West Nile virus (WNV) caused an unprecedented amount of arthropod-borne illness in Canada in 2002. Since then, we have been working on approaches to mosquito control: one of us (H.S.) has been involved in a cooperative group of southern Ontario public health departments to work through some of the issues in implementing mosquito control measures (the Central East West Nile Virus Work Group), and the other (S.M.) has been researching the effectiveness of pesticides in preventing the spread of WNV (Public Health Research, Education and Development Program, City of Hamilton). In this article, we comment on the general approach to WNV being taken by a number of public health units in southern Ontario.
The prevention of human illness from WNV involves far more than truck-mounted spraying of pesticides. A full response to WNV comprises 3 main components: public education, surveillance and mosquito control. At a local level these components need to be coordinated with elected officials, various city departments (roads, parks, public works, animal services, by-law and communications), conservation authorities, provincial and federal ministries of health, and regulatory agencies.
Public education is essential to help people understand WNV, to encourage people to eliminate mosquito breeding sites on their own property and to promote personal protective measures to avoid mosquito bites. Unfortunately, it is difficult to change personal behaviours, and thus the efficacy of personal protective measures will be limited.1 Education also plays a key role in helping people to understand what mosquito control is, how it works, why it is important and its potential health and environmental impacts.
Surveillance of bird deaths (e.g., of Corvidae birds such as crows and jays), adult mosquitoes, mosquito breeding sites and human cases of WNV will provide an indication of the extent and location of WNV activity throughout the season. This information will help guide local decisions to intensify activities during the WNV season, such as issuing an alert or increasing mosquito control in an area of high risk. In addition, surveillance data collected over a number of years will help to refine control strategies further.
The last component in the prevention of human WNV infection is mosquito control. Knowledge of mosquito biology and local conditions should be used to choose the best interventions (habitat modification, water management, sanitation or pesticides) on a site-specific basis.
The control measure that raises most public concern is pesticide use. Pesticides are designed to act at 2 of the stages in the mosquito life cycle (Fig. 1). Immature mosquitoes develop from eggs to larvae to pupae in standing water, and pupae develop into winged adults. Agents that work in standing water against mosquito larvae are termed larvicides, and those that work against winged mosquitoes are termed adulticides.
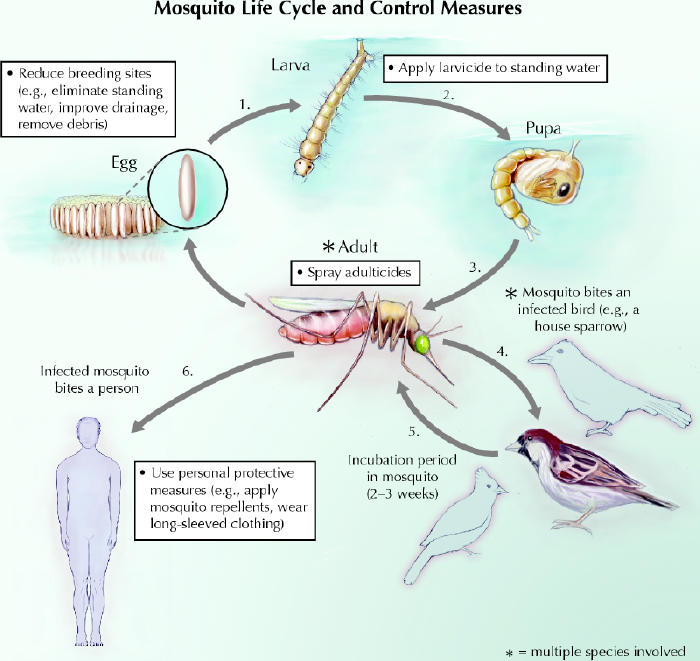
Fig. 1: Schematic of the mosquito life cycle and control measures. The development of immature mosquitoes from eggs to larvae to pupae occurs in standing water. These stages of the mosquito life cycle can be disrupted by eliminating standing water where possible and by applying larvicides to water bodies containing larvae. When the pupae develop into winged adults, mosquitoes acquire West Nile virus by biting infected birds. The incubation period in mosquitoes is about 2–3 weeks. An infected mosquito might then bite a person, passing the virus on. Many bird species act as reservoirs for the virus, and many mosquito species are involved in passing the infection from bird to bird, or from bird to human, or both. Populations of adult mosquitoes can be controlled by spraying adulticides. Individuals can reduce their exposure to mosquitoes by undertaking personal protective measures. Photo: Chesley Sheppard
Larvicides have a number of advantages over adulticides. Their use can be targeted to mosquito breeding sites, which avoids a wide application over an entire neighbourhood or city. They can be applied in solid form (e.g., pellets, granules and sand), which limits human exposure. Some larvicide agents are specific to mosquitoes when used according to directions and have relatively little impact on the environment and human health. Formulations are available that can prevent the emergence of adult mosquitoes for up to 1 month, which decreases labour costs.
Adulticides are most often applied as a very fine ultra-low-volume (ULV) droplet spray from a truck or aircraft. Adulticide operations dominate media coverage of WNV control, although they are only one part of control efforts. Adulticides applied as a ULV spray work by coming into direct contact with adult mosquitoes as they are flying. One criticism of adulticides is their transient effect: mosquito numbers return to pretreatment levels within a few days without repeat applications. The use of adulticides in many jurisdictions is reserved for response to human cases of WNV infection or environmental findings (WNV-positive birds or mosquitoes) that indicate a high level of human risk of WNV infection from adult mosquitoes.2,3
Every pesticide in Canada has to pass a science-based assessment by Health Canada's Pest Management Regulatory Agency (PMRA). The agency takes this information into account and provides reassurance as to the safety and potential usefulness of the pesticides when used as directed. However, critics of pesticide use have expressed concerns about their effectiveness and their impact on human health and the environment.
The effectiveness of pesticides for mosquito control has been reported in many different ways, from controlled experiments in a laboratory setting4 to descriptive reports of mosquito control programs over a wide geographic area.5 That pesticides kill mosquitoes (with varying levels of effectiveness depending on the product and species) when applied as larvicides to small, well-defined locations (e.g., ponds, catch basins, wetland areas) is supported by findings from controlled studies.6,7 Reports of before–after studies also provide evidence that mosquito control efforts lower the numbers of mosquitoes in a given neighbourhood or city.8 Unfortunately, randomized controlled trials of the effectiveness of mosquito control using human arboviral disease as an end point are not possible for practical reasons (e.g., the wide variety of local environmental conditions, the variety of mosquito species and the usually small numbers of human cases). This lack of evidence, especially as it concerns mitigation of human disease, is echoed by many experts.9,10 Nonetheless, mosquito control, especially the use of larvicides, is a recommended response to WNV by the US Centers for Disease Control and Prevention.2 Draft guidelines from Health Canada make similar recommendations but are not yet publicly available (Dr. Robbin Lindsay, National Microbiology Laboratory, Winnipeg: personal communication, 2003).
The 2 larvicides being considered by most public health units in Canada — methoprene and Bacillus thuringiensis subsp israelensis (commonly referred to as Bti) — have been used for many years and have well-documented track records of human and environmental safety.11,12,13 Both of these larvicides pose little risk to human health either through direct handling of the products or indirect exposure to them as a result of their use for mosquito control. Mild skin and eye irritation have been reported from direct contact with Bti.12
Methoprene has been shown to be toxic to some insects closely related to the mosquito, has very low toxicity to mammals, is moderately toxic to warm-water and freshwater fish, is slightly toxic to cold-water fish and is acutely toxic to some estuarine invertebrates.13 Toxicity of methoprene to insects and animals other than mosquitoes and blackflies occurs at concentrations much higher than those used for mosquito control.13 Bti is toxic to a lesser range of species than methoprene but is also effective against a lesser range of mosquito species.12 Both larvicides break down rapidly in the environment.
Unlike the application of larvicides, which is in standing water usually remote from people and is limited in scope, adulticides must be applied widely in the air, which puts other insects, birds, fish, crustaceans and mammals, including people, at increased risk of exposure to them.
Malathion is the main adulticide being considered for mosquito control in Ontario. Many of the products used for mosquito control in the United States (e.g., synergized pyrethroids such as sumithrin and piperonyl butoxide) are not available for use in Canada. Malathion is an organophosphate pesticide that has been used in Canada since 1953. In addition to its use for mosquito control, it has registered residential uses for insects on lawns, gardens and ornamental trees, shrubs and plants in Canada and the United States. An extensive re-evaluation of malathion was completed by the US Environmental Protection Agency in 2000.14 The PMRA has also re-evaluated malathion and approved its use as a mosquito adulticide.15 Among the available agents used for mosquito control, malathion has the most current and comprehensive safety information available. It works by inhibiting cholinesterase and is detoxified by carboxylesterases into polar, water-soluble compounds that are then excreted.16 Mammals have greater carboxylesterase activity than insects, which accounts for the selective toxicity of malathion toward insects. Cholinesterase inhibition in humans can overstimulate the nervous system and result in nausea, dizziness, confusion and, at very high exposures (e.g., accidents, major spills), respiratory paralysis and death.
A comprehensive literature review, risk assessment, and epidemiologic and attributable risk analyses of adulticides, including malathion, were performed by the Westchester County Board of Health, New York.11 The board concluded that no significant adverse human health effects would be expected from adulticides used in accordance with its mosquito control plan. It concluded that the active ingredients in the adulticides may cause short-term effects, such as skin irritation or respiratory effects for some sensitive individuals, but were not expected to increase asthma events or other respiratory effects significantly. Similar conclusions have been reached by the US Environmental Protection Agency14 and the PMRA.15
Malathion has low toxicity to birds and mammals and is not expected to pose a hazard to them.14 It degrades rapidly in the environment, especially in moist soils. But there are some environmental concerns with malathion. It is highly toxic to insects and to aquatic organisms, including fish. The toxic effects on aquatic organisms can be decreased by limiting drift of the adulticide around water.
That pesticides kill mosquitoes when used as directed is not much in doubt. The issue is whether mosquito numbers can be lowered enough to have a significant impact on human illness from WNV. In trying to prevent WNV infection, public health units face a challenge of balancing the risk of infection against the risk of human and environmental exposure to the pesticides used for mosquito control. Given some of the uncertainties surrounding WNV, owing to the recent arrival of the virus in North America and the newness of resulting mosquito control programs, it would be scientifically responsible to ensure that WNV control programs are evaluated using appropriate methods and the findings disseminated to the community.
β See related articles pages 1399, 1443 and 1455
Supplementary Material
Footnotes
Published at www.cmaj.ca on May 6, 2003
Contributors: Both authors contributed substantially to the writing of the article and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Howard Shapiro, Associate Medical Officer of Health, Peel Health, Ste. 102, 44 Peel Centre Dr., Brampton ON L6T 4B5; fax 905 789-1604
References
- 1.Zielinski-Gutierrez EC. Why don't people just use repellent? Barriers and facilitating factors to West Nile virus personal prevention. Fourth National Conference on West Nile virus in the United States; 2003 Feb 9–11; New Orleans. Available: www.cdc.gov/ncidod/dvbid/westnile/conf/February_2003.htm (accessed 2003 Apr 30).
- 2.Epidemic/epizootic West Nile virus in the United States: revised guidelines for surveillance, prevention, and control. Atlanta: US Centers for Disease Control and Prevention; 2001. Available: www.cdc.gov/ncidod/dvbid/westnile/resources/wnv-guidelines-apr-2001.pdf (accessed 2003 Apr 30).
- 3.Department of Health and Mental Hygiene, City of New York. Comprehensive mosquito surveillance and control plan 2003. New York: The Department; 2003. Available: www.ci.nyc.ny.us/html/doh/pdf/wnv/wnvplan2003.pdf (accessed 2003 Apr 30).
- 4.Ali A, Nayar JK, Xue RD. Comparative toxicity of selected larvicides and insect growth regulators to a Florida laboratory population of Aedes albopictus. J Am Mosq Control Assoc 1995;11:72-6. [PubMed]
- 5.Peavy JE, Dewlett HJ, Metzger WR, Bagby J. Epidemiology and aerial spray control of arthropod-borne viral encephalitis in Texas. Am J Public Health Nations Health 1967;57(12):2111. [DOI] [PMC free article] [PubMed]
- 6.Lawler SP, Jensen T, Dritz DA, Wichterman G. Field efficacy and nontarget effects of the mosquito larvicides temephos, methoprene, and Bacillus thuringiensis var. israelensis in Florida mangrove swamps. J Am Mosq Control Assoc 1999;15(4):446-52. [PubMed]
- 7.McCarry MJ. Efficacy and persistence of Altosid pellets against Culex species in catch basins in Michigan. J Amn Mosq Control Assoc 1996;12:144-6. [PubMed]
- 8.Andis MD, Sackett SR, Carroll MK, Bordes ES. Strategies for the emergency control of arboviral epidemics in New Orleans. J Am Mosq Control Assoc 1987;3(2):125-30. [PubMed]
- 9.West Nile virus list serve. West Nile virus. Ithica (NY): Cornell University, Center for the Environment; 2002.
- 10.Nasci RS, Newton NH, Terrillion GF, Parsons RE, Dame DA, Miller JR, et al. Interventions: vector control and public education: panel discussion. Ann N Y Acad Sci 2001;951:235-54. [DOI] [PubMed]
- 11.Westchester County Board of Health. Environmental review for the Comprehensive Mosquito-Borne Disease Surveillance and Control Plan. White Plains (NY): The Board; 2003. Available: www.westchestergov.com/planning/environmental/StingEIS/STING_DGEIS.htm (accessed 2003 Apr 30).
- 12.Glare TR, O'Callaghan M. Report for the New Zealand Ministry of Health: Environmental and health impacts of Bacillus thuringiensis israelensis. Lincoln (New Zealand): Biocontrol & Biodiversity, Grasslands Division, AgResearch; 1998. Available: www.moh.govt.nz/moh.nsf/c7ad5e032528c34c4c2566690076db9b/ff3b628d67e34963cc256ba3000d8476/$FILE/BacillusThuringiensisIsraelensis.pdf (accessed 2003 Apr 26).
- 13.Glare TR, O'Callaghan M. Report for the New Zealand Ministry of Health: Environmental and health impacts of the insect juvenile hormone analogue, S-methoprene. Lincoln (New Zealand): Biocontrol & Biodiversity, Grasslands Division, AgResearch; 1999. Available: www.moh.govt.nz/moh.nsf/c7ad5e032528c34c4c2566690076db9b/ff3b628d67e34963cc256ba3000d8476/$FILE/S-methoprene.pdf (accessed 2003 Apr 30).
- 14.US Environmental Protection Agency. Malathion: revised risk assessments. Washington: The Agency; 2000. Available: www.epa.gov/pesticides/op/malathion.htm (scroll down to “revised risk assessments” document) (accessed 2003 Apr 30).
- 15.Pest Management Regulatory Agency. Fact sheet on the use of malathion in mosquito control programs. Ottawa: The Agency; 2003. Available: www.hc-sc.gc.ca/pmra-arla/english/pdf/fact/fs_malathion-e.pdf (accessed 2003 Apr 30).
- 16.Agency for Toxic Substances and Disease Registry. Toxicological profile for malathion — draft for public comment. Atlanta: US Department of Health and Human Services, Public Health Service; 2001. Available: www.atsdr.cdc.gov/toxprofiles/tp154.html (updated 2002 Aug 5; accessed 2003 Apr 30).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


