Abstract
It is shown that the use of 5% acetonitrile or propionitrile in dichloromethane functions to increase the β-selectivity of a number of l-rhamnopyranosylation reactions conducted by the thioglycoside method with activation by the 1-benzenesulfinyl piperidine/trifluoromethanesulfonic anhydride couple. The use of more significant quantities of acetonitrile or propionitrile results in the formation of complex reaction mixtures containing little coupled product, but from which Ritter-type products can be isolated.
Keywords: Deoxy sugars, Acetonitrile, Nitrilium ion
1. Introduction
While significant advances have been made in the stereo-controlled formation of β-mannopyranosides in recent years, through both direct† and indirect methods,1–19 their 6-deoxy congenors, the β-rhamnopyranosides, continue to pose significant challenges. In the d-series, characterized by the scarcity of d-rhamnose itself,20 we have successfully devised methods based on stereochemically controlled β-mannosylation, followed by effcient deoxygenation at the 6-position by radical fragmentation of modified 4,6-O-acetals,21–23 but the l-series, while accessible by indirect methods,24–26 continues to pose significant challenges to direct stereocontrolled synthesis. We report here on our attempts to use the ‘nitrile effect’ to enhance the β-selectivity of solution-phase rhamnosylation reactions.
2. Results and discussion
As we have discussed,6 based on our determination that a transient contact oxacarbenium ion/triflate anion pair (CIP) is the reactive species in our benzylidene acetal mediated β-mannosylation systems,27 we considered the key to successful β-l-rhamnosylation to lie in the use of disarming non-participating protecting groups. The function of these groups is to destabilize the oxacarbenium ion, so as to shift the CIP/glycosyl triflate equilibrium toward the glycosyl triflate, which is the established resting state.6,28 In this manner, the concentration of the α-selective solvent-separated ion pair (SSIP), and of any free oxacarbenium ions is minimized resulting in enhanced β-selectivity (Scheme 1).
On this basis, following the early work of Schuerch,7 we surveyed of 2-O-sulfonyl protected rhamnosyl donors, ultimately arriving at the 4-O-benzoyl-2-O-sulfonyl system 1.6,29 A number of other non-participating electron-withdrawing protecting groups for O2, including phosphates, nitrate, cyanate, and a vinylous ester, were also surveyed but found to be less successful than the optimum sulfonate 1.6 Interestingly, 2,3-O-carbonyl and 2,3-O-alkylidene protected mannosyl and rhamnosyl thioglycosides were found to be highly α-selective in a number of homogeneous solution phase glycosylations.3,30–32 This contrasts with their use in heterogenous glycosylations in the form of glycosyl bromides with activation by insoluble silver salts when they are β-selective.33–37 We ascribe the α-selectivity of these 2,3-O-carbonates in homogeneous glycosylations to the half-chair conformation imposed on the pyranose ring, for which we have provided crystallographic evidence,38 which reduces the energy barrier to oxacarbenium ion formation: the β-directing effect observed with the bromides and insoluble silver salts is simply a function of the mode of adsorption onto the promoter surface.31 This understanding of the reactivity of the 2,3-O-carbonates led us to the 3,4-O-carbonyl protected thioglycoside 2, which exhibits comparable β-selectivity in our systems to sulfonate 1.31
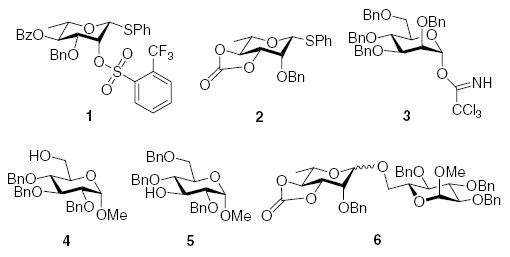
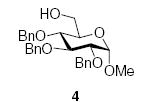
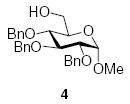
Although donors 1 and 2 gave moderate to excellent β-selectivity with simple secondary and tertiary alcohols, and with more reactive carbohydrate acceptors, they only afford modest selectivity with more typical secondary carbohydrate acceptors.6,29,31,39 As a consequence, we turned our attention to the well-known β-directing effect of acetonitrile in glycosylation reactions.40–44 We were encouraged in this endeavor by preliminary results from the Schmidt group in which it was found that 2,3,4,6-tetra-O-benzyl-α-d-mannopyranosyl trichloroacetimidate 3 gave approximately a 1:1 β:α mixture of anomers in good yield on coupling to methyl 2,3,4-tri-O-benzyl-α-d-glucopyranoside 4, and to methyl 2,4,6-tri-O-benzyl-α-d-glucopyranoside 5, in propionitrile at −80 °C on activation with trimethylsilyl triflate,44 when high α-selectivity would have been expected in non-participating solvents.45,46
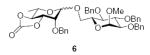
Working with donor 2 and coupling to acceptor 4, we surveyed the effect of propionitrile‡ under our BSP/Tf2O/TTBP conditions,3,4,47 with the formation of rhamnoside 6 (Table 1). In neat dichloromethane (Table 1, entry 1), 6 was formed in good yield with a 6:1 β:α ratio. By contrast, in neat propionitrile extensive decomposition of the donor was observed, most of the acceptor was recovered unchanged, and <10% of the coupled product 6 was discernible in the crude reaction mixture, albeit with a β:α ratio of at least 5:1 (Table 1, entry 2). This result was somewhat surprising as propionitrile had previously been demonstrated to be a satisfactory solvent for BSP type coupling reactions with other simple thioglycosides,3 and in the closely related sulfoxide couplings,48 but was nevertheless reproducible. Reducing the amount of propionitrile until it was simply an additive in the main solvent resulted in the restoration of efficient coupling, and with some increase in β-selectivity, with a maximum approaching 8:1 for the solvent ratio of 5/95 (v/v) propionitrile/dichloromethane (Table 1, entry 4). It was subsequently shown that comparable results could be obtained with low proportions of acetonitrile in dichloromethane (Table 1, entries 6–8), and because of the greater ease of removal, the mixed acetonitrile/dichloromethane system was selected for use in further coupling reactions.
Table 1.
The effect of varying quantities of propionitrile and acetonitrile on the formation of 6 from 2 and 4 at −60 °C
| Entry | Solvent (v/v) | Yield (%) 6 | β:α ratio |
|---|---|---|---|
| 1 | EtCN/CH2Cl2 0:100 | 81 | 6:1 |
| 2 | EtCN/CH2Cl2 100:0 | <10 | >5:1 |
| 3 | EtCN/CH2Cl2 10:90 | 69 | 6.8:1 |
| 4 | EtCN/CH2Cl2 5:95 | 80 | 7.9:1 |
| 5 | EtCN/CH2Cl2 2.5:97.5 | 78 | 6.6:1 |
| 6 | CH3CN/CH2Cl2 5:95 | 76 | 7.2:1 |
| 7 | CH3CN/CH2Cl2 2.5:97.5 | 80 | 8:1 |
| 8 | CH3CN/CH2Cl2 1.25:98.75 | 98 | 4.5:1 |
A further series of coupling reactions were then conducted with donor 2 in the 95/5 dichloromethane/acetonitrile solvent mixture with the results indicated in Table 2. In each a shift in the ratio of products, favoring the β-anomer, is observed on inclusion of acetonitrile, sometimes improving an already β-selective reaction, sometimes changing a previously α-selective reaction into a β-selective one, and other times reducing α-selectivity.
Table 2.
Coupling reactions with donor 2 in CH2Cl2/CH3CN 95/5 (v/v) and in pure CH2Cl2
| Entry | Acceptor | Product | Yielda (%) CH2Cl2 | (β:α ratio)b CH2Cl2/CH3CN (95/5) |
|---|---|---|---|---|
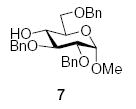
| 1 |
|
|
87 (5.8:1) | 76% (7.2:1) |
| 2 |
|
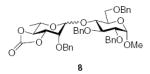
|
70 (1:1.1) | 55% (2.1:1) |
| 3 |
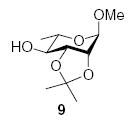
|
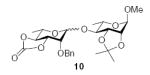
|
85 (1:8.1) | 72% (1:4.2) |
| 4 |
|
|
87 (1:2.1) | 77% (1:1.2) |
Yields refer to isolated products.
Ratios were determined on the crude reaction mixtures by integration of the 1H NMR spectra.
A similar series of experiments were conducted with donor 1 with similar consequences (Table 3). A comparison between the results presented in Tables 2 and 3 reveals donor 1 to be generally more β-selective than donor 2, whether the couplings are conducted in pure dichloromethane or with the inclusion of acetonitrile.
Table 3.
Further coupling reactions with donor 1 in CH2Cl2/CH3CN 95/5 (v/v) and in pure CH2Cl2
| Entry | Acceptor | Producta | Yielda (%) CH2Cl2 | (β:α ratio)b CH2Cl2/CH3CN (95/5) |
|---|---|---|---|---|
| 1 |
|
|
87 (12.7:1) | 88% (16:1) |
| 2 |
|
|
85 (1:1.4) | 78% (1.2:1) |
| 3 |
|
|
92 (2.6:1) | 88% (3.9:1) |
| 4 |
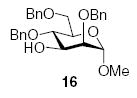
|
|
74 (2.4:1) | 42% (3.5:1) |
Ar = p-CF3C6H4
Yields refer to isolated products.
Ratios were determined on the crude reaction mixtures by integration of the 1H NMR spectra.
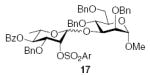
As is clear from the ensemble of results presented in Tables 1–3, the isolated yields of coupled products are generally lower when a nitrile is included in the solvent mixture, falling to a minimum with the use of pure propionitrile. In an attempt to shed further light on this problem, the coupling of donor 1 with the primary acceptor 4 was conducted in a 70/30 mixture of dichloromethane and acetonitrile. Examination of the crude reaction mixture by 1H NMR spectroscopy revealed the almost complete absence of the anticipated coupled product 13 and the formation of one major product, which was isolated by chromatography on silica gel in 30% yield, and tentatively assigned structure 18. This assignment is based on the rhamnose anomeric hydrogen signal at δ 5.13 whose broad singlet nature is typical for an α-rhamnoside in this series, and on the 3H singlet at δ 1.99, attributed to the imidate methyl group. On standing in deuteriochloroform, this unstable compound underwent decomposition to acetate 19, thereby providing further support for product 18, which is obviously formed by attack of the acceptor on the intermediate nitrilium ion 20. Unfortunately, attempts to detect the formation of nitrilium ion 20, or its congenor arising from donor 2, by low temperature NMR spectroscopy have so far been unsuccessful. Presumably, the increased concentration of acetonitrile leads to a stabilization of nitrilium ion 20 and diverts nucleophilic attack away from the anomeric center toward the sp-hydrolized carbon. In support of this mechanism, we note that a variety of glycosyl nitrilium ions have been captured intramolecularly by this ‘Ritter-like’ capture mode by various nucleophiles and that this chemistry has been applied in the synthesis of sugar β-peptide libraries.49,50
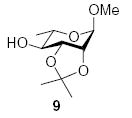
Finally, we note the unexpectedly high amounts of α-anomeric products 10α and 14α observed on coupling of acceptor 9 to donors 1 and, especially, 2. In our very extensive studies on the formation of the β-d-mannopyranosides,4 acceptor 9 typically delivers β-selectivities in excess of 10:1; certainly, it is usually a more β-selective acceptor than the glucose 4-OH derivative 7. However, comparison of Table 2, entries 2 and 3, reveals that the roles are reversed in coupling to l-rhamnopyranosyl donor 2. We believe this to be a manifestation of stereochemical matching/mismatching,51 a phenomenon that is gaining increasingly wide recognition in glycosidic bond forming reactions.52–54
3. Experimental
3.1. General methods
All solvents were dried and distilled by standard procedures. Trifluoromethanesulfonic anhydride was distilled over P2O5. Acceptors and donors were dried at 40 °C under vacuum for 2 h before use. BSP and TTBP were dried at room temperature under vacuum for 2 h before use. Optical rotations were determined with an Autopol III polarimeter for solutions in CHCl3. NMR spectra were recorded for CDCl3 solutions with a Bruker Avance spectrometer at 500 MHz (1H) or 125 MHz (13C). Chemical shifts are in parts per million downfield from tetramethylsilane. High resolution mass spectra were recorded with a Waters Q-TOF2 instrument. Microanalyses were conducted by Midwest Microlabs, Indianapolis, IN. The anomeric stereochemistry of all coupled products was assigned on the basis of the anomeric 1JCH coupling constants.55
3.2. General procedure for coupling reactions
Tf2O (0.40 mmol) was added dropwise to a stirred solution of donor (0.27 mmol), BSP (0.35 mmol), and TTBP (0.80 mmol) in CH3CN (0.5 mL) and CH2Cl2 (7.0 mL) under argon at −60 °C. After stirring for 1 h at −60 °C, the acceptor (0.80 mmol) in CH2Cl2 (2.5 mL) was added slowly over a period of 1 min. The reaction mixture was stirred for 3 h at −60 °C, then quenched at −60 °C with saturated NaHCO3, and extracted with CH2Cl2 (3 × 20 mL). The combined organic layer was dried (Na2SO4), and the solvent was removed under reduced pressure. Purification by silica gel column chromatography (EtOAc/hexane) afforded the corresponding α/β rhamnosides.
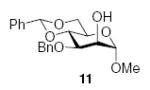
3.2.1. Methyl 6-O-(2-O-benzyl-3,4-O-carbonyl-α-l-rhamnopyranosyl)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (6α)
[α]D21 −3.6 (c 1.1, CHCl3), lit.31 −32.7; IR: 1813 cm−1; 1H NMR (CDCl3): δ 7.38–7.21 (m, 20H, arom H), 5.01 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.86 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.81–4.77 (m, 2H, –CH2Ph), 4.77 (s, 1H, H-1II), 4.74 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.68 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.60 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.54 (d, 1H, J = 4.0 Hz, H-1I), 4.50–4.48 (dd, 1H, J2,3 = 11.5 Hz, J3,4 3.0 Hz, H-3II), 4.46 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.41–4.36 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4II) 4.05 (m, 1H, H-5II), 4.02 (m, 1H, H-2II), 3.98 (t, 1H, J2,3, J3,4 = 10.0 Hz, H-3I), 3.83–3.81 (dd, 1H, J6a,6b = 10.5 Hz, J5,6a = 1.5 Hz, H-6I), 3.71–3.68 (m, 1H, H-5I), 3.54–3.50 (dd, 1H, J6a,6b = 11.5 Hz, J5,6b = 5.5 Hz, H-6I), 3.50–3.47 (dd, 1H, J2,3 = 9.5 Hz, J1,2 = 3.5 Hz, H-2I), 3.37 (t, 1H, J3,4, J4,5 = 10.0 Hz, H-4I), 3.32 (s, 3H, –OCH3), 1.34 (d, 3H, J5,6 = 6.5 Hz, H-6II); 13C NMR: δ 153.7, 138.6, 138.0, 136.8, 128.6, 128.52, 128.45, 128.18, 128.11, 128.00,127.98, 127.92, 127.80, 127.74, 127.71, 99.4 (1JCH = 172.3 Hz), 98.0 (1JCH = 167.1 Hz), 82.0, 80.5, 80.0, 77.9, 77.4, 75.8, 75.0, 74.1, 73.4, 72.9, 69.7, 68.6, 66.7, 55.2, 17.7; ESIMS m/z calcd for C42H46O11Na [M+Na]+: 749.2938. Found: 749.2938.
3.2.2. Methyl 6-O-(2-O-benzyl-3,4-O-carbonyl-β-l-rhamnopyranosyl)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (6β)
[α]D21 +46.6 (c 1.0, CHCl3), lit.31 +52.3; IR: 1810 cm−1; 1H NMR (CDCl3): δ 7.37–7.26 (m, 20H, arom H), 5.0 (d,1H, 2J = 11.0 Hz, –CH2Ph), 4.89–4.80 (m, 5H, –CH2Ph), 4.73 (d, 1H, 2J = 10.5 Hz, –CH2Ph), 4.68 (d, 1H, 2J = 10.5 Hz, –CH2Ph), 4.66 (s, 1H, H-1II), 4.58 (d, 1H, J = 3.5 Hz, H-1I), 4.48–4.44 (dd, 1H, J3,4 = 11.5 Hz, J4,5 = 9.0 Hz, H-4II), 4.29–4.27 (dd, 1H, J6a,6b = 11.5 Hz, J5,6a = 3.0 Hz, H-6I), 4.25 (d, 1H, J2,3 = 2.0 Hz, H-2II), 4.13–4.10 (dd, 1H, J3,4 = 12.0 Hz, J2,3 = 2.5 Hz, H-3II), 3.99 (t, 1H, J2,3, J3,4 = 9.0 Hz, H-3I), 3.76–3.69 (m, H-5I, H-6I, H-5II), 3.61 (t, 1H, J3,4, J4,5 = 8.5 Hz, H-4I), 3.46–3.43 (dd, 1H, J2,3 = 10.0 Hz, J1,2 = 4.0 Hz, H-2I), 3.36 (s, 3H, –OCH3), 1.39 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 153.9, 138.8, 138.3, 138.1 137.2, 128.5, 128.41, 128.39, 128.1, 128.0, 127.9, 127.8, 127.6, 101.2 (1JCH = 157.5 Hz), 98.3 (1JCH = 166.9 Hz), 81.8, 81.6, 80.0, 77.9, 77.6, 75.6, 75.2, 73.8, 73.5, 73.4, 71.0, 70.0, 68.0, 55.3, 17.8; ESIMS m/z calcd for C42H46O11Na [M+Na]+: 749.2938. Found: 749.2969.
3.2.3. Methyl 2,3,6-tri-O-benzyl-4-O-(2-O-benzyl-3,4-O-carbonyl-α-l-rhamnopyranosyl)-α-d-glucopyranoside (8α)
[α]D21−11.7 (c 1.0, CHCl3), lit.31 −19.6; IR: 1811 cm−1; 1H NMR (CDCl3): δ 7.33–7.21 (m, 20H, arom H), 5.09 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 5.0 (s, 1H, H-1II), 4.76 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.63–4.56 (m, 4H, H-1I –CH2Ph), 4.52 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.45–4.41 (m, 2H, –CH2Ph), 4.25–4.23 (m, 2H, H-3II, H-4II), 4.11–4.08 (m, 1H, H-5II), 3.93 (s, 1H, H-2II), 3.82–3.80 (m, 2H, H-3I, H-4I), 3.62 (m, 1H, H-5I), 3.58–3.53 (m, 2H, H-2I, H-6I), 3.41–3.38 (dd, 1H, J6a,6b = 11.0 Hz, J5,6a = 3.0 Hz, H-6I), 3.35 (s, 3H, –OCH3), 0.97 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 153.9, 138.2, 137.8, 137.5, 136.9, 128.7, 128.5, 128.4, 128.2, 128.08, 128.01, 127.90, 127.85, 127.75, 127.70, 98.6 (1JCH = 172.8 Hz), 97.9 (1JCH 166.1 Hz), 80.6, 80.2, 79.6, 78.2, 77.9, 77.5, 75.8, 74.1, 73.8, 73.6, 73.3, 72.6, 69.8, 69.0, 68.7, 55.3, 17.3; ESIMS m/z calcd for C42H46O11Na [M+Na]+: 749.2938. Found: 749.2941.
3.2.4. Methyl 2,3,6-tri-O-benzyl-4-O-(2-O-benzyl-3,4-O-carbonyl-β-l-rhamnopyranosyl)-α-d-glucopyranoside (8β)
[α]D21 +18.8 (c 1.0, CHCl3), lit31. +56.1; IR: 1811 cm−1; 1H NMR (CDCl3): δ 7.38–7.25 (m, 18H, arom H), 7.11 (m, 2H, arom H), 4.99 (d, 1H, 2J = 11.5 Hz, –CH2Ph), 4.76–4.65 (m, 6H, H-1I, H-1II, –CH2Ph), 4.61 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.52 (d,1H, 2J = 12.0 Hz, –CH2Ph), 4.31–4.25 (m, 2H, H-4II, –CH2Ph), 3.86 (t, 1H, J2,3, J3,4 = 9.0 Hz, H-3I), 3.82 (dd, 1H, J6a,6b = 10.5 Hz, J5,6a = 1.5 Hz, H-6I), 3.73 (m, 1H, H-6I), 3.69–3.62 (m, 2H, H-4I, H-5I), 3.53–3.48 (m, 4H, H-2I, H-2II, H-3II, H-5II), 3.44 (s, 3H, –OCH3), 1.24 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 154.0, 138.6, 138.4, 137.8, 137.5, 128.6, 128.5, 128.4, 128.2, 128.15, 128.09, 128.05, 128.00, 127.87, 127.85, 127.42, 127.37, 101.5 (1JCH = 165.0 Hz), 97.7 (1JCH = 163.1 Hz), 82.2, 81.5, 80.0, 77.9, 76.2, 75.7, 73.44, 73.37, 73.10, 72.98, 70.6, 69.5, 68.8, 55.5, 17.7; ESIMS m/z calcd for C42H46O11Na [M+Na]+: 749.2938. Found: 749.2923.
3.2.5. Methyl 4-O-(2-O-benzyl-3,4-O-carbonyl-α-l-rhamnopyranosyl)-2,3-O-isopropylidiene-α-l-rhamnopyranoside (10α)
[α]D21 −68.5 (c 1.0, CHCl3); IR: 1819 cm31; 1H NMR (CDCl3): δ 7.36–7.31 (m, 5H, arom H), 5.48 (d, 1H, J1,2 = 1.0 Hz, H-1II), 4.85 (s, 1H, H-1I), 4.76 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.70 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.45 (m, 2H, H-3II, H-4II), 4.13 (m, 1H, H-2II), 4.06 (m, 3H, H-2I, H-3I, H-5II), 3.58 (m, 1H, H-5I), 3.50 (m, 1H, H-4I), 3.36 (s, 3H, –OCH3), 1.51 (s, 3H, –C(CH3)2), 1.38 (d, 3H, J5,6 = 6.0 Hz, H-6II), 1.34 (s, 3H, –C(CH3)2), 1.26 (d, 3H, J5,6 = 6.0 Hz, H-6I); 13C NMR: δ 153.7, 136.9, 128.5, 128.1, 127.7, 109.6, 98.1 (1JCH = 174.9 Hz), 97.9 (1JCH = 167.4 Hz), 80.3, 78.4, 78.2, 77.9, 76.1, 74.6, 72.8, 69.2, 63.5, 54.9, 27.9, 26.3, 17.9, 17.8; ESIMS m/z calcd for C24H32O10Na [M+Na]+: 503.1893. Found: 503.1892.
3.2.6. Methyl 4-O-(2-O-benzyl-3,4-O-carbonyl-β-l-rhamno-pyranosyl)-2,3-O-isopropylidiene-α-l-rhamnopyranoside (10β)
[α]D21 +5.6 (c 0.7, CHCl3); IR: 1810 cm31; 1H NMR (CDCl3): δ 7.37–7.30 (m, 5H, arom H), 4.86–4.79 (q, 2H, 2J = 12.5 Hz, –CH2Ph), 4.83 (s, 1H, H-1I), 4.75 (d, 1H, J1,2 = 1.0 Hz, H-1II), 4.52–4.48 (dd, 1H, J4,5 = 11.5 Hz, J3,4 = 9.5 Hz, H-4II), 4.37 (t, 1H, J2,3, J3,4 = 6.0 Hz, H-3I), 4.21 (d, 1H, J2,3 = 1.5 Hz, H-2II), 4.13–4.11 (m, 2H, H-3II, H-2I), 3.76 (m, 1H, H-5II), 3.70 (m, 1H, H-5I), 3.39–3.36 (dd, 1H, J4,5 = 10.0 Hz, J3,4 = 7.0 Hz, H-4I), 3.37 (s, 3H, –OCH3), 1.48 (s, 3H, –C(CH3)2), 1.44 (d, 3H, J5,6 = 6.0 Hz H-6II), 1.33 (s, 3H, –C(CH3)2), 1.25 (d, 3H, J5,6 = 6.5 Hz, H-6I); 13C NMR: δ 153.9, 137.1, 128.4, 128.1, 128.0, 109.0, 100.9 (1JCH = 157.1 Hz), 98.2 (1JCH = 166.5 Hz), 83.4, 81.8, 77.9, 76.40, 75.8, 73.6, 73.2, 71.1, 63.9, 54.9, 28.0, 26.1, 17.8, 17.7; ESIMS m/z calcd for C24H32O10Na [M+Na]+: 503.1888. Found: 503.1885.
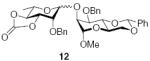
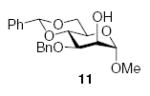
3.2.7. Methyl 3-O-benzyl-4,6-O-benzylidiene-2-O-(2-O-benzyl-3,4-O-carbonyl-α-l-rhamnopyranosyl)-α-d-manno-pyranoside (12α)
[α]D21 369.9 (c 0.9, CHCl3); IR: 1810 cm−1; 1H NMR (CDCl3): δ 7.52 (dd, 2H, J = 8.5 Hz, J = 2.5 Hz, arom H), 7.40–7.30 (m, 13H, arom H), 5.65 (s, 1H, H-7I), 4.87 (d, 1H, 2J = 11.5 Hz, –CH2Ph), 4.83 (s, 1H, H-1II), 4.82 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.67 (dd, 1H, J3,4 = 11.0 Hz, J2,3 = 2.5 Hz, H-3II), 4.66 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.63 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.49 (d, 1H, J1,2 = 2.0 Hz, H-1I), 4.45 (m, 1H, H-5II), 4.35 (dd, 1H, J4,5 = 11.5 Hz, J3,4 = 10.0 Hz, H-4II), 4.25 (dd, 1H, J6a,6b = 10.0 Hz, J5,6a = 4.5 Hz, H-6I), 4.21 (m, 1H, H-2II), 4.1 (dd, 1H, J2,3 = 3.5 Hz, J1,2 = 1.5 Hz, H-2I), 4.03 (t, 1H, J3,4, J4,5 = 10.0 Hz, H-4I), 3.94 (dd, 1H, J3,4 = 9.5 Hz, J2,3 = 3.0 Hz, H-3I), 3.84 (t, 1H, J5,6a, J6a,6b = 10.5 Hz, H-6I), 3.76 (m, 1H, H-5I), 3.35 (s, 3H, –OCH3), 1.11 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 153.8, 138.0, 137.4, 137.0, 129.0, 128.6, 128.4, 128.2, 127.9, 127.8, 126.0, 101.6 (1JCH = 162.4 Hz), 98.8 (1JCH = 165.9 Hz), 97.6 (1JCH 169.3 Hz), 80.2, 79.1, 77.9, 75.0, 74.4, 74.0, 73.8, 73.3, 68.9, 68.7, 63.9, 55.0, 17.5; ESIMS m/z calcd for C35H38O11Na [M+Na]+: 657.2307. Found: 657.2306.
3.2.8. Methyl 3-O-benzyl-4,6-O-benzylidiene-2-O-(2-O-benzyl-3,4-O-carbonyl-β-l-rhamnopyranosyl)-α-d-manno-pyranoside (12β)
[α]D21 +29.3 (c 0.6, CHCl3); IR: 1811 cm−1; 1H NMR (CDCl3): δ 7.49 (dd, 2H, J = 8.5 Hz, J = 3.0 Hz, arom H), 7.45 (d, 2H, J = 7.5 Hz, arom H), 7.40–7.37 (m, 4H, arom H), 7.34–7.31 (m, 4H, arom H), 7.27 (m, 3H, arom H), 5.56 (s, 1H, H-7I), 4.94 (m, 4H, H-1I, –CH2Ph), 4.79 (s, 1H, H-1II), 4.60 (d, 1H, 2J = 11.5 Hz, –CH2Ph), 4.44 (dd, 1H, J4,5 = 11.5 Hz, J3,4 = 9.5 Hz, H-4II), 4.32 (m, 1H, H-6I), 4.28 (d, 1H, J2,3 = 2.0 Hz, H-2II), 4.11 (t, 1H, J1,2, J2,3 = 2.5 Hz, H-2I), 4.0 (m, 2H, H-3I, H-4I), 3.92 (dd, 1H, J3,4 = 11.5 Hz, J2,3 = 2.5 Hz, , H-3II), 3.81 (m, 2H, H-5I, H-6I), 3.60 (m, 1H, H-5II), 3.38 (s, 3H, –OCH3), 1.42 (d, 3H, J5,6 = 6.5 Hz, H-6II); 13C NMR: δ 153.9, 138.5, 137.5, 137.4, 129.0, 128.5, 128.2, 128.0, 127.92, 127.86, 127.5, 126.0, 101.9 (1JCH = 164.8 Hz), 101.6 (1JCH = 160.5 Hz), 101.2 (1JCH = 174.8 Hz), 81.5, 79.4, 77.8, 76.4, 73.8, 73.7, 73.2, 71.0, 69.1, 63.7, 54.9, 17.8; ESIMS m/z calcd for C35H38O11Na [M+Na]+: 657.2307. Found: 657.2307.
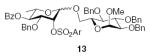
3.2.9. Methyl 6-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-tri-fluoromethylbenzenesulfonyl)-α-l-rhamnopyranosyl)]-2,3, 4-tri-O-benzyl-α-d-glucopyranoside (13α)
[α]D21 +31.9 (c 1.0, CHCl3), lit.29 −30.2; 1H NMR (CDCl3): δ 8.22 (d, 1H, J = 7.5 Hz, arom H), 7.95 (d, 2H, J = 8.5 Hz, arom H), 7.73 (d, 1H, J = 8.0 Hz, arom H), 7.59–7.26 (m, 17H, arom H), 7.14 (m, 1H, arom H), 7.06 (t, 2H, J = 8.0 Hz, arom H), 6.94 (d, 2H, J = 7.5 Hz, arom H), 5.28 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4II), 5.12 (d, 1H, J1,2 = 1.5 Hz, H-1II), 5.03–5.01 (m, 2H, H-2II, –CH2Ph), 4.94 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.85 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.83 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.72 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.62 (d, 1H, J1,2 = 3.0 Hz, H-1I), 4.55 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.30–4.23 (q, 2H, J = 12.5 Hz, –CH2Ph), 4.02 (t, 1H, J2,3, J3,4 = 9.5 Hz, H-3I), 3.90–3.83 (m, 3H, H-6I, H-3II, H-5II), 3.79–3.76 (m, 1H, H-5I), 3.60–3.57 (dd, 1H, J6a,6b = 11.0 Hz, J5,6a = 6.5 Hz, H-6I), 3.54 (dd, 1H, J2,3 = 9.5 Hz, J1,2 = 3.0 Hz, H-2I), 3.40–3.36 (m, 4H, H-4I, –OCH3), 1.19 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 165.3, 138.6, 138.0, 137.9, 137.0, 135.2, 133.5, 133.2, 132.0, 131.6, 129.8, 129.7, 128.52, 128.48, 128.37, 128.31, 128.26, 128.20, 128.16, 128.08, 128.07, 128.00, 127.96, 127.92, 127.8, 127.6, 97.9 (1JCH 172.9 Hz), 97.8 (1JCH 157.3 Hz), 82.0, 79.9, 77.8, 76.8, 75.9, 75.2, 73.6, 73.3, 72.5, 71.5, 70.2, 67.0, 66.8, 55.2, 17.3; ESIMS m/z calcd for C55H55O13F3NaS [M+Na]+: 1035.3213. Found: 1035.3218.
3.2.10. Methyl 6-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-tri-fluoromethylbenzenesulfonyl)-β-l-rhamnopyranosyl)]-2,3, 4-tri-O-benzyl-α-d-glucopyranoside (13β)
[α]D21 +59.0 (c 1.0, CHCl3), lit.29 +22.9; 1H NMR (CDCl3): δ 8.22 (d, 1H, J = 7.5 Hz, arom H), 7.96–7.94 (dd, 2H, J = 7.5 Hz, J 0.5 Hz, arom H), 7.66 (d, 1H, J = 8.0 Hz, arom H), 7.59 (t, 1H, J = 7.0 Hz, arom H), 7.46–7.18 (m, 15H, arom H), 7.11 (t, 2H, J = 7.5 Hz, arom H), 7.04 (d, 2H, J = 7.0 Hz, arom H), 5.41 (d, 1H, J2,3 = 3.0 Hz, H-2II), 5.29 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4II), 4.98 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.86–4.82 (m, 2H, –CH2Ph), 4.75 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.66 (s, 1H, H-1II), 4.64 (d, 1H, J1,2 = 3.5 Hz, H-1I), 4.56–4.49 (m, 3H, –CH2Ph), 4.37 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.27–4.24 (dd, 1H, J6a,6b = 11.5 Hz, J5,6a = 3.5 Hz, H-6I), 3.91 (t, 1H, J2,3, J3,4 = 9.0 Hz, H-3I), 3.69–3.63 (m, 3H, H-5I, H-6I, H-3II), 3.56–3.52 (m, 2H, H-2I, H-5II), 3.43 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4I), 3.36 (s, 3H, –OCH3), 1.26 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 165.2, 139.1, 138.6, 138.4, 136.8, 136.5, 133.24, 133.22, 131.9, 130.9, 129.8, 129.6, 128.5, 128.4, 128.34, 128.26, 128.25, 128.17, 128.0, 127.92, 127.86, 127.74, 127.55, 127.52, 98.3 (1JCH 165.9 Hz), 97.8 (1JCH 156.8 Hz), 81.7, 79.6, 77.4, 77.2, 75.6, 74.9, 73.4, 72.5, 71.0, 70.9, 69.9, 67.1, 55.2, 17.6; ESIMS m/z calcd for C55H55O13F3NaS [M+Na]+: 1035.3213. Found: 1035.3209.
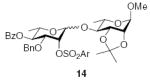
3.2.11. Methyl 4-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-trifluoromethylbenzenesulfonyl)-α-l-rhamnopyranosyl)]-2,3-O-isopropylidiene-α-l-rhamnopyranoside (14α)
[α]D21 +21.5 (c 0.5, CHCl3), lit.29 −55.2; 1H NMR (CDCl3): δ 8.26 (d, 1H, J = 7.5 Hz, arom H), 7.94 (d, 2H, J = 7.5 Hz, arom H), 7.76 (d, 1H, J = 7.5 Hz, arom H), 7.58–7.50 (m, 3H, arom H), 7.42 (t, 2H, J = 8.0 Hz, arom H), 7.17 (t, 1H, J = 7.5 Hz, arom H), 7.1 (t, 2H, arom H), 6.99 (d, 2H, J = 7.5 Hz, arom H), 5.48 (d, 1H, J1,2 = 1.5 Hz, H-1II), 5.28 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4II), 5.19 (t, 1H, J1,2, J2,3 = 2.5 Hz, H-2II), 4.86 (s, 1H, H-1I), 4.39 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.30 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.12–4.11 (m, 2H, H-2I, H-3I), 3.91–3.86 (m, 2H, H-3II, H-5II), 3.66–3.63 (m, 1H, H-5I), 3.48–3.45 (m, 1H, H-4I), 3.39 (s, 3H, –OCH3), 1.55 (s, 3H, –C(CH3)2), 1.37 (s, 3H, –C(CH3)2), 1.27 (d, 3H, J5,6 = 6.5 Hz, H-6I), 1.22 (d, 3H, J5,6 = 6.5 Hz, H-6II); 13C NMR: δ 165.2, 137.1, 135.5, 133.3, 133.1, 131.9, 131.7, 129.8, 129.7, 128.3, 128.24, 128.20, 128.1, 127.7, 127.5, 109.7, 98.0 (1JCH = 165.4 Hz), 97.2 (1JCH = 171.6 Hz), 79.3, 77.8, 77.1, 76.0, 73.7, 72.4, 71.4, 67.7, 63.9, 55.0, 28.0, 26.4, 17.7, 17.5. Anal calcd for C37H41F3O12S: C, 57.96; H, 5.39. Found: C, 57.66; H, 5.23.
3.2.12. Methyl 4-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-trifluoromethylbenzenesulfonyl)-β-l-rhamnopyranosyl)]-2,3-O-isopropylidiene-α-l-rhamnopyranoside (14β)
[agr;]D21 +43.6 (c 0.7, CHCl3), lit.29 +30.7; 1H NMR (CDCl3): δ 8.28 (m, 1H, arom H), 7.96 (d, 2H, J = 8.0 Hz, arom H), 7.74 (m, 1H, arom H), 7.59–7.52 (m, 3H, arom H), 7.43 (t, 2H, J = 7.5 Hz, arom H), 7.16 (t, 1H, J = 7.0 Hz, arom H), 7.08 (t, 2H, J = 7.5 Hz, arom H), 7.0 (d, 2H, J = 7.5 Hz, arom H), 5.34 (d, 1H, J2,3 = 3.0 Hz, H-2II), 5.30 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4II), 4.80 (s, 2H, H-1I, H-1II), 4.82 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.34 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.16 (t, 1H, J2,3, J3,4 = 6.5 Hz, H-3I), 4.05 (d, 1H, J2,3 = 6.0 Hz, H-2I), 3.70–3.68 (dd, 1H, J3,4 = 10.0 Hz, J2,3 = 3.0 Hz, H-3II), 3.56 (m, 1H, H-5II), 3.49 (m, 1H, H-5I), 3.42–3.39 (m, 1H, H-4I), 3.34 (s, 3H, –OCH3), 1.50 (s, 3H, –C(CH3)2), 1.31 (s, 3H, –C(CH3)2), 1.30 (d, 3H, J5,6 = 6.0 Hz, H-6II), 1.26 (d, 3H, J5,6 = 6.5 Hz, H-6I); 13C NMR: δ 165.2, 136.83, 136.80, 133.3, 132.7, 131.8, 131.2, 129.8, 129.7, 128.4, 128.2, 128.0, 127.85, 127.80, 127.75, 127.70, 127.65, 127.61, 108.9, 98.0 (1JCH = 166.6 Hz), 97.6 (1JCH = 155.1 Hz), 82.8, 78.0, 76.3, 76.1, 75.7, 72.6, 71.06, 71.05, 63.8, 54.7, 28.1, 26.1, 17.7, 17.5. Anal calcd for C37H41F3O12S: C, 57.96; H, 5.39. Found: C, 58.03; H, 5.39.
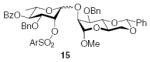
3.2.13. Methyl 3-O-benzyl-4,6-O-benzylidiene-2-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-trifluoromethylbenzenesulfonyl)-α-l-rhamnopyranosyl)]-α-d-mannopyranoside (15α)
[α]D21 −24.3 (c 1.1, CHCl3), lit.29 −35.6; 1H NMR (CDCl3): δ 8.27 (d, 1H, J = 7.5 Hz, arom H), 7.92 (d, 2H, J = 7.5 Hz, arom H), 7.75 (d, 1H, J = 7.5 Hz, arom H), 7.57–7.39 (m, 10H, arom H), 7.34 (d, 2H, J = 7.0 Hz, arom H), 7.26–7.19 (m, 4H, arom H), 7.14 (t, 2H, J = 7.5 Hz, arom H), 6.99 (d, 2H, J = 7.0 Hz, arom H), 5.66 (s, 1H, H-7I), 5.30 (t, 1H, J3,4, J4,5 = 10.0 Hz, H-4II), 5.18 (d, 1H, J1,2 = 1.0 Hz, H-1II), 5.11 (t, 1H, J1,2, J2,3 = 3.0 Hz, H-2II), 4.82 (d, 1H, 2J = 11.5 Hz, –CH2Ph), 4.72 (s, 1H, H-1I), 4.70 (d, 1H, 2J = 11.5 Hz, –CH2Ph), 4.39–4.30 (m, 4H, H-6I, H-5II, –CH2Ph), 4.19 (s, 1H, H-2I), 4.02–3.98 (m, 3H, H-3I, H-4I, H-3II), 3.88 (t, 1H, J5,6a, J6a,6b = 10.0 Hz, H-6I), 3.81 (m, 1H, H-5I), 3.41 (s, 3H, –OCH3), 1.07 (d, 3H, J5,6 = 6.5 Hz, H-6II); 13C NMR: δ 165.4, 138.0, 137.5, 137.1, 135.0, 133.5, 133.1, 132.0, 131.7, 129.9, 129.7, 129.0, 128.4, 128.32, 128.26, 128.23, 128.18, 127.7, 127.6, 126.1, 101.6 (1JCH = 162.3 Hz), 99.0 (1JCH = 170.5 Hz), 96.5 (1JCH = 170.6 Hz), 78.7, 74.5, 74.1, 73.8, 73.1, 72.4, 71.7, 68.8, 67.2, 63.9, 55.1, 17.2; ESIMS m/z calcd for C48H47F3O13NaS [M+Na]+: 943.2587. Found: 943.2598.
3.2.14. Methyl 3-O-benzyl-4,6-O-benzylidiene-2-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-trifluoromethylbenzenesulfonyl)-β-l-rhamnopyranosyl)]-α-d-mannopyranoside (15β)
[α]D21 +51.1 (c 1.0, CHCl3), lit.29 +25.6; 1H NMR (CDCl3): δ 8.35 (m, 1H, arom H), 7.94–7.92 (dd, 2H, J = 8.0 Hz, 1.0 Hz, arom H), 7.77 (m, 1H, arom H), 7.59–7.26 (m, 15H, arom H), 7.16 (t, 1H, J = 7.5 Hz, arom H), 7.07 (t, 2H, J = 7.5 Hz, arom H), 6.96 (d, 2H, J = 7.0 Hz, arom H), 5.69 (s, 1H, H-7I), 5.47 (d, 1H, J2,3 = 2.5 Hz, H-2II), 5.26 (t, 1H, J3,4, J4,5 = 10.0 Hz, H-4II), 4.98 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.89 (s, 1H, H-1II), 4.67 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.63 (d, 1H, J1,2 = 1.5 Hz, H-1I), 4.44 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.31 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4I), 4.25–4.19 (m, 2H, H-6I, –CH2Ph), 4.15 (m, 1H, H-2I), 4.00–3.98 (dd, 1H, J3,4 = 9.5 Hz, J2,3 2.5 Hz, H-3I), 3.81 (t, 1H, J5,6a, J6a,6b = 10.0 Hz, H-6I), 3.75 (m, 1H, H-5I), 3.49–3.46 (dd, 1H, J3,4 = 9.5 Hz, J2,3 = 2.5 Hz, H-3II), 3.42 (m, 1H, H-5II), 3.33 (s, 3H, –OCH3), 1.29 (d, 3H, J5,6 = 6.5 Hz, H-6II); 13C NMR: δ 165.2, 138.9, 137.8, 137.0, 136.9, 133.2, 132.6, 132.0, 131.1, 129.8, 129.7, 128.8, 128.5, 128.4, 128.20, 128.17, 127.95, 127.91, 127.86, 127.81, 127.72, 127.65, 127.60, 127.57, 126.1, 101.5 (1JCH = 164.5 Hz), 100.9 (1JCH = 171.8 Hz), 98.6 (1JCH = 161.0 Hz), 79.0, 77.6, 76.6, 76.5, 75.9, 73.6, 72.3, 71.0, 70.8, 68.9, 64.0, 54.7, 17.6; ESIMS m/z calcd for C48H47F3O13NaS [M+Na]+: 943.2587. Found: 943.2542.
3.2.15. Methyl 3-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-tri-fluoromethylbenzenesulfonyl)-α-l-rhamnopyranosyl)]-2,4, 6-tri-O-benzyl-α-d-mannopyranoside (17α)
[α]D21 +6.9 (c 0.7, CHCl3), lit.29 −23.1; 1H NMR (CDCl3): δ 8.16 (d, 1H, J = 8.5 Hz, arom H), 7.72 (m, 3H, arom H), 7.56–7.07 (m, 21H, arom H), 7.01 (t, 2H, J = 7.5 Hz, arom H), 6.88 (d, 2H, J = 7.5 Hz, arom H), 5.36 (s, 1H, H-1II), 5.30 (t, 1H, J3,4, J4,5 10.0 Hz, H-4II), 5.03 (s, 1H, H-2II), 4.85 (s, 1H, H-1I), 4.81–4.71 (m, 3H, –CH2Ph), 4.62–4.55 (m, 2H, –CH2Ph), 4.45 (d, 1H, 2J = 11.0 Hz, –CH2Ph), 4.21–4.10 (m, 3H, H-3I, –CH2Ph), 4.03 (m, 1H, H-5II), 4.00–3.93 (m, 3H, H-2I, H-4I, H-3II), 3.82 (m, 1H, H-6I), 3.78–3.72 (m, 2H, H-5I, H-6I), 3.34 (s, 3H, –OCH3), 1.13 (d, 3H, J5,6 = 6.0 Hz, H-6II); 13C NMR: δ 165.2, 138.4, 138.3, 138.0, 136.7, 134.9, 133.5, 133.1, 131.9, 131.7, 129.7, 129.6, 128.5, 128.3, 128.1, 127.74, 127.71, 127.69, 127.64, 127.59, 127.54, 127.50, 127.3, 98.7 (1JCH 169.8 Hz), 93.5 (1JCH 171.6 Hz), 76.9, 75.0, 74.8, 73.9, 73.8, 73.6, 73.4, 73.2, 72.2, 71.5, 71.3, 69.0, 67.1, 54.9, 17.3; ESIMS m/z calcd for C55H55O13F3KS [M+K]+: 1051.2953. Found: 1051.2914.
3.2.16. Methyl 3-O-[(4-O-benzoyl-3-O-benzyl-2-O-(2-tri-fluoromethylbenzenesulfonyl)-β-l-rhamnopyranosyl)]-2,4, 6-tri-O-benzyl-α-d-mannopyranoside (17β)
[α]D21 +68.9 (c 1.1, CHCl3), lit.29 +35.2; 1H NMR (CDCl3): δ 8.23 (d, 1H, J = 7.0 Hz, arom H), 7.90 (dd, 2H, J = 8.0 Hz, J = 0.5 Hz, arom H), 7.67 (d, 1H, J = 9.0 Hz, arom H), 7.56 (t, 1H, J = 7.0 Hz arom H), 7.45–7.24 (m, 19H, arom H), 7.15 (t, 1H, J = 7.5 Hz, arom H), 7.06 (t, 2H, J = 7.5 Hz, arom H), 6.90 (d, 2H, J = 7.5 Hz, arom H), 5.27 (t, 1H, J3,4, J4,5 = 9.0 Hz, H-4II), 5.13 (d, 1H, J2,3 = 2.5 Hz, H-2II), 4.88 (d, 1H, 2J = 13.0 Hz, –CH2Ph), 4.77 (t, 2H, 2J = 10.5 Hz, –CH2Ph), 4.72 (s, 1H, H-1II), 4.71 (d, 1H, 2J = 12.0 Hz, –CH2Ph), 4.65 (d, 1H, J1,2 = 1.5 Hz, H-1I), 4.60–4.57 (m, 2H, –CH2Ph), 4.30 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.13 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.02 (dd, 1H, J3,4 = 9.5 Hz, J2,3 = 2.5 Hz, H-3I), 3.94 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-4I), 3.89 (m, 1H, H-2I), 3.79–3.75 (m, 3H, H-5I, H-6I), 3.48 (m, 1H, H-5II), 3.41–3.39 (dd, 1H, J3,4 = 9.5 Hz, J2,3 = 3.0 Hz, H-3II), 3.32 (s, 3H, –OCH3), 1.26 (d, 3H, J5,6 = 6.5 Hz, H-6II); 13C NMR: δ 165.2, 139.1, 139.0, 138.4, 136.7, 136.5, 133.2, 132.7, 131.8, 131.0, 129.7, 129.6, 128.40, 128.37, 128.32, 128.25, 128.17, 128.10, 127.9, 127.8, 127.6, 127.55, 127.47, 127.2, 100.2 (1JCH = 155.4 Hz) (1JCH = 164.5 Hz), 83.4, 78.2, 76.7, 76.2, 75.0, 74.8, 73.5, 73.1, 72.3, 71.9, 71.2, 70.8, 69.4, 54.7, 17.7; ESIMS m/z calcd for C55H55O13F3KS [M+Na]+: 1035.3213. Found: 1035.3236.
3.3. Methyl 6-O-acetyl-2,3,4-tri-O-benzyl-α-d-gluco-pyranoside
Tf2O (0.027 mL, 0.16 mmol) was added dropwise to a stirred solution of donor 1 (70 mg, 0.11 mmol) and BSP (29 mg, 0.14 mmol), in CH3CN (1.2 mL) and CH2Cl2 (1.8 mL) under argon at −60 °C. After stirring for 1 h at −60 °C, acceptor 4 (150 mg, 0.32 mmol) in CH2Cl2 (1.0 mL) was added over a period of 1 min. The reaction mixture was stirred for 3 h at −60 °C, then quenched at −60 °C with saturated aqueous NaHCO3, and extracted with CH2Cl2. (3 × 15 mL). The combined organic layer was dried (Na2SO4), and the solvent was removed under reduced pressure. Examination of the crude reaction mixture by 1H NMR spectroscopy revealed the formation of one very major product, tentatively assigned as the imidate (18). Purification by silica gel column chromatography (EtOAc/hexane) afforded the unstable 18 (30 mg, 27%) and acetate 19 (10 mg, 6%). Compound 18 rapidly decomposed on standing in CDCl3 to a mixture of 19 and several other compounds. It was characterized by the following signals: 1H NMR (300 MHz, CDCl3): δ 5.13 (s, 1H, H-1II), 4.62 (d, 1H, J1,2 = 3.6 Hz, H-1I), 3.42 (s, 3H, –OCH3), 1.99 (s, 3H, CH3C(OR)=N), 1.17 (d, 3H J5,6 = 6.3 Hz, H-6II).
3.3.1. Methyl 6-O-acetyl-2,3,4-tri-O-benzyl-α-d-gluco-pyranoside (19)
[α]D21 +18.0 (c 0.8, CHCl3), lit.56 +28.6; 1H NMR (CDCl3): δ 7.37–7.26 (m, arom H), 5.09 (d, 1H, 2J = 10.5 Hz, –CH2Ph), 4.89–4.79 (m, 3H, –CH2Ph), 4.68 (d, 1H, 2J = 12.5 Hz, –CH2Ph), 4.59–4.55 (m, 2H, –CH2Ph), 4.26–4.24 (m, 2H, H-6), 4.01 (t, 1H, J3,4, J4,5 = 9.5 Hz, H-3), 3.81 (m, 1H, H-5), 3.55–3.52 (dd, 1H, J2,3 = 9.5 Hz, J1,2 = 3.5 Hz, H-2), 3.48 (t, 1H, J4,5, J3,4 = 9.0 Hz, H-4), 3.37 (s, 3H, –OCH3), 2.02 (s, 3H, CH3CO); 13C NMR: δ 170.7, 138.6, 138.0, 137.8, 128.50, 128.48, 128.45, 128.13, 128.09, 128.01, 127.99, 127.92, 127.7, 98.1, 82.0, 79.8, 75.8, 75.0, 73.4, 68.5, 63.0, 55.2, 20.9.
Supplementary Material
Scheme 1.
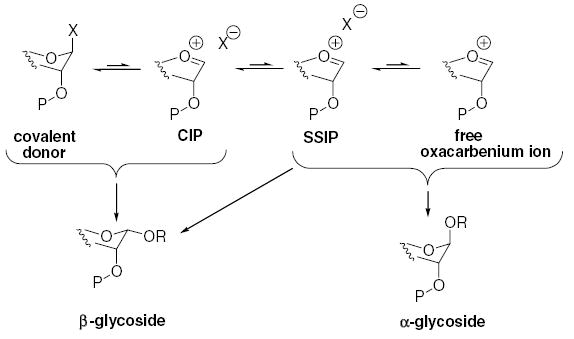
Abbreviated glycosylation mechanism.
Acknowledgments
We thank the NIH (GM59335) for support of this work, and John Picione for first recognizing the α-selective coupling of donor 2 with acceptor 9.
Footnotes
We distinguish between direct methods, which afford the desired glycosidic bond in a single step, and indirect methods, requiring either prior attachment of the acceptor to the donor, or correction of stereochemistry after formation of the glycosidic bond.
Propionitrile has a lower freezing point (−93 °C) than acetonitrile (−48 °C), making it the nitrile of choice when used in significant proportion in low temperature glycosylations. It was demonstrated by Schmidt that propionitrile and acetonitrile have similar directing effects in other glycosylation reactions.44
Supplementary data
Copies of the 1H and 13C NMR spectra for disaccharides. Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.carres.2006.03.036.
References
- 1.Crich D, Sun S. J Org Chem. 1997;62:1198–1199. [Google Scholar]
- 2.Crich D, Sun S. Tetrahedron. 1998;54:8321–8348. [Google Scholar]
- 3.Crich D, Smith M. J Am Chem Soc. 2001;123:9015–9020. doi: 10.1021/ja0111481. [DOI] [PubMed] [Google Scholar]
- 4.Crich D, Lim LBL. Org React. 2004;64:115–251. [Google Scholar]
- 5.Crich D. J Carbohydr Chem. 2002;21:663–686. [Google Scholar]
- 6.Crich D, Hutton TK, Banerjee A, Jayalath P, Picione J. Tetrahedron: Asymmetry. 2005;16:105–119. [Google Scholar]
- 7.Srivastava VK, Schuerch C. J Org Chem. 1981;46:1121–1126. [Google Scholar]
- 8.Ito Y, Kanie O, Ogawa T. Angew Chem, Int Ed. 1996;35:2510–2512. [Google Scholar]
- 9.Ito Y, Ogawa T. Angew Chem, Int Ed Engl. 1994;33:1765–1767. [Google Scholar]
- 10.Barresi F, Hindsgaul O. Can J Chem. 1994;72:1447–1465. [Google Scholar]
- 11.Cumpstey I, Chayajarus K, Fairbanks AJ, Redgrave AJ, Seward CMP. Tetrahedron: Asymmetry. 2004;15:3207–3221. [Google Scholar]
- 12.Ziegler T, Lemanski G. Angew Chem, Int Ed. 1998;37:3129–3132. doi: 10.1002/(SICI)1521-3773(19981204)37:22<3129::AID-ANIE3129>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Stork G, La Clair JJ. J Am Chem Soc. 1996;118:247–248. [Google Scholar]
- 14.Abdel-Rahman AA-H, El Ashry ESH, Schmidt RR. Carbohydr Res. 2002;337:195–206. doi: 10.1016/s0008-6215(01)00306-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaji E, Lichtenthaler FW. Trends Glycosi Glycotechnol. 1993;5:121–142. [Google Scholar]
- 16.Hodosi G, Kovác P. J Am Chem Soc. 1997;119:2335–2336. [Google Scholar]
- 17.El Ashry ESH, Rashed N, Ibrahim ESI. Curr Org Synth. 2005;2:175–213. [Google Scholar]
- 18.Pozsgay, V. In Carbohydrate in Chemistry and Biology; Ernst, B., Hart, G. W., Sinaÿ, P., Eds.; Wiley-VCH: Weinheim, 2000; Vol. 1, pp 319–343.
- 19.Ito, Y.; Ohnishi, Y. In Glycoscience: Chemistry and Chemical Biology; Fraser-Reid, B., Kuniaki, T., Thiem, J., Eds.; Springer: Berlin, 2001; Vol. 2, pp 1589–1619.
- 20.Ramm M, Lobe M, Hamburger M. Carbohydr Res. 2003;338:109–112. doi: 10.1016/s0008-6215(02)00353-1. [DOI] [PubMed] [Google Scholar]
- 21.Crich D, Yao Q. J Am Chem Soc. 2004;126:8232–8236. doi: 10.1021/ja048070j. [DOI] [PubMed] [Google Scholar]
- 22.Crich, D.; Bowers, A. A. J. Org. Chem., in press.
- 23.Kwon YT, Lee YJ, Lee K, Kim KS. Org Lett. 2004;6:3901–3904. doi: 10.1021/ol048648u. [DOI] [PubMed] [Google Scholar]
- 24.Schüle G, Ziegler T. Liebigs. 1996:1599–1607. [Google Scholar]
- 25.Lau R, Schüle G, Schwaneberg U, Ziegler T. Liebigs. 1995:1745–1754. [Google Scholar]
- 26.Lichtenthaler FW, Metz TW. Tetrahedron Lett. 1997;38:5477–5480. [Google Scholar]
- 27.Crich D, Chandrasekera NS. Angew Chem, Int Ed. 2004;43:5386–5389. doi: 10.1002/anie.200453688. [DOI] [PubMed] [Google Scholar]
- 28.Crich D, Sun S. J Am Chem Soc. 1997;119:11217–11223. [Google Scholar]
- 29.Crich D, Picione J. Org Lett. 2003;5:781–784. doi: 10.1021/ol0340890. [DOI] [PubMed] [Google Scholar]
- 30.Crich D, Cai W, Dai Z. J Org Chem. 2000;65:1291–1297. doi: 10.1021/jo9910482. [DOI] [PubMed] [Google Scholar]
- 31.Crich D, Vinod AU, Picione J. J Org Chem. 2003;68:8453–8458. doi: 10.1021/jo035003j. [DOI] [PubMed] [Google Scholar]
- 32.Codée JDC, Litjens REJN, den Heeten R, Overkleeft HS, van Boom JH, van der Marel GA. Org Lett. 2003;5:1519–1522. doi: 10.1021/ol034312t. [DOI] [PubMed] [Google Scholar]
- 33.Gorin PAJ, Perlin AS. Can J Chem. 1961;39:2474–2485. [Google Scholar]
- 34.Iversen T, Bundle DR. Carbohydr Res. 1980;84:C13–C15. [Google Scholar]
- 35.Paulsen H, Kutschker W, Lockho3 O. Chem Ber. 1981;114:3233–3241. [Google Scholar]
- 36.Backinowsky LV, Balan NF, Shashkov AS, Kochetkov NK. Carbohydr Res. 1980;84:225–235. [Google Scholar]
- 37.Barresi, F.; Hindsgaul, O. In Modern Methods in Carbohydrate Synthesis; Khan, S. H., O’Neill, R. A., Eds.; Harwood Academic Publishers: Amsterdam, 1996; pp 251–276.
- 38.Crich D, Vinod AU, Picione J, Wink DJ. ARKIVOC. 2005;6:339–344. [PMC free article] [PubMed] [Google Scholar]
- 39.Bedini E, Carabellese A, Barone G, Parrilli M. J Org Chem. 2005;70:8064–8070. doi: 10.1021/jo051153d. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar, D.; Boons, G.-J. In Handbook of Reagents for Organic Synthesis: Reagents for Glycoside, Nucleotide, and Peptide Synthesis; Crich, D., Ed.; Wiley: Chichester, 2005; pp 11–15.
- 41.Pougny JR, Sinaÿ P. Tetrahedron Lett. 1976;17:4073–4076. [Google Scholar]
- 42.Ratcliffe AJ, Fraser-Reid B. J Chem Soc, Perkin Trans 1. 1990:747–750. [Google Scholar]
- 43.Braccini J, Derouet C, Esnault J, de Penhoat CH, Mallet JM, Michon V, Sinaÿ P. Carbohydr Res. 1993;246:23–41. [Google Scholar]
- 44.Schmidt RR, Behrendt M, Toepfer A. Synlett. 1990:694–696. [Google Scholar]
- 45.Fügedi P, Liptak A, Nanasi P, Neszmelyi A. Carbohydr Res. 1982;107:C5–C8. [Google Scholar]
- 46.Uriel C, Gomez AM, Lopez JC, Fraser-Reid B. J Carbohydr Chem. 2005;24:665–675. [Google Scholar]
- 47.Crich D, Smith M, Yao Q, Picione J. Synthesis. 2001:323–326. [Google Scholar]
- 48.Kahne D, Walker S, Cheng Y, Engen DV. J Am Chem Soc. 1989;111:6881–6882. [Google Scholar]
- 49.Schweizer F, Lohse A, Otter A, Hindsgaul O. Synlett. 2001:1434–1436. [Google Scholar]
- 50.Lohse A, Schweizer F, Hindsgaul O. Comb Chem High Throughput Screening. 2002;5:389–394. doi: 10.2174/1386207023330228. [DOI] [PubMed] [Google Scholar]
- 51.Masamune S, Choy W, Peterson JS, Sita LR. Angew Chem, Int Ed Engl. 1985;24:1–76. [Google Scholar]
- 52.Paulsen, H. In Selectivity a Goal for Synthetic Efficiency; Bartmann, W., Trost, B. M., Eds.; Verlag Chemie: Weinheim, 1984; pp 169–190.
- 53.Spijker NM, van Boeckel CAA. Angew Chem, Int Ed Engl. 1991;30:180–183. [Google Scholar]
- 54.Fraser-Reid B, López JC, Gómez AM, Uriel C. Eur J Org Chem. 2004:1387–1395. [Google Scholar]
- 55.Bock K, Pedersen C. J Chem Soc, Perkin Trans 2. 1974:293–297. [Google Scholar]
- 56.Prosperi D, Ronchi S, Lay L, Rencurosi A, Russo G. Eur J Org Chem. 2004:395–405. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


