Abstract
The factors explaining interspecific differences in prevalences of blood parasites in birds are poorly known. We simultaneously assessed 20 social, ecological, life history, and sampling-related variables that could influence hemoparasite prevalences among diurnal birds of prey in Spain. Our results show that multiple factors are responsible for the studied host–parasite association. We confirmed for the first time that prevalence is inversely correlated to the embryonic development period, and thus probably to immune performance, even among closely related birds. Macrohabitat features related to vector availability are also important, prevalences being higher in species breeding in forested habitats. Finally, prevalence is positively correlated with the host’s world geographic range. We hypothesize that larger geographic ranges offered more opportunities for host-vector-hemoparasite associations to become established. The results from our multivariate analyses differ from those obtained through univariate ones, showing that all potential factors should be assessed jointly when testing any ecological or evolutionary hypothesis dealing with parasites.
The study of avian blood parasites has received much attention during the last century. Bennett et al. (1) compiled 5,640 papers published on avian hematozoa since the discovery of these parasites in 1885, most of which deal with the taxonomy and distribution of blood parasites. More recently, Hamilton and Zuk (2) triggered a renewed interest in avian hematozoa among behavioral and evolutionary ecologists, who have greatly increased their research on the effects of these and other parasites on different traits of avian hosts (3, 4). However, relatively few studies examined the epizootiology of parasites and the factors explaining patterns in host–parasite associations, especially in the case of avian blood parasites (5).
Hamilton and Zuk (2) concluded that plumage brightness is positively correlated with rates of parasitism by hematozoa, but they did not attempt to explain why variability in blood parasitemias exists. However, the large number of conflicting results obtained during tests of this hypothesis prompted researchers to look for factors that could explain such variability, both at intra- and interspecific levels, and that could obscure the patterns originally showed by Hamilton and Zuk (2). The prevalence of hemoparasites is not a species-specific constant; in addition to seasonal variations associated with the host’s breeding cycle (6), blood parasitemias also vary between years (7, 8) and populations (9, 10), presumably because of ecological factors affecting both host condition and vector abundance. Interspecific differences in avian blood parasitemias, both at macrohabitat (11) and microhabitat scales (12), might be influenced by habitat-dependent distribution of vectors (hematophagous arthropods). Migratory bird species may have a higher risk of parasitization by being exposed to a wider array of parasites and vectors (13, 14). Plumage coloration could influence the risk of parasitization if vectors were differentially attracted by colors (15). Host immunity also could play an important role, as suggested by Ricklefs (16), who showed that prevalences of hematozoan infections among nonraptorial, altricial birds are inversely correlated with embryonic development period. Prevalences of blood parasites vary between taxonomic groups (17), mainly at the family level (16).
Most studies of differential prevalences of hematozoa in birds have considered only a few explanatory variables. When different variables have been included in multivariate analyses, inconsistent results were obtained (12, 18–21). Moreover, some factors that are known to affect other bird–parasite associations (5) have rarely or never been assessed in studies of blood parasites. For instance, the richness of helminth parasites in birds is positively correlated with their geographical ranges (22). A similar positive correlation with blood parasite prevalences is likely if larger ranges offer more opportunities for host–parasite associations.
Determining the sources of variability in blood parasitemias is important not only for the understanding of host–parasite associations, but also for identifying confounding variables when testing evolutionary hypotheses (9, 10, 15). The objective of this study was to assess simultaneously all factors that have been identified as possible causes of interspecific differences in the prevalences of blood parasites in birds. We sampled closely related birds (diurnal raptors) that breed in Spain to reduce the importance of geographic and phylogenetic confounding variables. We used an improved generalized linear model (GLM) procedure that permits analysis of data sets in which prevalences were obtained from unequal sample sizes, a widespread problem in the blood parasite literature (23).
METHODS
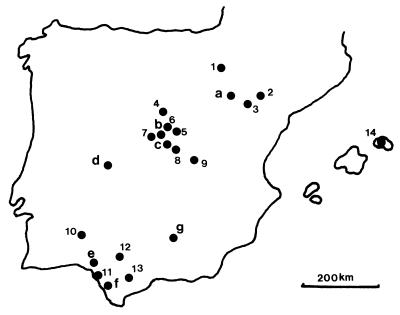
We sampled diurnal birds of prey between 1993 and 1998 in 14 different areas of Spain. These areas are at least 150 km away from each other for populations of each sampled species and represent a wide array of habitats (see Fig. 1). We sampled as many species as possible to represent different life histories and ecological requirements. We captured birds by using bal-chatri, do-ghaza, and padded leg-hold traps, cannon nets and mist-nets, and by hand at nests. Adult birds were sampled during their breeding seasons, when infections are expected to peak (24). We also sampled nestlings (39.4% of the individuals) because nestlings of at least two European raptors are parasitized at higher rates than adults (25, 26); several passerines are also highly infected in Spain at 13 days of age (ref. 27; J. A. Fargallo, J.L.T., and G.B., unpublished data). In addition, some birds (13.6% of the total) were sampled when they arrived at rehabilitation centers (Fig. 1). Birds sampled during a previous study of blood parasites at a rehabilitation center [n = 25 birds (28)] are included in our analyses because prevalences within species did not differ from those found by us (χ2 tests, Ps > 0.05). Because we could not sex most birds, we cannot determine whether parasitism was sex-biased (ref. 29; but see ref. 8 for sex-biases inverted between years). Because no yearly or geographic differences in hematozoan prevalences were detected (χ2 tests, Ps > 0.05), individuals within species were pooled in all analyses.
Figure 1.
Location (and main habitats) of the sampled areas (numbers) and rehabilitation centers (letters). 1, Bardenas Reales (native steppe, pine forests, and cereal cultives); 2, Monegros (pseudosteppe and extensive cereal cultures); 3, Ebro valley (irrigated crops); 4, Riaza River (canyons in steppe highlands); 5, Henares river (riparian forest); 6, Meco (cereal cultives); 7, Campo Azálvaro (montane grasslands); 8, Manzanares and Jarama rivers (riparian forests); 9, Tarancon area (cereal cultives); 10, Sierra Pelada (oak forests and Mediterranean scrubland); 11, Doñana National Park (marshes and oak and pine forests); 12, Guadalquivir Valley (irrigated crops); 13, Cádiz mountains (Mediterranean forests); 14, Menorca (Mediterranean forests); a, La Alfranca; b, GREFA; c, Zoo of Madrid; d, Las Cansinas; e, El Acebuche; f, Zoo of Jerez; g, CREA.
Blood Parasites.
A drop of blood was taken from the brachial vein and was smeared, air-dried, ethanol fixed, and Giemsa stained. We searched for extracellular parasites (trypanosomes, microfilariae) by scanning whole smears under low magnification (×40), and then we examined 100 microscopic fields under oil immersion (×1,000) for intraerythrocytic hematozoa, going from one end of the slide to the other to compensate for differences in blood thickness (30). This method clearly underestimates the presence of trypanosomes, but the bias is consistent among all samples (9, 10, 27). Prevalence is expressed as the percentage of birds that are infected by any hematozoan species.
Variables.
We included all variables that could possibly cause interspecific differences in prevalences of avian hematozoa or other parasites, whether or not previous studies reported significant results (for justification of the chosen variables, see references above and in this section). The variables we used are (i) phylogeny, family level comparison [Falconidae or Accipitridae (31)]; (ii) brightness, scored in three categories (12) but also including the color of bare parts (32); (iii) plumage color attractiveness for vectors, the scoring method of Yezerinac and Weatherhead (15); (iv) egg size (from ref. 33); (v) incubation period (from ref. 33); (vi) incubation period index, I (16), the residuals from a log-log regression analysis of the length of the incubation period on the size of the egg (r = 0.95, P < 0.0001); (vii) body size, the average body mass (from ref. 33); (viii) habitat, forest, open woodland, or open habitats; (ix) nest stratum, ground or above ground [we did not consider nest-height above ground (12) because it is highly variable within the species sampled whereas the former categorical variable clearly separates nest stratum for each species]; (x) nest substrate, ground, trees, cliffs, or buildings (34); (xi) nest reuse (35); (xii) sociability, territorial, clumped, or colonial breeders (5, 35); (xiii) migratory status, sedentary, partially migratory, or migratory (if a species occurred in more than one category for variables viii–xiii, we placed it in the one in which it occurred most frequently in our study area); (xiv) breeding geographic range in our study area (Spain), the number of 1:50,000 Lambert squares occupied by each species (36); (xv) world breeding geographic range, measured with a grid 2 × 2 mm on maps provided by Cramp and Simmons (33) and considering the most recent classification of raptor species (31); and (xvi) world geographic range, combined breeding and wintering ranges. We did not include some variables analyzed by other authors, such as diet and breeding sex-roles (15), because they do not vary significantly among the species studied here (33). Regarding variability in mating systems (18, 19), all species we analyzed are monogamous, or alternative mating systems are shared by <5% of the individuals in Spain (37–49). Finally, a set of variables was defined to control for potential sampling biases: (xvii) sample size (although the GLM procedure we used actually controlled for sample sizes, this variable was included for assessing possible interactions with other variables); (xviii) number of sampling sites; (xix) percentage of birds sampled as adults; and (xx) percentage of birds sampled at rehabilitation centers.
Analyses.
First, we analyzed the data univariately. Each variable was related to the prevalence of blood parasites through Spearman correlations for continuous variables and χ2 tests with Yates correction for categorical ones (12). Second, we used GLM modeling to assess simultaneously which explanatory variables and/or their interactions better explain the interspecific differences in hematozoan prevalences. For analyzing prevalence data, a GLM with binomial error and a logistic link function is the most appropriate statistical tool (12, 40). Instead of using the percentage of infected birds, as is usually done, which loses information on the sample size from which the proportion was estimated, this procedure uses the number of infected birds as the response variable and the number of birds examined as the binomial denominator (40). We fitted each explanatory variable to the observed data by using the program glim (40), following the Forward Stepwise Branching Modeling Procedure (41). When data suggested no linear trends, explanatory variables were transformed and fitted again trying to improve their contribution to the models. The robustness of the final model was assessed following Crawley (40).
RESULTS
A total of 1,264 different individuals, representing 20 of 24 species of diurnal birds of prey breeding in Spain, was examined for blood parasites. We found only three hemoparasite species, and their prevalences were low, ranging between 0 and 40% for different host species (see Table 1). Univariate analyses showed that prevalences were related only to 3 of the 20 examined variables. Individuals of solitary species were slightly more parasitized (3.27%) than semicolonial (0.64%) and colonial (1.95%) species (χ2 = 5.82, df = 2, P = 0.05) whereas those of sedentary species were more parasitized (4.7%) than migratory (1.48%) and partially migratory species (0%) (χ2 = 13.58, df = 2, P = 0.001). A stronger trend was associated with habitat; individuals of species breeding in forested habitats were more parasitized (37.5%) than those breeding in woodland (4.25%) and open habitats (1.31%) (χ2 = 59.55, df = 2, P < 0.0001). The same trend was marginally significant when considering species instead of individuals (χ2 = 5.24, df = 2, P = 0.07).
Table 1.
Species of diurnal birds of prey sampled for this study
| Bird species | No. of infected birds | No. of sampled birds | Parasite species |
|---|---|---|---|
| Neophron percnopterus | 0 | 111 | |
| Gyps fulvus | 0 | 124 | |
| Aegypius monachus | 2 | 64 | Leucozytozoon toddi |
| Pernis apivorus | 1 | 3 | L. toddi |
| Circaetus gallicus | 0 | 12 | |
| Aquila adalberti | 0 | 4 | |
| Aquila chrysaetos | 0 | 12 | |
| Hieraaetus pennatus | 0 | 6 | |
| Hieraaetus fasciatus | 0 | 18 | |
| Milvus migrans | 0 | 224 | |
| Milvus milvus | 0 | 46 | |
| Buteo buteo | 6 | 35 | Haemoproteus elani |
| Circus aeroginosus | 0 | 3 | |
| Circus cyaneus | 0 | 7 | |
| Circus pygargus | 0 | 26 | |
| Accipter gentilis | 2 | 5 | L. toddi |
| Falco peregrinus | 0 | 10 | |
| Falco subbuteo | 0 | 3 | |
| Falco naumanni | 12 | 490 | Haemoproteus tinnunculi |
| Falco tinnunculus | 2 | 61 | H. tinnunculi |
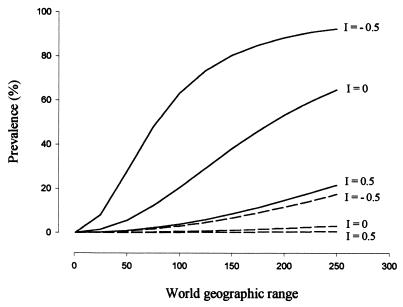
The multivariate analysis showed a very different picture. We obtained a unique GLM model in which only three variables entered: habitat, the log-transformed world geographic range, and the incubation period index (I). However, the factor levels 1 (open areas) and 2 (open woodland) of the variable habitat were not significantly different from one another in their parameter estimates. We derived a simplified model by grouping both levels that was not statistically different from the first one (change in deviance = 2.4, df = 1, P > 0.1). This final model (Table 2) indicated that the prevalence of blood parasites in Spanish raptors increased as its world geographic range increased (31.85% of the explained deviance) and decreased as its embryonic development period was shorter (i.e., smaller I) (39.65% of the explained deviance). Both trends were stronger in species breeding in forested habitats (habitat accounting for 28.5% of the explained deviance) (Fig. 2).
Table 2.
GLM model for prevalence of blood parasites in Spanish diurnal birds of prey, using binomial error and logistic link (total deviance = 54.905)
| Parameter estimate | Standard error | |
|---|---|---|
| Constant | −15.310 | 2.936 |
| Incubation period index (I) | −3.793 | 1.222 |
| Habitat | 4.056 | 0.766 |
| Log world geographic range | 2.148 | 0.566 |
| Residual deviance | 13.706 | |
| df | 16 |
Figure 2.
Prevalence of blood parasites (percent of infected individuals) in Spanish diurnal birds of prey in relation to their breeding habitat, world geographic range, and incubation period index (I) as predicted by the GLM model. Continuous lines, species breeding on forests; discontinuous lines, species breeding on open or open-woodland habitats. Values for world geographic range and I cover the variability observed in the studied species. For units, see Methods.
Despite the small number of host species studied, most of which were not parasitized by hematozoa, our model was very robust. The appearance of many zeroes in the response variable could reduce the original deviance, thereby reducing the likelihood of detecting significant explanatory variables. However, in our case (Table 2), the inclusion of a large number of zeroes was not a statistical problem. None of the variables that could reflect potential sampling biases, nor their interactions with other variables, entered even in the first steps of the GLM modeling procedure. Considerable care must be exercised when interpreting binomial GLM models based on marginally significant parameters or when they explain a very small fraction of the total deviance (40). However, the three variables in the final model entered at P < 0.001. This model accounted for most of the original deviance (75%), without evidence of overdispersion (residual deviance/residual df = 0.85). Finally, omitting the only potentially influential point (Pernis apivorus) did not change the model (change in deviance = 2.13, df = 1, P > 0.1), and the parameter estimates only changed by 0.02–4.45%.
DISCUSSION
Ricklefs (16), by analyzing blood parasites in a large number of nonraptorial, altricial birds, found that prevalence was inversely related to the relative length of the incubation period. He argued convincingly that such a correlation could arise from a direct relationship between immunocompetence and period of embryonic growth. Species with longer incubation periods with respect to egg size might have more cycles of proliferation of B stem cells in the bursa of Fabricius during embryonic development, allowing for a greater diversification of the variable region of Ig light chain genes before their expression as antibody. The resulting enhanced immune system should help to prevent and/or control hemoparasite infections during postnatal development and probably even throughout life (16). Our study extends the scope of Ricklefs’ results. First, we confirmed the hypothesis by using a group of birds (diurnal raptors) not studied by Ricklefs (16). Second, the relationship remains significant even after controlling for a larger number of potential confounding variables. Finally, in addition to the relationship found by Ricklefs (16) by comparing families of birds, we have shown that this trend is also evident even at a smaller phylogenetic scale (i.e., within two closely related families).
The prevalence of blood parasites in Spanish birds of prey also was related to macrohabitat characteristics, being highest in species breeding in forested habitats. This finding agrees with previous studies suggesting that the likelihood of hemoparasite infections varies among habitats, probably because vector availability is lower in treeless habitats (42) and areas that are heavily human-transformed (10). Our multivariate analysis suggests that, at least in our study area, macrohabitat constraints on hematozoan transmission may be more important than some potential microhabitat effects (e.g., nest-stratum within habitats; see ref. 12) or local differences between sampling areas (9, 10). Macrohabitat differences at a larger scale also could explain satisfactorily the overall low prevalence of blood parasites in Spain, where habitats are drier, less forested, and thus less suitable for hemoparasite vectors than in temperate or boreal forested areas, where the same or closely related host species are heavily parasitized (8, 43, 44). Finally, our study offers a scenario for a potential habitat segregation by birds in relation to their immunocompetence in addition to that recently suggested by Piersma (11). If blood parasites impose important costs for their avian hosts (although their degree of pathogenity is variable and still debated; see refs. 1 and 45–48), species with poor immunocompetence may be selected for, or limited to, open habitats in which the prevalence of hematozoa is low.
Another result is the positive relationship we found between hematozoa prevalence and the world geographic range of hosts. A positive correlation between the number of parasite species per host and host geographic range is known for some parasite–host associations (4, 49); however, there are not previous reports dealing with prevalences. The fact that prevalence is related to the world-wide range of the host but not to the host’s range in Spain may indicate that the evolutionary history of hosts and parasites, rather than current conditions, governs this relationship. Hemoparasite–host associations are largely mediated by complex vector–host interactions (45). Apart from ecological and geographical barriers (48), avian hosts have a variety of physiological, immunological, and behavioral mechanisms that act as barriers to vectors and parasites (50, 51), thus limiting both host’s susceptibility and exposure. It is plausible that avian species with larger geographic ranges have more variation in their “barriers,” offering greater opportunities for fitting together the life cycles of parasites, vectors, and hosts. Once the host–parasite association has been established, it could expand geographically over time and be expressed at reduced spatial scales. The evolution of host–parasite systems, however, is highly complex (52, 53), and the interactions between phylogenetic history, temporal association, and ecological factors complicate the development of causal explanations (53). Furthermore, the feeding activities of vectors and life cycles of both vectors and parasites are unknown for most hematozoa–bird associations (45, 51). Clearly no simple mechanistic explanation exists; however, our intention in presenting these results and speculations is to stimulate further research on this surprising relationship between host range and parasite prevalence.
Because a combination of life history traits (embryonic development) and present (habitat) and, presumably, historic (world geographic range) conditions influences the observed patterns of parasite distribution among hosts, host–parasite associations can be determined by a wide array of ecological and evolutionary forces. Ecological pressures and host life histories can vary greatly among host–parasite associations, and other variables may be important for other avian lineages. Including many potential factors in multivariate analyses, instead of testing hypotheses on a one-at-a-time basis, should be necessary to identify these relationships. Univariate analyses may mask a genuine relationship or provide incorrect results. GLM modeling is a powerful tool for simultaneously assessing multiple socioecological and life history traits of birds that may affect their blood parasitemias while controlling for potential sampling biases, even when studying small numbers of host species that show low overall prevalences.
Acknowledgments
We are indebted to O. Ceballos, R. López, M. de la Riva, and E. Mínguez for their help during trapping wild birds and to M. Máñez, M. Cabrera, J. Colás, P. Prieto, M. A. Quevedo, and the staff of Las Cansinas for their assistance getting samples from their rehabilitation centers. The Servicio de Diagnóstico de Fauna Silvestre, Facultad de Veterinaria, Universidad of Zaragoza allowed us to work in their laboratories. While writing, J.L.T. was supported by a postdoctoral grant of the Spanish Ministerio de Educatión y Ciencia. G. R. Bortolotti, R. D. Dawson, J. Potti, G. H. Orians, and two anonymous referees greatly improved the manuscript.
ABBREVIATION
- GLM
generalized linear model
References
- 1.Bennett G F, Peirce M A, Ashford R W. J Nat Hist. 1993;27:993–1001. [Google Scholar]
- 2.Hamilton W D, Zuk M. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 3.Loye J E, Zuk M, editors. Bird–Parasite Interactions: Ecology, Evolution and Behavior. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 4.Clayton D H, Moore J, editors. Host–Parasite Evolution: General Principles and Avian Models. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 5.Gregory R D. In: Host–Parasite Evolution: General Principles and Avian Models. Clayton D H, Moore J, editors. Oxford: Oxford Univ. Press; 1997. pp. 198–211. [Google Scholar]
- 6.Weatherhead P J, Bennett G F. Can J Zool. 1991;69:2352–2359. [Google Scholar]
- 7.Allander K, Bennett G F. J Avian Biol. 1994;25:69–74. [Google Scholar]
- 8.Wiehn J, Korpimäki E. Proc R Soc London Ser B. 1998;265:1197–1201. [Google Scholar]
- 9.Bennett G F, Squires-Parsons D, Siikamäki P, Huhta E, Allander K, Hillström L. J Avian Biol. 1995;26:33–38. [Google Scholar]
- 10.Merilä J, Björklund M, Bennett G F. Can J Zool. 1995;73:1798–1804. [Google Scholar]
- 11.Piersma T. Oikos. 1997;80:623–631. [Google Scholar]
- 12.Garvin M C, Remsen J V. Auk. 1997;114:179–191. [Google Scholar]
- 13.Peirce M A, Mead C J. J Nat Hist. 1978;12:337–340. [Google Scholar]
- 14.Valkiûnas G A. Ecologiya. 1993;2:57–65. [Google Scholar]
- 15.Yezerinac S, Weatherhead P J. J Animal Ecol. 1995;64:528–537. [Google Scholar]
- 16.Ricklefs R E. Proc Natl Acad Sci USA. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett G F. Syst Parasitol. 1993;26:39–43. [Google Scholar]
- 18.Read A F. Am Nat. 1991;138:434–459. [Google Scholar]
- 19.Zuk M. In: Bird–Parasite Interactions: Ecology, Evolution, and Behavior. Loye J E, Zuk M, editors. New York: Oxford Univ. Press; 1991. pp. 317–327. [Google Scholar]
- 20.John J L. Oikos. 1995;72:395–401. [Google Scholar]
- 21.Underhill L G, Kalejta-Summers B. Ostrich. 1995;66:10–14. [Google Scholar]
- 22.Gregory R D. Func Ecol. 1990;4:645–654. [Google Scholar]
- 23.John J L. Int J Parasitol. 1997;11:1269–1288. doi: 10.1016/s0020-7519(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Allander K, Sundberg J. J Avian Biol. 1997;28:325–330. [Google Scholar]
- 25.Ashford R W, Green E E, Holmes P R, Lucas A J. J Nat Hist. 1991;25:269–277. [Google Scholar]
- 26.Toyne E P, Ashford R W. J Raptor Res. 1997;31:81–83. [Google Scholar]
- 27.Merino S, Potti J, Moreno J. Proc Natl Acad Sci USA. 1996;93:5726–5730. doi: 10.1073/pnas.93.12.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peirce M A, Greenwood A G, Cooper J E. Avian Pathol. 1983;12:443–446. doi: 10.1080/03079458308436189. [DOI] [PubMed] [Google Scholar]
- 29.McCurdy D G, Shutler D, Mullie A, Forbes M R. Oikos. 1998;82:303–312. [Google Scholar]
- 30.Korpimäki E, Tolonen P, Bennett G F. Ecoscience. 1995;2:335–343. [Google Scholar]
- 31.Del Hoyo J, Elliot A, Sargatal J. Handbook of the Birds of the World. Vol. 2. Barcelona: Lynx Edicions; 1994. [Google Scholar]
- 32.Negro J J, Bortolotti G R, Tella J L, Fernie K J, Bird D M. Func Ecol. 1998;12:307–312. [Google Scholar]
- 33.Cramp S, Simmons K E L. The Birds of the Western Palearctic. II. Oxford: Oxford Univ. Press; 1980. [Google Scholar]
- 34.Greiner E C, Mundy P J. J Parasitol. 1979;65:147–153. [PubMed] [Google Scholar]
- 35.Moller A P, Erritzoe J. Evolution. 1996;50:2066–2072. [Google Scholar]
- 36.SEO/BidLife. Atlas de las aves de España (1975–1995) Barcelona: Lynx Editions; 1997. [Google Scholar]
- 37.Arroyo B. J Raptor Res. 1996;30:100–102. [Google Scholar]
- 38.Tella J L. J Raptor Res. 1993;27:119–120. [Google Scholar]
- 39.Tella J L, Negro J J, Villarroel M, Kuhnlein U, Hiraldo F, Bird D M. Auk. 1996;113:262–265. [Google Scholar]
- 40.Crawley M J. GLIM for Ecologists. Oxford: Blackwell; 1993. [Google Scholar]
- 41.Bustamante J. Biol Conserv. 1997;80:153–160. [Google Scholar]
- 42.Bennett G F, Montgomerie R, Seutin G. Condor. 1992;94:289–292. [Google Scholar]
- 43.Greiner E C, Bennett G F, White E M, Coombs R F. Can J Zool. 1975;53:1762–1787. doi: 10.1139/z75-211. [DOI] [PubMed] [Google Scholar]
- 44.Wiehn J, Korpimäki E, Bildstein K L, Sorjonen J. Ethology. 1997;103:304–317. [Google Scholar]
- 45.Atkinson C T, van Ripper C., III . In: Bird–Parasite Interactions: Ecology, Evolution, and Behavior. Loye J E, Zuk M, editors. New York: Oxford Univ. Press; 1991. pp. 19–48. [Google Scholar]
- 46.Hunter D B, Rohner C, Currie D C. J Wildl Dis. 1997;33:486–491. doi: 10.7589/0090-3558-33.3.486. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura K, Mitarai Y, Tanimura N, Hara H, Ikeda A, Shimada J, Isobe T. J Parasitol. 1997;83:325–327. [PubMed] [Google Scholar]
- 48.Shutler D, Ankney C D, Dennis D G. J Wildl Manage. 1996;60:569–580. [Google Scholar]
- 49.Poulin R. Annu Rev Ecol Syst. 1997;28:341–358. [Google Scholar]
- 50.Hart B L. In: Host–Parasite Evolution: General Principles and Avian Models. Clayton D H, Moore J, editors. Oxford: Oxford Univ. Press; 1997. pp. 59–77. [Google Scholar]
- 51.Scott T W, Edman J D. In: Bird–Parasite Interactions: Ecology, Evolution, and Behavior. Loye J E, Zuk M, editors. New York: Oxford Univ. Press; 1991. pp. 179–204. [Google Scholar]
- 52.Brooks D R. Syst Zool. 1990;39:14–30. [Google Scholar]
- 53.Hoberg E P, Brooks D R, Siegel-Causey D. In: Host–Parasite Evolution: General Principles and Avian Models. Clayton D H, Moore J, editors. Oxford: Oxford Univ. Press; 1997. pp. 212–235. [Google Scholar]