The question implicit in my title was explicitly framed by Alan Guttmacher and colleagues1 in an article entitled “Genomic Medicine: Who Will Practice It?” While this question is a general one, my particular concern is with what the roles of medical genetics and medical geneticists will be in what is being referred to as genomic medicine. To answer these questions, it is necessary to define what we mean by “medical genetics and medical geneticists” and by “genomic medicine.” Although this might appear to be a semantic exercise, that is not my intention. Rather, if we are to look at the future role of medical genetics in the practice of medicine, I believe that we really need to understand what we are talking about. I shall start with medical genetics and medical geneticists.
Medical Genetics
The term “medical genetics” has been variously defined as the science of human biological variation as it relates to health and disease2; the study of the etiology, pathogenesis, and natural history of diseases and disorders that are at least partially genetic in origin3; and the application of genetics to medicine4 or to medical practice.5 Furthermore, medical genetics services have been defined as “an integrated clinical and laboratory service provided for those with [or] concerned about a disorder with a significant genetic component and their families (this includes inherited and sporadic genetic disorders)….”6 These definitions embody the tension between medical genetics as a science and as a clinical area or specialty of medicine, but, clearly, medical genetics is really both.
However it is defined, medical genetics initially grew up within human genetics, with an agenda consisting largely of research and only a modest clinical component. Many of the founders of the field, or at least of the American version of it—Arno Motulsky, Victor McKusick, James Neel, Kurt Hirschhorn—were internists by training, as were many of their early disciples. However, with the progress in medical genetics that was made possible by the conceptual and technological advances of the 1950s and 60s—particularly in cytogenetics and biochemical genetics7—and with the growth of dysmorphology in the 1960s and 70s, medical geneticists became more clinical and more pediatric in orientation. This shift was of great concern to at least some medical geneticists, and in his 1977 Presidential Address to the American Society of Human Genetics, Motulsky issued the following warning:
Medical geneticists need to broaden their fields of interest to encompass other fields than those of pediatric interest alone. We need to attract more basic scientists. Our field is evolving from a largely research oriented science to a service-oriented specialty. This logical development is a sign of increasing maturity and makes available to the public the results of our research. The resulting stresses and strains need careful watching to prevent their slowing the momentum of our science….8
Despite this warning, the demands of clinical service created increasing inroads into the more basic research endeavors of medical geneticists, and medical genetics rapidly became virtually synonymous with clinical genetics and pediatrics.
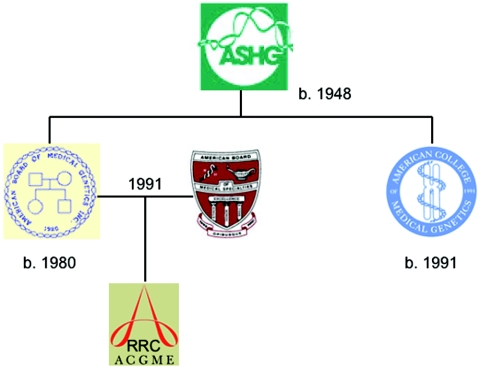
This change in identity was carved into stone by a series of events that began in 1980, when the American Society of Human Genetics spun off the American Board of Medical Genetics to certify medical geneticists and genetic counselors and to accredit training programs (fig. 1). Both clinical and laboratory-based medical geneticists were certified, with those who were physicians being certified in clinical genetics. The American College of Medical Genetics was created about 10 years later, and this relieved the Society of responsibilities to clinical genetics and geneticists and permitted it to return to the research and education agenda that was and still is its principal concern. The full legitimization of medical genetics as a clinical specialty took place in 1991, when the American Board of Medical Genetics was admitted, without the genetic counselors, to the American Board of Medical Specialties. Medical Genetics thus became the 24th primary specialty of medicine, and the Residency Review Committee (RRC) for Medical Genetics, which accredits clinical medical genetics training programs, was formed the following year under the aegis of the Accreditation Council for Graduate Medical Education.
Figure 1. .
Pedigree of the organizations that constitute the medical genetics establishment.
Medical genetics now had in place the four pillars (fig. 2) of a conventional medical specialty: a research society, a college (or academy), a certifying board, and an RRC—each officially independent of the other, but in reality highly interrelated. However, by its very success in becoming institutionalized, medical genetics defined itself principally in terms of its clinical content—which is really clinical genetics—and not of its science. This might give the impression that the fortunes of medical genetics as a scientific discipline rise and fall with the success of medical genetics as a clinical specialty, but this is not really the situation. The science of medical genetics, the application of human genetics to medicine, is in fact more robust and thriving than ever and has truly entered the mainstream of medical science. The meetings of the American Society of Human Genetics, at which the science is presented, are very well attended, and the pages of the American Journal of Human Genetics and of many other journals, where it is published, testify to the continued and increasing vigor of the field. What has changed, however, is that much of medical genetics research is now being done by physicians and scientists who, while using the tools and principles of genetics to study human genetic diseases, do not consider themselves to be medical geneticists or, for the most part, even human geneticists. Fortunately, as far as the science is concerned, this is really of no great consequence. Medical genetics research is being done and continues to contribute to medicine, genomic or otherwise, and this is what is important.
Figure 2. .
Quadripartite structure of the medical genetics edifice.
Medical Geneticists
Turning again to the clinical side of medical genetics, it is instructive to view how it is currently formulated. This is perhaps best summarized in the Program Requirements of the RRC for Medical Genetics, which describe what medical geneticists do and specify how medical genetics training programs are to operate.
Clinical medical geneticists are physicians who provide comprehensive diagnostic, management, and genetic counseling services for patients with genetic, or possibly genetic, disorders…[and] plan and coordinate large-scale screening programs….Clinical medical geneticists are able to (a) diagnose and manage genetic disorders; (b) provide patient and family counseling; (c) use their knowledge of heterogeneity, variability, and natural history of genetic disorders in patient-care decision making; (d) elicit and interpret individual and family medical histories; (e) interpret clinical genetic and specialized laboratory testing information; (f) explain the causes and natural history of genetic disorders and genetic risk assessment; and (g) interact with other health-care professionals in the provision of services for patients with genetically influenced disorders.
[Training] Programs must provide…education in the basic sciences and clinical areas pertinent to medical genetics, including mendelian genetics, cytogenetics, diagnosis and treatment of inborn errors of metabolism, molecular diagnosis, syndrome identification and dysmorphology, teratology, reproductive genetics, congenital malformations, multifactorial disorders, mental retardation and developmental disabilities, genetic screening, social and ethical issues in medical genetics, genetic counseling, and quantitative human genetics.9
These descriptions and specifications are, of course, quite broad, and not every medical geneticist is expert in or practices all of the components listed. In fact, whether formally or informally defined, there are areas of specialization within clinical genetics, the three most important being dysmorphology, metabolic diseases, and prenatal diagnosis. Nevertheless, all medical geneticists are expected to have a general knowledge of all of the areas specified in the program requirements.
Genomic Medicine
With this understanding of what medical geneticists are being trained to do, I turn now to consider what is meant by “genomic medicine” (or by the more or less interchangeable terms “genetic medicine” and “molecular medicine”). Below are two definitions.
In the description of a course on the subject, the Harvard-MIT Division of Health Sciences & Technology defines genomic medicine as
…the use of industrialized methods of data acquisition and analysis to improve medical care, including prognostics, diagnostics, preventive intervention, therapeutic selection, and individualized treatment based on the complex interaction between inherited and acquired elements of human variation. Genomic medicine will have a transformative role in healthcare, whose emphasis can be anticipated to dramatically shift from disease treatment to health maintenance.10
In a similar vein, Willard defined genomic medicine as the application of the science of genomes, as opposed to genes, to the practice of medicine. As such,
It goes well beyond the traditional boundaries of genetics in medicine, as articulated by the medical specialty of Medical Genetics…that branch of human genetics that concerns itself with disease….Genomic medicine…is an approach that will build on the comprehensive nature of the genome sciences….As a clinical paradigm, genomic medicine will provide global, comprehensive, and multidimensional treatment and management strategies based on the science now emerging from the study of genomes.11
He outlined the purpose for all of this in the following manner:
The prospect of examining a person’s entire genome (or at least a large fraction of it) in order to make individualized risk predictions and treatment decisions is a tantalizing one….Having access to the entire human sequence is a necessary but insufficient prerequisite for genomic medicine. What is equally important is having the technology at hand to reliably visualize individual genomes (and their derivatives, the transcriptome, proteome and metabolome) for health information that, in combination with clinical data, can contribute to assessment of individual risks and guide clinical management and decision-making. [This makes] the prospect for developing truly individualized care…even more real.12
These two definitions are quite broad. They certainly encompass the “medicine” side of “genomic medicine,” but there is also emphasis in the first definition on “industrialized methods of data acquisition and analysis” and in the second on “global, comprehensive, and multidimensional…strategies” and “the technology to reliably visualize individual genomes.” It is clear that what is really new in genomic medicine is the application of genomic technology on a large scale to virtually every problem of medicine. We might call this the “process” of genomic medicine. However, these definitions also make it clear that the intent of genomic medicine is what has been variously described as preventive intervention, individualized treatment, prospective medicine,13 or personalized medicine14 and medicines (referring, of course, to pharmacogenetics15). Weston and Hood16 combine all of these together as the 3 Ps: predictive, preventive, and personalized medicine.
Key to all of this, of course, will be risk assessment based on testing, and there are, indeed, many forms of testing already in use and being developed—biochemical, genetic, genomic, proteomic, metabolomic, and pharmacogenetic (or pharmacogenomic). From the point of view of public perception, testing is where things are headed when we talk about genomic medicine. Indeed, as I have written elsewhere,17 there appears to be a pervasive belief in both scientific and public circles that genetic testing or profiling is going to be the cornerstone of much, if not all, of genomic medicine—in fact, all of medicine—in the future. In that article, I outlined the debate swirling around this notion, and I will not repeat that entire discussion here. However, a few points are worth repeating: genetic risk assessment will attain sufficient predictive power to be of use if—and only if—analyses of many genetic loci are combined with evaluations of nongenetic lifestyle and environmental factors. The results of such risk assessments will not be absolutely definitive. The information will be probabilistic, and we shall always be dealing with estimates of risk, not predictions of certain outcomes. That risk assessment or profiling can be done does not mean that it actually will or, indeed, should be done. This will ultimately be determined by whether testing really offers more than currently available forms of risk assessment in terms of predictive power and securing compliance—in other words, by whether it will have greater clinical utility and would enhance a person’s motivation to comply with recommendations for therapy and changes in lifestyle.
Medical Geneticists and Genomic Medicine
I have no doubt that medical genetics and medical geneticists will play an important role in the development of genomic medicine, however that development ultimately plays out. But what role will clinical medical geneticists play in its implementation and applications? (I focus here on clinical geneticists because laboratory-based geneticists, by virtue of what they do, will be intimately involved in testing, unless the process becomes so industrialized as to exclude even them—a scenario that is certainly not impossible to imagine.) It has been said, and rightly, that genomic medicine will transcend the current boundaries of medical genetics11 and will be applicable to the health care of the many or all rather than just the few.1,18 Whether, given the current inequities in the health-care system, the latter situation will ever be realized in full19 will have to await future determination. However, it is certainly true that genomic medicine, as defined earlier, is a far cry from clinical medical genetics, as currently conceptualized in the Program Requirements of the RRC for Medical Genetics presented earlier. The question is what this means for the future of clinical medical genetics. This and related issues are currently the subject of active debate in the official organizations concerned with medical genetics,20 and, at this point, I can only give my own view of the situation.
The easiest thing, of course, would be for clinical medical geneticists to continue to do what they have been doing for the past 35 years or so. They could maintain the status quo and continue to function as experts on Mendelian disorders, dysmorphology, chromosomal disorders, inherited metabolic diseases, and perhaps the genetic aspects of diseases, such as breast and ovarian cancer, that are caused by high-penetrance susceptibility genes, with the degree of emphasis on some versus others being a matter of personal preferences and/or institutional requirements. In fact, I believe that it will certainly be necessary for clinical geneticists, at least some proportion of them, to continue to do many of these things—but I also think that they will need to be able to do even more in the future. What is lacking from this approach, and from the Program Requirements of the RRC, is a concern for and involvement with the wide range of conditions referred to as the “common adult diseases” or “complex traits,” and it just these disorders that are the targets of genomic medicine. Although they are generally not Mendelian in origin, these conditions have their origins in the interactions of genetic factors (some or many, depending on the particular condition) with one another and with the environment. Their genetics is, therefore, truly polygenic and multifactorial—but it is still genetics nonetheless, and the fact that genomic tools will make it possible to acquire the knowledge required and to carry out the necessary testing procedures, whether genomewide or otherwise, does not make the approach any less genetic.
The issue, then, is whether medical geneticists acting in their clinical roles can and should be part of genomic medicine. With regard to the can, I do not believe that the training being provided at present to clinical genetics residents is, in most instances, sufficient to allow this to happen.21 What is lacking is a greater and more intensive instruction in population genetics and epidemiology, genetic and otherwise; pharmacogenetics; bioinformatics; the principles of risk assessment; and the common adult diseases and complex traits. There is no question in my mind that training programs can be reformulated to permit this to occur, and discussions about how this might be done are currently under way in organizations within medical genetics. So, if we assume that they would have the knowledge to do so, should the clinical geneticists get involved with genomic medicine?
It has been argued in several quarters that the principal role for the realization of the promise of genomic medicine will belong to primary-care physicians and other health-care personnel rather than to medical geneticists1 (for fuller discussion, see Epstein17). Although the therapy, preventive and otherwise, of common/adult/complex diseases will (and should, in most instances) remain firmly in the domain of primary-care physicians and relevant specialists, I think it unlikely that these physicians will be able to handle the complexities of comprehensive genetic testing and risk assessment alone, unless the ways in which medicine is practiced are drastically changed. They just won’t have the time or expertise to do so. It might be argued that all of the testing and risk assessment that genomic medicine embodies will be a highly automated operation, perhaps even a largely online operation. In this model, the testing will, of course, be completely automated, and only a blood sample will be required. The acquisition of historical, demographic, and environmental information from the interested person him- or herself will be computer-based, as will the calculation of risks and formulation of recommendations. All in all, it will be a black-box procedure, but both the physician and the patient him- or herself will still have to deal with the probabilistic nature of the results. As experience with risk assessment for breast and ovarian cancer has already taught us, they will need to have knowledgeable people to turn to.
The major issues for geneticists and nongeneticists alike are time, knowledge, and money. Given the present organization of medical services, especially in the United States, nongeneticist primary-care providers and specialists will have very little time to take on the broad responsibilities of personal risk assessment and management. The danger is that these functions will become somewhat mindless operations driven by commercial testing companies and passed down to less-qualified personnel. Furthermore, most primary-care providers and specialists do not really have the knowledge base that will be required to permit meaningful assessments to be made. Although efforts are being made by professional organizations, such as the American Academy of Family Practice and the American College of Physicians, to educate their members about genetics, fundamental changes will have to be made to integrate genetics fully into medical school curricula and residency training programs if the necessary concepts and knowledge base are to be inculcated. Despite much talk, this has been slow to evolve in medical training, and it has not yet even begun to occur in a serious way at the residency level, where the attitudes that will influence practice for the rest of life are really developed. And, with regard to money, there will have to be a major shift in how medical services are paid for if physicians are to be adequately reimbursed for the time that meaningful risk assessments and preventive interventions will require. The same impediments becoming involved in genomic medicine—time, knowledge, and money—also apply to medical geneticists.
So, why should medical geneticists get involved? Why don’t medical geneticists just continue to do what they are doing? For me, the answer is quite straightforward—in fact, there are two answers. The first is that I just can’t imagine medical geneticists not being intimately involved in the expanded role of genetics in medicine. Genomic medicine in some form is ultimately going to happen—it has already begun—and medical geneticists ought to and need to be part of it. The second answer to why they should is that it will be critical to the survival of medical genetics as a vital and exciting profession. How ironic it would be if medical genetics, possibly the last of the primary clinical specialties of medicine to be formally recognized, were to wither away just at the time that it is being finally acknowledged that genetics does have a major part to play in all aspects of medicine and health.
When they issued their call to open arms—that is, for the medical geneticists to open their arms and bring the primary-care providers into the genetics fold—Guttmacher et al.1 asserted that the expansion of nongenetic specialist providers’ use of genetics “will not relegate genetic specialists to the dustbin of medical history, but instead will redefine their roles.” Although this was intended to be reassuring, the phrase “dustbin of medical history” certainly conjured up a terrible image. Nevertheless, I think that we have now truly reached the point of the redefinition of roles of which they wrote. If the needs of the general public are to be properly served, primary-care physicians, nongeneticist specialists, and/or testing laboratories will not be able to go it alone in the era of wide-scale presymptomatic risk assessment and preventive management and pharmacogenetic testing when it eventually comes. Clinical geneticists and genetic counselors will have to be part of the mix. There will be enough for everyone to do, once they understand what is required. If medical geneticists remember their scientific heritage and become less passive, broaden their perspectives, expand their training, and interact more closely with primary-care providers and other specialists, medical genetics will assuredly become an essential part of the genomic medicine of the 21st century. The real task then will be to organize the health-care system to facilitate rather than hinder the integration of medical genetics into genomic medicine.
Footnotes
This article is based on a talk given at the Symposium on the Future of Human and Medical Genetics, held in Seattle on May 19, 2004, in honor of the establishment of the Arno G. Motulsky Endowed Professorship at the University of Washington School of Medicine.
References
- 1.Guttmacher AE, Jenkins J, Uhlmann WR (2001) Genomic medicine: who will practice it? A call to open arms. Am J Med Genet (Semin Med Genet) 106:216–222 [DOI] [PubMed] [Google Scholar]
- 2.Gelehrter TD, Collins FS (1990) Principles of medical genetics. Williams & Wilkins, Baltimore, p v [Google Scholar]
- 3.American Heritage Stedman's Medical Dictionary, 2nd ed. (2004) Houghton Mifflin, Boston [Google Scholar]
- 4.Wikipedia (2006) Medical genetics (http://en.wikipedia.org/wiki/Medical_genetics) (accessed April 17, 2006)
- 5.Jorde LB, Carey JC, Bamshad MJ, White RL (1999) Medical genetics, 2nd ed. Mosby, St. Louis, p 1 [Google Scholar]
- 6.Department of Health (2001) Specialized services national definition set, 2nd ed. (http://www.phgu.org.uk/ecard?link_ID=1013) (accessed April 17, 2006)
- 7.Vogel F, Motulsky AG (1997) Human genetics: problems and approaches, 3rd ed. Springer Verlag, Berlin, pp 19–22 [Google Scholar]
- 8.Motulsky AG (1977) Medical and human genetics 1977: trends and directions. Am J Hum Genet 30:123–131 [PMC free article] [PubMed] [Google Scholar]
- 9.Program Requirements for Graduate Medical Education in Medical Genetics (2004) (http://www.acgme.org/acWebsite/downloads/RRC_progReq/130pr703_u704.pdf) (accessed April 17, 2006)
- 10.Harvard-MIT Division of Health Sciences & Technology (2004) Genomic medicine. HST 512/513 (https://hstdev.mit.edu/servlet/ControllerServlet?handler=PublicHandler&action=viewCourse&courseID=HST+512&term=2004SP) (accessed April 17, 2006)
- 11.Willard HF (2004) Message from the director. (http://www.genome.duke.edu/genomelife/glarchive/issue9/dirmessage) (accessed April 17, 2006)
- 12.Willard HF, Angrist M, Ginsburg, GS (2005) Genomic medicine: genetic variation and its impact on the future of health care. Phil Trans R Soc B 360:1543–1550 10.1098/rstb.2005.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyderman R, Williams RS (2003) Prospective medicine: the next health care transformation. Acad Med 78:1079–1084 10.1097/00001888-200311000-00002 [DOI] [PubMed] [Google Scholar]
- 14.Ginsburg GS, Donahue MP, Newby LK (2005) Prospects for personalized cardiovascular medicine: the impact of genomics. J Am Coll Cardiol 46:1615–1627 10.1016/j.jacc.2005.06.075 [DOI] [PubMed] [Google Scholar]
- 15.Royal Society (2005) Personalised medicines: hopes and realities. (http://www.royalsoc.ac.uk/displaypagedoc.asp?id=15874) (accessed April 17, 2006)
- 16.Weston AD, Hood L (2004) Systems biology, proteomics, and the future of heath care: toward predictive, preventative, and personalized medicine. J Proteome Res 3:179–196 10.1021/pr0499693 [DOI] [PubMed] [Google Scholar]
- 17.Epstein CJ (2004) Genetic testing: hope or hype? Genet Med 6:165–172 [DOI] [PubMed] [Google Scholar]
- 18.Khoury MJ (2003) Genetics and genomics in practice: the continuum from genetic disease to genomic information in health and disease. Genet Med 5:261–268 [DOI] [PubMed] [Google Scholar]
- 19.Qureshi N, Kai J (2005) Genomic medicine for underserved minority populations in family medicine. Am Fam Physician 72:386–387 [PubMed] [Google Scholar]
- 20.Korf BR, Feldman G, Wiesner GL (2005) Report of Banbury Summit meeting on training of physicians in medical genetics, October 20–22, 2004. Genet Med 7:433–438 [DOI] [PubMed] [Google Scholar]
- 21.Epstein CJ (2005) Medical geneticists in the 21st century. Genet Med 7:375–379 [DOI] [PubMed] [Google Scholar]