Abstract
“Cone dystrophy with supernormal rod electroretinogram (ERG)” is an autosomal recessive disorder that causes lifelong visual loss combined with a supernormal ERG response to a bright flash of light. We have linked the disorder to a 0.98-cM (1.5-Mb) region on chromosome 9p24, flanked by rs1112534 and rs1074449, using homozygosity mapping in one large consanguineous pedigree. Analysis of one gene within this region, KCNV2, showed a homozygous nonsense mutation. Mutations were also found in 17 alleles of 10 other unrelated families with the same disorder. In situ hybridization demonstrated KCNV2 expression in human rod and cone photoreceptors. The precise function of KCNV2 in human photoreceptors remains to be determined, although this work suggests that mutations might perturb or abrogate IKX, the potassium current within vertebrate photoreceptor inner segments, which has been shown to set their resting potential and voltage response.
The mechanism by which the sensitivity of the visual system is regulated has been a focus of study for many years. “Cone dystrophy with supernormal rod electroretinogram (ERG)” is unusual, in that affected individuals show a delayed and reduced cone and rod ERG response that switches at higher light levels in rods to an exaggerated or supernormal response. Since the rod ERG b-wave is derived predominantly from bipolar cells in the retina, the ERG phenotype suggests a dysfunction affecting the first synapse of photoreception. The identification of the causative gene(s) would be expected, therefore, to provide insights into the control mechanisms that occur during signal transmission to these second-order neurons.
The disorder was first described in 1983,1 with five subsequent case series.2–6 It shows an autosomal recessive pattern of inheritance and is characterized by reduced visual acuity, photoaversion, night blindness, and abnormal color vision. At an early age, the retina shows subtle depigmentation at the macula and, later, more obvious areas of atrophy (fig. 1). Electroretinography is characteristic and is required to make a specific diagnosis (fig. 1). All patients in this study underwent a full ophthalmological examination. Blood samples were taken from affected individuals and family members, for DNA extraction and mutation screening. The protocol of this study adhered to the provisions of the Declaration of Helsinki.
Figure 1. .
Autofluorescence (A) and color image (B) of right and left retina of affected patient aged 59 years (family 4), showing areas of central atrophy of retinal pigment epithelium (RPE) and choroid. C, Color image of right and left retina of affected patient aged 24 years (family 1), showing subtle RPE depigmentation around fovea and crystals at the macula. D, Full-field ERGs (International Society for Clinical Electrophysiology of Vision [ISCEV]) in an unaffected subject (top row) and in a representative patient from family 9 (bottom row). In the patient, dark-adapted responses to the dimmest flash (0.002 candela [cd]-s/m2) are undetectable. Increasing stimulus intensity (“rod” 0.012 cd-s/m2) produces an abrupt increase in amplitude and a delayed rod ERG. At higher flash energies (“standard” 3.0 cd-s/m2 and “maximum” 11.5 cd-s/m2), the a-wave commences normally but develops a broadened trough before a high-amplitude, sharply rising b-wave that approaches the upper limit of normality (supernormal). Flicker and photopic single-flash ERGs were performed after 10 min of light adaptation. ISCEV-standard 30-Hz flicker ERGs show delay and marked reduction. The photopic single-flash ERG is delayed and subnormal, with a simplified waveform and delayed recovery after the beta-wave. Broken lines replace blink artifacts, frequently seen after the ERGs with strong flashes.
An altered phosphodiesterase activity within photoreceptors, which leads to an elevation in cyclic guanosine monophosphate (cGMP) levels, has been suggested as a possible disease mechanism.1,3,7 However, the only change reported so far is a G→C nucleotide transversion in the 5′ UTR of the cone cGMP-phosphodiesterase γ subunit (PDE6H) gene, which caused a significant increase in reporter-gene transcription.8 We screened the 5′ UTR and coding region of PDE6H in a panel of six unrelated patients of varied ethnic origin, using the primer pairs listed in table 1, but failed to detect any changes. Two flanking STR markers (D12S320 and D12S1669) and two intragenic SNPs (rs11056264 and rs3748304) flanking PDE6H also failed to cosegregate with disease in a family containing multiple affected members (data not shown). We conclude, therefore, that PDE6H is not the disease gene in our study sample.
Table 1. .
PCR Primer Pairs for PDE6H, KCNV2, KCNB1, KCNC1, and KCNF1
| Primer(5′→3′) |
||||
| Gene and Primer Pair |
Exon | Size (bp) |
Forward | Reverse |
| PDE6H: | ||||
| A | 1 | 290 | GGTCCCCATGACTTGAAAGATC | AAACAAAAGCAGGTTTTGTGGG |
| B | 2 | 159 | TCTCCATGTGAGTGACTCCAA | GCAGAACTCCAAGTGCGAAGT |
| C | 3 | 328 | AGGAGTGAAGTGTCTCTGCCT | CAGTAAGAGAACTCTTAGTAG |
| KCNV2: | ||||
| A | 1 | 1,133 | TAGAGGCAGTGAGCAGGTGA | CACTCGAGCAGCAGCTGA |
| B | 1 | 826 | TAGAGGCAGTGAGCAGGTGA | TCTCCATGAGGTTCCAGAGG |
| C | 1 | 1,184 | GCTGGACTACTGCGAGCTG | ATCCATCGCCGTTTTGTTAG |
| D | 1 | 822 | AGGAACTCTTCCGCGACAT | ATCCATCGCCGTTTTGTTAG |
| E | 2 | 448 | GCTTGCTCCTCTCCCTTTCT | CATTTCCTTTTGCTGCCAAT |
| F | 1 | 731 | GCTGGACTACTGCGAGCTG | CACTCGAGCAGCAGCTGA |
| Ga | 1 | 427 | GCTGGACTACTGCGAGCTG | TCTCCATGAGGTTCCAGAGG |
| KCNB1: | ||||
| H | … | 424 | GGGAGCGGGAAGGCGAGGAGTT | CTGGACCACGCGGCGGACATTC |
| KCNC1: | ||||
| I | … | 566 | TGGCCTTCGCTTCCCTCTTCTTCA | GCTCACTGGCGCTGGGGTCATTG |
| KCNF1: | ||||
| J | … | 536 | TGCTGGCCATCCTCCCCTTCTACG | CGCCCCCGCTGCTGGAGTTGAG |
Primer pair G was used for the amplification of a KCNV2 gene fragment, for the generation of riboprobes for in situ hybridization.
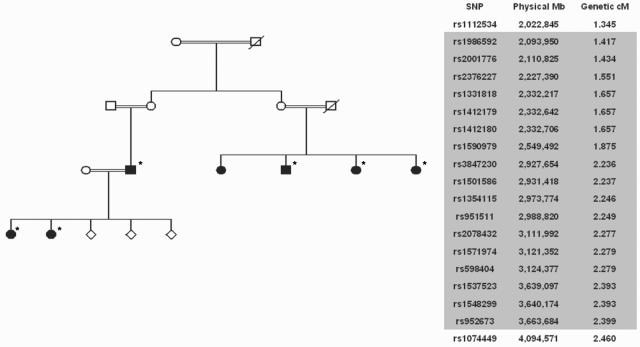
To extend the search for the disease gene, we performed homozygosity mapping on a consanguineous family with six affected members, using the GeneChip Human Mapping 10K SNP Array (Affymetrix). The family was of Middle Eastern ethnicity, and the affected individuals were from three sibships of two generations, each sibship having parents who were first or second cousins (family 1 in figs. 2 and 3). A region containing the largest number of contiguous identical homozygous genotypes for all five affected individuals consisted of 17 SNPs within 0.98 cM (1.5 Mb) on chromosome 9p24, flanked by rs1112534 and rs1074449. Identical homozygous genotypes occurred for 2,579 of 9,769 autosomal SNPs, and contiguity of 17 would not be expected to occur by chance in <5,000 experiments; all other contiguous regions contained ⩽10 SNPs. This finding supported the hypothesis of autozygosity in affected individuals for a disease locus at 9p24. The region contained seven known human expressed sequences (Ensembl version 37), including an attractive candidate—a voltage-gated K+ channel subunit gene, family V, member 2 (KCNV2 [MIM 607604]; synonyms are Kv8.2, Kv11.1, and MGC120515). A BLAST analysis of KCNV2 mRNA against human dbEST identified 18 matching ESTs derived from ocular tissue, a higher proportion than in any other organ.
Figure 2. .
Linkage analysis. A, Pedigree of consanguineous family used for mapping with the GeneChip Human Mapping 10K SNP Array (Affymetrix). Individuals included in the analysis are marked by an asterisk. B, Contiguous homozygous SNPs at chromosome 9q24 (shaded), with flanking SNPs above and below.
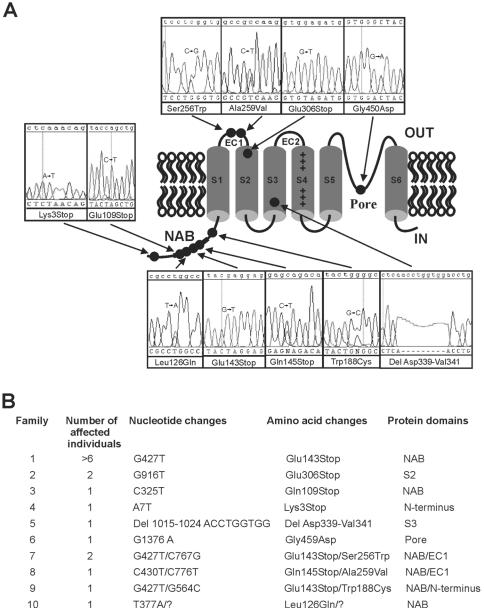
Figure 3. .
Mutations in KCNV2 channel protein. A, Domain structure of KCNV2, with the approximate position of mutations (circles) linked to electropherograms showing sequence in patient DNA. B, KCNV2 disease-associated mutations. Affected individuals from families 1–6 were homozygotes for the variant indicated. Only a single missense mutation was found in family 10; the “missing” mutation is indicated by a quotation mark. Additionally, the proband of family 4 was heterozygous for L533V, and the proband of family 8 also had D147F in phase with Q145X.
The two exons and intron-exon boundaries of KCNV2 were amplified using the primer pairs listed in table 1. Direct sequencing of the amplified fragments was performed on an ABI 3100 with the BigDye Terminator v3.1 Cycle Sequencing Kit (PE Applied Biosystems). Genomic DNA from probands from 11 unrelated families who showed typical clinical features of the disorder (a detailed clinical description of a subset of these patients has been presented elsewhere5) was sequenced to determine variants. The ethnicities of the families were as follows: white, from the United Kingdom (families 4, 7, 8, 10, and 11); Middle Eastern (families 1 and 9); and South Asian (families 2, 3, 5, and 6). Numerous sequence variants were found, including five distinct nonsense variants, one inframe deletion of 9 nt, and six distinct missense variants (fig. 3). Family 8 was heterozygous for three variants—a nonsense (Gln145Stop) and two missense (Asp147Phe and Ala259Val) changes—and cloning of a fragment amplified from exon 1 into pGEM-T-Easy vector, followed by sequencing with the use of vector-specific primers, showed Asp147Phe to be in cis with the nonsense mutation. One proband (family 10) showed only a single heterozygous change. Finally, one proband (family 11) showed no sequence variants, and it remains to be seen whether this family has undetected mutations or represents genetic heterogeneity for this disorder. Appropriate segregation was demonstrated in three families (1, 2, and 7) in which multiple members were affected and were available for analysis. Detailed electrophysiology was performed with the two heterozygous parents of family 6, and no subclinical abnormalities were detected, suggesting that heterozygosity for mutation in KCNV2 does not produce a clinical effect.
KCNV2 (Kv11.1) was identified elsewhere, through a homology search of the human genome for voltage-gated K+ channel subunit genes.9 The KCNV2 protein comprises 545 aa and, in common with other voltage-gated K+ channel subunits,9,10 comprises an N-terminal A and B box (NAB) or T1 domain and six transmembrane (TM) domains (S1–S6), with a K+ selective motif, GlyTyrGly, in the pore-forming loop (P loop) between S5 and S6. A highly charged S4 segment, which bears a regular array of positively charged amino acids (Arg or Lys) in every fourth position, is the principal structural element responsible for voltage sensing. A BLASTN search of genomes with the human KCNV2 protein sequence identified orthologues in seven other mammals (two primates, dog, cow, mouse, rat, and opossum), in chicken, in frog, and in three fish species. The two-exon structure and the position of the single intron was preserved in all. A ClustalW analysis of all 13 sequences demonstrated a high degree of amino acid identity with the human sequence, ranging from 95% with the other primates to 60% with fish.
All five nonsense mutations occur in exon 1, and mRNA would be predicted to succumb to nonsense-mediated decay. If translated, the mutated sequences all would lead to termination before four of the TM domains and the P loop (fig. 3). The Gly459Asp variant, found on both alleles of a proband of Pakistani origin (family 6), disrupts the first amino acid of the highly conserved GlyTyrGly K+ selective motif in the P loop. The four missense variants—Leu126Gln, Trp188Cys, Ser256Trp, and Ala259Val—and the first two residues of the AspLeuVal deletion occur at sites that are conserved across all 13 vertebrate orthologues and that are each highly conserved in other voltage-gated K+ subunits.10 Two other missense variants, Asp147Phe and Leu533Val, are not conserved to the same degree.
The portion of exon 1 harboring the variants between codons 126 and 188 was sequenced in 72 control samples (144 control chromosomes) derived from unexamined, anonymous U.K. blood donors (European Collection of Cell Cultures [ECACC] human control panel Q5671). All 144 alleles had Leu at codon 126 and Trp at codon 188. Codon 147 was polymorphic; two alleles showed an Asp→Gly (GAC→GGC) change, and one allele showed an Asp→Glu (GAC→GAG) change. No other variants were seen. Specific assays were designed to test for Ser256Trp and Ala259Val (KBiosciences Amplifluor) and were run on 2×96 ECACC unexamined human control panels. The wild-type variant was present in all 384 alleles. Finally, the Gly459Asp variant was tested with 50 unexamined control individuals of Pakistani ethnicity, and all 100 chromosomes showed the wild-type allele. Given the sample sizes used in the examination of controls, this would give an 80% probability of detecting one or more alleles with minor frequency 0.016 (100 chromosomes), 0.011 (144 chromosomes), and 0.004 (384 chromosomes), calculated using the binomial distribution. The Leu533Val variant, found as a heterozygous change in the proband of family 4, is a known coding-region SNP (rs12352254). The Asp147Phe variant is also likely to be a rare polymorphism, since this codon was found to be polymorphic in 3 of 144 alleles, and is likely to be in cis with a second rare variant Gln145Stop (see above).
Presence of the KCVN2 transcript has been demonstrated, with the use of RT-PCR, in a variety of human tissues,10 although, to our knowledge, retinal tissue has not been tested previously. We amplified the coding sequence from human retinal cDNA and confirmed the expression of KCNV2 (fig. 4). Within the human retina, in situ hybridization with a KCNV2 antisense riboprobe showed a positive signal in the inner segments of both cone and rod photoreceptors, which was not seen with the control sense probe (fig. 5).
Figure 4. .
Gel image showing PCR products of KCNV2, KCNB1, KCNC1, and KCNF1 amplified from human retinal cDNA. The fragments were amplified using primer pairs G, H, I, and J (table 1), were cloned into the pGEM-T-Easy vector, and were sequenced to confirm identity.
Figure 5. .
In situ hybridization of human retina probed with antisense and sense KCNV2 riboprobes. Sections 10 μm thick, from fixed and cryoprotected human retinal tissue, were probed with either antisense or sense digoxigenin (DIG)–labeled riboprobes generated from a 427-bp gene fragment, cloned into pGemT Easy vector, that spans the central portion of exon 1. Retinal sections were prepared for hybridization with the use of standard methods. Hybridization and washing was performed at 65°C. Signal was resolved using anti-DIG antibody at a 1:2,000 dilution, followed by color development. OS = Photoreceptor outer segment; IS = photoreceptor inner segment; ONL = outer nuclear layer.
Although mutations in genes encoding voltage-gated K+ channel subunits have been shown elsewhere to underlie other human diseases,11–13 this is the first report of a disorder affecting the visual system. Such channels are formed as a tetramer, and KCNV2 belongs to the group of electrically silent channels that do not produce a K+ current as a homotetramer but interact with other K+ channel subunits—such as KCNB1, KCNC1, and KCNF1—to form functional heterotetramers.10 It is, therefore, likely that KCNV2 binds with at least one other subunit to form channels in photoreceptors. Gene-specific primers (table 1) successfully amplified KCNB1, KCNC1, and KCNF1 fragments from human retinal cDNA (fig. 4), and KCNB1 subunits have been localized previously to inner segments of rods.14 All three subunits are, therefore, candidates for interaction with KCNV2, although other subunits remain to be tested.
A K+ current, IKX, which is present in vertebrate rod photoreceptors and deactivates slowly when the cell is hyperpolarized, figures prominently in setting the dark resting potential and accelerating the voltage response to small photocurrents.15,16 The process that underlies this current may be perturbed or abrogated by the loss of KCNV2 subunits. The presence of cone dysfunction in our patients would imply that both cones and rods require functional voltage-gated K+ channels, and further studies should improve our understanding of the exact role of KCNV2 in phototransduction and in disease pathogenesis.
Acknowledgments
The work was supported by the Foundation Fighting Blindness (United States), Fight for Sight (United Kingdom), European Vision Institute Genoret (European Union), and Guide Dogs for the Blind Association. We are grateful to the patients and family members who took part in this study. Dr. Lynda Erskine (UCL Institute of Ophthalmology) kindly provided technical guidance for the in situ hybridizations. We thank Phil Hykin (Moorfields Eye Hospital) and Dr. Mohammed M. Yousef Nazari (Al Baraha Hospital, Dubai), for their help with recruiting patients and family members; Mostafa Elgohary (Moorfields Eye Hospital), for invaluable English-Arabic translation; and Bev Scott (UCL Institute of Ophthalmology), for technical assistance. We also thank London IDEAS, for the use of the genotyping facility for the full-genome screen of the large consanguineous family, and Professor Pete Scambler and Kerra Pearce (UCL Institute of Child Health), for their technical advice and expertise.
Web Resources
The URLs for data presented herein are as follows:
- BLAST, http://www.ncbi.nlm.nih.gov/BLAST/
- ClustalW, ftp://ftp.ebi.ac.uk/pub/software/unix/clustalw/
- ECACC, http://www.ecacc.org.uk/ (for control DNAs from anonymous white donors)
- Ensembl, http://www.ensembl.org/
- International Society for Clinical Electrophysiology of Vision (ISCEV), http://www.iscev.org/
- London IDEAS, http://www.londonideas.org/internet/professionals/genotyping_service/index.html
References
- 1.Gouras P, Eggers HM, MacKay CJ (1983) Cone dystrophy, nyctalopia, and supernormal rod responses: a new retinal degeneration. Arch Ophthalmol 101:718–724 [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg T, Simonsen SE (1993) Retinal cone dysfunction of supernormal rod ERG type: five new cases. Acta Ophthalmol (Copenh) 71:246–255 [DOI] [PubMed] [Google Scholar]
- 3.Sandberg MA, Miller S, Berson EL (1990) Rod electroretinograms in an elevated cyclic guanosine monophosphate-type human retinal degeneration: comparison with retinitis pigmentosa. Invest Ophthalmol Vis Sci 31:2283–2287 [PubMed] [Google Scholar]
- 4.Kato M, Kobayashi R, Watanabe I (1993) Cone dysfunction and supernormal scotopic electroretinogram with a high-intensity stimulus: a report of three cases. Doc Ophthalmol 84:71–81 10.1007/BF01203284 [DOI] [PubMed] [Google Scholar]
- 5.Michaelides M, Holder GE, Webster AR, Hunt DM, Bird AC, Fitzke FW, Mollon JD, Moore AT (2005) A detailed phenotypic study of “cone dystrophy with supernormal rod ERG.” Br J Ophthalmol 89:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hood DC, Cideciyan AV, Halevy DA, Jacobson SG (1996) Sites of disease action in a retinal dystrophy with supernormal and delayed rod electroretinogram b-waves. Vision Res 36:889–901 10.1016/0042-6989(95)00174-3 [DOI] [PubMed] [Google Scholar]
- 7.Pawlyk BS, Sandberg MA, Berson EL (1991) Effects of IBMX on the rod ERG of the isolated perfused cat eye: antagonism with light, calcium or L-cis-diltiazem. Vision Res 31:1093–1097 10.1016/0042-6989(91)90035-4 [DOI] [PubMed] [Google Scholar]
- 8.Piri N, Gao YQ, Danciger M, Mendoza E, Fishman GA, Farber DB (2005) A substitution of G to C in the cone cGMP-phosphodiesterase gamma subunit gene found in a distinctive form of cone dystrophy. Ophthalmology 112:159–166 10.1016/j.ophtha.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 9.Shealy RT, Murphy AD, Ramarathnam R, Jakobsson E, Subramaniam S (2003) Sequence-function analysis of the K+-selective family of ion channels using a comprehensive alignment and the KcsA channel structure. Biophys J 84:2929–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottschytsch N, Raes A, Van Hoorick D, Snyders DJ (2002) Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci USA 99:7986–7991 10.1073/pnas.122617999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating MT, Sanguinetti MC (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104:569–580 10.1016/S0092-8674(01)00243-4 [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W (2003) KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 299:251–254 10.1126/science.1077771 [DOI] [PubMed] [Google Scholar]
- 13.Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JPA, Nolte D, Mock AF, Evidente VGH, Fee DB, Müller U, Dürr A, Brice A, Papazian DM, Pulst SM (2006) Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 38:447–451 10.1038/ng1758 [DOI] [PubMed] [Google Scholar]
- 14.Pinto LH, Klumpp DJ (1998) Localization of potassium channels in the retina. Prog Retin Eye Res 17:207–230 10.1016/S1350-9462(97)00011-6 [DOI] [PubMed] [Google Scholar]
- 15.Beech DJ, Barnes S (1989) Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron 3:573–581 10.1016/0896-6273(89)90267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes S (1994) After transduction: response shaping and control of transmission by ion channels of the photoreceptor inner segments. Neuroscience 58:447–459 10.1016/0306-4522(94)90072-8 [DOI] [PubMed] [Google Scholar]