figure 2. (A) Nuclear import kinetics of DTAF–INmut protein in the absence (solid line) or presence (dashed line) of 0.025% CHAPS as indicated. The nuclear import kinetics of DTAF–INWT is included for comparison. (B) The ability of INWT and INmut to bind DNA was assessed using a gel shift assay as described in the Methods section. Plasmid DNA (500 ng) was pre-incubated with the indicated concentrations of either INWT or INmut and complexes were resolved on an agarose gel. Binding interactions are indicated by the upward shift, and eventual complete retardation, of the migrating DNA band. DNA did not shift in the presence of non-binding proteins such as GST and GFP (results not shown). (C) The nuclear accumulation of YOYO-labelled pNL4-3 DNA was analysed in vitro as described in Figure 7 in the absence or presence of 4 μM unlabelled INWT or INmut as indicated and import kinetics were determined.
DISCUSSION
Although the potential of IN as a karyophilic protein has been debated for some time, there has been little information regarding the nuclear import mechanism of IN. The nuclear targeting ability of IN also appears to be influenced by the type and location of fusion proteins [21,22,30,42]. We therefore chose to use purified, unconjugated IN protein to circumvent these issues and utilized specific nuclear import and Imp binding assays to avoid the complications associated with in vivo analyses.
Binding experiments using His–IN protein indicate the potent ability of IN to bind to the classical mediators of nuclear import, Imp α and β. Our observation that IN is recognized directly by Imp α as well as the Imp α/β heterodimer is consistent with that of others [13], but the recognition of IN by Imp β alone is a novel finding. Whereas Imp β is capable of mediating protein nuclear import alone [43], the lack of accumulation observed in the presence of antibodies to Imp α indicates that IN import requires both Imp α and Imp β, almost certainly in the form of the Imp α/β heterodimer. Imp βs have been reported to play a chaperone-like role for basic, DNA/RNA binding proteins, including a number of ribosomal proteins [44], and could be serving such a role here to shield the highly basic DNA-binding domains of IN.
The results obtained from the in vitro transport assay indicate that IN is imported into the nucleus by the Imp α/β heterodimer in a process that involves the Ran GTP cycle but does not require ATP. Our results indicate a fundamental requirement for Imp α and β in IN nuclear import, but we observed no significant reduction in accumulation following the addition of antibodies to Imp 7. Although others have shown using isolated nuclei that the Imp 7–Imp β heterodimer is capable of mediating IN nuclear import [25], our results suggest that this is not the primary pathway for IN import and, in the context of a full complement of import factors, Imp α/β-mediated import predominates.
The lack of requirement for ATP observed in our system for IN, although in contrast with that seen by others [23,24], is attributable to the fact that translocation of the complex through the NPC is a passive process [45]. The requirement of ATP observed by others may be attributable to the method of ATP depletion used, as common ATP inhibitors are also known to reduce levels of free GTP [46], which prevents the recycling of Imp receptors required for sustained nuclear import (see [47]). Indeed, our results indicate that perturbing the nuclear to cytoplasmic ratio of Ran GTP, through the use of the non-hydrolysable Ran GTP[S], inhibits IN nuclear import.
Having established that IN is capable of rapid and efficient nuclear import, we used our in vitro system to assess whether the import signals contained within IN remained functional when bound to DNA and if these signals were sufficient to mediate nuclear import of an IN–DNA complex. The fact that IN retained its high-affinity interaction with Imps when complexed with DNA supported the potential for IN to facilitate DNA nuclear import within the cell. Indeed, the results obtained from the in vitro transport assay show that pre-incubation of HIV-1 DNA with unlabelled IN protein resulted in an increased nuclear accumulation of DNA. The inability of Tag NLS-β-gal to mediate the import of DNA confirms that the enhanced uptake observed is not a non-specific phenomenon, while the observation that antibodies to Imp α and Imp β abolished this effect confirms that the DNA is imported via an Imp α/β-mediated, and thus IN-specific, mechanism. Interestingly, this enhancement of DNA import was not specific for HIV-1 DNA, as IN was also able to facilitate import of an unrelated DNA plasmid. Although this approach requires optimization, these results suggest that IN is capable of binding DNA and mediating its entry into the nucleus.
As mentioned above, the precise regions of IN involved in nuclear import remain controversial and no specific NLS has been defined. However, there is evidence to suggest that lysine-rich regions within IN, including amino acids 186–188, may be involved in nuclear import [13,28]. We therefore chose to mutate one of these lysine-rich regions, K186RK, to create an NLS mutant (INmut). In vitro analysis showed that this mutant is indeed partially defective for nuclear import but retains full DNA binding potential. In the present study, INmut showed a reduced ability to import DNA into the nucleus, confirming the specificity of the enhancement observed with INWT.
The regions of IN required for integration activity are well characterized, and the ability of IN mutants with defects in non-integration-related functions to complement for integration-defective IN mutants in trans (see [16]) implies that the integrative and non-integrative roles of IN are discrete. Therefore an IN mutant defective for integration, but which retained full DNA binding and nuclear import potential, appears highly plausible and would represent an ideal mediator for the nuclear delivery of therapeutic DNA within gene therapy applications, overcoming the problem of insertional mutagenesis associated with current retroviral-based methods.
Given the potent nucleophilic and DNA-importing abilities demonstrated here, it seems possible that IN may act as a key mediator of cDNA nuclear import and integration during HIV-1 infection. Our study indicates that IN can perform such a function in vitro and, although the in vivo situation is likely to be significantly more complicated, our results imply a potential for IN to play a key role in nuclear import of the HIV-1 cDNA. Such a theory does not, of course, preclude a role for other viral components such as matrix and Vpr, which may have a cumulative affect on cDNA nuclear import or may play a role prior to import, such as aiding the passage of the PIC through the cytoplasm. The possibility that IN plays such a fundamental role in cDNA nuclear import and subsequent infection of non-dividing cells will be valuable knowledge in identifying new and innovative drug targets to halt the progression of HIV/AIDS and may also prove useful for the development of effective DNA delivery methods for therapeutic gene therapy.
Acknowledgments
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pINSD.His.Sol was from Dr Robert Craigie and pNL4-3 was from Dr Malcolm Martin. We acknowledge the support of the National Health and Research Council, Australia (fellowship no. 143790/no. 333013 and project grant no. 143710/no. 22274).
References
- 1.Weinberg J. B., Matthews T. J., Cullen B. R., Malim M. H. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 1991;174:1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quimby B. B., Corbett A. H. Nuclear transport mechanisms. Cell. Mol. Life Sci. 2001;58:1766–1773. doi: 10.1007/PL00000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pemberton L. F., Paschal B. M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky M. I., Sharova N., McDonald T. L., Pushkarskaya T., Tarpley W. G., Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassati A., Goff S. P. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J. Virol. 2001;75:3626–3635. doi: 10.1128/JVI.75.8.3626-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zennou V., Petit C., Guetard D., Nerhbass U., Montagnier L., Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 7.Dvorin J. D., Bell P., Maul G. G., Yamashita M., Emerman M., Malim M. H. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 2002;76:12087–12096. doi: 10.1128/JVI.76.23.12087-12096.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piller S. C., Caly L., Jans D. A. Nuclear import of the pre-integration complex (PIC): the Achilles heel of HIV? Curr. Drug Targets. 2003;4:409–429. doi: 10.2174/1389450033490984. [DOI] [PubMed] [Google Scholar]
- 9.Bukrinsky M. I., Haggerty S., Dempsey M. P., Sharova N., Adzhubel A., Spitz L., Lewis P., Goldfarb D., Emerman M., Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells [see comment] Nature (London) 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Schwedler U., Kornbluth R. S., Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Rouzic E., Mousnier A., Rustum C., Stutz F., Hallberg E., Dargemont C., Benichou S. Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1. J. Biol. Chem. 2002;277:45091–45098. doi: 10.1074/jbc.M207439200. [DOI] [PubMed] [Google Scholar]
- 12.Freed E. O., Martin M. A. HIV-1 infection of non-dividing cells. Nature (London) 1994;369:107–108. doi: 10.1038/369107b0. [DOI] [PubMed] [Google Scholar]
- 13.Gallay P., Hope T., Chin D., Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouchier R. A., Meyer B. E., Simon J. H., Fischer U., Malim M. H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrake M. D., Skalka A. M. Retroviral integrase, putting the pieces together. J. Biol. Chem. 1996;271:19633–19636. doi: 10.1074/jbc.271.33.19633. [DOI] [PubMed] [Google Scholar]
- 16.Esposito D., Craigie R. HIV integrase structure and function. Adv. Virus Res. 1999;52:319–333. doi: 10.1016/s0065-3527(08)60304-8. [DOI] [PubMed] [Google Scholar]
- 17.Lu R., Limon A., Devroe E., Silver P. A., Cherepanov P., Engelman A. Class II integrase mutants with changes in putative nuclear localization signals are primarily blocked at a postnuclear entry step of human immunodeficiency virus type 1 replication. J. Virol. 2004;78:12735–12746. doi: 10.1128/JVI.78.23.12735-12746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu K., Dobard C., Chow S. A. Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase. J. Virol. 2004;78:5045–5055. doi: 10.1128/JVI.78.10.5045-5055.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelman A., Englund G., Orenstein J. M., Martin M. A., Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 1995;69:2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin C. G., Taddeo B., Haseltine W. A., Farnet C. M. Genetic analysis of the human immunodeficiency virus type 1 integrase protein. J. Virol. 1994;68:1633–1642. doi: 10.1128/jvi.68.3.1633-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kukolj G., Jones K. S., Skalka A. M. Subcellular localization of avian sarcoma virus and human immunodeficiency virus type 1 integrases. J. Virol. 1997;71:843–847. doi: 10.1128/jvi.71.1.843-847.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devroe E., Engelman A., Silver P. A. Intracellular transport of human immunodeficiency virus type 1 integrase. J. Cell Sci. 2003;116:4401–4408. doi: 10.1242/jcs.00747. [DOI] [PubMed] [Google Scholar]
- 23.Depienne C., Mousnier A., Leh H., Le Rouzic E., Dormont D., Benichou S., Dargemont C. Characterization of the nuclear import pathway for HIV-1 integrase. J. Biol. Chem. 2001;276:18102–18107. doi: 10.1074/jbc.M009029200. [DOI] [PubMed] [Google Scholar]
- 24.Armon-Omer A., Graessmann A., Loyter A. A synthetic peptide bearing the HIV-1 integrase 161–173 amino acid residues mediates active nuclear import and binding to importin alpha: characterization of a functional nuclear localization signal. J. Mol. Biol. 2004;336:1117–1128. doi: 10.1016/j.jmb.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 25.Fassati A., Gorlich D., Harrison I., Zaytseva L., Mingot J. M. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22:3675–3685. doi: 10.1093/emboj/cdg357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherepanov P., Maertens G., Proost P., Devreese B., Van Beeumen J., Engelborghs Y., De Clercq E., Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003;278:372–381. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- 27.Maertens G., Cherepanov P., Pluymers W., Busschots K., De Clercq E., Debyser Z., Engelborghs Y. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J. Biol. Chem. 2003;278:33528–33539. doi: 10.1074/jbc.M303594200. [DOI] [PubMed] [Google Scholar]
- 28.Petit C., Schwartz O., Mammano F. The karyophilic properties of human immunodeficiency virus type 1 integrase are not required for nuclear import of proviral DNA. J. Virol. 2000;74:7119–7126. doi: 10.1128/jvi.74.15.7119-7126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouyac-Bertoia M., Dvorin J. D., Fouchier R. A., Jenkins Y., Meyer B. E., Wu L. I., Emerman M., Malim M. H. HIV-1 infection requires a functional integrase NLS. Mol. Cell. 2001;7:1025–1035. doi: 10.1016/s1097-2765(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 30.Limon A., Devroe E., Lu R., Ghory H. Z., Silver P. A., Engelman A. Nuclear localization of human immunodeficiency virus type 1 preintegration complexes (PICs): V165A and R166A are pleiotropic integrase mutants primarily defective for integration, not PIC nuclear import. J. Virol. 2002;76:10598–10607. doi: 10.1128/JVI.76.21.10598-10607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins T. M., Engelman A., Ghirlando R., Craigie R. A soluble active mutant of HIV-1 integrase: involvement of both the core and carboxyl-terminal domains in multimerization. J. Biol. Chem. 1996;271:7712–7718. doi: 10.1074/jbc.271.13.7712. [DOI] [PubMed] [Google Scholar]
- 32.Hubner S., Xiao C. Y., Jans D. A. The protein kinase CK2 site (Ser111/112) enhances recognition of the simian virus 40 large T-antigen nuclear localization sequence by importin. J. Biol. Chem. 1997;272:17191–17195. doi: 10.1074/jbc.272.27.17191. [DOI] [PubMed] [Google Scholar]
- 33.Baliga B. C., Colussi P. A., Read S. H., Dias M. M., Jans D. A., Kumar S. Role of prodomain in importin-mediated nuclear localization and activation of caspase-2. J. Biol. Chem. 2003;278:4899–4905. doi: 10.1074/jbc.M211512200. [DOI] [PubMed] [Google Scholar]
- 34.Xiao C. Y., Hubner S., Elliot R. M., Caon A., Jans D. A. A consensus cAMP-dependent protein kinase (PK-A) site in place of the CcN motif casein kinase II site simian virus 40 large T-antigen confers PK-A-mediated regulation of nuclear import. J. Biol. Chem. 1996;271:6451–6457. doi: 10.1074/jbc.271.11.6451. [DOI] [PubMed] [Google Scholar]
- 35.Jans D. A., Jans P., Briggs L. J., Sutton V., Trapani J. A. Nuclear transport of granzyme B (fragmentin-2). Dependence of perforin in vivo and cytosolic factors in vitro. J. Biol. Chem. 1996;271:30781–30789. doi: 10.1074/jbc.271.48.30781. [DOI] [PubMed] [Google Scholar]
- 36.Wagstaff K. M., Jans D. A. Intramolecular masking of nuclear localization signals: analysis of importin binding using a novel AlphaScreen-based method. Anal. Biochem. 2005;348:49–56. doi: 10.1016/j.ab.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Adam S. A., Sterne-Marr R., Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]
- 38.Engelman A., Hickman A. B., Craigie R. The core and carboxyl-terminal domains of the integrase protein of human immunodeficiency virus type 1 each contribute to nonspecific DNA binding. J. Virol. 1994;68:5911–5917. doi: 10.1128/jvi.68.9.5911-5917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Efthymiadis A., Shao H., Hubner S., Jans D. A. Kinetic characterization of the human retinoblastoma protein bipartite nuclear localization sequence (NLS) in vivo and in vitro. A comparison with the SV40 large T-antigen NLS. J. Biol. Chem. 1997;272:22134–22139. doi: 10.1074/jbc.272.35.22134. [DOI] [PubMed] [Google Scholar]
- 40.Steggerda S. M., Paschal B. M. Regulation of nuclear import and export by the GTPase Ran. Int. Rev. Cytol. 2002;217:41–91. doi: 10.1016/s0074-7696(02)17012-4. [DOI] [PubMed] [Google Scholar]
- 41.Salman H., Zbaida D., Rabin Y., Chatenay D., Elbaum M. Kinetics and mechanism of DNA uptake into the cell nucleus. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7247–7252. doi: 10.1073/pnas.121067698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pluymers W., Cherepanov P., Schols D., De Clercq E., Debyser Z. Nuclear localization of human immunodeficiency virus type 1 integrase expressed as a fusion protein with green fluorescent protein. Virology. 1999;258:327–332. doi: 10.1006/viro.1999.9727. [DOI] [PubMed] [Google Scholar]
- 43.Lam M. H., Briggs L. J., Hu W., Martin T. J., Gillespie M. T., Jans D. A. Importin beta recognizes parathyroid hormone-related protein with high affinity and mediates its nuclear import in the absence of importin α. J. Biol. Chem. 1999;274:7391–7398. doi: 10.1074/jbc.274.11.7391. [DOI] [PubMed] [Google Scholar]
- 44.Jakel S., Mingot J. M., Schwarzmaier P., Hartmann E., Gorlich D. Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. EMBO J. 2002;21:377–386. doi: 10.1093/emboj/21.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribbeck K., Kutay U., Paraskeva E., Gorlich D. The translocation of transportin-cargo complexes through nuclear pores is independent of both Ran and energy. Curr. Biol. 1999;9:47–50. doi: 10.1016/s0960-9822(99)80046-3. [DOI] [PubMed] [Google Scholar]
- 46.Schwoebel E. D., Ho T. H., Moore M. S. The mechanism of inhibition of Ran-dependent nuclear transport by cellular ATP depletion. J. Cell Biol. 2002;157:963–974. doi: 10.1083/jcb.200111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuersten S., Ohno M., Mattaj I. W. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 2001;11:497–503. doi: 10.1016/s0962-8924(01)02144-4. [DOI] [PubMed] [Google Scholar]