Abstract
We have investigated the mechanism of COX-2 (cyclo-oxygenase 2)-dependent inhibition of apoptosis in liver, a key pathway underlying proliferative actions of COX-2 in liver cancers, cirrhosis, chronic hepatitis C infection and regeneration after partial hepatectomy. Stable expression of COX-2 in CHL (Chang liver) cells induced proliferation, with an increase in the proportion of cells in S-phase, but no other significant changes in cell-cycle distribution. This was associated with a marked inhibition of the apoptotic response to serum deprivation, an effect mimicked by treating empty-vector-transfected control cells (CHL-V cells) with prostaglandin E2 and prevented in COX-2-expressing cells (CHL-C cells) treated with selective inhibitors of COX-2. Serum-deprived CHL-V cells displayed several indicators of activation of intrinsic apoptosis: caspases 9 and 3 activated within 6 h and caspase 8 within 18 h, Bax expression was induced, cytochrome c was released to the cytosol, and PARP-1 [poly(ADP-ribose) polymerase 1] cleavage was evident in nuclei. COX-2 expression blocked these events, concomitant with reduced expression of p53 and promotion of Akt phosphorylation, the latter indicating activation of survival pathways. CHL cells were resistant to stimulation of the extrinsic pathway with anti-Fas antibody. Moreover, in vivo expression of GFP (green fluorescent protein)-labelled COX-2 in mice by hydrodynamics-based transient transfection conferred resistance to caspase 3 activation and apoptosis induced by stimulation of Fas.
Keywords: apoptosis, cyclo-oxygenase (COX), hepatocyte, hydrodynamic transfection, liver, prostaglandin
Abbreviations: AA, arachidonic acid; ALT, alanine aminotransferase; CHL, Chang liver; CHL-C cell, cyclo-oxygenase-2-expressing CHL cell; CHL-V cell, empty-vector-transfected control CHL cell; COX, cyclo-oxygenase; COXIB, selective COX-2 inhibitor; DFU, 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulfonyl)phenyl-2(5H)-furanone; DMEM, Dulbecco's modified Eagle's medium; FBS, foetal bovine serum; GFP, green fluorescent protein; HCC, hepatocellular carcinoma; IAP, inhibitor of apoptosis; NF-κB, nuclear factor κB; PARP-1, poly(ADP-ribose) polymerase 1; PG, prostaglandin; PI, propidium iodide; PI3K, phosphoinositide 3-kinase; RT, reverse transcription; Sp1, specificity protein 1; TNF, tumour necrosis factor; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; XIAP, X-linked IAP
INTRODUCTION
COX (cyclo-oxygenase) enzymes catalyse the first step in the biosynthesis of PGs (prostaglandins) and thromboxanes [1,2]. The COX-1 isoform is constitutively expressed in many tissues [3], whereas COX-2 is induced by growth factors, tumour promoters and cytokines [2,4,5]. The role of COX-2 in the production and development of neoplasic tumours is well established, especially in colon cancer [6]. Moreover, several authors have reported that increased tissue COX-2 expression and PG production may contribute to the development of other cancers, including HCC (hepatocellular carcinoma) [7]. Expression of COX-2 has been demonstrated in many contexts related to liver cell pathologies: regeneration after partial hepatectomy [8,9], animal models of cirrhosis [10], human hepatoma cell lines [11,12], chronic hepatitis C infection [7,13] and HCC [14,15].
Failure of apoptosis is an important event in tumour progression and resistance to cytotoxic therapy, and a principal mechanism of COXIBs (selective COX-2 inhibitors) is their pro-apoptotic activity. Apoptosis is mediated by activation of caspases, a family of cysteine proteases [16]. Caspase activation occurs by at least two mechanisms: the extrinsic or death receptor pathway, initiated by agonists of the TNF (tumour necrosis factor) superfamily such as TNFα, Fas ligand and Apo2 ligand/TRAIL (TNF-related apoptosis-inducing ligand) [17], and the intrinsic or mitochondrial pathway [18]. In the mitochondrial pathway, caspase activation principally occurs as a result of the release of cytochrome c from the organelle, a process closely regulated by the Bcl-2 family of proteins. Cytochrome c in the cytosol associates with Apaf-1 (apoptotic protease-activating factor 1) and ATP and pro-caspase 9 in a multiprotein complex called the apoptosome. Once activated in the apoptosome, caspase 9 in turn activates downstream executioner caspases, such as caspase 3 and caspase 7 [19]. In liver, the extrinsic and intrinsic apoptotic mechanisms are both operative. Constitutive expression of Fas is found in mouse and human liver, and this pathway appears to be very important in executing apoptosis in healthy hepatocytes and in the pathogenesis of diseases including liver injury, viral hepatitis and cirrhosis [20]. However, HCC is one of the tumours known to be resistant to Fas-mediated apoptosis, because Fas expression is down-regulated and the Fas-activated signalling pathway is altered [21].
We have used two approaches to express COX-2 in liver cells in order to elucidate the mechanisms implicated in PGE2-dependent inhibition of apoptosis. In the first, we generated liver cell lines expressing COX-2 protein by stable transfection with a vector containing the human COX-2 cDNA. In the second, we used hydrodynamics-based transient transfection to systemically administer mice with a pcDNA3hCOX-2-GFP plasmid. Our results show that both in vitro and in vivo expression of COX-2 directly inhibits apoptosis in hepatocytes through mechanisms that involve potent inhibition of caspases 3 and 9, a decrease in p53 and Bax expression, and an increase in the survival pathway through activation of Akt.
MATERIALS AND METHODS
Chemicals and reagents
Antibodies were from Santa Cruz Laboratories, BD Biosciences, R&D Systems, Cayman Chemical, Alexis and Cell Signaling Technologies. DFU [5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulfonyl)phenyl-2(5H)-furanone] was from Merck. PGs were from Calbiochem EMD Biosciences. Fluorescent probes were from Molecular Probes (Invitrogen) and Calbiochem. Tissue culture media were from BioWhittaker. Electrophoresis reagents were obtained from Bio-Rad, and other reagents were from Roche Diagnostics or Sigma Chemical Co.
Cell culture and stable transfection
The human liver cell line CCL-13 (Chang liver, CHL), an immortalized non-tumour cell line derived from normal liver, and the hepatoma cell line HepG2 were purchased from the American Type Culture Collection (A.T.C.C.). The A.T.C.C. catalogue states that the CCL-13 (Chang liver, CHL) cell line was derived from normal liver tissue but has HeLa markers. To evaluate the hepatocyte nature of the clones, we analysed the expression of the hepatocyte markers α-fetoprotein and albumin, which were highly expressed (results not shown) as described previously [22]. Cells were grown on Falcon tissue culture dishes in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FBS (foetal bovine serum) and antibiotics (50 μg/ml each of penicillin, streptomycin and gentamicin) at 37 °C in a humidified 5% CO2 atmosphere. For plasmid transfections, attached CHL and HepG2 cells at 50% confluence were exposed for 24 h to FuGENE 6® reagent containing COX-2 expression vector (full-length human COX-2 cDNA cloned into pcDNA3) or control vector (pcDNA3). At the end of this period of transfection, media were replaced with fresh medium containing 10% FBS, and, on the second day after transfection, the cells were plated at 25% confluence in medium supplemented with 500 μg/ml G418 sulfate. Single colonies of resistant cells were detectable after approx. 12 days and were selected and subcultured for 30 days. Subsequent cultures of selected cells were grown in the presence of G418. Cells stably expressing pcDNA3hCOX-2 and empty pcDNA3 vector were termed CHL-C and CHL-V respectively. At 18 h before experiments, the culture medium was replaced with fresh medium containing 1% (v/v) FBS.
Characterization of COX-2 vectors
pcDNA3 and pEGFP-N1 were obtained from Invitrogen and BD Biosciences (Clontech) respectively. Human full-length COX-2 cDNA [23] in pcDNA1/Amp was a gift from Dr S. Prescott (Huntsman Cancer Institute, Salt Lake City, UT, U.S.A.). The COX-2 cDNA was subsequently cloned into the pcDNA3 expression vector. COX-2–GFP (green fluorescent protein) was obtained by inserting 720 bp of the GFP open reading frame (excluding the initiator methionine and the termination codon) from pEGFP-N1 vector into the BspEI site of the human COX-2 cDNA. This inserts the GFP at the C-terminal end of COX-2 polypeptide, as described previously [24]. The orientation and integrity of the constructs were determined by sequencing. Large-scale preparation of plasmids was performed using EndoFree plasmid mega kit (Qiagen).
Cell-cycle distribution and cell counting
Cell counts were performed by initially plating 1.6×105 cells. At the indicated times, cells were trypsinized and stained with Trypan Blue, and the cells that excluded Trypan Blue were counted under the microscope. For the analysis of cell-cycle distribution, cells were resuspended in PBS and fixed in 70% ethanol. PI (propidium iodide) staining was performed after incubation of the cells with 0.05% PI and 0.1 mg/ml RNase for 30 min which were then analysed in a CyAn MLE-R cytometer (DakoCytomation).
Immunocytochemistry
Cells (5×104 per well) were cultured in 24-well plates on glass coverslips and maintained overnight in DMEM containing 1% (v/v) FBS. Cells were then fixed for 15 min with 4% (w/v) paraformaldehyde, pH 7.0, washed with PBS and permeabilized with methanol for 10 min at −20 °C. After blocking with 3% (w/v) BSA for 1 h at room temperature (25 °C), the cells were incubated for 2 h at room temperature with the corresponding monoclonal anti-COX-2 antibodies diluted 1:150 in PBS containing 1% (w/v) BSA. After three washes with PBS, the coverslips were incubated with a 1:500 dilution of fluorescent secondary antibody (IgG-Cy3, DakoCytomation), washed three times with PBS and treated with Hoechst 33258 for 30 min at room temperature. Fluorescence was visualized and quantified using a MRC 1024 microscope (Bio-Rad) and Lasersharp software. For tissue immunohistochemistry, liver samples were snap-frozen in 2-methylbutane immersed in liquid N2, and serial 10 μm-thick sections were cut on to gelatinized glass with a Microm HM550 sledge microtome. After fixing and blocking, the sections were incubated overnight with GFP antiserum (diluted 1:50; Molecular Probes) in 5% (v/v) horse serum at 4 °C. After three washes with PBS, the sections were incubated with a 1:500 dilution of fluorescent secondary antibody (IgG–Alexa Fluor® 488), washed and analysed as above. For the detection and quantification of apoptosis, the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) commercial kit for cell death detection (Roche) was used in accordance with the manufacturer's instructions. Histological examination was performed on 4% (w/v) paraformaldehyde-fixed mouse liver sections prepared by routine histological staining (haematoxylin/eosin) procedures.
Caspase assays
Cell extracts were prepared by lysing in 10 mM Hepes, pH 7.9, 1 mM EGTA, 1 mM EDTA, 120 mM NaCl, 1 mM dithiothreitol, 0.5 mM PMSF, 2 μg/ml aprotinin, 10 μg/ml leupeptin, 2 μg/ml Tos-Lys-CH2Cl (‘TLCK’, tosyl-lysylchloromethane), 5 mM NaF, 1 mM NaVO4, 10 mM Na2MoO4 and 0.5% Nonidet P-40 (buffer A). After centrifugation of the cell lysate at 15700 g for 5 min, the supernatant was stored at −80 °C (cytosolic extract), and protein content was assayed with the Bio-Rad protein reagent. The activities of caspases 3, 8 and 9 in cytosolic extracts were determined with the fluorogenic substrates N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin, N-acetyl-Ile-Glu-Thr-Asp-7-amino-4-trifluoromethylcoumarin, and N-acetyl-Leu-Glu-His-Asp-7-amino-4-trifluoromethylcoumarin respectively, and in accordance with the supplier's instructions (Calbiochem). The linearity of caspase assays was determined over a 30 min reaction period [25].
Cell extracts and Western blotting
Cells [(2–3)×106] or tissue samples (100 mg) were homogenized in a lysis buffer containing 10 mM Tris/HCl, pH 7.5, 1 mM MgCl2, 1 mM EGTA, 10% (v/v) glycerol, 0.5% (w/v) CHAPS, 1 mM 2-mercaptoethanol and 0.1 mM PMSF. Extracts were vortex-mixed for 30 min at 4 °C and, after centrifuging for 20 min at 13000 g, the supernatants were stored at −20 °C (cytosolic extracts). To obtain nuclear extracts, the pellets were processed in the same volume with 20% (v/v) glycerol and 0.4 M KCl and mixed for 30 min at 4 °C as described previously [8]. To determine the release of cytochrome c from the mitochondria to the cytosol, cell extracts were obtained by controlled lysis of the plasma membrane as described previously [25]. For Western blot analysis, whole-cell extracts were boiled for 5 min in Laemmli sample buffer, and equal amounts of protein (20–30 μg) were separated by SDS/PAGE (10–12% gels). The relative amounts of each protein were determined in total, cytosolic or nuclear cellular extracts as appropriate with polyclonal or monoclonal antibodies against the following: COX-2 and COX-1 (Cayman), IAPs (inhibitors of apoptosis) (R&D Systems), Bcl-2 family proteins (Santa Cruz), cytochrome c and Fas (BD Pharmingen), p53 (Santa Cruz), PARP-1 [poly(ADP-ribose) polymerase 1] (Alexis), and Akt/phospho-Akt (Ser473) (Cell Signaling Technologies) After incubation with the corresponding anti-rabbit or anti-mouse horseradish-peroxidase-conjugated secondary antibody, blots were developed using the ECL® (enhanced chemiluminescence) protocol (Amersham Biosciences). Target protein band densities were normalized by calculating the ratio to the corresponding densities of β-actin (whole-cell/cytosolic extracts) or Sp1 (specificity protein 1) (nuclear extracts). Different exposure times were performed on each blot to ensure linearity of the band intensities. Densitometric analysis was expressed in arbitrary units.
Determination of metabolites
PGE2 levels were determined in culture media by specific immuno-assay (Amersham Biosciences). ALT (alanine aminotransferase) was assayed spectrophotometrically in plasma [26]. Protein levels were determined using the Bradford reagent.
RNA isolation and RT (reverse transcription)–PCR
Total RNA was extracted from liver with TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed with 50 units of expand reverse transcriptase and pd(N)6 random hexamer as primer (Amersham Biosciences). The resulting cDNAs were amplified with the following oligonucleotide sequences: COX-2, 5′-CAGAGTTGGAAGCACTCTATGG-3′ (sense) and 5′-CTGTTTTAATGAGCTCTGGATC-3′ (antisense); and 18 S rRNA, 5′-GCAATTATTCCCCATGAACGA-3′ (sense) and 5′-CAAAGGGCAGGGACTTAATCAA-3′ (antisense).
Hydrodynamic transient transfection experiments
Plasmid (100 μg) dissolved in 2 ml of isotonic NaCl was injected into the tail veins of 18–22 g adult male Swiss CD1 mice (Charles River) over 8 s (hydrodynamic injection) [27]. Eight animals were used for each condition. At 24 h after injection, animals were injected intraperitoneally with a single dose of purified hamster anti-(mouse Fas) monoclonal antibody Jo2 (0.3 μg/g of body mass) freshly dissolved in 0.9% NaCl. This dose was chosen on the basis of previous studies [28]. Animals were killed 5 h later, and livers were rapidly removed and freeze-clamped in liquid N2. Animals were treated in accordance with Institutional Care Instructions.
Data analysis
Data are expressed as means±S.D. Statistical significance of differences between the CHL-C and CHL-V groups was evaluated by the Mann–Whitney U test. All tests were calculated two-tail, and significance was considered at P<0.05.
RESULTS
Characterization of CHL cells expressing COX-2
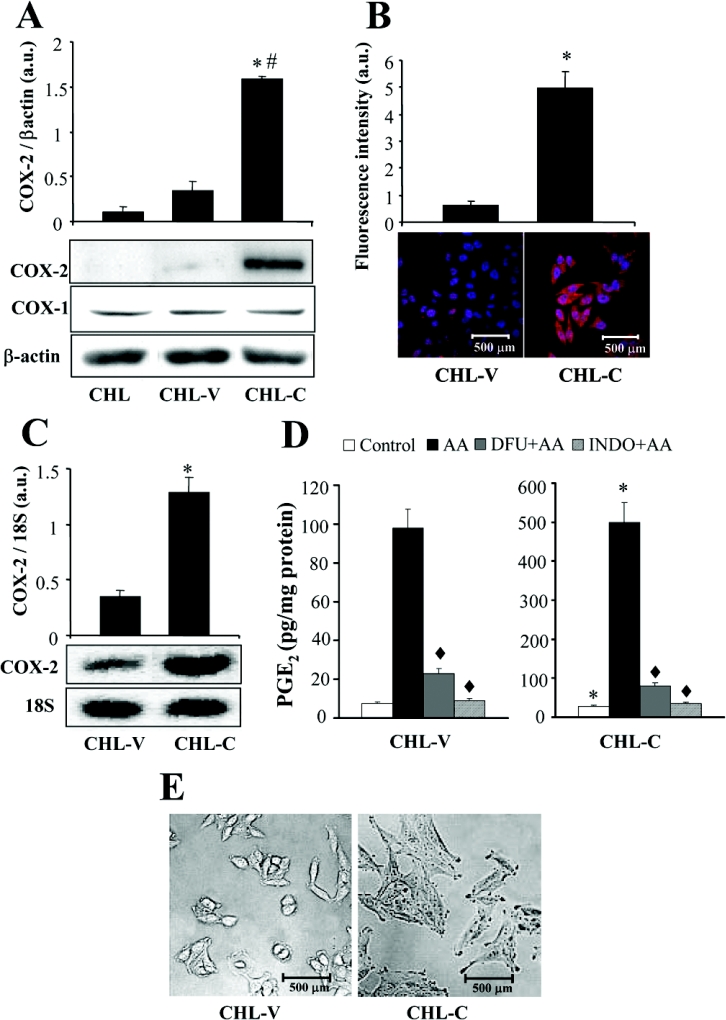
The human CHL cell line was transfected with the COX-2 expression vector pcDNA3hCOX-2 or the control vector pcDNA3 and selected with 500 μg/ml G418. Among 20 clones analysed, we selected two clones: a control clone CHL-V, transfected with the empty vector; and a COX-2 clone, CHL-C, transfected with pcDNA3hCOX-2 and selected on the basis of its high COX-2 expression. These clones were used for all subsequent experiments. As shown in Figure 1(A), COX-2 protein was significantly expressed in CHL-C cells, as determined by Western blot, but was not expressed in CHL or CHL-V cells. COX-1 protein was expressed at low levels and without significant differences in CHL and in the transfected cells. Immunocyto-chemistry revealed clear staining for COX-2 in the endoplasmic reticulum and perinuclear membranes (Figure 1B). RT–PCR of COX-2 mRNA confirmed increased COX-2 mRNA expression in CHL-C cells (Figure 1C). COX-2 expression in liver cells was functional: Figure 1(D) shows that CHL-C cells released significantly greater quantities of PGE2 into the culture medium than did CHL-V (3.7-fold increase). This PGE2 synthesis was markedly increased after incubation of the cells for 30 min with 30 μM AA (arachidonic acid). The contribution of COX-2 to this process was confirmed by inhibition (>90%) of AA-inducible PGE2 synthesis after treatment with the COXIB DFU (5 μM). When the cells were incubated in the presence of indomethacin (50 μM), PGE2 levels returned to the control (or even lower) values. These data indicate that COX-2 is the major contributor to PGE2 synthesis, whereas the contribution of COX-1 could be evaluated in less than 5% of the PGE2 levels in CHL-C cells. Figure 1(E) shows the clear morphological differences between CHL-V and CHL-C cells, with CHL-C being larger and exhibiting a less rounded shape than the CHL-V control cells.
Figure 1. COX-2 expression in CHL cells increases PGE2 production.
CHL cells were stably transfected with empty pcDNA3 vector (CHL-V) or pcDNA3hCOX-2, encoding human COX-2 (CHL-C). (A) COX-2 and COX-1 protein expression in whole-cell extracts was detected by Western blot. COX-2 and COX-1 expression were normalized by calculating the ratios of COX-2 and β-actin band densities, expressed in arbitrary units (a.u.). Representative blots are shown. (B) Immunocytochemical analysis of COX-2 by confocal microscopy. COX-2 staining is in red and nuclear staining with Hoechst 33258 in blue. Relative fluorescence intensities are shown in arbitrary units (a.u.). (C) RT–PCR analysis of COX-2 mRNA expression. COX-2 mRNA amounts were calculated as arbitrary units (a.u.) normalized to the expression of 18 S rRNA, to control for the quantity and integrity of total RNA. (D) PGE2 production by COX-2-expressing CHL cells is five times that in control cells. PGE2 concentrations were determined by ELISA in the culture medium of untreated cells or cells treated at 37 °C for 30 min with 30 μM AA±5 μM COXIB DFU and ±50 μM non-selective COX inhibitor indomethacin (INDO). (E) Light microscopy showing morphological changes in CHL cells expressing COX-2. All data presented in the histograms are the means±S.D. for five independent experiments. #P<0.05 compared with CHL-V cells. *P<0.05 compared with CHL-V cells. ◆P<0.05 compared with paired AA-treated cells without DFU or indomethacin treatment.
COX-2 expression in liver cells promotes an increase in S-phase cells and inhibits apoptosis
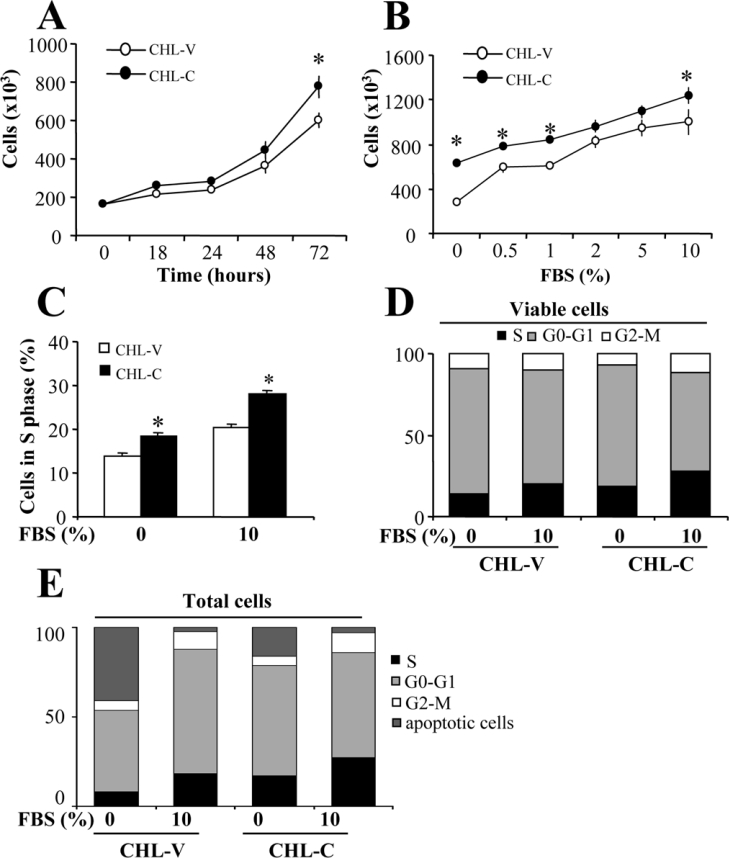
The CHL-C cell number after 72 h of culture in 10% (v/v) FBS was greater (129%) than that of CHL-V (Figure 2A). To assess the role of COX-2 in cell proliferation, we analysed the dependence of CHL-V and CHL-C cell number on FBS concentration. As Figure 2(B) shows, COX-2-dependent proliferation was evident in the absence and presence of serum (229 and 129% more CHL-C cells compared with CHL-V cells respectively). Analysis of cell-cycle distribution by PI staining and FACS revealed that COX-2-expression is associated with an increase in the number of cells in S-phase at both 0 and 10% FBS (133% more CHL-C cells compared with CHL-V cells, Figure 2C), with no significant changes in the G0/G1 and G2/M populations observed, when considering non-apoptotic cells (Figure 2D). Figure 2(E) shows the distribution of apoptotic and non-apoptotic cells in the different steps of the cell cycle.
Figure 2. Effect of COX-2 expression on cellular growth and cell-cycle distribution.
(A) Time-dependent proliferation of CHL-V and CHL-C cells. Cells were seeded at 1.6×105 on 6-cm plates in medium containing 10% (v/v) FBS, and after the indicated times in culture, cells were trypsinized, stained with Trypan Blue and counted (Trypan Blue-negative cells). (B) Effect of FBS concentration on cell growth at 72 h. Cells were seeded as in (A), but in medium containing differing concentrations of FBS as indicated. (C) Flow-cytometric analysis of the proportion of CHL-V and CHL-C cell populations in S-phase at 0 and 10% (v/v) FBS (72 h). (D) Cell-cycle analysis by FACS of CHL-V and CHL-C cells at 72 h. (E) The same experiment as in (D), but considering also apoptotic cells. Cells were resuspended in PBS, fixed with 70% ethanol, stained with PI and analysed in a CyAn MLE-R cytometer. Results are means±S.D. for four independent experiments. *P<0.05 compared with CHL-V cells.
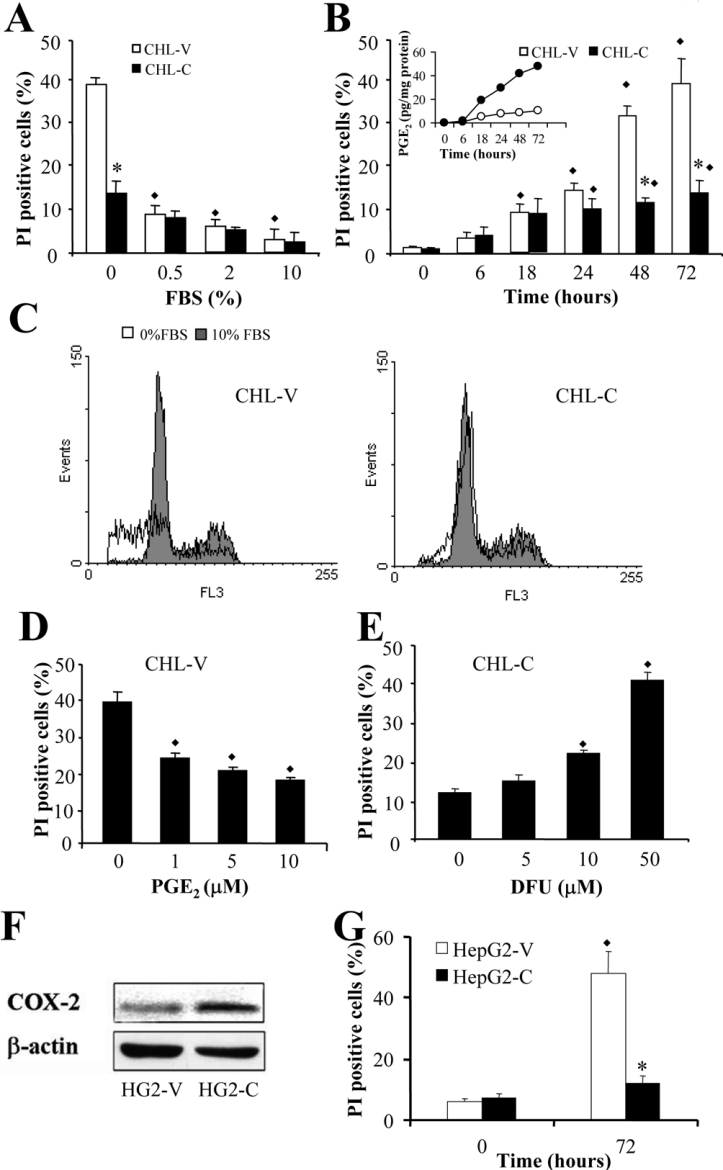
Flow cytometry of PI-stained cells revealed an inhibition of apoptosis in CHL-C cells cultured in the absence of FBS (Figure 3A–3C). After 72 h of culture, only 14% of CHL-C cells were apoptotic, compared with 40% of CHL-V cells (Figure 3B). In order to correlate the number of PI-positive cells induced after FBS removal with COX-2 expression, the levels of PGE2 were measured at various times, and, as shown in Figure 3B (inset), a correlation exists between an increase in PGE2 synthesis and a decrease in the number of PI-positive cells. The inhibition of apoptosis could be mimicked in CHL-V control cells by treating with exogenous PGE2 (Figure 3D), and the action of COX-2 metabolites in the decrease in apoptosis in CHL-C cells was confirmed by treatment with increasing concentrations of DFU, which progressively increased the number of apoptotic cells (Figure 3E). CHL-C cells were also resistant to the induction of apoptosis by 10 nM staurosporine, camptothecin or a combination of TNFα and actinomycin D, with 30% fewer cells in apoptosis compared with CHL-V cells (results not shown). We also overexpressed COX-2 in HepG2 cells, as a different cell line with a basal constitutive COX-2 expression (Figure 3F) obtaining the same results as in CHL cells. COX-2 overexpression, but not the constitutive level, caused a decrease in the number of apoptotic cells after serum deprivation (Figure 3G).
Figure 3. COX-2 expression inhibits PI staining in liver cells.
Cells resuspended in PBS were PI-stained, and cell-cycle analysis was determined by flow cytometry. (A) Effect of FBS concentration on the percentage of apoptotic CHL-V and CHL-C cells after 72 h of culture. (B) Time-dependent apoptosis and PGE2 concentrations in CHL-V and CHL-C cells cultured without FBS. The inset shows the level of PGE2 in each cell type over the same time period. (C) Representative DNA histograms and G1 profiles showing the PI staining from CHL-V and CHL-C cells cultured at 0 and 10% (v/v) FBS for 72 h. (D) Effect of exogenous PGE2 on the percentage of apoptotic cells. (E) Effect of DFU on the percentage of apoptotic CHL-C cells (no FBS, 72 h of culture). (F) Western blot analysis of COX-2 expression in HepG2-V and HepG2-C. (G) Apoptosis of HepG2-V and HepG2-C cells without FBS after 72 h of culture. Results are means±S.D. for four independent experiments. *P<0.05 compared with paired CHL-V cells. ◆P<0.05 compared with cells at zero time (B) or untreated cells (D and E).
COX-2 expression leads to altered expression and activity of apoptosis markers
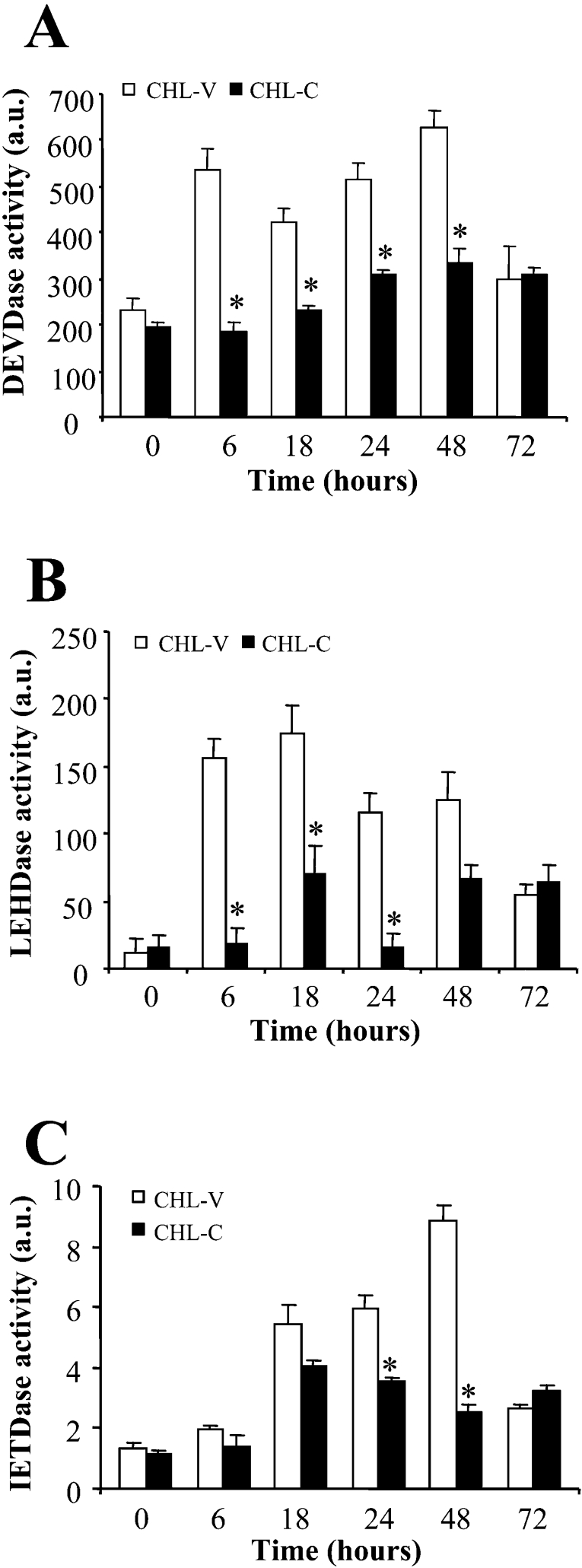
The data presented so far provide convincing evidence that PGE2 synthesized by COX-2 suppresses apoptotic signals in CHL-C cells. To investigate the mechanisms through which this occurs we first analysed the activities of caspases 3, 8 and 9 by fluorimetry (measured as DEVDase, IETDase and LEHDase activities respectively). As shown in Figures 4(A) and 4(B), the activities of caspases 3 and 9 were markedly lower in CHL-C cells than in CHL-V cells. Caspase 8 activity was also inhibited in CHL-C cells, but to a lesser extent and with a delay with respect to caspases 3 and 9 (Figure 4C). These results are in agreement with data obtained in gastric mucosal cells [29], where COX-2 expression also suppresses the activation of caspases 8 and 9 in addition to caspase 3.
Figure 4. Expression of COX-2 suppresses caspase-like activities.
Activities of caspases 3 (A), 9 (B) and 8 (C), measured as DEVDase, IETDase and LEHDase activities respectively, in CHL-V and CHL-C cells cultured without FBS. Caspase activities were measured by fluorimetric assay with specific fluorogenic substrates (see the Materials and methods section). One unit (a.u.) of protease activity was defined as the amount of enzyme required to release 1 pmol of AMC (aminomethylcoumarin)/min. Results are means±S.D. for five independent experiments. *P<0.05 compared with the corresponding CHL-V cells.
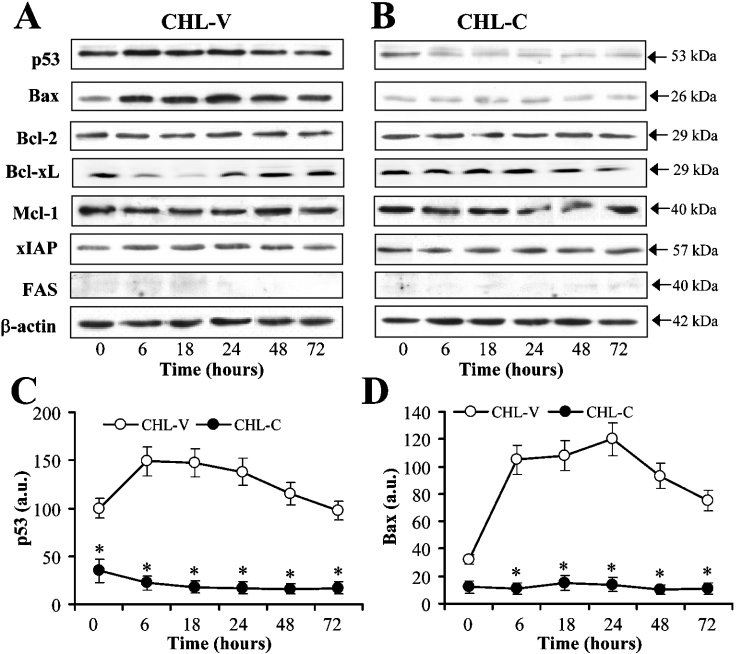
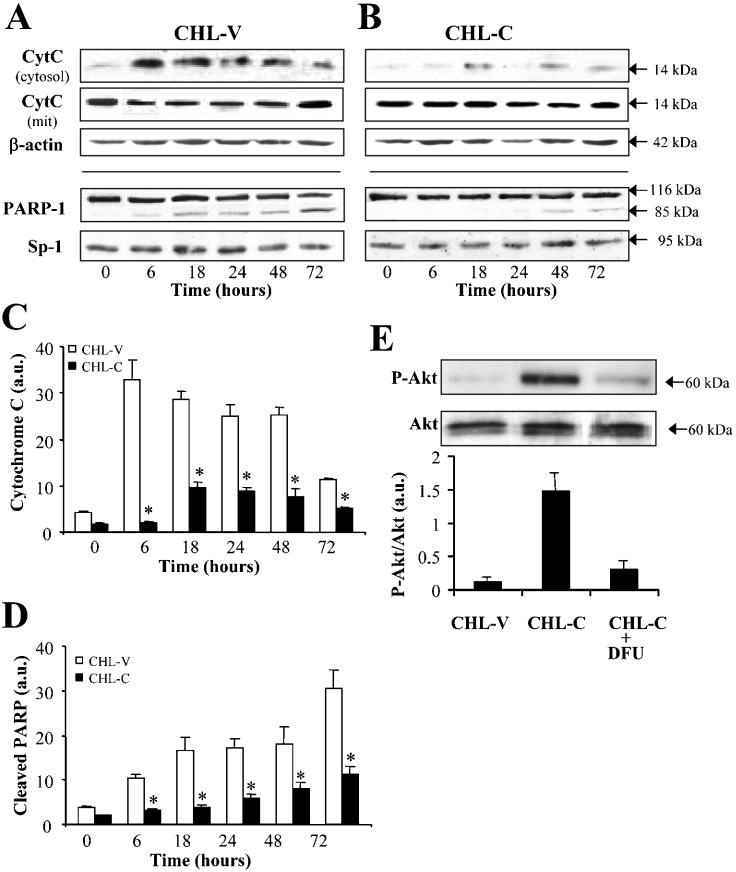
Figure 5 shows the effect of COX-2 expression on the levels of several proteins governing apoptosis fate. As Figures 5(A)–5(C) show, p53 expression declined notably in CHL-C cells at zero time and within 6 h of culture in medium without FBS, whereas it was maintained in CHL-V controls. Bax expression, scarcely detected in both cell lines at 10% serum, was increased in CHL-V cells from 6 h without FBS, but was not observed even after 72 h in CHL-C cells (Figure 5D). The protein levels of Bcl-2, XIAP (X-linked IAP) and Mcl-1 did not vary between CHL-V or CHL-C cells; however, Bcl-XL decreased in CHL-V cells from 6 to 48 h of culture in medium without FBS, whereas it was maintained in CHL-C cells (Figure 5). Release of cytochrome c to the cytosol with the corresponding decrease in the mitochondria and cleavage of PARP-1 were also observed only in CHL-V cells (Figures 6A and 6B).
Figure 5. Expression of apoptosis indicators is altered in COX-2-expressing liver cells.
Representative Western blots showing the expression of p53, Bax, Bcl-2, Bcl-XL, Mcl-1, XIAP and Fas in CHL-V cells and CHL-C cells cultured without FBS (A, B). Densitometric analysis of the expression of p53 and Bax (C, D). The expression of target proteins was normalized to that of β-actin (whole-cell and cytosolic extracts), and the ratios are presented in arbitrary units (a.u.). Results in (C) and (D) are the means±S.D. for five independent experiments. *P<0.05 compared with the corresponding CHL-V cells. a.u., arbitrary units.
Figure 6. Cytochrome c, PARP-1 and phospho-Akt changes in CHL-V and CHL-C cells.
The presence of cytochrome c in the cytosolic and mitochondrial fractions after controlled lysis of the cells, and the levels of PARP-1 were analysed by Western blotting (A–D). Blots were normalized with β-actin (whole-cell and cytosolic extracts) or Sp1 (nuclear extracts). Total and phospho-Akt levels were determined in the same total cell extract in the presence or absence of 5 μM DFU (E). Representative Western blots are shown (n=3), and data are the means±S.D. for five independent experiments. *P<0.05 compared with the corresponding CHL-V cells. a.u., arbitrary units.
The PI3K (phosphoinositide 3-kinase)/Akt pathway plays a central role in integrating diverse survival signals. The levels of phospho-Akt (Ser473) increased in cells expressing COX-2. Moreover, when the CHL-C cells were incubated with DFU to inhibit COX-2, the increase in phospho-Akt was not observed (Figure 6E), suggesting a role for COX-2 in liver cell survival. The same mechanism occurs in HepG2 COX-2-expressing cells (results not shown).
These results indicate that the anti-apoptotic effect induced by COX-2/PGE2 is mediated by the inhibition of the mitochondrial intrinsic pathway. But the inhibition of caspase 8 (Figure 4C) suggested the possible involvement of death receptor signalling, known to be important in hepatocyte apoptosis and in the pathogenesis of liver diseases. As shown in Figure 5(A), the levels of Fas protein were scarcely detected in either cell line. The abovementioned failure of anti-Fas antibody to induce apoptosis (results not shown) indicates that CHL-V and CHL-C cells are resistant to Fas-mediated apoptosis, as occurs in many liver cell lines and in HCC [21].
Transient expression of COX-2 inhibits liver apoptosis in vivo
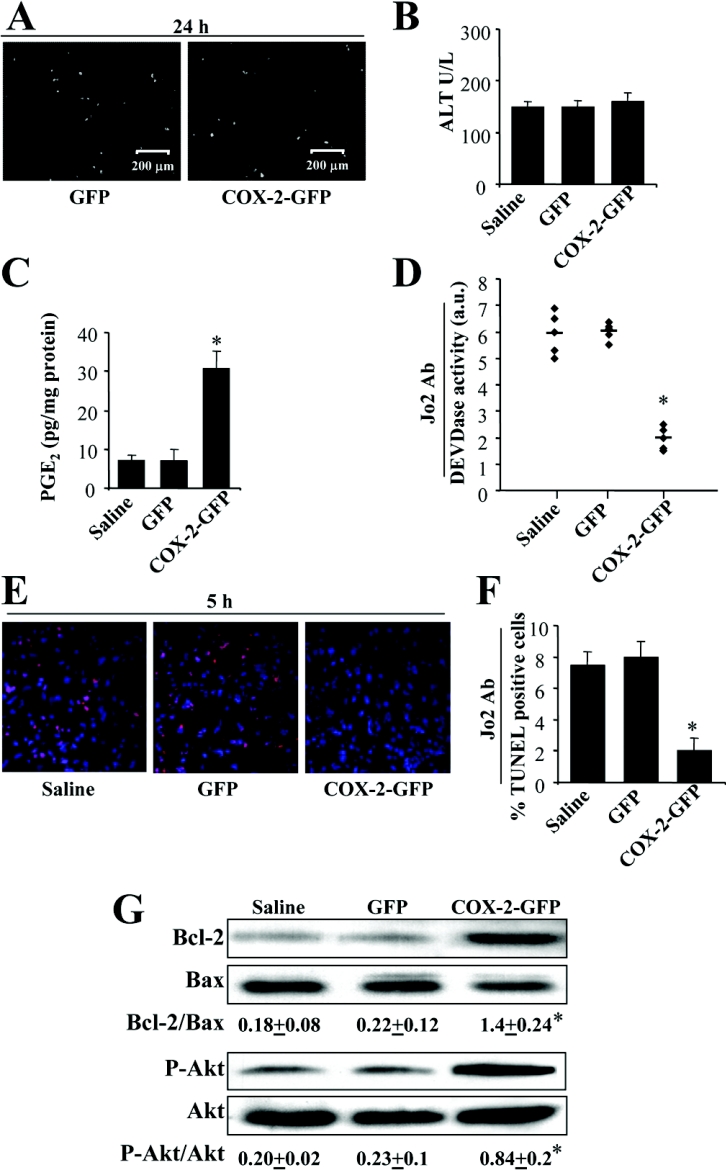
To examine whether COX-2 expression inhibits liver cell apoptosis in living animals, we developed an in vivo model to deliver the COX-2 gene to the liver via hydrodynamics-based transfection. COX-2 was expressed in approx. 35% of the liver cells of animals transfected with a construct encoding GFP–COX-2, as indicated by the associated GFP fluorescence (Figure 7A). Under these conditions, the levels of ALT determined 24 h after transfection were similar between animals transfected with GFP or with GFP–COX-2, and were in the range of the animals receiving saline (Figure 7B). The transfected COX-2 was active, since intrahepatic levels of PGE2 increased in the GFP–COX-2 mice, but not in the GFP animals (Figure 7C). As Figures 7(D)–7(F) show, transfection of liver with GFP–COX-2 impaired Fas-dependent apoptosis as indicated by the inhibition of caspase 3 activity and reduced staining in TUNEL analysis. Moreover, the analysis of apoptosis markers in the liver of GFP–COX-2 mice demonstrates an increase in the levels of phospho-Akt and in the Bcl-2/Bax ratio (Figure 7G).
Figure 7. Hydrodynamic transfection of mice with GFP–COX-2 inhibits Fas-induced liver apoptosis.
Animals were transfected with 100 μg of plasmid encoding GFP or GFP–COX-2. Control animals were treated with saline. (A) GFP fluorescence in liver sections taken 24 h after transfection to verify the expression of GFP or GFP–COX-2 fusion protein. (B) ALT serum activity determined as an index of transfection-induced liver injury after 24 h. (C) Intrahepatic PGE2 concentrations were determined 24 h after transfection. (D) Caspase 3 activity (DEVDase) in liver extracts taken 5 h after intraperitoneal injection with the Fas-activating antibody (Ab) Jo2 (0.3 μg/g of body mass). (E) Apoptosis in liver sections taken 5 h after injection with Jo2. The fluorograms show representative results of TUNEL analysis (red fluorescence) and nuclear staining with Hoechst 33258 (blue). The bar chart shows the percentage of TUNEL-positive cells (F). Protein levels of phospho-Akt and Bcl-2/Bax ratio in liver extracts from saline, GFP and GFP–COX-2 mice (G). All data are means+S.D. for eight animals per condition. *P<0.05 compared with GFP-transfected animals. a.u., arbitrary units.
DISCUSSION
The important role of COX-2 as inhibitor of apoptotic pathways has been demonstrated in vivo in transgenic mouse models of COX-2 expression in several tissues. Transgenic mice expressing COX-2 in mammary gland developed tumours, and these animals had reduced levels of the pro-apoptotic proteins Bax and Bcl-XL and elevated levels of Bcl-2 [30]. In basal keratinocytes, the results are conflicting, since both epidermal hyperplasia and suppression of tumour development have been described [31,32]. Transgenic mice expressing COX-2 in neurons develop age-dependent cognitive deficiencies due to an increase in neuronal apoptosis [33]. A recent study demonstrated hyperplastic gastric tumours in transgenic mice expressing COX-2 and microsomal PGE synthase [34].
Although a number of experimental studies have demonstrated a clear positive correlation in vitro between COX-2 expression and the inhibition of apoptosis, the underlying molecular mechanisms are still not fully understood. Most of this work has been done in colon or gastric carcinoma cell lines, where Tsujii and DuBois [35] demonstrated that RIE (rat intestinal epithelial) cells stably transfected with COX-2 were resistant to butyrate-induced apoptosis. The same authors reported that these cells exhibit a delayed G1 transit [35], and this may be related to the resistance to apoptosis. Our results demonstrate that COX-2 expression in liver cells is not associated with significant changes in cell-cycle distribution, but is related to the inhibition of cellular apoptosis. Cell-cycle arrest in G0/G1 has been described in COX-2-expressing endothelial, NIH 3T3, COS-7 and HEK-293 (human embryonic kidney) cells [24], but this effect did not require the synthesis of PGs. We did not find significant changes in G0/G1 or G2/M cell populations, but the inhibition of apoptosis in COX-2-expressing liver cells was dependent on PGE2 synthesis.
COXIBs provide an indirect means of establishing a relationship between COX-2-dependent PGs and apoptosis. However, the doses which induce apoptosis are much higher than those needed to inhibit COX-2 activity, indicating that these inhibitors may be acting via COX-2-independent effects, depending on the compound used and the cell type. Some NSAIDs (non-steroidal anti-inflammatory drugs), such as sulindac, potently induce apoptosis in human gastric cancer cells [36], while others, such as SC-58125, induce both apoptosis and cell-cycle arrest in HCA-7 cells [37]. A prostate apoptosis response gene (Par-4) was found to be up-regulated following exposure of HCA-7 cells to NS-398 [38]. Sulindac, salicylate and aspirin have been shown to inhibit NF-κB (nuclear factor κB) signalling by directly blocking the activity of IκB (inhibitor of NF-κB) kinase, leading to reconstituted sensitivity of cancer cells to apoptosis [39]. Additionally, indomethacin, a non-selective COX inhibitor, can act as a direct ligand of PPARγ (peroxisome-proliferator-activated receptor γ), a suppressor of tumour cell proliferation [40].
In liver, increased COX-2 expression has been demonstrated in patients with HCC, especially in tissue with cirrhosis as well as in well-differentiated tumour tissue [41]. In HCC, COX-2 expression seems to be up-regulated at early stages and down-regulated in advanced stages. Silencing of the COX-2 gene by promoter hypermethylation has been reported to regulate the growth of some HCC cell lines [42]. However, in vitro studies revealed that NS-398, celecoxib and sulindac effectively inhibited growth of human hepatoma cell lines [11,12]. In mice implanted with hepatoma, nimesulide inhibits tumour growth by inducing apoptosis and overexpression of Bax over Bcl-2 [43]. Moreover, combinations of COX-2 and MEK (mitogen-activated protein kinase/extracellular-signal-regulated kinase kinase) inhibitors synergistically increase apoptosis in human HCC [44].
Several signalling pathways have been proposed as mediators of COX-2-dependent inhibition of apoptosis. PGE2 inhibits apoptosis in gastric mucosa cells via the mitochondrial pathway and PKA (cAMP-dependent protein kinase) activation [29]. In cholangiocarcinoma cells, celecoxib suppressed Akt phosphorylation, released cytochrome c to the cytosol and activated caspases 9 and 3 [45]. There are a few reports which describe the direct effect of COX-2 expression on apoptosis in liver or HCC cells. Leng et al. [46] reported an enhanced phosphorylation of Akt in Hep 3B cells transiently and permanently transfected with a COX-2 expression vector but Bcl-2 is not induced. Celecoxib reduced Akt activation and induced caspase 9 and 3 activation with a concomitant release of cytochrome c. Our results clearly demonstrate that caspase 3 and 9 activities are inhibited in COX-2-expressing cells and the activity of caspase 8 is also decreased in CHL-C cells. The absence of cytochrome c in the cytosol of CHL-C cells after exposure to apoptotic stimulus clearly implies that COX-2 PG products act on the mitochondrial pathway to prevent apoptosis. We did not find significant changes in the expression of IAP proteins. This is in agreement with results obtained in cholangiocarcinoma cells, although cAMP-dependent induction of c-IAP2 (cellular IAP 2) has been reported to mediate the anti-apoptotic action of PGE2 synthesized by COX-2 in intestinal epithelial cells [47]. The levels of Bcl-2 remained unaffected by COX-2 expression in our experiments, in agreement with previous findings in liver cells [12].
COX-2 has been reported to promote survival of human lung adenocarcinoma cells by up-regulating the level of the anti-apoptotic protein Mcl-1 and activating the PI3K/Akt-dependent pathway [43,48]. Our results demonstrate that PGE2 produced by COX-2 does not change the levels of Mcl-1, but induces a significant increase in Akt phosphorylation, indicating a role for Akt in COX-2-dependent survival of HCC cells. p53 activation plays an important role in the induction of apoptosis by various agents. Once activated, p53 negatively regulates cell-cycle progression and induces apoptosis by regulating the pro-apoptotic gene Bax and several death-promoting molecules in many cell lines [49]. The regulation of p53 is complex and implicates different mechanisms, such as stability, nuclear accumulation and activity. COX-2 expression negatively affects p53 activity via mechanism that could involve physical interaction, and COX-2-positive prostate cancer cells exhibited a decrease p53 stability, nuclear accumulation and activity after hypoxia, both via direct effects on p53 activation and via activation of Mdm2 (murine double minute 2), the primary negative regulator of p53 [50]. In this sense, our results showed an important decrease in p53 levels in CHL-C cells.
The results obtained in vivo from mice hydrodynamically transfected with COX-2 clearly demonstrated that PGs produced by COX-2 expression protected the liver against Fas-mediated apoptosis in agreement with the results obtained from the in vitro liver cell lines. Interestingly, the levels of Bcl-2 that are very low in liver were up-regulated significantly after hydrodynamic transfection of COX-2.
In summary, the results of the present study indicate that PGs produced by COX-2 in liver, both in vitro and in vivo, inhibit apoptosis dependent on the intrinsic mitochondrial pathway. Given the frequent expression of COX-2 in HCC, COX-2 would be expected to provide these cancers with a survival advantage. This is an important finding and may explain both the anti-apoptotic hepatoprotective effect of PGE2 after acute liver injury and the important contribution of COX-2 to the development of tumorigenesis through increased resistance to apoptosis in HCC.
Acknowledgments
A. F. M. and R. M. were supported by CNIC (Centro Nacional de Investigaciones Cardiovasculares)/Bancaja fellowships. This work was supported by grants SAF2003-00342, SAF2004-00957 and SAF2005-03022 from CICYT (Comisión Interministerial de Ciencia y Tecnología), Spain and by a grant from Instituto de Salud Carlos III (Red de Centros C03/01).
References
- 1.Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeWitt D. L. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim. Biophys. Acta. 1991;1083:121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- 3.Pilbeam C. C., Kawaguchi H., Hakeda Y., Voznesensky O., Alander C. B., Raisz L. G. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J. Biol. Chem. 1993;268:25643–25649. [PubMed] [Google Scholar]
- 4.Fletcher B. S., Lim R. W., Varnum B. C., Kujubu D. A., Koski R. A., Herschman H. R. Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. J. Biol. Chem. 1991;266:14511–14518. [PubMed] [Google Scholar]
- 5.Feng L., Xia Y., Garcia G. E., Hwang D., Wilson C. B. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J. Clin. Invest. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai N., Tsujii M., Tsuji S. Cyclooxygenases and colon cancer. Prostaglandins Other Lipid Mediators. 2002;68–69:187–196. doi: 10.1016/s0090-6980(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 7.Kondo M., Yamamoto H., Nagano H., Okami J., Ito Y., Shimizu J., Eguchi H., Miyamoto A., Dono K., Umeshita K., et al. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin. Cancer Res. 1999;5:4005–4012. [PubMed] [Google Scholar]
- 8.Casado M., Callejas N. A., Rodrigo J., Zhao X., Dey S. K., Boscá L., Martín-Sanz P. Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. FASEB J. 2001;15:2016–2018. doi: 10.1096/fj.01-0158fje. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Martínez A., Callejas N. A., Casado M., Boscá L., Martín-Sanz P. Thioacetamide-induced liver regeneration involves the expression of cyclooxygenase 2 and nitric oxide synthase 2 in hepatocytes. J. Hepatol. 2004;40:963–970. doi: 10.1016/j.jhep.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto H., Kondo M., Nakamori S., Nagano H., Wakasa K., Sugita Y., Chang-De J., Kobayashi S., Damdinsuren B., Dono K., et al. JTE-522, a cyclooxygenase-2 inhibitor, is an effective chemopreventive agent against rat experimental liver fibrosis1. Gastroenterology. 2003;125:556–571. doi: 10.1016/s0016-5085(03)00904-1. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A. S., Chan H. L., Leung W. K., Wong N., Johnson P. J., Sung J. J. Specific COX-2 inhibitor, NS-398, suppresses cellular proliferation and induces apoptosis in human hepatocellular carcinoma cells. Int. J. Oncol. 2003;23:113–119. [PubMed] [Google Scholar]
- 12.Kern M. A., Schubert D., Sahi D., Schoneweiss M. M., Moll I., Haugg A. M., Dienes H. P., Breuhahn K., Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- 13.Núñez O., Fernández-Martínez A., Majano P. L., Apolinario A., Gómez-Gonzalo M., Benedicto I., López-Cabrera M., Boscá L., Clemente G., Garcia-Monzón C., Martín-Sanz P. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665–1672. doi: 10.1136/gut.2003.038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae S. H., Jung E. S., Park Y. M., Kim B. S., Kim B. K., Kim D. G., Ryu W. S. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin. Cancer Res. 2001;7:1410–1418. [PubMed] [Google Scholar]
- 15.Koga H., Sakisaka S., Ohishi M., Kawaguchi T., Taniguchi E., Sasatomi K., Harada M., Kusaba T., Tanaka M., Kimura R., et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 16.Talanian R. V., Brady K. D., Cryns V. L. Caspases as targets for anti-inflammatory and anti-apoptotic drug discovery. J. Med. Chem. 2000;43:3351–3371. doi: 10.1021/jm000060f. [DOI] [PubMed] [Google Scholar]
- 17.Ashkenazi A., Dixit V. M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 18.Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X., Wang X. Cytochrome c-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 20.Kondo T., Suda T., Fukuyama H., Adachi M., Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 21.Lee S. H., Shin M. S., Lee H. S., Bae J. H., Lee H. K., Kim H. S., Kim S. Y., Jang J. J., Joo M., Kang Y. K., et al. Expression of Fas and Fas-related molecules in human hepatocellular carcinoma. Hum. Pathol. 2001;32:250–256. doi: 10.1053/hupa.2001.22769. [DOI] [PubMed] [Google Scholar]
- 22.Lee J. S., Thorgeirsson S. S. Functional and genomic implications of global gene expression profiles in cell lines from human hepatocellular cancer. Hepatology. 2002;35:1134–1143. doi: 10.1053/jhep.2002.33165. [DOI] [PubMed] [Google Scholar]
- 23.Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J. Biol. Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- 24.Trifan O. C., Smith R. M., Thompson B. D., Hla T. Overexpression of cyclooxygenase-2 induces cell cycle arrest: evidence for a prostaglandin-independent mechanism. J. Biol. Chem. 1999;274:34141–34147. doi: 10.1074/jbc.274.48.34141. [DOI] [PubMed] [Google Scholar]
- 25.Hortelano S., Zeini M., Castrillo A., Alvarez A. M., Boscá L. Induction of apoptosis by nitric oxide in macrophages is independent of apoptotic volume decrease. Cell Death Differ. 2002;9:643–650. doi: 10.1038/sj.cdd.4401017. [DOI] [PubMed] [Google Scholar]
- 26.Martín-Sanz P., Cascales C., Gomez A., Brindley D. N., Cascales M. Effect of a rhodium complex on alterations of hepatic function in thioacetamide-induced hyperplastic noduligenesis in rats. Carcinogenesis. 1987;8:1685–1690. doi: 10.1093/carcin/8.11.1685. [DOI] [PubMed] [Google Scholar]
- 27.Liu F., Song Y., Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 28.Guidotti J. E., Mallet V. O., Parlier D., Mitchell C., Fabre M., Jaffray P., Lambert M., Kahn A., Gilgenkrantz H. Fas/CD95 pathway induces mouse liver regeneration and allows for highly efficient retrovirus-mediated gene transfer. Hepatology. 2001;33:10–15. doi: 10.1053/jhep.2001.20678. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino T., Tsutsumi S., Tomisato W., Hwang H. J., Tsuchiya T., Mizushima T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J. Biol. Chem. 2003;278:12752–12758. doi: 10.1074/jbc.M212097200. [DOI] [PubMed] [Google Scholar]
- 30.Liu C. H., Chang S. H., Narko K., Trifan O. C., Wu M. T., Smith E., Haudenschild C., Lane T. F., Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 31.Neufang G., Furstenberger G., Heidt M., Marks F., Muller-Decker K. Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7629–7634. doi: 10.1073/pnas.121574098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bol D. K., Rowley R. B., Ho C. P., Pilz B., Dell J., Swerdel M., Kiguchi K., Muga S., Klein R., Fischer S. M. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62:2516–2521. [PubMed] [Google Scholar]
- 33.Andreasson K. I., Savonenko A., Vidensky S., Goellner J. J., Zhang Y., Shaffer A., Kaufmann W. E., Worley P. F., Isakson P., Markowska A. L. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J. Neurosci. 2001;21:8198–8209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshima H., Oshima M., Inaba K., Taketo M. M. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsujii M., Dubois R. N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y. L., Sun B., Zhang X. J., Wang S. N., He H. Y., Qiao M. M., Zhong J., Xu J. Y. Growth inhibition and apoptosis induction of Sulindac on human gastric cancer cells. World J. Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams C. S., Mann M., Dubois R. N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z., Dubois R. N. Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterology. 2000;118:1012–1017. doi: 10.1016/s0016-5085(00)70352-0. [DOI] [PubMed] [Google Scholar]
- 39.Yin M. J., Yamamoto Y., Gaynor R. B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature (London) 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 40.Wick M., Hurteau G., Dessev C., Chan D., Geraci M. W., Winn R. A., Heasley L. E., Nemenoff R. A. Peroxisome proliferator-activated receptor-γ is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol. Pharmacol. 2002;62:1207–1214. doi: 10.1124/mol.62.5.1207. [DOI] [PubMed] [Google Scholar]
- 41.Koga H. Hepatocellular carcinoma: is there a potential for chemoprevention using cyclooxygenase-2 inhibitors? Cancer. 2003;98:661–667. doi: 10.1002/cncr.11576. [DOI] [PubMed] [Google Scholar]
- 42.Murata H., Tsuji S., Tsujii M., Sakaguchi Y., Fu H. Y., Kawano S., Hori M. Promoter hypermethylation silences cyclooxygenase-2 (Cox-2) and regulates growth of human hepatocellular carcinoma cells. Lab. Invest. 2004;84:1050–1059. doi: 10.1038/labinvest.3700118. [DOI] [PubMed] [Google Scholar]
- 43.Li X. H., Li J. J., Zhang H. W., Sun P., Zhang Y. L., Cai S. H., Ren X. D. Nimesulide inhibits tumor growth in mice implanted hepatoma: overexpression of Bax over Bcl-2. Acta Pharmacol. Sin. 2003;24:1045–1050. [PubMed] [Google Scholar]
- 44.Schmidt C. M., Wang Y., Wiesenauer C. Novel combination of cyclooxygenase-2 and MEK inhibitors in human hepatocellular carcinoma provides a synergistic increase in apoptosis. J. Gastrointest. Surg. 2003;7:1024–1033. doi: 10.1016/j.gassur.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z., Lai G. H., Sirica A. E. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. Hepatology. 2004;39:1028–1037. doi: 10.1002/hep.20143. [DOI] [PubMed] [Google Scholar]
- 46.Leng J., Han C., Demetris A. J., Michalopoulos G. K., Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 47.Nishihara H., Kizaka-Kondoh S., Insel P. A., Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8921–8926. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin M. T., Lee R. C., Yang P. C., Ho F. M., Kuo M. L. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells: involvement of phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2001;276:48997–49002. doi: 10.1074/jbc.M107829200. [DOI] [PubMed] [Google Scholar]
- 49.Levine A. J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 50.Liu X. H., Kirschenbaum A., Yu K., Yao S., Levine A. C. Cycloxygenase-2 suppresses hypoxia-induced apoptosis via a combination of direct and indirect inhibition of p53 activity in a human prostate cancer cell line. J. Biol. Chem. 2004;280:3817–3823. doi: 10.1074/jbc.M406577200. [DOI] [PubMed] [Google Scholar]