Abstract
H+/OH− permeation through lipid bilayers occurs at anomalously high rates and the determinants of proton flux through membranes are poorly understood. Since all life depends on proton gradients, it is important to develop a greater understanding of proton leak phenomena. We have used stopped-flow fluorimetry to probe the influence of two lipid raft components, chol (cholesterol) and SM (sphingomyelin), on H+/OH− and water permeability. Increasing the concentrations of both lipids in POPC (palmitoyl-2-oleoyl phosphatidylcholine) liposomes decreased water permeability in a concentration-dependent manner, an effect that correlated with increased lipid order. Surprisingly, proton flux was increased by increasing the concentration of chol and SM. The chol effect was complex with molar concentrations of 17.9, 33 and 45.7% giving 2.8-fold (P<0.01), 2.2-fold (P<0.001) and 5.1-fold (P<0.001) increases in H+/OH− permeability from a baseline of 2.4×10−2 cm/s. SM at 10 mole% effected a 2.8-fold increase (P<0.01), whereas 20 and 30 mole% enhanced permeability by 3.6-fold (P<0.05) and 4.1-fold respectively (P<0.05). Supplementing membranes containing chol with SM did not enhance H+/OH− permeability. Of interest was the finding that chol addition to soya-bean lipids decreased H+/OH− permeability, consistent with an earlier report [Ira and Krishnamoorthy (2001) J. Phys. Chem. B 105, 1484–1488]. We speculate that the presence of proton carriers in crude lipid extracts might contribute to this result. We conclude that (i) chol and SM specifically and independently increase rates of proton permeation in POPC bilayers, (ii) domains enriched in these lipids or domain interfaces may represent regions with high H+/OH− conductivity, (iii) H+/OH− fluxes are not governed by lipid order and (iv) chol can inhibit or promote H+/OH− permeability depending on the total lipid environment. Theories of proton permeation are discussed in the light of these results.
Keywords: cholesterol, diffusion, leak, lipid bilayer, sphingomyelin, water and proton permeability
Abbreviations: AQP1, aquaporin 1; CF, 5,6-carboxyfluorescein; chol, cholesterol; DPH, diphenylhexatriene; PC, phosphatidylcholine; DPPC, dipalmitoyl PC; NEFA, non-esterified fatty acid; POPC, palmitoyl-2-oleoyl PC; SBPL, soya-bean polar lipids; SM, sphingomyelin
INTRODUCTION
Phospholipid bilayers are selectively permeable to water and other small non-electrolytes but virtually impermeable to ions. This impermeability arises because of the low dielectric in the hydrocarbon interior of the membrane, resulting in a prohibitive Born energy requirement for ions to leave the aqueous environment and enter the membrane [1,2]. Ions such as Na+ and Cl− therefore tend to be relatively impermeant in the absence of specific channels. However, protons (H+) appear to be exceptional in that they permeate five to six orders of magnitude more rapidly than other univalent cations [3–6]. Protons exhibit further anomalous behaviour in that permeability appears to be insensitive to pH, even when conditions are varied from highly acidic (pH 1) to highly alkaline (pH 11) [2]. These results suggest that the mechanism of proton movement across membranes is likely to be unique to the special properties of hydrogen nuclei.
Although the mechanism of proton permeation across membranes is still unclear, there have been several theories that attempt to explain it: these include water wires, water clusters and weak acids. The water-wire hypothesis predicts that protons are transported across the membrane via a Grotthus-type conductance. In this mechanism, chains of transient hydrogen-bonded water molecules termed water ‘wires’ are produced by thermally induced defects in lipid packing. At one end of the water wire, a proton hydrogen-bonds with water to form a hydronium (H3O+) ion. The excess proton is then free to hop from water molecule to water molecule ultimately to be released from the other end of the wire and in the process induce an orientational switch in the alignment of water molecules.
Haines [7] proposed a ‘cluster-contact’ model for proton permeation, in which small clusters of water molecules within the bilayer, some of which might stabilize and carry an excess proton, could donate that proton to other clusters and in that way facilitate its transfer from one side of the bilayer to the other. This concept has been partly validated in a recent computational modelling study which adds important new information to the debate about mechanism. Tepper and Voth [8] showed that a single excess proton can stabilize a network of hydrogen-bonded water molecules (clusters) that span the membrane. The stabilized network exhibited lifetimes of hundreds of picoseconds, many times longer than that reported for single-file water wires and of sufficient duration to potentially account for elevated permeation rates.
The weak acid hypothesis stipulates that there are molecular species present within or on one side of the bilayer which can become protonated, translocate or diffuse to the other side and then release a proton. Potential candidates for such a mechanism include lipid hydrolysis products or contaminants such as NEFAs (non-esterified fatty acids) [3–6]. Water wires, water clusters or weak acids could therefore provide a conduit for proton translocation which is not available to other ionic species.
If water wires, water clusters or NEFA shuttles mediate proton flux, then we would predict that increasing the concentrations of chol (cholesterol) and SM (sphingomyelin), lipids that order the bilayer and reduce water permeability, should reduce proton permeability. For Grotthus conductance, increased lipid order should reduce the likelihood that water wires (and water clusters) will form in defects and kinks within the bilayer. In the case of the weak acid shuttle, increased lipid order should slow the diffusion of weak acids from one side of the bilayer to the other, as it occurs with the flux of small non-electrolytes such as urea and glycerol [9].
Of interest from a physiological standpoint is how proton permeability is regulated in cellular membranes. Most of the biophysical studies on proton permeation have been performed in planar bilayers or simple liposomal systems with a view to understanding the mechanisms of proton flux and the basis for the anomalously high proton permeability. These studies have often used readily available pure lipids such as DPPC [dipalmitoyl PC (phosphatidylcholine)] or lipid mixtures extracted from natural sources such as soya beans or Escherichia coli plasma membrane. There have been few attempts to systematically examine the role of physiologically important lipids such as chol and SM in influencing proton permeation. We have attempted to partially redress this by using a model system based on a pure PC lipid [POPC (palmitoyl-2-oleoyl PC)] with one unsaturated acyl chain that renders the bilayer fluid at room temperature (23 °C) and should be essentially free of lipid breakdown products or other contaminants.
Chol and SM are known to form self-associating lipid domains that have been termed rafts [10–13]. They are also present in high concentrations in the outer leaflet of apical membranes of barrier epithelia where they function to increase lipid order and reduce membrane permeability [9,14]. In a recent study from our laboratory in which the inner and outer leaflet of the MDCK (Madin–Darby canine kidney) apical membrane was reconstituted in liposomes, it was observed that while water permeability was 18 times lower in outer leaflet liposomes than in inner leaflet lipids, proton permeability was 4-fold higher [15]. Lipid subtraction experiments implicated both chol and SM as facilitators of proton conduction. Since both of the major theories of proton permeation would predict that increasing chol should result in a decrease in proton flux rates, this finding prompted us to examine systematically the effect of increasing chol and SM concentrations in POPC vesicles. Surprisingly, chol and SM each increased proton permeability. There was a complex concentration-dependent behaviour, which in general demonstrated a pronounced enhancement of proton permeation in the presence of chol and a moderate enhancement with SM. There was no synergistic effect when both were present simultaneously under conditions that might be expected to promote raft formation. Potential explanations for these findings and the implications for cellular membranes are discussed.
EXPERIMENTAL
Liposome preparation
Liposomes were prepared from the following lipids: POPC, Avanti Polar Lipids catalogue no. 850457C; chol, Sigma catalogue no. C-8667; brain SM, Avanti catalogue no. 860062; and SBPL (soya-bean polar lipids), Avanti catalogue no. 541602. Liposomes were also prepared using POPC obtained from Matreya (catalogue no. 1437-1). Proton flux rates were not different between liposomes prepared from POPC sourced from either company, so Avanti POPC was used for the experiments presented.
Liposomes made from POPC, chol, SBPL and SM were prepared essentially as described in [15]. In that earlier study, we confirmed using isotopically labelled lipids that all of the lipids were present in their correct molar ratios. Lipids were dissolved in chloroform/methanol (2:1, v/v) to generate 10 mg/ml stock solutions. The lipids were divided into aliquots (300 μl) by volume into glass vials and the solvent was evaporated under a stream of nitrogen for 30 min. Lipids were then placed in a vacuum chamber for 2 h at −90 kPa (27 in. Hg). Since it has been reported that small amounts of organic solvent can dramatically affect proton permeation rates [1,16], we tested drying times between 0.5 and 12 h. Proton flux rates did not vary with drying times of 2 h or longer; therefore 2 h was routinely used for all experiments. Vials containing fully dried lipids not for immediate use were flushed with nitrogen, sealed and stored at −20 °C.
POPC liposomes were prepared by adding a buffer containing 2 mM CF (5,6-carboxyfluorescein), 150 mM NaCl, 1 mM dithiothreitol and 10 mM Hepes (pH 7.4) followed by vortex-mixing and sonication. Soya-bean liposomes were prepared in 1 mM CF. Lipids were probe sonicated on ice for 30 s over 2 min intervals, typically between five and ten times total. Vesicle sizes (average radius) were determined by quasi-elastic light scattering using an LSR (light scattering reader) Dyna Pro particle sizer (Protein Solutions) and DYNAMICS data collection and analysis software as per the manufacturer's instructions. If most of the particles were larger than 250 nm in radius, liposomes were sonicated again for 10 s and/or extruded through a 200 nm polycarbonate membrane. PD10 desalting columns (Amersham Biosciences) were used to remove extravesicular CF. CF antibody (Molecular Probes, Eugene, OR, U.S.A.) was added to quench any residual extravesicular CF. The quantity of antibody required for complete quenching of all extravesicular CF was determined on an Aminco Bowman Series 2 luminescence spectrometer.
MS
To ensure that our results were not affected by lipid degradation as a result of repeated sonication cycles, the fatty acid content of lipids/liposomes was examined with MS. Lipids to which 500 μl of buffer was added were vortex-mixed, or vortex-mixed and then sonicated, as described above. The samples were then dried down at 37 °C under a stream of nitrogen. MS was performed at the University of Pittsburgh Mass Spectrometry Facility (Molecular Medicine Institute, Pittsburgh, PA, U.S.A.). Samples were extracted, the organic phase was removed and dried over nitrogen. The dried sample was then brought up in 100 μl of chloroform/methanol (1:2, v/v) prior to analysis. Samples were analysed by electrospray ionization MS on a triple quadrupole instrument (Micromass). Samples were scanned in the range of 200–700 m/z for mass ions pertaining to palmitic acid (C16:0, m/z 255) and oleic acid (C18:1, m/z 281) in the negative ion mode. Samples were quantified against internal and external standards.
Water permeability measurements
Water permeability of liposomal membranes was measured at 25 °C as described in [9,15,17] using stopped-flow fluorimetry (on an SX.18MV Applied Photophysics stopped-flow reaction analyser). In brief, liposomes prepared in a buffer containing 2 mM CF were rapidly exposed to a hyperosmotic gradient. Equal volumes of vesicles and an identical buffer whose osmolality was three times higher (due to addition of sucrose) were rapidly mixed. Buffer osmolalities were determined on a Precision Systems Osmette A osmometer. Vesicle shrinkage results in self-quenching of CF fluorescence. The rate of vesicle shrinkage in response to the gradient was measured as a reduction in fluorescence with time. The resulting curves (8–10 experiments) were averaged and single exponential curves were fitted to the data. Mathcad software (MathSoft) was used to calculate Pf (osmotic water permeability coefficient) as a function of the applied osmotic gradient, average vesicle radius and the measured rate of CF quenching.
Proton permeability measurements
CF fluorescence at 2 mM is both concentration-dependent and pH-dependent. We used this pH-dependence to directly measure the dissipation of an imposed pH gradient on the liposomes. Since the solutions to be mixed are osmotically balanced, there are no volume-related changes reported by the dye. Proton permeability coefficients (PH+) were measured at 25 °C as described in [9,15,17]. Stopped-flow measurements involved rapid mixing of equal volumes of CF-containing liposomes in pH 7.4 buffer, with acidified buffer. This results in a pH gradient of 0.3–0.5 pH units, which has been shown not to induce the formation of limiting potentials from an accumulation of positive charge. In prior studies with valinomycin-treated liposomes and K+-containing buffer to shunt charge accumulation, we obtained identical acidification rates in the absence or presence of valinomycin [15,18]. It appears that measurements of proton flux involving small gradients near neutral pH do not require the use of charge-dissipating ionophores [19]. Resulting curves (8–10 experiments) were averaged and fit with a single exponential function. In some experiments, a small linear component remained after quenching appeared to be complete; these curves were fit by adjusting the fit range or applying a single exponential fit with a linear component. Internal buffer capacities of the liposomes were determined using an Aminco Bowman Series 2 luminescence spectrometer by adding 0.5 μl aliquots of 1 M HCl to liposomes to calibrate the change in pH to change in fluorescence. Addition of sodium acetate (10 mM final) to fresh liposomes results in an acidification of the interior of the liposomes which can be quantified and allows calculation of the internal buffer capacity. PH+ was calculated as a function of the internal buffer capacity and the rate of proton flux using the following equation:
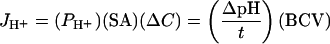 |
where JH+ represents proton flux in mol/s, SA is the surface area of a veside, ΔC is the initial difference in proton concentration inside and outside the vesicles, ΔpH is the change in pH when time equals τ, the time constant of the single exponential curve describing the initial change of fluorescence as a function of time, and BCV is the buffer capacity of an individual vesicle in mol·l−1·pH unit−1.
Fluorimetry
Fluorimetry was performed on an Aminco Bowman Series 2 luminescence spectrometer at excitation and emission wavelengths of 490 and 520 nm. Fluorescence anisotropy measurements were performed on liposomes that had been equilibrated with 2 μM DPH (diphenylhexatriene; Molecular Probes) for 30 min at room temperature in the dark. Fluorescence polarization measurements were conducted on an Aminco Bowman Series 2 luminescence spectrometer equipped with automated prism polarizers at emission and excitation wavelengths of 354 and 428 nm. Data collection and anisotropy analysis were performed on AB2 software supplied by Spectronic Unicam.
RESULTS
Gramicidin
As Deamer and Nichols [1] have noted previously, it is impossible to differentiate between the movement of protons into a vesicle and the movement of hydroxide anion in the opposite direction. This is certainly true when experiments are performed close to neutral pH. As a consequence of this uncertainty, we have chosen to use the term ‘proton flux’ in the present study to describe the dissipation of pH gradients as measured by pH-dependent quenching of a fluorescent indicator. We remain aware, however, that the permeating species could be OH−.
Proton permeabilities through lipid bilayers have been measured using a range of techniques and have produced widely varying permeability coefficients even for bilayers of the same composition [2] – therefore an attempt was made to validate independently the permeability coefficients obtained in these experiments. Gramicidin is an antibiotic peptide pore that conducts both water and protons. Since the water and proton conductances of gramicidin are known [20,21], it offered us a means to validate our assay system. We measured the osmotic water permeability of liposomes into which gramicidin was inserted and, since the single channel conductance for water is known, an estimate of the number of channels per liposome could be arrived at. Since we can estimate the number of channels as well as the number of liposomes, our measurements of proton permeability, which rely upon pH-dependent fluorescence quenching of intravesicular CF, then allowed us to calculate a single channel proton conductance for gramicidin. That number was compared with a previously determined value for proton conductance measured at a similar pH [22].
Liposomes were prepared from 0.9 mg of POPC (7.128×1017 molecules) and then 10 or 1 μM gramicidin was added. To determine the total water conductance of these liposomes, we calculated the number of liposomes.
Average liposome radius was 148 nm; therefore the surface area/liposome was 2.753×10−9 cm2. Assuming each phospholipid occupies an average interfacial area of 72.5 Å2 (1 Å=0.1 nm) [23], the number of lipids per liposome is 7.59×105. Therefore the total number of liposomes was estimated to be 9.39×1011. The total surface area of this many liposomes is 2584 cm2. This compares well with the value of approx. 2200 cm2/mg lipid estimated by Deamer and Nichols [1]. We multiplied this value by the measured water permeability coefficient (Pf=3.1×10−2 cm/s) with background membrane permeability (Pf=6.3×10−3 cm/s) subtracted, to obtain a total water conductance through gramicidin channels of 63.8 cm3/s.
The single channel water conductance for a gramicidin pore has been estimated at either 1×10−14 cm3/s [21] or 6×10−14 cm3/s [20] in two separate studies. Therefore the number of conducting gramicidin dimers/liposome is the total conductance divided by the product of the single channel water conductance for gramicidin and the number of liposomes. Using both estimates for single channel conductance, we obtained either 6797 or 1133 channels per liposome.
Liposomes containing CF were exposed to a pH gradient (ΔpH=0.29) and the rate of proton flux was derived from the rate of vesicle acidification. With passive membrane flux subtracted, JH+=5.24×10−20 mol·s−1·liposome−1. The amount of current per liposome was determined as the proton flux multiplied by Faraday's constant, and since 1 pH unit is equivalent to 0.059 V, we derived the conductance per liposome:
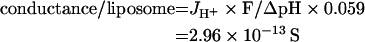 |
Therefore single channel proton conductance for gramicidin in our liposomes will be either 4.35×10−17 or 2.61×10−16 S. The single channel proton conductance of a gramicidin pore according to Krishnamoorthy [22] is (2.1±0.5)×10−16 S for H+ at pH 7.5. Since our assay system gives us a value which is in excellent agreement with one estimate of single channel water conductance and not more than 5-fold different from the other estimate, we believe that our proton permeability coefficients under the specific conditions employed are likely to be reasonably accurate. When 1 μM gramicidin was used instead of 10 μM, conductances of 2.67×10−17 and 1.60×10−16 S were obtained – also in very good agreement with the Krishnamoorthy [22] value.
Fatty acid contaminants or degradation products in sonicated liposomes
Fatty acids were analysed in lipids that had been resuspended in a buffer by vortex-mixing and in liposomes that had been prepared by multiple cycles of sonication. This analysis was performed for two reasons. Firstly, to ensure that the commercially available POPC did not have significant levels of NEFAs, which have been proposed to function as ‘protonophores’ by facilitating proton binding and translocation across the bilayer. Secondly, to check that the method used for preparing liposomes was not responsible for generating high levels of additional fatty acids during sonication. We employed MS to quantify the levels of palmitate and oleate in pure POPC and POPC to which 45.7 mole% chol was added. The masses of palmitate and oleate respectively in vortex-mixed POPC were 14.3 and 46.7 ng respectively in 5 mg of lipid (means for duplicate samples). On a weight basis, this represents 0.0003 and 0.0009% respectively or alternatively on a molar basis, 8.5 and 25.1 p.p.m. Liposomes prepared using seven cycles of sonication and cooling (described in the Experimental section) showed a slight increase in fatty acid levels. The increases in palmitate and oleate for POPC liposomes were 56 and 24% respectively and for POPC/chol liposomes were 21 and 9% respectively (based on the means for duplicate analyses for each condition). If we take the data in aggregate, the mean increase in fatty acid levels due to sonication is approx. 27.5%. Therefore the concentration of palmitate and oleate increases to 10.8 and 32 p.p.m. respectively. There was no significant difference between the pure POPC and POPC/chol liposomes in terms of the effects of sonication. These levels are exceptionally low. It seems unlikely that 40 NEFAs for every million phospholipids would contribute in a significant way to the proton fluxes we measure.
Water permeability
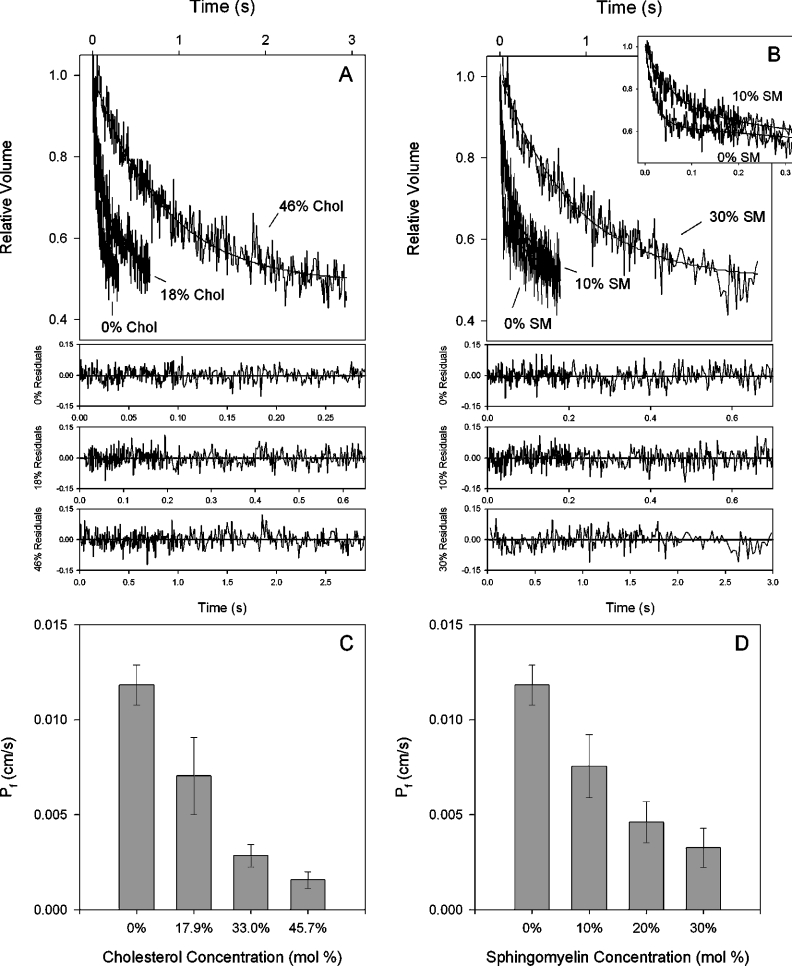
Although these experiments were primarily aimed at studying the effects of chol and SM on proton permeability of PC bilayers, we also measured the effects of altering lipid compositions on water permeability. These water flux measurements permitted us to show, on the same membranes as were used for proton fluxes, that increased lipid order decreased water permeation. An intuitive prediction of the proton wire hypothesis is that a more highly water-permeable membrane should have a greater number of hydrogen-bonded chains. In Figure 1, stopped-flow data show the effect of increasing chol (Figure 1A) and increasing SM (Figure 1B) concentrations on liposomal water efflux kinetics. With increasing concentrations of either lipid, the vesicles shrank more slowly in response to an osmotic gradient. The inset in Figure 1(B) shows on an expanded scale the difference between 0 and 10% SM. Single exponential curves have been fitted to the normalized fluorescence data and the residuals of the fitted curves are shown below. The raw data are clearly not ideal single exponential functions as assessed by deviations from the fit. This is anticipated on the basis that we are measuring the average permeability of a population of liposomes with varying sizes and potentially compositions (see the Discussion section regarding microdomain formation). However, deviations from the fit are not pronounced and flux rates are fitted well by the assumption of a single exponential. For reasons that are unclear, some experiments generated curves that exhibited a slow steady-state ramp following initial shrinkage, as may be seen in Figure 1(B) (0 and 10% SM). The results from three to five separate liposomal preparations are shown in Figures 1(C) and 1(D) for chol and SM respectively. It is clear that the presence of increasing chol or increasing SM in the vesicle membrane decreased water permeability in a concentration-dependent fashion. The concentrations of chol and SM chosen were designed to cover the range found in cell membranes. With the addition of 46 mole% chol, water permeability decreased by 87% relative to pure POPC liposomes. Liposomes with 30% SM exhibited a 72% reduction in water permeability relative to pure POPC liposomes. These results are consistent with prior studies [9,15] and are due to the known membrane ordering properties of chol and SM [24,25].
Figure 1. Water permeability of POPC liposomes with varying amounts of chol and SM.
Liposomes were prepared with 2 mM entrapped CF and rapidly exposed to a doubling of external osmolality. (A) Effect of increasing chol on water permeation kinetics. Stopped-flow tracings (average of eight to ten trials) show quenching of CF as vesicles shrink. Fluorescence was converted into relative volume and single exponential curves were fitted to the data. The residuals from those fits are shown for each composition below (A, B). (B) Effect of increasing SM on water permeation kinetics. The inset shows more detail of the curves for 0 and 10 mole% SM. (C) Osmotic water permeability of liposomes with varying chol concentrations. (D) Osmotic water permeability of liposomes with varying SM concentrations. Results in (C, D) are from three to five different liposomal preparations. Results shown are the means±S.E.M.
Proton permeability
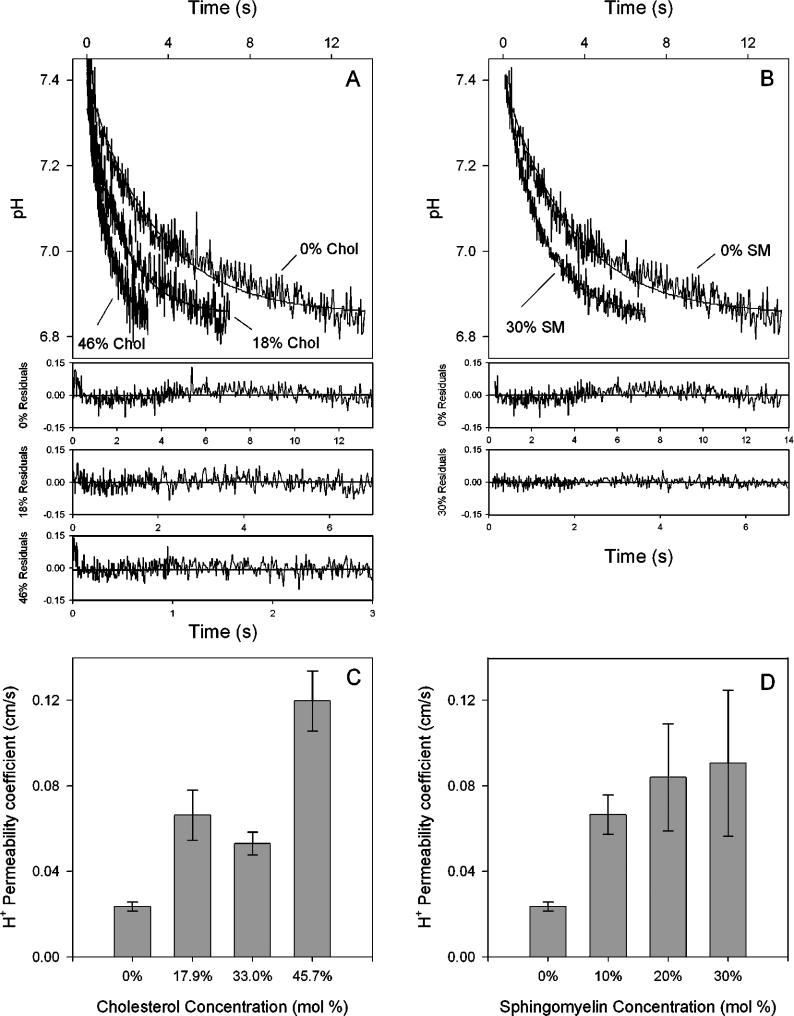
Proton fluxes in POPC liposomes with varying chol and SM concentrations were measured upon exposure of liposomes to a pH gradient of 0.3–0.5 pH unit. Stopped-flow tracings in Figures 2(A) and 2(B) show the quenching of intravesicular fluorescence upon internal acidification. In Figure 2(A), it can be seen that increasing chol to 17.9 and 45.7 mole% accelerated proton flux rates across the membrane. The same phenomenon was noted when SM was present at 30 mole% (Figure 2B). The residuals of the single exponential fits are shown below and reveal somewhat greater deviations from ideal kinetics than was observed for water permeation. It is realistic to conclude that the single exponentials shown approximate to what may be a multicomponent process. The conservative approach to analysing these kinetics permits the use of a single exponential function to approximate the process. The results from experiments on three to five separate liposome preparations are shown in Figures 2(C) and 2(D). In Figure 2(C), it can be seen that proton permeability of liposomes with 45.7 mole% chol increased 5-fold, from 0.024±0.002 to 0.12±0.01 cm/s (P<0.001), while 30% SM increased permeability 3.8-fold to 0.091±0.034 cm/s (P<0.05). The chol-mediated increase was not a simple concentration–response relationship however. At 33 mole%, the permeability was not statistically different from 17.9%, possibly reflecting a different lipid phase behaviour at this concentration (see the Discussion section). The SM response was also complex in that 10% SM increased proton permeability by 280% (P<0.01) but 20 and 30% SM did not significantly increase it further. These results suggest that chol is more potent than SM at facilitating proton transfer and that SM at relatively low molar concentrations is able to induce a 3-fold increase in proton permeation.
Figure 2. Proton permeability of POPC liposomes with varying amounts of chol and SM.
Liposomes were prepared with 2 mM entrapped CF and rapidly exposed to a pH gradient of 0.3–0.5 pH unit. (A) Effect of increasing chol on proton permeation kinetics. Stopped-flow tracings (average of eight to ten trials) show quenching of CF as intravesicular pH decreases. Fluorescence was converted into pH and single exponential curves were fitted to the data. The residuals from those fits are shown for each composition below (A, B). (B) Effect of increasing SM on proton permeation kinetics. (C) Proton permeability of liposomes with varying chol concentrations. (D) Proton permeability of liposomes with varying SM concentrations. Results in (C, D) are from three to five different liposomal preparations. Results shown are the means±S.E.M.
Flux behaviour of liposomes
To eliminate the possibility that our methods for drying lipids and for preparing liposomes were influencing the results ‘artefactually’, we dissolved POPC and chol at different ratios, in chloroform alone, and dried the lipids as described in the Experimental section. The concern was that the lipids might demix as solvents were evaporated and that as a result the liposomes would not be compositionally uniform. The lipids were dried for 14 h under vacuum and then prepared by extrusion through a 200 nm polycarbonate filter with no sonication. Proton permeability coefficients on liposomes prepared in this way showed no significant differences from the data presented in Figure 2(C), with a baseline value of 0.014±0.008 cm/s for POPC liposomes with no chol and increases of 2.2-fold at 17.9% chol and 6.1-fold at 45.7% chol. We conclude that our preparative methods do not result in liposomes with macroscopic heterogeneity and our measurements of proton flux are accurate as confirmed by the gramicidin experiment.
Fluorescence anisotropy
Water permeability showed a clear concentration dependence on chol and SM levels and this was likely to be due to the ordering properties of these lipids [15,25,26]. However, proton permeability in the presence of increasing concentrations of chol and SM showed complex behaviour which was not dose-dependent. To verify that lipid order was varying in a consistent manner with the inclusion of chol and SM in our liposomes, measurements of steady-state fluorescence anisotropy of the membrane-permeant probe, DPH, were made (Figure 3). This technique, while unable to report directly on membrane microviscosity as once thought, can provide indications of relative lipid order, since the probe is more sensitive to order (half cone angle of motion) than to rate of probe rotation (membrane viscosity) within the bilayer [27,28].
Figure 3. Relative membrane order for different membrane compositions assessed by steady-state fluorescence anisotropy of DPH.
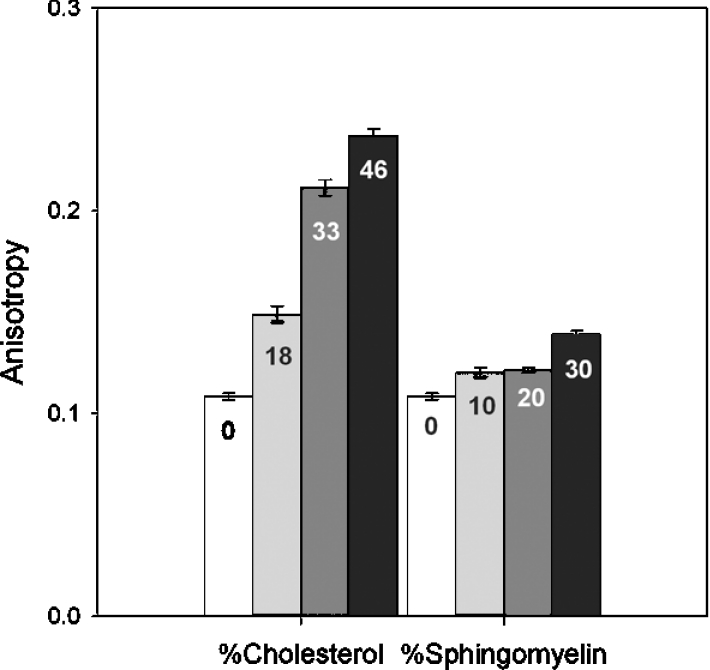
Chol and SM percentages are indicated on the bars and have been rounded up to the nearest whole number. The higher the anisotropy, the more ordered the membrane.
Increasing either chol or SM concentration (mole% shown in bars in Figure 3) increased anisotropy, supporting the contention that more chol or SM in the membrane caused the lipids to pack more tightly in a concentration-dependent manner. Higher anisotropy values correspond to increased membrane order. Therefore incrementally increasing order in the membrane with higher concentrations of chol or SM results in decreased water permeability. The relationship between water permeability and anisotropy is shown in Figure 4. It is of interest to note that while water permeabilities are reduced by comparable amounts at approx. 30 mole% of either lipid (Figures 1C and 1D), the anisotropy exhibited by each membrane is very different (Figures 3 and 4). This difference in anisotropic behaviour displayed by DPH probably reflects differences in probe motion within bilayers of different composition as noted by Kaiser and London [29].
Figure 4. Relationship between osmotic water permeability and anisotropy for liposomes with varying chol and SM content.
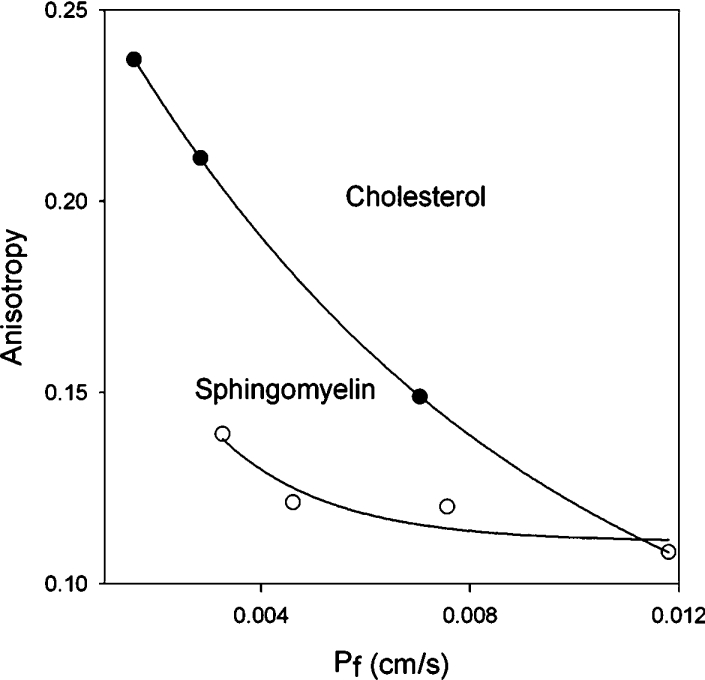
Whereas water permeability is reduced, proton permeability is not similarly restricted. Therefore, unlike most non-electrolytes that exhibit higher permeation rates with decreased membrane order, proton permeation exhibits no such correlation.
Are there synergistic effects of chol and SM on water and proton permeation?
We wished to test the hypothesis that specific hydrogen-bonding interactions thought to occur between chol and SM [11,12,26] would further enhance proton permeation. Initially we measured water permeability and anisotropy of membranes containing combinations of chol and SM. It can be seen in Figure 5(A) that at a fixed level of 17.9 mole% chol, addition of SM tended to reduce water permeability moderately and slightly increased membrane order (Figure 5B). At 46 mole% chol, there was very low water permeability, which was essentially unaffected by addition of SM and these membranes exhibited higher anisotropy (Figure 5B). These results suggest that at lower chol concentrations, SM has some ability to reduce water permeability further, but at the higher level, SM has little further impact on lipid ordering.
Figure 5. Effect of different combinations of chol and SM on water permeability (A) and membrane order (B).
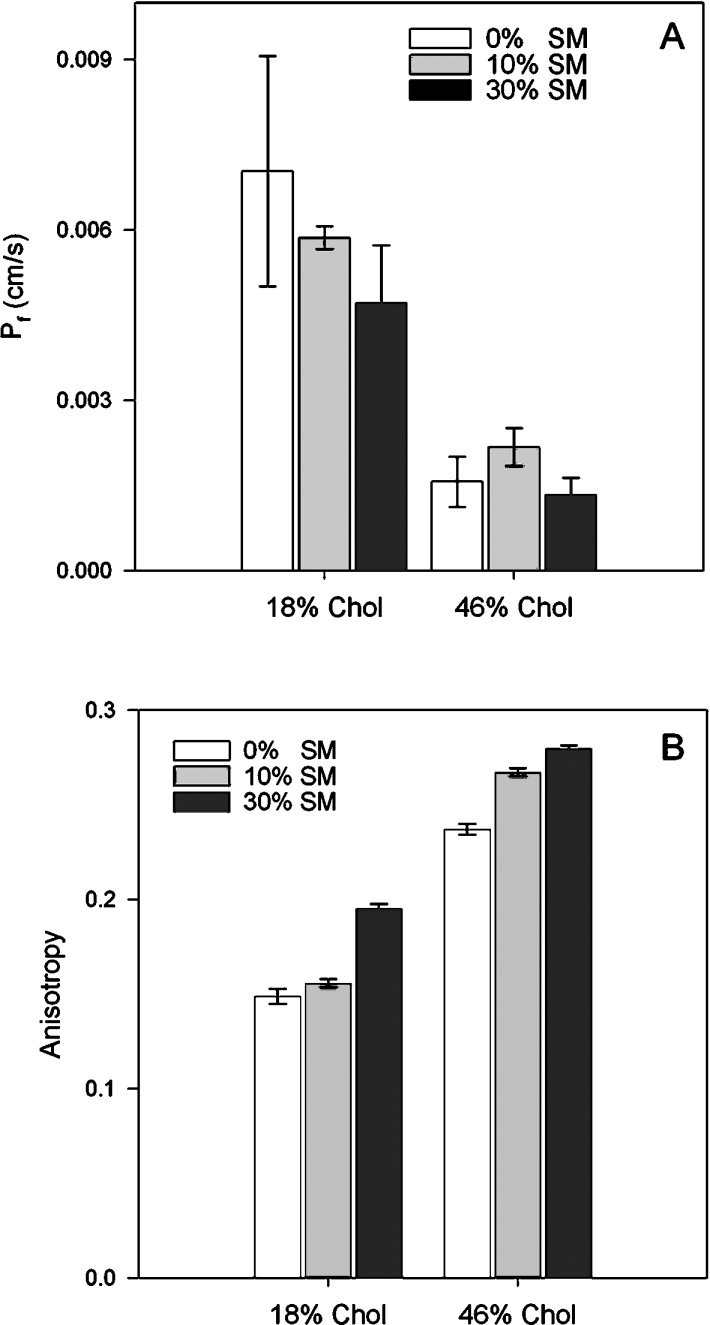
Chol percentages have been rounded up to a whole number.
Addition of SM to membranes that contained 46 mole% chol resulted in no significant increases in H+ permeability (Figure 6, black bars). With membranes containing 18 mole% chol, there was a reduction of proton permeability upon addition of 10 or 30% SM which was not concentration-dependent. These results indicated that SM in combination with chol had little additional effect on proton permeability and certainly did not facilitate a further increase in proton flux rates. Therefore chol and SM in combination appear not to synergize their effects on proton permeability.
Figure 6. Effect of different combinations of chol and SM on proton permeability.
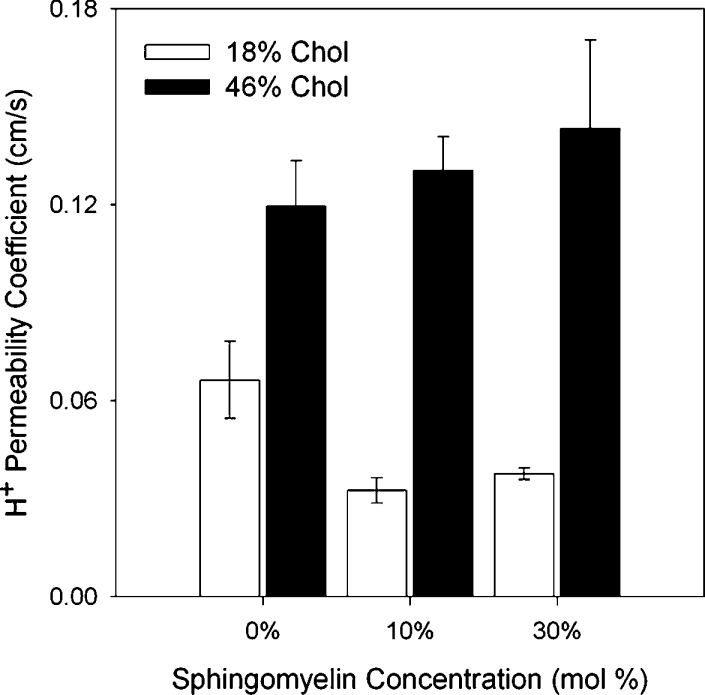
Chol percentages have been rounded up to a whole number.
Proton permeability of soya-bean lipid liposomes
Ira and Krishnamoorthy [30] have shown that addition of chol reduces proton permeability in SBPL liposomes. Since this observation was at odds with our POPC data and there were several methodological differences between our study and theirs, we decided to test SBPLs in our system. The results shown in Figure 7 recapitulate the findings of their original study. Proton flux rates progressively decreased as chol was increased in this lipid background. Figure 7(A) shows the stopped-flow curves normalized to the change in intravesicular pH and Figure 7(B) shows the reduction in both flux rate and proton permeability coefficients with increasing chol in one experiment. The phenomenology was the same whether we used our buffer system (shown) or the Ira and Krishnamoorthy [30] buffers, which contained potassium chloride and potassium phosphate. This result implies that the effect of chol on H+/OH− permeation is context-dependent.
Figure 7. Effect of chol on proton permeability in soya-bean lipid liposomes.
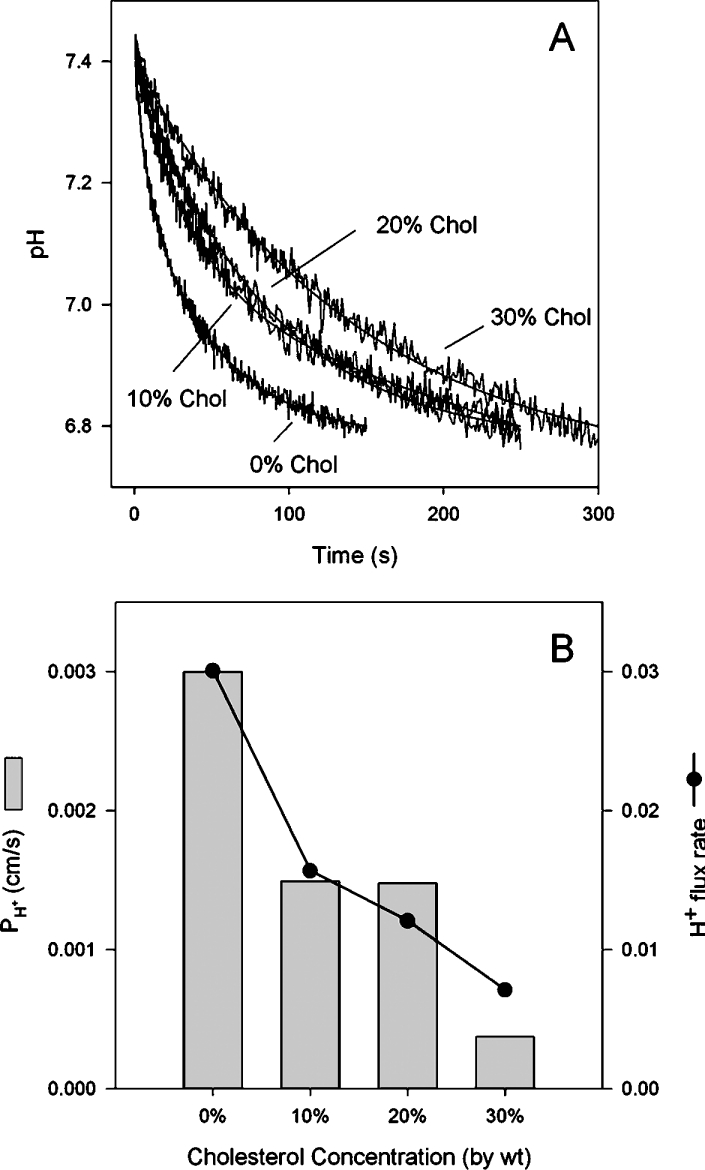
(A) Stopped-flow tracings showing pH gradient dissipation for liposomes with differing concentrations of chol. Increasing chol leads to slower proton flux rates. (B) Flux rate and permeability coefficients for liposomes of different compositions. Results shown are from one experiment (of three).
DISCUSSION
Current models for proton flux (water wires, water clusters and weak acid/base shuttle) predict that as membrane order increases, proton permeability should fall. Increasing lipid order reduces the water permeability of bilayers [9] and therefore likely reduces the quantity of water within the bilayer. Intuitively, it seems reasonable that less bilayer water should reduce the probability of water wire formation. Increased order should also impede the diffusion of weak acids, as it does for urea and other small non-electrolytes. However, earlier results in which we modelled the leaflets of a barrier epithelial membrane and examined their permeabilities suggested that this was not always true [15]. Specifically, liposomes enriched in PC, sphingolipids and chol (outer leaflet composition) had lower water and urea permeabilities but higher proton permeabilities than the inner leaflet lipids. Furthermore, removing chol and sphingolipids from outer leaflet liposomes reduced proton permeability, suggesting specific facilitative influences of these lipids on the ability of protons to permeate. To test these models further, we decided to focus on the lipids that are known to preferentially self-associate in the outer leaflet of many cell membranes, i.e. PC, SM and chol. These lipids have been used in equimolar ratios to model lipid rafts and appear to approximately recapitulate the lipid composition of detergent-insoluble microdomains extracted from cells [31]. In these experiments, we made liposomes from a pure PC species (POPC, C16:0–C18:1) which is fluid at room temperature, has been well characterized in combination with SM and chol for its phase separation behaviour [32–34] and has a commonly occurring 1-saturated, 2-unsaturated motif. We investigated the effects on H+/OH− permeation of adding chol and SM at physiological concentrations.
Because proton permeabilities have been reported to vary from 10−9 to 10−1 cm/s [2] and the results appear to be conditional on the experimental system used, we attempted to validate our proton flux measurements using the well-studied proton- and water-conducting antibiotic peptide, gramicidin. Our method for estimating the quantity of functional gramicidin pores used measurements of bulk water flux and the estimated single channel water conductance of gramicidin. Our water permeability assay has been validated extensively [9,17,35–40] and measurements of AQP1 (aquaporin 1) conductance in reconstituted proteoliposomes matched native AQP1 water fluxes in the intact red blood cell very well [41]. In addition, our measurements of liposomal water permeability were independently validated in planar bilayers using scanning Na+-sensitive electrodes to measure Na+-dilution within the unstirred layer [42].
The agreement between the single channel proton conductance that we arrived at and the value reported by Krishnamoorthy [22] at pH 7.5 indicates that our proton permeability coefficients are reasonable. For pure POPC liposomes, PH+ was 2.36×10−2 cm/s. It should be noted, however, that one of the anomalous aspects of proton permeability is an apparent insensitivity to pH, i.e. bulk [H+] [1]. This has led to discussion on whether the derivation of permeability coefficients is appropriate for protons. Since permeation theory predicts that proton flux should be proportional to the rate at which protons encounter the membrane and that rate should be proportional to the bulk concentration of protons, the insensitivity to pH is problematic [2]. Potential explanations include the ‘antenna’ effect of the membrane surface whereby negatively charged head groups can concentrate protons at the membrane interface [43,44] and therefore alter the effective local concentration. Increasing the negative surface charge of the membrane has indeed been shown to increase proton permeability [45]. Conversely, proton fluxes through gramicidin have been shown to depend on bulk [H+] and the parameter PH+ is relatively invariant with pH as it should be [46]. We believe therefore that our assay system is accurately measuring proton flux rates across different membranes because of the reasonable agreement with gramicidin's conductance properties; however, it is possible that PH+ may not accurately describe the intrinsic proton permeability of these membranes. Nonetheless, it offers a useful benchmark for comparing permeabilities at the same pH.
Our results clearly indicate that in POPC bilayers the addition of chol or SM results in facilitated proton flux, while water flux rates are restricted. A number of studies have noted that there appears to be little or no correlation between the water and proton permeability of membranes. Gutknecht [6] demonstrated no correlation between water and proton permeability in planar bilayers made from bacterial phosphatidylethanolamine, diphytanoyl PC or egg PC+chol. Lande et al. [9] showed that liposomes deliberately constructed to exhibit varying fluidities demonstrated water permeabilities that varied 70-fold, while proton permeabilities only changed 3-fold. These results and others argue that there are very different mechanisms by which water and protons permeate bilayers. However, it has still been widely accepted that membranes that drastically reduce water permeability will also tend to reduce proton permeation. Ira and Krishnamoorthy [30] specifically examined this question and found that soya-bean lipid liposomes had progressive reductions in proton permeability with increasing mole% of chol. This effect was interpreted as being due to decreasing water content based on lifetime measurements of the polarity-sensitive fluorescent probe, Nile Red. Ira and Krishnamoorthy [30] concluded that chol caused a reduction in membrane water and therefore reduced the probability of proton wire formation. The results presented in our study are in direct contrast with these and led us to measure proton leaks in SBPL liposomes (Figure 7). We found the same phenomenology described by Ira and Krishnamoorthy [30], leading to the conclusion that proton fluxes are dependent on the overall lipid milieu. Chol in and of itself does not ‘control’ proton flux rates. We speculate, however, that soya-bean phospholipids, which represent a natural mixture of lipids, may contain appreciable quantities of NEFAs. Some estimates put the levels of fatty acids as high as 1% in commercially available products [5]. Consistent with the weak acid hypothesis, these ‘contaminants’ could be responsible for transporting protons and the addition of chol would be expected to reduce flip-flop rates and hence proton flux, consistent with [30] and our findings. It is also possible that proton permeation rates may be highly dependent on specific lipid head group chemistries.
For the binary (SM/POPC and chol/POPC) and ternary (SM/POPC/chol) bilayers used in the present study, it is worth considering whether phase separation might play a role. The combination of a low Tm (‘melting’ temperature) phospholipid (POPC, −2.9 °C) with either a high Tm SM (natural SMs exhibit phase transitions in the range of 30–45 °C [26]) and/or chol will often result in micrometre scale phase separation depending on the precise lipid ratios and the temperature [33,34]. This creates the potential for coexisting liquid ordered (lo) and liquid disordered (ld) domains that protons may permeate through differentially and also for interfacial regions which will have different physical properties from the bilayer proper. A close look at the binary and ternary phase diagrams for POPC, palmitoyl SM and chol [33] reveals that some of the lipid combinations tested by us have coexisting phases, while others do not. At 25 °C, SM and POPC do not exhibit multiphase behaviour until the mole fraction of SM reaches 33%. Thus all of our SM/POPC liposomes exist in the ld state, although it should be noted that 30% SM lies close to a phase boundary. We conclude that the SM-induced increases in proton permeability were not due to domain formation.
For POPC/chol liposomes, the situation is more complex. Liposomes with no chol are uniformly ld, 17.9 and 33% chol have coexisting ld and lo domains, while 45.7% chol lies very close to, if not on, the lo boundary [33]. Chol at all three concentrations results in increases to proton permeability, although at 33%, permeability was lower that at 17.9% (Figure 2C). At 17.9% chol, the predominant phase is ld, although there is some lo present; at 33%, the proportions of ld and lo are likely to be similar, while at 45.7% most of the membrane surface area will be lo. The anisotropy data shown in Figure 3 support the conclusion that large changes in lipid order and phase states occur for chol-containing bilayers but not for SM-containing bilayers. The results suggest that the progression from a fully disordered to a mostly ordered bilayer is not strictly correlated with proton permeability. The same statement holds true for the presence of more interfacial area between domains. In other words, protons are not simply leaking through the interfaces between domains. If this were the explanation for chol-induced increases in permeability, then fluxes at 33% should be higher than at 17.9% chol.
The addition of varying amounts of SM to 17.9% chol/POPC results in membranes that have coexisting lo and ld domains. At 30% SM, these membranes lie close to a tri-phasic boundary which includes a solid phase. Domain sizes have been shown to vary depending on the composition of the membrane [32]. In areas of the phase diagram closer to the tri-phase boundary, lo rafts are much larger, while at lower SM or chol concentrations the rafts are much smaller. Despite these changes in domain structure, there is no difference in proton permeability between 10 and 30% SM (Figure 6), suggesting that domain behaviour is not a significant determinant. At 45.7% chol, the membrane is in a predominantly ordered state regardless of the concentration of SM and proton permeation kinetics was unchanged for these membranes, as is seen in Figure 6. Despite being in a fully ordered state, the proton fluxes are 2–4-fold higher for membranes with the higher chol concentrations. The results overall argue against a significant role for microdomains in determining proton flux. However, the possibility that phase state and interfacial fluctuations may contribute in some measure to the regulation of proton permeability is intriguing and deserves further investigation.
What other explanations might account for the chol and SM effects that we observed? It is known that the addition of chol increases hydration at the interfacial head group region of the bilayer [47]; therefore the presence of greater numbers of hydrogen-bonded water molecules in this region might facilitate proton movement into the bilayer at the critical interfacial juncture. Indeed, Chen and Li [48] demonstrated such a phenomenon when they showed that cardiolipin increased the proton permeability of DPPC liposomes by increasing surface hydration. Since head group spacing does not seem to be affected by the incorporation of chol [49], it would seem unlikely that proton permeation is enhanced as a result of charge dilution. This is also supported by the finding that SM increases proton permeability. With the same head group as PC, we believe dipole-mediated effects are unlikely to be responsible.
Both chol and SM reduce water permeability by increasing lipid packing order, a condition that presumably reduces the water content of the bilayer. As far as proton permeation is concerned, it may not be so much the quantity of the water in the bilayer as the quality. By this we mean that in a more condensed bilayer containing chol (or SM), the water molecules/clusters which ‘blaze a trail’ [2] across a bilayer may be constrained from a following a ‘random walk’ and be forced instead into a more ‘directed walk’. The single file of water molecules necessary for Grotthus conductance or Tepper and Voth's [8] ‘concerted mechanism’ in a rigidified membrane may be analogous to water molecules in a gramicidin channel and exhibits enhanced lifetimes or higher formation frequency when compared with more fluid membranes.
The lack of synergism when chol and SM are both present suggests that the predicted hydrogen-bonding between chol and SM [11] (but see [50] for an alternative viewpoint) is not important for proton permeation.
Physiologically, the effects of chol and SM on cellular pH homoeostasis may be important. We recently showed that isolated caveolae, a specialized form of lipid rafts, have high proton permeability (8.8×10−2 cm/s [51]). Since this represents proton permeation across a naturally occurring membrane rich in chol and SM, it provides support for the notion that raft lipids are physiologically important in regulating proton leak. Epithelia with barrier membranes such as those found facing the lumen of the stomach, bladder and kidney collecting duct are known to have high concentrations of PC, chol and SM in the outer leaflet of the apical membrane [15]. Proton leaks across membranes with these components may be part of an unavoidable energy cost to cells with a need to otherwise rigidify these membranes for functional and protective purposes. It is also interesting to note that acidified organelles such as lysosomes, endosomes and the trans-Golgi network have low levels of chol [52–54]. Haines [7] has argued that the main function of sterols may be to reduce Na+ leaks in mammalian cells and H+ leaks in prokaryotes and that chol can be considered an ‘energy saver’ molecule. The evidence presented in this study would tend to contradict this model, although it must be stated that much remains to be learned. Liposomes do not recapitulate the complexity of cellular membranes. However, the finding that chol and to a lesser degree SM appear to enhance proton permeation may require a re-evaluation of current thinking in regard to the link between water and proton permeability.
Acknowledgments
This work was supported by the National Institutes of Health grants DK-43955 and DK-48217 (to M.L.Z.) and the SmithKline Beecham Young Investigator grant of the National Kidney Foundation (to W.G.H.). We thank Dr John Mathai (Renal Division, Department of Medicine, University of Pittsburgh) for helpful discussions and suggestions on this paper, and Dr Andrew Amoscato of the University of Pittsburgh Mass Spectrometry Facility for analyses and advice.
References
- 1.Deamer D. W., Nichols J. W. Proton flux mechanisms in model and biological membranes. J. Membr. Biol. 1989;107:91–103. doi: 10.1007/BF01871715. [DOI] [PubMed] [Google Scholar]
- 2.Decoursey T. E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 3.Gutknecht J. Proton/hydroxide conductance through phospholipid bilayer membranes: effects of phytanic acid. Biochim. Biophys. Acta. 1987;898:97–108. doi: 10.1016/0005-2736(87)90028-9. [DOI] [PubMed] [Google Scholar]
- 4.Gutknecht J. Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J. Membr. Biol. 1988;106:83–93. doi: 10.1007/BF01871769. [DOI] [PubMed] [Google Scholar]
- 5.Gutknecht J. Proton conductance through phospholipid bilayers: water wires or weak acids? J. Bioenerg. Biomembr. 1987;19:427–442. doi: 10.1007/BF00770028. [DOI] [PubMed] [Google Scholar]
- 6.Gutknecht J. Proton/hydroxide conductance and permeability through phospholipid bilayer membranes. Proc. Natl. Acad. Sci. U.S.A. 1987;84:6443–6446. doi: 10.1073/pnas.84.18.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines T. H. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 2001;40:299–324. doi: 10.1016/s0163-7827(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 8.Tepper H. L., Voth G. A. Protons may leak through pure lipid bilayers via a concerted mechanism. Biophys. J. 2005;88:3095–3108. doi: 10.1529/biophysj.104.056184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lande M. B., Donovan J. M., Zeidel M. L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 1995;106:67–84. doi: 10.1085/jgp.106.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown D. A., London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 11.Bittman R., Kasireddy C. R., Mattjus P., Slotte J. P. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 1994;33:11776–11781. doi: 10.1021/bi00205a013. [DOI] [PubMed] [Google Scholar]
- 12.Smaby J. M., Momsen M., Kulkarni V. S., Brown R. E. Cholesterol-induced interfacial area condensations of galactosylceramides and sphingomyelins with identical acyl chains. Biochemistry. 1996;35:5696–5704. doi: 10.1021/bi953057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons K., Ikonen E. Functional rafts in cell membranes. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 14.Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 15.Hill W. G., Zeidel M. L. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J. Biol. Chem. 2000;275:30176–30185. doi: 10.1074/jbc.M003494200. [DOI] [PubMed] [Google Scholar]
- 16.Cafiso D. S., Hubbell W. L. Electrogenic H+/OH− movement across phospholipid vesicles measured by spin-labeled hydrophobic ions. Biophys. J. 1983;44:49–57. doi: 10.1016/S0006-3495(83)84276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivers R., Blanchard A., Eladari D., Leviel F., Paillard M., Podevin R. A., Zeidel M. L. Water and solute permeabilities of medullary thick ascending limb apical and basolateral membranes. Am. J. Physiol. 1998;274:F453–F462. doi: 10.1152/ajprenal.1998.274.3.F453. [DOI] [PubMed] [Google Scholar]
- 18.Hill W. G., Kaetzel M. A., Kishore B. K., Dedman J. R., Zeidel M. L. Annexin A4 reduces water and proton permeability of model membranes but does not alter aquaporin 2-mediated water transport in isolated endosomes. J. Gen. Physiol. 2003;121:413–425. doi: 10.1085/jgp.200308803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deamer D. W., Nichols J. W. Proton-hydroxide permeability of liposomes. Proc. Natl. Acad. Sci. U.S.A. 1983;80:165–168. doi: 10.1073/pnas.80.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dani J. A., Levitt D. G. Binding constants of Li+, K+, and Tl+ in the gramicidin channel determined from water permeability measurements. Biophys. J. 1981;35:485–499. doi: 10.1016/S0006-3495(81)84804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg P. A., Finkelstein A. Water permeability of gramicidin A-treated lipid bilayer membranes. J. Gen. Physiol. 1978;72:341–350. doi: 10.1085/jgp.72.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamoorthy G. Temperature jump as a new technique to study the kinetics of fast transport of protons across membranes. Biochemistry. 1986;25:6666–6671. doi: 10.1021/bi00369a051. [DOI] [PubMed] [Google Scholar]
- 23.Nagle J. F., Tristram-Nagle S. Structure of lipid bilayers. Biochim. Biophys. Acta. 2000;1469:159–195. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lentz B. R., Hoechli M., Barenholz Y. Acyl chain order and lateral domain formation in mixed phosphatidylcholine–sphingomyelin multilamellar and unilamellar vesicles. Biochemistry. 1981;20:6803–6809. doi: 10.1021/bi00527a010. [DOI] [PubMed] [Google Scholar]
- 25.Straume M., Litman B. J. Influence of cholesterol on equilibrium and dynamic bilayer structure of unsaturated acyl chain phosphatidylcholine vesicles as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry. 1987;26:5121–5126. doi: 10.1021/bi00390a034. [DOI] [PubMed] [Google Scholar]
- 26.Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim. Biophys. Acta. 1980;604:129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- 27.Kinosita K., Jr, Kataoka R., Kimura Y., Gotoh O., Ikegami A. Dynamic structure of biological membranes as probed by 1,6-diphenyl-1,3,5-hexatriene: a nanosecond fluorescence depolarization study. Biochemistry. 1981;20:4270–4277. doi: 10.1021/bi00518a006. [DOI] [PubMed] [Google Scholar]
- 28.Lee A. G. Lipids and their effects on membrane proteins: evidence against a role for fluidity. Prog. Lipid Res. 1991;30:323–348. doi: 10.1016/0163-7827(91)90002-m. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser R. D., London E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry. 1998;37:8180–8190. doi: 10.1021/bi980064a. [DOI] [PubMed] [Google Scholar]
- 30.Ira, Krishnamoorthy G. Probing the link between proton transport and water content in lipid membranes. J. Phys. Chem. B. 2001;105:1484–1488. [Google Scholar]
- 31.Edidin M. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 32.de Almeida R. F., Loura L. M., Fedorov A., Prieto M. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J. Mol. Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 33.de Almeida R. F., Fedorov A., Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veatch S. L., Keller S. L. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Chang A., Hammond T. G., Sun T. T., Zeidel M. L. Permeability properties of the mammalian bladder apical membrane. Am. J. Physiol. 1994;267:C1483–C1492. doi: 10.1152/ajpcell.1994.267.5.C1483. [DOI] [PubMed] [Google Scholar]
- 36.Coury L. A., Mathai J. C., Prasad G. V., Brodsky J. L., Agre P., Zeidel M. L. Reconstitution of water channel function of aquaporins 1 and 2 by expression in yeast secretory vesicles. Am. J. Physiol. 1998;274:F34–F42. doi: 10.1152/ajprenal.1998.274.1.F34. [DOI] [PubMed] [Google Scholar]
- 37.Grossman E. B., Harris H. W., Jr, Star R. A., Zeidel M. L. Water and nonelectrolyte permeabilities of apical membranes of toad urinary bladder granular cells. Am. J. Physiol. 1992;262:C1109–C1118. doi: 10.1152/ajpcell.1992.262.5.C1109. [DOI] [PubMed] [Google Scholar]
- 38.Lavelle J. P., Negrete H. O., Poland P. A., Kinlough C. L., Meyers S. D., Hughey R. P., Zeidel M. L. Low permeabilities of MDCK cell monolayers: a model barrier epithelium. Am. J. Physiol. 1997;273:F67–F75. doi: 10.1152/ajprenal.1997.273.1.F67. [DOI] [PubMed] [Google Scholar]
- 39.Negrete H. O., Rivers R. L., Goughs A. H., Colombini M., Zeidel M. L. Individual leaflets of a membrane bilayer can independently regulate permeability. J. Biol. Chem. 1996;271:11627–11630. doi: 10.1074/jbc.271.20.11627. [DOI] [PubMed] [Google Scholar]
- 40.Negrete H. O., Lavelle J. P., Berg J., Lewis S. A., Zeidel M. L. Permeability properties of the intact mammalian bladder epithelium. Am. J. Physiol. 1996;271:F886–F894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 41.Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992;31:7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- 42.Krylov A. V., Pohl P., Zeidel M. L., Hill W. G. Water permeability of asymmetric planar lipid bilayers: leaflets of different composition offer independent and additive resistances to permeation. J. Gen. Physiol. 2001;118:333–340. doi: 10.1085/jgp.118.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garlid K. D., Beavis A. D., Ratkje S. K. On the nature of ion leaks in energy-transducing membranes. Biochim. Biophys. Acta. 1989;976:109–120. doi: 10.1016/s0005-2728(89)80219-1. [DOI] [PubMed] [Google Scholar]
- 44.Georgievskii Y., Medvedev E. S., Stuchebrukhov A. A. Proton transport via the membrane surface. Biophys. J. 2002;82:2833–2846. doi: 10.1016/S0006-3495(02)75626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norris F. A., Powell G. L. Characterization of CO2/carbonic acid mediated proton flux through phosphatidylcholine vesicles as model membranes. Biochim. Biophys. Acta. 1992;1111:17–26. doi: 10.1016/0005-2736(92)90269-r. [DOI] [PubMed] [Google Scholar]
- 46.de Godoy C. M., Cukierman S. Modulation of proton transfer in the water wire of dioxolane-linked gramicidin channels by lipid membranes. Biophys. J. 2001;81:1430–1438. doi: 10.1016/s0006-3495(01)75798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasenkiewicz-Gierula M., Rog T., Kitamura K., Kusumi A. Cholesterol effects on the phosphatidylcholine bilayer polar region: a molecular simulation study. Biophys. J. 2000;78:1376–1389. doi: 10.1016/S0006-3495(00)76691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q. P., Li Q. T. Effect of cardiolipin on proton permeability of phospholipid liposomes: the role of hydration at the lipid–water interface. Arch. Biochem. Biophys. 2001;389:201–206. doi: 10.1006/abbi.2001.2319. [DOI] [PubMed] [Google Scholar]
- 49.Plank L., Dahl C. E., Ware B. R. Effect of sterol incorporation on head group separation in liposomes. Chem. Phys. Lipids. 1985;36:319–328. doi: 10.1016/0009-3084(85)90039-8. [DOI] [PubMed] [Google Scholar]
- 50.Holopainen J. M., Metso A. J., Mattila J. P., Jutila A., Kinnunen P. K. Evidence for the lack of a specific interaction between cholesterol and sphingomyelin. Biophys. J. 2004;86:1510–1520. doi: 10.1016/S0006-3495(04)74219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill W. G., Almasri E., Ruiz W. G., Apodaca G., Zeidel M. L. Water and solute permeability of rat lung caveolae: high permeabilities explained by acyl chain unsaturation. Am. J. Physiol. Cell Physiol. 2005;289:C33–C41. doi: 10.1152/ajpcell.00046.2005. [DOI] [PubMed] [Google Scholar]
- 52.Lange Y., Ye J., Steck T. L. Circulation of cholesterol between lysosomes and the plasma membrane. J. Biol. Chem. 1998;273:18915–18922. doi: 10.1074/jbc.273.30.18915. [DOI] [PubMed] [Google Scholar]
- 53.Bretscher M. S., Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- 54.Hoekstra D., van IJzendoorn S. C. D. Lipid trafficking and sorting: how cholesterol is filling gaps. Curr. Opin. Cell Biol. 2000;12:496–502. doi: 10.1016/s0955-0674(00)00122-8. [DOI] [PubMed] [Google Scholar]