Abstract
Stickler syndrome is characterized by ophthalmic, articular, orofacial, and auditory manifestations. It has an autosomal dominant inheritance pattern and is caused by mutations in COL2A1, COL11A1, and COL11A2. We describe a family of Moroccan origin that consists of four children with Stickler syndrome, six unaffected children, and two unaffected parents who are distant relatives (fifth degree). All family members were clinically investigated for ear, nose, and throat; ophthalmologic; and radiological abnormalities. Four children showed symptoms characteristic of Stickler syndrome, including moderate-to-severe sensorineural hearing loss, moderate-to-high myopia with vitreoretinopathy, and epiphyseal dysplasia. We considered the COL9A1 gene, located on chromosome 6q13, to be a candidate gene on the basis of the structural association with collagen types II and XI and because of the high expression in the human inner ear indicated by cDNA microarray. Mutation analysis of the coding region of the COL9A1 gene showed a homozygous R295X mutation in the four affected children. The parents and four unaffected children were heterozygous carriers of the R295X mutation. Two unaffected children were homozygous for the wild-type allele. None of the family members except the homozygous R295X carriers had any signs of Stickler syndrome. Therefore, COL9A1 is the fourth identified gene that can cause Stickler syndrome. In contrast to the three previously reported Stickler syndrome–causing genes, this gene causes a form of Stickler syndrome with an autosomal recessive inheritance pattern. This finding will have a major impact on the genetic counseling of patients with Stickler syndrome and on the understanding of the pathophysiology of collagens. Mutation analysis of this gene is recommended in patients with Stickler syndrome with possible autosomal recessive inheritance.
Stickler syndrome was first described in 1965 by Stickler et al.1 and is characterized by ophthalmic, articular, orofacial, and auditory manifestations. Until now, it has been reported as an autosomal dominant inherited syndrome that is caused by mutations in COL2A1 (Stickler syndrome type I [MIM 108300]), COL11A1 (Stickler syndrome type II [MIM 604841]), and COL11A2 (Stickler syndrome type III [MIM 184840]).2–4 Despite this heterogeneity, the majority of patients with Stickler syndrome have mutations in the COL2A1 gene, which is positioned on chromosome 12q13.5,6
The most conspicuous manifestations in the syndrome are ophthalmologic, including myopia, vitreoretinal degeneration, cataracts, and, often, retinal detachment. It is the most common inherited cause of rhegmatogeneous retinal detachment in childhood.7 Typical facial changes are midface hypoplasia, broad nasal bridge, micrognathia, and cleft palate. These facial features often become less pronounced with age. Osteoarthritis develops typically in the 3rd or 4th decade. In most cases, there is radiographic evidence of irregularity of articular surfaces suggestive of epiphyseal dysplasia. Sensorineural, mixed, and conductive hearing loss has been reported, with conductive hearing loss often a secondary effect of craniofacial anomalies.
Collagens are important in the maintenance of structural strength. In all tissues, collagen molecules are composed of three polypeptide chains that have a triple-stranded helical structure. On the basis of their function and structure, collagens have been classified into fibril-forming collagens, fibril-associated collagens, and collagens that form structures unrelated to fibrils. A further subdivision can be made on the basis of the characteristics of their precursor molecules.8
Currently, at least 40 different genes have been identified that encode for at least 27 different collagens. In the eye, collagens play an important role in the maintenance of the three-dimensional structure that provides a correct optical environment for vision. At least 22 different collagens have been detected in the embryonic or mature eye.9 Six distinct collagen types seem to be characteristic of the eye and cartilage and are absent in the majority of other adult tissues. Most of the collagen fibrils in the cartilage and vitreous are heterotypic, containing more than one collagen type. These collagens include types II, V/XI, VI, IX, and XXVII. Types II, V/XI, and XXVII are fibril-forming collagens that mainly provide the structural strength of the vitreous and cartilage. Type VI is a member of the short-chain collagens that are thought to serve a function in stabilizing different fibrillar structures. Type IX is one of the fibril-associated collagens with interrupted triple helices (FACIT), which do not form fibrils themselves but are found attached to the surfaces of pre-existing fibrils.10
Until now, only mutations in the fibrillar collagen types XI (encoded by COL11A1 and COL11A2) and II (encoded by COL2A1) have been found to cause Stickler syndrome. There is large clinical variability in Stickler syndrome,11 and no exact correlations exist with specific mutations. A clinical subclassification can be made on the basis of the ocular phenotype. Mutations in COL2A1 and COL11A1 cause ocular signs, whereas mutations in COL11A2 do not. This can be explained by the absence of COL11A2 in the vitreous, where it is replaced by COL5A2.12,13 Patients with COL2A1 mutations have a characteristic “membranous,” or type I, vitreous phenotype, whereas patients with COL11A1 mutations show a “beaded,” or type II, vitreous phenotype.14
Type IX collagen fibrils are regularly aligned along the surface of the fibrils composed of collagen types II and XI.15 Mutations in all three collagen type IX genes have been associated with autosomal dominant multiple epiphyseal dysplasia, a relatively mild and clinically variable osteochondrodysplasia, affecting ∼1 in 10,000 individuals (for review, see the work of Briggs and Chapman16).
Type IX collagen is a FACIT, consisting of three collagenous domains (COL1–COL3) interspersed among four noncollagenous domains (NC1–NC4). Type IX collagen fibrils are heterotrimers of α1(IX), α2(IX), and α3(IX) chains encoded by the COL9A1, COL9A2, and COL9A3 genes.17,18 The collagen acts as a tissue stabilizer through interactions between the fibrillar surface and the extrafibrillar matrix by its NC4 domain.19
Like the collagen types II and XI, type IX collagen is also present in the cochlea20,21 but only in limited regions—that is, in the cartilaginous rests of the enchondral bone, the tectorial membrane, and the Reissner’s membrane. Type XI collagen is mainly expressed in the same structures as type IX collagen, except for the Reissner’s membrane. At all places in the cochlea, type IX collagen colocalizes with type II collagen.20 However, type II collagen is also expressed in other structures, making it the major protein in the cochlea.
We describe a family of Moroccan origin in which four children have symptoms characteristic of Stickler syndrome. Mutation analysis of the COL9A1 gene was performed on the basis of the structural association with collagen types II and XI and because of the high expression in the human inner ear indicated by cDNA microarray.22 Mutation analysis of the COL9A1 gene, located on chromosome 6q13, showed a homozygous R295X mutation in the four affected children. In contrast to the dominant inheritance pattern of all previously described Stickler syndrome mutations, this family shows an autosomal recessive inheritance pattern. The unaffected parents are distant relatives (fifth degree) and are both heterozygous carriers of the R295X mutation.
Material and Methods
Clinical Examination
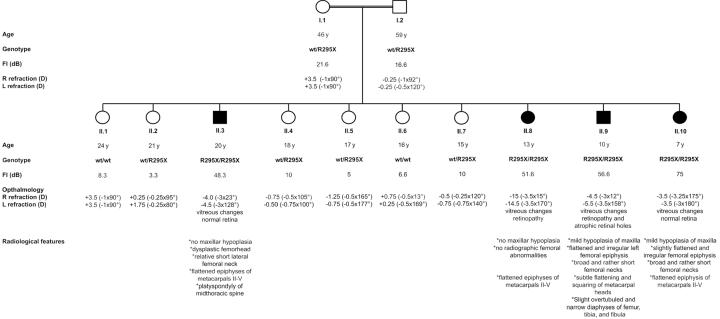
In this study, we investigated a Moroccan family with autosomal recessive Stickler syndrome. All 12 family members (fig. 1) were investigated through general examination by specialists of ear, nose, and throat; ophthalmology; radiology; and genetics. Vitreous examination and cycloplegic refraction were performed to investigate the ophthalmologic phenotype. The audiological phenotype was investigated by measuring air and bone conduction pure-tone audiometry. Radiographs were taken of the skull, the spine, and the lower and upper extremities to investigate the skeletal characteristics of all family members, including the parents. Serum parameters were also investigated, including levels of alkaline phosphatase, parathyroid hormone, calcium, phosphorus, creatinine, urea, and magnesium.
Figure 1. .
Pedigree of the family with autosomal recessive Stickler syndrome. Blackened symbols represent individuals with Stickler syndrome, and unblackened symbols represent individuals with a normal phenotype. Individual II.2 is a girl with hypophosphatemic rickets thought to be unrelated to Stickler syndrome. For all family members, the age when the clinical investigations were performed; the genotype; the PTA, or Fletcher index (FI), of the better ear; and the refractions for the right (R) and left (L) eyes are given below each symbol. For the affected individuals, extra clinical data on the ophthalmologic and radiological findings are also shown. wt = wild type; y = years.
Genetic Analysis
Blood samples for DNA extraction were collected from all 12 family members with informed consent. DNA was extracted from leukocytes according to standard methods. Primers were designed for the amplification of the coding region and exon-intron boundaries of the COL9A1 gene. Sequencing of the PCR products was performed by standard procedures and was analyzed on an ABI 3110 DNA sequencer (Applied Biosystems).
Audiological Examination
Pure-tone air and bone thresholds were determined at frequencies of 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz, with intensities up to 120 dB. The pure-tone average (PTA) was calculated for all family members, by use of the average of the hearing thresholds at 500, 1,000, and 2,000 Hz.
Results
General Examination
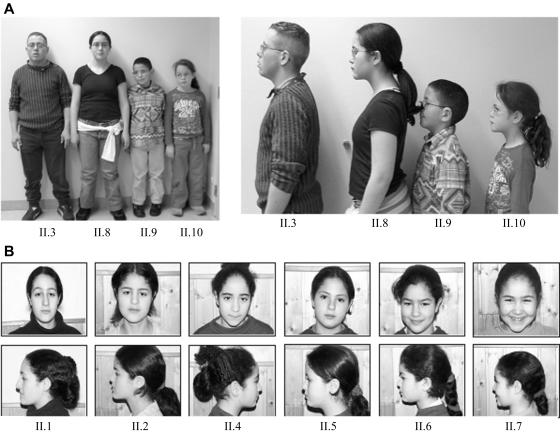
All family members had normal blood parameters, except for family member II.2. The serum parameters of II.2 were consistent with a known diagnosis of hypophosphatemic rickets. A general characteristic of the family was the relatively short stature of all family members. Most children were between the 10th and 25th percentiles. Remarkably, the height of all patients (II.3, II.8, II.9, and II.10) was <3rd percentile. This short stature was not disproportionate, and no severe abnormalities in the skeleton were noted. The height of II.2 was also <3rd percentile, most likely because of the hypophosphatemic rickets. Unaffected family member II.4 was also very short (∼3rd percentile). All patients had genua valga. Patient II.8 had a Beighton score of 7 (out of 9 total), indicating hypermobility of the joints. The oldest patient, II.3, had painful knees, which may indicate arthritis. All family members showed a relatively flat face, which was not more pronounced in the affected family members (fig. 2). None of the patients was born with a cleft palate or micrognathia.
Figure 2. .
A, Pictures of the four affected family members. The height of all patients is <3rd percentile. They all have a relatively flat face. All patients wear glasses because of amblyopia with high myopia and astigmatism. They all have genua valga. B, Pictures of the six unaffected children. The facial features between unaffected and affected individuals are indistinguishable.
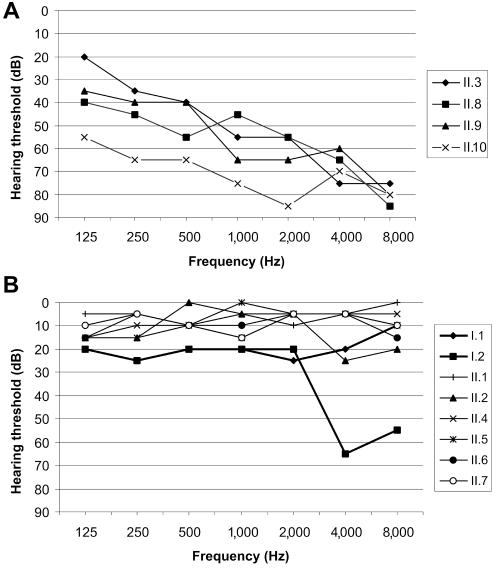
Audiology
The best PTA value at 500, 1,000, and 2,000 Hz for every individual is shown in figure 1. The father had normal hearing for his age (PTA=16.6 dB), with a bilateral sensorineural dip at 4,000 Hz (fig. 3B), probably caused by known professional noise exposure. The mother (age 46 years) had mild hearing loss (PTA=21.6 dB), with a flat audiometric configuration of unknown origin (fig. 3B). The four heterozygous carriers of the mutation (II.2, II.4, II.5, and II.7) and the two homozygous wild-type children (II.1 and II.6) did not have any hearing loss (fig. 3B). All four patients (II.3, II.8, II.9, and II.10) had moderate-to-severe hearing impairment, with a mildly down-sloping audiogram (fig. 3A). No air-bone gap was present in patients II.3, II.9, and II.10, which points to sensorineural hearing loss. Air and bone audiograms were symmetrical in these three patients. The air conduction threshold of the left ear of patient II.8 was below the bone conduction level in that ear (PTA=105 dB), but the bone conduction levels of this patient were symmetrical in both ears (PTA=51.6 dB). The asymmetrical hearing loss was due to chronic otitis media in the left ear.
Figure 3. .
A, Most-recent air conduction thresholds of the better ear for each of the four patients. The hearing loss is moderate to severe, with a mildly down-sloping audiogram. B, Air conduction thresholds of the better ear for each of the parents (bold lines) and unaffected children. The father has normal hearing for his age, with a bilateral sensorineural dip at 4,000 Hz, probably caused by known professional noise exposure. The mother has mild hearing loss of unknown cause. None of the unaffected children has hearing loss.
Two earlier audiograms were available for patients II.3 and II.8, taken 6 years before this study. The progression of the PTA values in these patients was 1.4 dB/year. However, this limited information did not allow a definitive conclusion about the progression of hearing loss in all four patients.
Ophthalmologic Findings
The four patients showed amblyopia with moderate-to-high myopia and astigmatism. The refractions for the right and left eyes for all family members are included in figure 1. All patients presented vitreous changes consisting of syneresis of an optically empty vitreous with some fibrillar condensates. Important to note was that the vitreous phenotype did not resemble the membranous, beaded, and nonfibrillar phenotypes that have been described elsewhere for Stickler syndrome. It resembled an aged vitreous, the composition of which is degenerated because of progressive gel liquefaction. Patients II.3 and II.10 had normal retinas, whereas patients II.8 and II.9 showed retinopathy. Atrophic retinal holes were noted in the left eye of patient II.9, whereas patient II.8 exhibited circumferential and perivascular pigmentary degeneration of the retina. The mean (±SD) refractive power of the ocular lens of the four patients was significantly higher (26.9 ± 2.83 diopters [D]) than that of the other family members (18.5 ± 1.79 D). None of the unaffected family members demonstrated vitreous alterations, significant refractive error, or high refractive power of the lens or amblyopia.
Radiological Findings
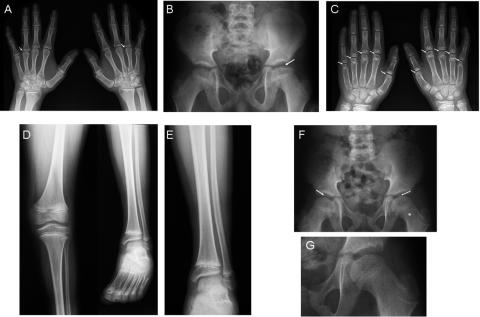
The radiological features observed in all four patients varied, but, in general, they were rather subtle (fig. 4). The two youngest patients (II.9 and II.10) had the most pronounced radiological features. They had mild hypoplasia of the maxilla, whereas the maxilla was radiologically normal in the two oldest patients. This may be explained by the fact that midface hypoplasia often becomes less conspicuous with advancing age. The femoral epiphyses of patients II.9 and II.10 were flattened and irregular, and the femoral necks were very broad and relatively short. The oldest patient (II.3, age 20 years) showed dysplasia of the femoral head, with a relative broadening of the femoral neck. Remarkably, patient II.8 did not have any radiographic femoral abnormalities. The four patients showed flattened epiphyses of metacarpals II–V. Patient II.3 showed platyspondyly of the midthoracic spine. Eight members of the family (all members except I.2, II.5, II.9, and II.10) showed irregular delineation of the vertebral end plates, resembling Scheuermann disease. This spinal abnormality has been associated with Stickler syndrome.23 However, since patients, carriers, and noncarriers in this family had Scheuermann-like changes, the changes were probably not associated with Stickler syndrome in this family.
Figure 4. .
A, Standard radiograph of the hands and wrists of individual II.3 at age 20 years. Flattening, underdevelopment, and squaring of the heads of the metacarpal bones, particularly at metacarpal IV bilaterally, are indicated (arrows). Also note bilateral clinodactyly as an incidental finding. B–E, Radiographs of individual II.9 at age 10 years. B, Standard radiograph of the pelvis. Flattening and irregular delineation of the left femoral epiphysis are indicated (arrow). Also note the broadening of the femoral neck, particularly at the left side. C, Standard radiograph of the hands and wrists. Subtle flattening and squaring of the metacarpal heads are indicated (arrows). D, Standard radiograph of the left knee and lower leg. Note slight overtubulation and narrowness of the diaphyses of the femur, tibia, and fibula. E, Detail of the left tibia and fibula. F, Standard radiograph of the pelvis of individual II.10 at age 7 years. Slight flattening and irregularity of the femoral epiphyses are indicated (arrows). Also note the broadening of the femoral neck, particularly at the left side (asterisk [*]). G, Spot view of the slightly flattened and irregular left femoral epiphysis of individual II.10.
Genetic Analysis
The family was incorporated into an ongoing project in which mutation analysis was performed on genes with high expression in the inner ear, including COL9A1. Neither a linkage genome scan nor an analysis of other Stickler syndrome genes was done. Sequencing of all COL9A1 coding exons in patients II.9 and II.10 revealed homozygous nonsense mutation R295X in exon 9, which encodes for the first triple-helix repeat domain of COL9A1. The mutated exon 9 was sequenced in all other family members. The four affected individuals (II.3, II.8, II.9, and II.10) were homozygous for the mutation, and both parents and four unaffected children (II.2, II.4, II.5, and II.7) were heterozygous. The mutation was not found in two family members (II.1 and II.6) and in 100 random Belgian control persons. One other sequence variant, c.1862A→G, was identified in exon 28 of the gene, but this variant has already been reported as polymorphism rs1135056 (dbSNP).
Discussion
COL11A2 is the only Stickler syndrome–causing gene in which homozygous mutations have been reported. These mutations are almost all predicted to cause complete absence of α2(XI) chains and to cause otospondylomegaepiphyseal dysplasia (OSMED)24 and, recently, also nonsyndromic hearing impairment (DFNB53).25 This is a skeletal dysplasia accompanied by severe hearing loss. The phenotype overlaps Stickler syndrome but can be distinguished by disproportionately short limbs, severe hearing loss, and lack of ocular involvement. Also, in this family with recessive Stickler syndrome, the nonsense mutation R295X will probably lead to absence of type IX collagen. The milder skeletal and auditory phenotype, in comparison with OSMED, can be explained by the fact that COL9A1 is a FACIT and not a fibril-forming collagen.
Mutations in all three collagen type IX genes result in multiple epiphyseal dysplasia. All mutations in COL9A2 and COL9A3 are clustered at the splice-site junctions of exon 3.18,26–30 This results in an aberrant mRNA with an in-frame 36-bp deletion of exon 3 and produces a protein lacking 12 aa from the COL3 domain. There is only a single mutation in COL9A1 that causes multiple epiphyseal dysplasia (MED). It is a splice-site mutation that results in an in-frame deletion of exon 8 and/or exon 10.31 Since COL9A1 contains six additional exons, compared with COL9A2 and COL9A3, exon 9 of COL9A1 corresponds to exon 3 in the other two COL9 genes. Therefore, it was surprising that the splice-site mutation in COL9A1 that causes MED did not result in skipping of exon 9. The reason why mutations group around the donor/acceptor sites of exon 3 in COL9A2 and COL9A3 and around exon 8 in COL9A1 is not yet clear. It is plausible that the resulting deletion of amino acids from this domain of type IX collagen may disrupt interactions with other components of the cartilage. This may be caused by the deletion of a binding site within the COL3 domain. Another possibility is that the orientation of the α1(IX) NC4 domain relative to the type II/XI collagen fibril is changed by the mutation.
In the vitreous type IX collagen is a proteoglycan with a single chondroitin sulphate side chain covalently linked to the collagenous protein core.32 This side chain may be important for the maintenance of spacing between adjacent collagen fibrils.33,34 It was shown that type IX collagen shields type II collagen from exposure to the surface of the vitreous fibrils.35 With aging, the vitreous collagen fibrils are predisposed to fusion, because of the diminished shielding due to loss of type IX collagen. These changes are thought to underlie vitreous liquefaction and weakening of vitreoretinal adhesion. Because type IX collagen is most likely not present in the patients of the family studied here, the fibrillar collagens in their vitreous may not be shielded, possibly resulting in a rapidly aging vitreous due to liquefaction and degeneration. It is not surprising that this vitreous phenotype has not been described previously in patients with Stickler syndrome with collagen type II or XI mutations, because of the different function of type IX collagens compared with the fibril-forming collagen types II and XI.
All three genes that elsewhere were shown to cause autosomal dominant Stickler syndrome have been studied extensively by the creation of transgenic mice. Homozygous COL2 knockout mice died perinatally with severe chondrodysplasia. The chondrodysplasia (cho) mouse model is a functional null allele of Col11a1 that causes lethal chondrodysplasia in cho/cho newborn mice and osteoarthritis in cho/+ heterozygotes.36 In humans, no recessive diseases are known to be caused by COL2A1 and COL11A1. Similar to the homozygous knockout mice of these genes, homozygosity in humans might not be compatible with life. Both homozygous and heterozygous Col11a2 knockout mice had hearing loss due to disorganized collagen fibrils in the tectorial membrane.37 Interestingly, the phenotype of the Col11a2 knockout mice was milder than that of the cho/cho mice.
A functional knockout mouse of the Col9a1 gene has been created.38 The animals did not produce α1(IX) mRNA and were born without conspicuous skeletal abnormalities. However, they developed early-onset osteoarthritis. Histological analysis of the eyes did not show apparent abnormalities, even in older animals with articular changes. The deficiency in α1(IX) chains was shown to lead to a functional knockout of all three chains of collagen type IX.39 Therefore, the synthesis of α1(IX) polypeptides is essential for the assembly of heterotrimeric collagen type IX molecules. Although the knockout mice lacked collagen type IX, they had normal cartilage fibrils and banding patterns. Therefore, it was proposed that collagen type IX is not essential and may be functionally redundant in the fibrillinogenesis in cartilage.39 The auditory function of the Col9a1 knockout mice, however, was impaired. We recently showed that homozygous α1(IX) collagen knockout mice had progressive hearing loss that was due to the disorganization of the collagen fibres.40
Early recognition of the Stickler syndrome phenotype is important for accurate counseling and possible treatment. Treatment includes cryotherapy and laser therapy for retinal detachment, the use of hearing aids, and joint replacements. The patients in this Moroccan family did not show cataract or retinal detachment that needed surgical treatment, but close ophthalmologic follow-up will be necessary, since atrophic retinal holes and pigmentary degeneration of the retina were already present in patients II.8 and II.9, respectively. Progressive degeneration of the vitreous results in a collapse of the entire gel-like structure. As a consequence, the vitreous is detached first from the areas of the weakest attachments, located at the posterior retina and optic nerve. In areas of firm localized attachments, retinal holes or retinal ruptures develop. Because of the young age and the lack of early follow-up of the affected siblings in this family, the risk of rhegmatogeneous retinal detachment cannot be determined yet. It also could not be traced whether the refractive error was congenital or developed during childhood. They all had bilateral amblyopia that probably was due to uncorrected refractive anomaly during early childhood, because no macular abnormalities were observed. The abnormalities in the ocular lens power that were seen in this family have not been reported elsewhere in Stickler syndrome.
In conclusion, COL9A1 is the fourth identified gene that can cause Stickler syndrome. In contrast to the three previously identified Stickler syndrome–causing genes, this gene causes a form of Stickler syndrome that has an autosomal recessive inheritance pattern. This finding will have a major impact on the genetic counseling of patients with Stickler syndrome and on the understanding of the pathophysiology of collagens. Mutation analysis of this gene is recommended for patients with Stickler syndrome with possible autosomal recessive inheritance. In addition, the ophthalmologic examination of patients with clinical symptoms and signs of Stickler syndrome could be used as the main distinction among the different collagen genes known to cause this syndrome (table 1). The vitreous phenotype on its own, however, is not always conclusive for deciding which gene caused the Stickler syndrome. Parentin et al.41 reported a patient with a type I vitreous phenotype for which linkage to COL2A1 could be excluded. Linkage to COL11A1, however, was not convincing, since the LOD score at θ=0 was only 0.58. McLeod et al.42 reported a patient with a mutation in COL2A1 in whom the vitreous phenotype shifted from type II to type I. The first child of this patient showed a type I vitreous phenotype in the left eye and a type II phenotype in the right eye. The clinical observations in both articles could be explained by posterior vitreous detachment. However, these reports show that the vitreous phenotype should be interpreted with caution.
Table 1. .
Ocular Symptoms in Stickler Syndrome Caused by Mutations in Different Collagen Genes
| Gene | Ocular Symptoms |
| COL2A1 | Membranous or type I vitreous phenotype, afibrillar phenotype |
| COL11A1 | Beaded or type II vitreous phenotype |
| COL11A2 | No ocular symptoms |
| COL9A1 | Vitreous syneresis with an optically empty vitreous due to progressive gel liquefaction |
Acknowledgment
This study was supported by the European Commission FP6 Integrated Project EUROHEAR grant LSHG-CT-2004-512063.
Web Resource
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/OMIM (for Stickler syndrome types I, II, and III)
References
- 1.Stickler GB, Belau PG, Farrell FJ, Jones JD, Pugh DG, Steinberg AG, Ward LE (1965) Hereditary progressive arthro-ophthalmopathy. Mayo Clin Proc 40:433–455 [PubMed] [Google Scholar]
- 2.Williams CJ, Ganguly A, Considine E, McCarron S, Prockop DJ, Walsh-Vockley C, Michels VV (1996) A-2→G transition at the 3′ acceptor splice site of IVS17 characterizes the COL2A1 gene mutation in the original Stickler syndrome kindred. Am J Med Genet 63:461–467 [DOI] [PubMed] [Google Scholar]
- 3.Richards AJ, Yates JR, Williams R, Payne SJ, Pope FM, Scott JD, Snead MP (1996) A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in α1(XI) collagen. Hum Mol Genet 5:1339–1343 10.1093/hmg/5.9.1339 [DOI] [PubMed] [Google Scholar]
- 4.Vikkula M, Mariman EC, Lui VC, Zhidkova NI, Tiller GE, Goldring MB, van Beersum SE, de Waal Malefijt MC, van den Hoogen FHJ, Ropers H-H, Mayne R, Cheah KSE, Olsen BR, Warman ML, Bruuner HG (1995) Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell 80:431–437 10.1016/0092-8674(95)90493-X [DOI] [PubMed] [Google Scholar]
- 5.Snead MP, Payne SJ, Barton DE, Yates JR, al-Imara L, Pope FM, Scott JD (1994) Stickler syndrome: correlation between vitreoretinal phenotypes and linkage to COL2A1. Eye 8:609–614 [DOI] [PubMed] [Google Scholar]
- 6.Snead MP, Yates JR, Williams R, Payne SJ, Pope FM, Scott JD (1996) Stickler syndrome type 2 and linkage to the COL11A1 gene. Ann N Y Acad Sci 785:331–332 [DOI] [PubMed] [Google Scholar]
- 7.Scott JD (1980) Congenital myopia and retinal detachment. Trans Ophthalmol Soc UK 100:69–71 [PubMed] [Google Scholar]
- 8.Valkkila M, Melkoniemi M, Kvist L, Kuivaniemi H, Tromp G, Ala-Kokko L (2001) Genomic organization of the human COL3A1 and COL5A2 genes: COL5A2 has evolved differently than the other minor fibrillar collagen genes. Matrix Biol 20:357–366 10.1016/S0945-053X(01)00145-7 [DOI] [PubMed] [Google Scholar]
- 9.Ihanamaki T, Pelliniemi LJ, Vuorio E (2004) Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res 23:403–434 10.1016/j.preteyeres.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 10.Myllyharju J, Kivirikko KI (2001) Collagens and collagen-related diseases. Ann Med 33:7–21 [DOI] [PubMed] [Google Scholar]
- 11.Zlotogora J, Sagi M, Schuper A, Leiba H, Merin S (1992) Variability of Stickler syndrome. Am J Med Genet 42:337–339 10.1002/ajmg.1320420316 [DOI] [PubMed] [Google Scholar]
- 12.van Steensel MA, Buma P, de Waal Malefijt MC, van den Hoogen FH, Brunner HG (1997) Oto-spondylo-megaepiphyseal dysplasia (OSMED): clinical description of three patients homozygous for a missense mutation in the COL11A2 gene. Am J Med Genet 70:315–323 [DOI] [PubMed] [Google Scholar]
- 13.Mayne R, Brewton RG, Mayne PM, Baker JR (1993) Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem 268:9381–9386 [PubMed] [Google Scholar]
- 14.Martin S, Richards AJ, Yates JR, Scott JD, Pope M, Snead MP (1999) Stickler syndrome: further mutations in COL11A1 and evidence for additional locus heterogeneity. Eur J Hum Genet 7:807–814 10.1038/sj.ejhg.5200377 [DOI] [PubMed] [Google Scholar]
- 15.Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P (1989) Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol 108:191–197 10.1083/jcb.108.1.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs MD, Chapman KL (2002) Pseudoachondroplasia and multiple epiphyseal dysplasia: mutation review, molecular interactions, and genotype to phenotype correlations. Hum Mutat 19:465–478 10.1002/humu.10066 [DOI] [PubMed] [Google Scholar]
- 17.Pihlajamaa T, Prockop DJ, Faber J, Winterpacht A, Zabel B, Giedion A, Wiesbauer P, Spranger J, Ala-Kokko L (1998) Heterozygous glycine substitution in the COL11A2 gene in the original patient with the Weissenbacher-Zweymuller syndrome demonstrates its identity with heterozygous OSMED (nonocular Stickler syndrome). Am J Med Genet 80:115–120 [DOI] [PubMed] [Google Scholar]
- 18.Paassilta P, Pihlajamaa T, Annunen S, Brewton RG, Wood BM, Johnson CC, Liu J, Gong Y, Warman ML, Prockop DJ, Mayne R, Ala-Kokko L (1999) Complete sequence of the 23-kilobase human COL9A3 gene: detection of Gly-X-Y triplet deletions that represent neutral variants. J Biol Chem 274:22469–22475 10.1074/jbc.274.32.22469 [DOI] [PubMed] [Google Scholar]
- 19.Jacenko O, Olsen BR (1995) Transgenic mouse models in studies of skeletal disorders. J Rheumatol Suppl 43:39–41 [PubMed] [Google Scholar]
- 20.Slepecky NB, Cefaratti LK, Yoo TJ (1992) Type II and type IX collagen form heterotypic fibers in the tectorial membrane of the inner ear. Matrix 12:80–86 [DOI] [PubMed] [Google Scholar]
- 21.Thalmann I (1993) Collagen of accessory structures of organ of Corti. Connect Tissue Res 29:191–201 [DOI] [PubMed] [Google Scholar]
- 22.Abe S, Katagiri T, Saito-Hisaminato A, Usami S, Inoue Y, Tsunoda T, Nakamura Y (2003) Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet 72:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose PS, Ahn NU, Levy HP, Ahn UM, Davis J, Liberfarb RM, Nallamshetty L, Sponseller PD, Francomano CA (2001) Thoracolumbar spinal abnormalities in Stickler syndrome. Spine 26:403–409 10.1097/00007632-200102150-00017 [DOI] [PubMed] [Google Scholar]
- 24.Melkoniemi M, Brunner HG, Manouvrier S, Hennekam R, Superti-Furga A, Kaariainen H, Pauli RM, van Essen T, Warman ML, Bonaventure J, Miny P, Ala-Kokko L (2000) Autosomal recessive disorder otospondylomegaepiphyseal dysplasia is associated with loss-of-function mutations in the COL11A2 gene. Am J Hum Genet 66:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Kahrizi K, Meyer NC, Riazalhosseini Y, Van Camp G, Najmabadi H, Smith RJ (2005) Mutation of COL11A2 causes autosomal recessive non-syndromic hearing loss at the DFNB53 locus. J Med Genet 42:e6 10.1136/jmg.2004.028514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muragaki Y, Mariman EC, van Beersum SE, Perala M, van Mourik JB, Warman ML, Hamel BC, Olsen BR (1996) A mutation in COL9A2 causes multiple epiphyseal dysplasia (EDM2). Ann N Y Acad Sci 785:303–306 [DOI] [PubMed] [Google Scholar]
- 27.Holden P, Canty EG, Mortier GR, Zabel B, Spranger J, Carr A, Grant ME, Loughlin JA, Briggs MD (1999) Identification of novel pro-α2(IX) collagen gene mutations in two families with distinctive oligo-epiphyseal forms of multiple epiphyseal dysplasia. Am J Hum Genet 65:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnemann CG, Cox GF, Shapiro F, Wu JJ, Feener CA, Thompson TG, Anthony DC, Eyre DR, Darras BT, Kunkel LM (2000) A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy. Proc Natl Acad Sci USA 97:1212–1217 10.1073/pnas.97.3.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohiniva J, Paassilta P, Seppanen U, Vierimaa O, Kivirikko S, Ala-Kokko L (2000) Splicing mutations in the COL3 domain of collagen IX cause multiple epiphyseal dysplasia. Am J Med Genet 90:216–222 [DOI] [PubMed] [Google Scholar]
- 30.Nakashima E, Kitoh H, Maeda K, Haga N, Kosaki R, Mabuchi A, Nishimura G, Ohashi H, Ikegawa S (2005) Novel COL9A3 mutation in a family with multiple epiphyseal dysplasia. Am J Med Genet A 132:181–184 [DOI] [PubMed] [Google Scholar]
- 31.Czarny-Ratajczak M, Lohiniva J, Rogala P, Kozlowski K, Perälä M, Carter L, Spector TD, Kolodziej L, Seppänen U, Glazar R, Królewski J, Latos-Bielenska A, Ala-Kokko L (2001) A mutation in COL9A1 causes multiple epiphyseal dysplasia: further evidence for locus heterogeneity. Am J Hum Genet 69:969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop PN, Crossman MV, McLeod D, Ayad S (1994) Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem J 299:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott JE (1990) Proteoglycan-collagen interactions and sub-fibrillar structure in collagen fibrils: implications in the development and remodelling of connective tissues. Biochem Soc Trans 18:489–490 [DOI] [PubMed] [Google Scholar]
- 34.Scott JE (1992) The chemical morphology of the vitreous. Eye 6:553–555 [DOI] [PubMed] [Google Scholar]
- 35.Bishop PN, Holmes DF, Kadler KE, McLeod D, Bos KJ (2004) Age-related changes on the surface of vitreous collagen fibrils. Invest Ophthalmol Vis Sci 45:1041–1046 10.1167/iovs.03-1017 [DOI] [PubMed] [Google Scholar]
- 36.Li SW, Takanosu M, Arita M, Bao Y, Ren ZX, Maier A, Prockop DJ, Mayne R (2001) Targeted disruption of Col11a2 produces a mild cartilage phenotype in transgenic mice: comparison with the human disorder otospondylomegaepiphyseal dysplasia (OSMED). Dev Dyn 222:141–152 10.1002/dvdy.1178 [DOI] [PubMed] [Google Scholar]
- 37.McGuirt WT, Prasad SD, Griffith AJ, Kunst HP, Green GE, Shpargel KB, Runge C, Huybrechts C, Mueller RF, Lynch E, King MC, Brunner HG, Cremers CW, Takanosu M, Li SW, Arita M, Mayne R, Prockop DJ, Van Camp G, Smith RJ (1999) Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nat Genet 23:413–419 10.1038/70516 [DOI] [PubMed] [Google Scholar]
- 38.Fassler R, Schnegelsberg PN, Dausman J, Shinya T, Muragaki Y, McCarthy MT, Olsen BR, Jaenisch R (1994) Mice lacking α1(IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci USA 91:5070–5074 10.1073/pnas.91.11.5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagg R, Hedbom E, Mollers U, Aszodi A, Fassler R, Bruckner P (1997) Absence of the α1(IX) chain leads to a functional knock-out of the entire collagen IX protein in mice. J Biol Chem 272:20650–20654 10.1074/jbc.272.33.20650 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki N, Asamura K, Kikuchi Y, Takumi Y, Abe S, Imamura Y, Hayashi T, Aszodi A, Fassler R, Usami S (2005) Type IX collagen knock-out mouse shows progressive hearing loss. Neurosci Res 51:293–298 10.1016/j.neures.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 41.Parentin F, Sangalli A, Mottes M, Perissutti P (2001) Stickler syndrome and vitreoretinal degeneration: correlation between locus mutation and vitreous phenotype. Apropos of a case. Graefes Arch Clin Exp Ophthalmol 239:316–319 [DOI] [PubMed] [Google Scholar]
- 42.McLeod D, Black GCM, Bishop PN (2002) Vitreous phenotype: genotype correlation in Stickler syndrome. Graefes Arch Clin Exp Ophtalmol 240:63–65 [DOI] [PubMed] [Google Scholar]