Abstract
Maternal touch profoundly regulates infant neural and behavioral development, and supports learned odor associations necessary for infant attachment. Endogenous opioids are well characterized to mediate the calming and analgesic properties of maternal touch; yet their role in learned odor-touch associations is unknown. We administered naltrexone, an opioid receptor antagonist, before or immediately following classical conditioning with peppermint odor and tactile stimulation (stroking) in rat neonates. Results indicate odor-stroke conditioning produces odor preferences facilitated by endogenous opioids during acquisition and memory consolidation. These results provide additional evidence for the modulatory role of opioids in neonate learning and memory. Disturbances to this system may alter the impact of touch on infant development, particularly in the realm of learning necessary for attachment.
Keywords: opioids, learning, memory, neonate, odor, stroke
Sensory stimuli associated with the mother (touch, odor, milk, warmth) are substantial regulators of infant physiology and behavior (Hofer, 1994; Myers et al., 2004; Stanton, Wallstrom, & Levine, 1987). Indeed, studies of maternal separation in rats and primates highlight the importance of mother-infant interactions, as isolation of an infant from the mother compromises both brain and behavioral development (Brake, Zhang, Diorio, Meaney, & Gratton, 2004; Carden, Tempel, Hernandez, & Hofer, 1996; Gunnar, 2003; Ladd et al., 2000; Meaney et al., 1996; Schanberg, Ingledue, Lee, Hannun, & Bartolome, 2003; van Oers, de Kloet, Whelan, & Levine, 1998, Zimmerberg, Kim, Davidson, & Rosenthal, 2003). Studies using various forms of supplemental tactile stimulation of both rat and human neonates further demonstrate the importance of maternal touch during development. Such treatment in nondeprived rats significantly alters hormones (particularly those involved in stress responses) and gene expression, and in deprived pups is sufficient to reverse many of the neural and behavioral severities generated by maternal separation (Jutapakdeegul, Casalotti, Govitrapong, & Kotchabhakdi, 2003; Lucion, Pereira, Winkelman, Sanvitto & Anselmo-Franci, 2003; Schanberg & Field, 1987; van Oers et al., 1998). In humans, stroking and skin-to skin contact causes positive changes in the neonate’s physiology and behavior, especially in preterm or low-birth weight infants (Anisfeld, Casper, Nozyce, & Cunningham, 1990; Bystrova et al., 2003; Ferber & Makhoul, 2004; Pelaez-Nogueras, Field, Hossain, & Pickens, 1996; Schanberg & Field, 1987; Vickers, Ohlsson, Lacy, & Horsley, 2000). Overall, these studies demonstrate the importance of maternal tactile stimulation in normal infant development.
Tactile stimulation also serves as a reward and supports learning in newborns. Specifically, human infants can associate tactile stimulation with an odor during the first few hours of life (Sullivan et al., 1991), indicative that the neural mechanisms necessary for affective learning and memory are already present and functional. Similar associative conditioning has been well documented in rat neonates (Dominguez, Lopez, & Molina, 1999; McLean, Darby-King, Sullivan, & King, 1993; Sullivan & Hall, 1988; Weldon, Travis, & Kennedy, 1991). Neurochemical systems involved in mediating the neonate’s response to touch include cholecystokinin (Weller & Feldman, 2003), opioids (Panksepp, Herman, Vilberg, Bishop & DeEskinazi, 1980), oxytocin (Insel, 1997; Nelson & Panksepp, 1996), and serotonin (McLean et al., 1993), although norepinephrine (NE, Sullivan & Wilson, 1994) has a particularly prominent role in mediating learned odor-stroke associations in rat neonates. It is well known that the calming (attenuation of stress) and analgesic effects of mother-infant interaction is mediated through endogenous opioids (Blass, Fillion, Weller, & Brunson, 1990; Gray, Watt, & Blass, 2000; Weller & Feldman, 2003); however, their role in learned odor-touch associations has not been examined. The goal of this research was to examine the role of opioids in the associative learning and memory of an odor preference following odor-stroke conditioning in neonatal rats.
METHODS
Subjects
All procedures were approved by the University of Oklahoma Institutional Animal Care and Use Committee and follow NIH guidelines. Subjects were both male and female pups, born of Long-Evans rats (Harlan Sprague-Dawley, IN) in the animal vivarium at the University of Oklahoma. Mothers were housed in polypropylene cages lined with aspen wood shavings, and kept in an environment with controlled temperature (20°C) and light (12 hr: 12 day), with food and water available ad libitum. The day of parturition was termed Day 0 of age, and litters were culled to five males and five females Day 1–2 later.
Behavioral Training Procedures
On the day of training, 7–8-day-old pups were randomly assigned to a training condition: (1) Paired odor-stroke, (2) Unpaired odor-stroke, and (3) Odor only. After being marked for identification (indelible ink), weighed, and placed in individual 600 ml glass beakers, pups were given a 10 min habituation period to recover from handling. During a 1 hr training session, pups received 14 presentations of a 30 s peppermint odor and stroke, with an intertrial interval of 4 min. Paired odor-stroke subjects received 14 pairings of the 30 s odor with stroke during the last 20 s of the odor presentation, while Unpaired odor-stroke subjects received stroking approximately 2 min after an odor presentation. Odor-only subjects received only the peppermint odor presentations. Peppermint odor was presented with a flow-dilution olfactometer at 2 L/min and at a concentration of 1:10 peppermint vapor. Using a small painter’s brush, strokes were delivered in a rostral-caudal direction on the dorsal surface of the pup.
To assess pups’ learning during odor-shock training, limb movements (0, no movement−5, movement of all limbs) 10 s prior to an odor presentation and during the odor presentation were recorded (Hall, 1979). This rating scale allows us to measure generalized behavioral activity and to provide a general assessment of learning during training. Following training, pups were returned to the mother.
Drug Treatment
For Experiment 1, pups received Naltrexone (NTX; Naltrexone Hydrochloride, Sigma, St. Louis, MO) or equal volume saline subcutaneously at the nape of the neck. Pups received 0.5 mg/kg of NTX (Kehoe & Blass, 1986a; Roth & Sullivan, 2001, 2003) and were given 15 min to recover undisturbed in a 30°C incubator before beginning odor-stroke conditioning. For Experiment 2, pups received 0.5 mg/kg of NTX or equal volume saline (SAL; subcutaneous at the nape of the neck) immediately following the end of odor-stroke conditioning. After injections, pups were placed in a 30°C incubator for 15 min before they were returned to their home cage.
Behavioral Testing Procedures
One day after training, pups were removed from the mother and tested using a Y-maze. The Y-maze consisted of a start box (7 cm long and 9 cm wide) and 2 alleys at 45° angles (22 cm long and 9 cm wide). The start box was separated from the alleys via removable doors. One arm of the maze contained the familiar aspen wood nest odor (20 ml of clean, aspen shavings in a petri dish), while the other arm contained the peppermint odor (25 μl of peppermint extract placed on a Kim wipe that had been placed in a ventilation hood for 5 min). Each pup was placed in the starting box and given 5 s for habituation before the doors to the alleys were removed. Each subject had 60 s to make a choice, which required the pup to enter the alley. Each subject was given five trials, and the floor was wiped clean (using a cloth with distilled water) between each trial. A 30 s intertrial interval was used between testing trials, and the orientation of the pup was counterbalanced between trials when placed in the habituation chamber. Observations of each pup were made blind to the training condition.
Data Analysis
We used the analysis of variance (ANOVA) and post-hoc Fisher tests to analyze differences between training conditions and drug treatment groups for both experiments. To analyze learning curves, we used repeated measures ANOVAs and post-hoc Fisher tests. For all statistical tests, significance was set at p < .05.
RESULTS
Odor-Stroke Learning
In Experiment 1, pups received NTX prior to conditioning to assess whether disruption of the endogenous opioid system impairs pups’ ability to form odor-stroke associations. ANOVA analysis of behavior during conditioning in Experiment 1 indicates that all pups had similar behavior before an odor presentation, F (12, 246) =1.37, p = .18 (data not shown). Analysis of behavior during odor presentations indicates there was a training condition × drug interaction effect, F (12, 246) = 2.59, p <.01 (Fig. 1A). Only SAL treated pups in the Paired condition demonstrated significant acquisition (learning) during the course of the training session, p < .05. Pups given NTX within the Paired condition were not different from control pups, p > .05. As illustrated in Figure 1B, ANOVA analysis of Y-maze results revealed a main effect of training condition, F (2,41) = 5.45, p < .01, and a training condition × drug interaction, F (2, 41) = 3.86, p < .03. Post-hoc analysis indicates that pups that received Paired presentations of odor and stroke learned an odor preference (p < .05), while pups that received NTX prior to Paired presentations of odor and shock failed to learn the preference. NTX had no observable effect in either the Unpaired or Odor Only conditions.
FIGURE 1.
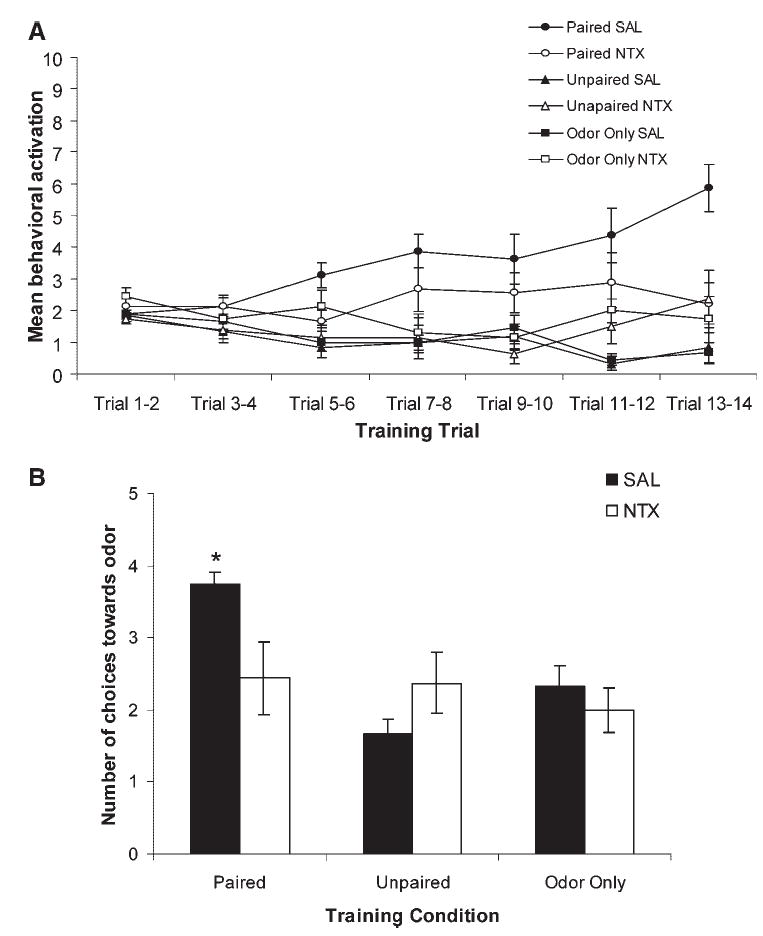
Opioid receptor antagonism blocks the learning of a stroke-induced odor preference in rat neonates. (A) Behavioral acquisition in response to the odor during conditioning for rat neonates receiving NTX or SAL prior to paired (NTX n = 9; SAL n = 8) or unpaired (NTX n = 8; SAL n = 6) presentations of odor-stroke, or odor only presentations (NTX n = 7; SAL n = 9) on postnatal Day 7 or 8. Each point represents the mean of two summated trials; vertical lines indicate SEM. (B) Number of approaches toward the conditioned odor in the Y-maze test. Bars represent mean values; vertical lines indicate SEM. SAL, saline; NTX, naltrexone. *indicates p < .05.
Odor-Stroke Memory Consolidation
In Experiment 2, pups received NTX immediately following conditioning to assess whether disruption of the endogenous opioid system impairs pups’ ability to form memories of learned odor-stroke associations. For Experiment 2, ANOVA analysis of behavior during conditioning indicates that all pups had similar behavior before an odor presentation, F (12, 252) = .75, p = .70 (data not shown). Analysis of behavior during odor presentations indicates there was a significant effect of the training condition, F (12, 252) = 4.07, p < .01 (Fig. 2A). All pups in the Paired condition demonstrated significant acquisition (learning) during the course of the training session, and were significantly different from control pups, p < .05. ANOVA analysis of Y-maze results (Fig. 2B) revealed a main effect of drug treatment, F (2, 42) = 4.69, p < .04, and a training condition × drug interaction, F (2, 42) = 5.44, p < .01. Post hoc analysis revealed that pups that received Paired presentations of odor and stroke had an odor preference, p < .05. NTX post-training blocked the formation of an odor preference, as these pups did not differ from controls. NTX had no observable effect in either the Unpaired or Odor Only conditions.
FIGURE 2.
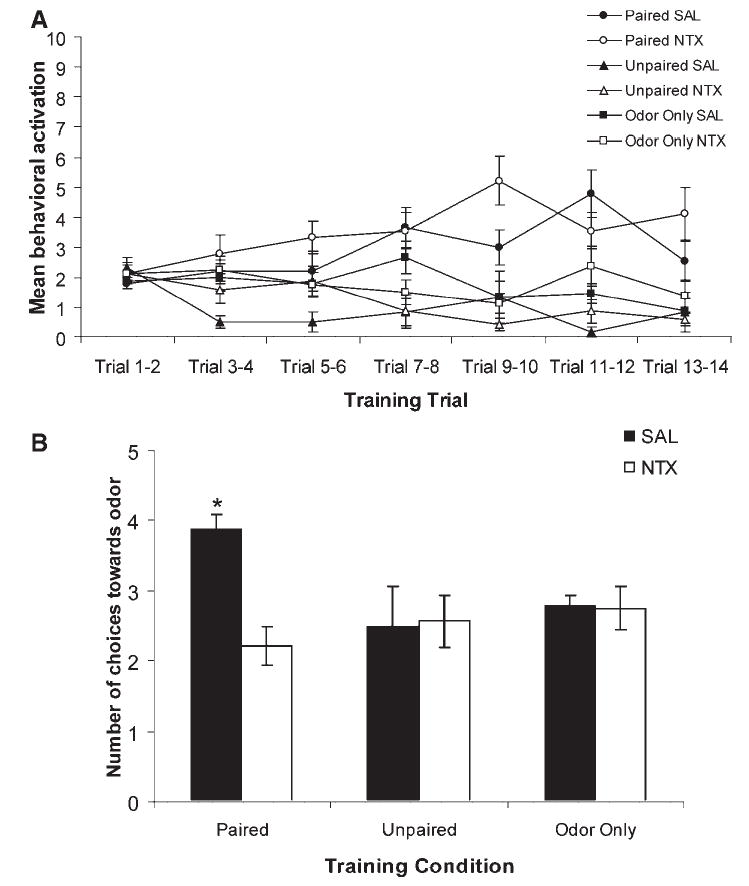
Opioid receptor antagonism blocks the consolidation of an odor preference following odor-stroke conditioning in rat neonates. (A) Behavioral acquisition in response to the odor during conditioning for rat neonates receiving NTX or SAL immediately following paired (NTX n = 9; SAL n = 9) or unpaired (NTX n = 7; SAL n = 6) presentations of odor-stroke, or odor only presentations (NTX n = 8; SAL n = 9) on postnatal Day 7 or 8. Each point represents the mean of 2 summated trials; vertical lines indicate SEM. (B) Number of approaches toward the conditioned odor in the Y-maze test. Bars represent mean values; vertical lines indicate SEM. SAL, saline; NTX, naltrexone. *indicates p < .05.
DISCUSSION
The present study examined the role of opioids in odor-stroke associative learning and memory in rat neonates. Blockade of opioid receptors during acquisition prevented pups from learning, as indicated by their failure to display learning curves and an odor preference during the Y-maze test. Likewise, administration of an opioid receptor antagonist immediately following training prevented memory consolidation of the odor association. Overall, these results demonstrate that opioids facilitate the acquisition and memory consolidation of neonate learned odor-touch associations.
Early odor learning is important in securing mother-infant bonding in the postnatal environment (Hofer & Sullivan, 2001). Endogenous opioids have been shown to play a prominent role in the postnatal attachment process. Specifically in the rat neonate, opioids facilitate odor preference learning (Barr & Rossi, 1992; Kehoe & Blass, 1986a; Panksepp, Nelson, & Siviy, 1994; Randall, Kraemer, Dose, Carbary, & Bardo, 1992; Roth & Sullivan, 2001,2003; Shide & Blass, 1991), and nipple-milk conditioning (Petrov, Varlinskaya, Becker, & Smotherman, 1998, Petrov, Varlinskaya, & Smotherman, 2000; Robinson, Arnold, Spear, & Smotherman, 1993; Robinson & Smotherman, 1997). Suggestive of their rewarding value in neonates, opioids are sufficient to alleviate separation distress (Carden, Barr, & Hofer, 1991; Goodwin, Molina, & Spear, 1994; Kehoe & Blass, 1986b; Panksepp, Herman, Conner, Bishop, & Scott, 1978). Additionally, Moles, Kieffer, and D’Amota (2004) have recently demonstrated that mice neonates lacking μ-opioid receptors fail to show preferences toward maternal odor and do not show distress when separated from the mother, indicative that opioids play a prominent role in modulating the rewarding experience of mother-infant interactions.
We have previously demonstrated that opioids also play a critical role when neonates learn odor preferences despite pain. Specifically, in rat neonates up to PN9, presentations of odor and shock (0.5 mA) result in learned odor preferences (Camp & Rudy, 1988; Roth & Sullivan, 2001; Sullivan, Landers, Yeaman, & Wilson, 2000). Indicative of the aversive nature of this conditioning paradigm, a shock intensity of 0.5 mA is similar to that commonly used in adult fear conditioning experiments to effectively elicit avoidance (e.g., LaLumiere, Buen, & McGaugh, 2003; Paschall & Davis, 2002; Wilensky, Schafe, & LeDoux, 1999). Additionally, threshold to shock does not appear to change developmentally, and 0.5 mA shock elicits both pain vocalizations (White, Adox, Reddy, & Barfield, 1992) and escape responses (Emerich, Scalzo, Enters, Spear & Spear, 1985; Stehouwer & Campbell, 1978; Sullivan et al., 2000). Thus, neonates display unique learning characteristics about pain. Using this paradigm, we have shown that opioid receptor antagonism during neonatal odor-shock conditioning prevents acquisition of a conditioned odor preference (Roth & Sullivan, 2001). In sharp contrast, opioid receptor antagonism immediately following the odor-shock conditioning produces an odor aversion instead of the typical odor preference (Roth & Sullivan, 2001, 2003). Given these results from our previous studies, one might also expect opioid receptor antagonism post odor-stroke conditioning to elicit an odor aversion. However, results from the present study indicate this is not the case, and rather disruption of the endogenous opioid system following odor-stroke conditioning only blocks consolidation of an odor preference. We suggest that the discrepancy between these studies appears to reflect an important role of endogenous opioids in the neonate’s hedonic response to stimuli. Though our understanding of the neural circuitry mediating infant perception of rewarding and aversive stimuli remains premature, our present behavioral results and those from odor-shock conditioning suggest that neonates can perceive and assign hedonic value to the stimuli, as we are only able to induce a neonate odor aversion when using an aversive conditioning paradigm with opioid receptor manipulation.
Together, results from our previous behavioral studies and the present indicate that opioids play a pivotal role in ensuring learned odor preferences, especially in response to aversive stimuli. Indeed, neonates do not readily learn avoidances or aversions typically produced from passive avoidance, active avoidance, or inhibitory conditioning (reviewed in Roth, Wilson, & Sullivan, 2004). The limitations on aversive learning corresponds to a developmental period when the pups are confined to the nest (Bolles & Woods, 1965) and learned odor aversions would hinder proximity seeking of the mother, and thus the nutrition, warmth, and protection necessary for survival (Hofer & Sullivan, 2001). And the infant learning circuitry appears optimized to support readily learned odor preferences (Sullivan, 2003). Specifically, brain areas activated by odor-stroke, odor-milk, or odor-shock conditioning in rat neonate are the locus coeruleus, olfactory bulb, and piriform cortex (reviewed in Roth et al., 2004; Roth & Sullivan, 2005). Additionally, learning during odor-stroke or odor-shock conditioning in neonates does not appear to involve significant participation of the amygdala (Sullivan & Wilson, 1993; Sullivan et al., 2000; Roth & Sullivan, 2005). An understanding of the role of the endogenous opioid system in this neural circuitry and behavior should provide a better understanding of how the infant brain is optimized for attachment behavior and the mechanisms of mother-infant interactions on infant neural and emotional development.
Overall, our results indicate that infant learning of both rewarding and aversive stimuli engages the endogenous opioid system, and its participation ultimately secures the learning and memory necessary for attachment-learned odor preferences and not aversions. Furthermore, our results suggest that disturbances to the normal development of the endogenous opioid system, such as maternal drug use or postnatal stress and abuse, will impact neonatal attachment mechanisms.
Acknowledgments
NOTES
This work was funded by grants HHS-PHS NRSA F31 DA06082 to T.L.R. and NICHD-HD33402 and NSF-IBN0117234 to R.M.S. We thank Gordon A. Barr, Joseph A. Bastian, Douglas D. Gaffin, and Donald A. Wilson for comments on the manuscript.
References
- Anisfeld E, Casper V, Nozyce M, Cunningham N. Does infant carrying promote attachment? An experimental study of the effects of increased physical contact on the development of attachment. Child Development. 1990;61:1617–1627. doi: 10.1111/j.1467-8624.1990.tb02888.x. [DOI] [PubMed] [Google Scholar]
- Barr GA, Rossi G. Conditioned place preference from ventral tegmental injection of morphine in neonatal rats. Developmental Brain Research. 1992;66:133–136. doi: 10.1016/0165-3806(92)90149-q. [DOI] [PubMed] [Google Scholar]
- Blass EM, Fillion TJ, Weller A, Brunson L. Separation of opioid from nonopioid mediation of affect in neonatal rats: Nonopioid mechanisms mediate maternal contact influences. Behavioral Neuroscience. 1990;104:625–636. doi: 10.1037//0735-7044.104.4.625. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Animal Behavior. 1965;12:427–441. [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Bystrova K, Widstsrom AM, Matthiesen AS, Ransjo-Arvidson AB, Welle Nystrom B, Wassberg C, Vorontsov I, Uvnas-Moberg K. Skin-to-skin contact may reduce negative consequences of “the stress of being born”: A study on temperature in newborn infants, subjected to different ward routines in St. Petersburg. Acta Paediatrica. 2003;92:320–326. doi: 10.1080/08035250310009248. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Brain Research Development Brain Research. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Developmental Brain Research. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carden SE, Tempel A, Hernandez N, Hofer MA. Isolation alters striatal met-enkephalin immunoreactivity in rat pups. Physiology & Behavior. 1996;60:51–53. doi: 10.1016/0031-9384(95)02243-0. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Interactions between perinatal and neonatal associative learning defined by contiguous olfactory and tactile stimulation. Neurobiology of Learning & Memory. 1999;71:272–288. doi: 10.1006/nlme.1998.3882. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear N, Spear L. Effects of 6-hydroxydopamine-induced catecho-lamine depletion on shock-precipitated wall climbing of infant rat pups. Developmental Psychobiology. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Ferber SG, Makhoul IR. The effect of skin-to-skin contact (kangaroo care) shortly after birth on the neurobehavioral responses of the term newborn: A randomized, controlled trial. Pediatrics. 2004;113:858–865. doi: 10.1542/peds.113.4.858. [DOI] [PubMed] [Google Scholar]
- Goodwin GA, Molina VA, Spear LP. Repeated exposure of rat pups to isolation attenuates isolation-induced ultrasonic vocalization rates: Reversal with naltrexone. Developmental Psychobiology. 1994;27:53–64. doi: 10.1002/dev.420270106. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Annals of the New York Academy of Science. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105:e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- Hall WG. The ontogeny of feeding in rats: I. Ingestive and behavioral responses to oral infusions. Journal of Comparative Physiological Psychology. 1979;93:977–1000. doi: 10.1037/h0077771. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatrica Supplement. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hofer, M. A., & Sullivan, R. M. (2001). Toward a neurobiology of attachment. In C. A. Nelson, & M. Luciana (Eds.), Handbook of developmental cognitive neuroscience (pp. 599–616). Cambridge: MIT Press.
- Insel TR. A neurobiological basis of social attachment. American Journal of Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Jutapakdeegul N, Casalotti SO, Govitrapong P, Kotchabhakdi N. Postnatal touch stimulation acutely alters corticosterone levels and glucocorticoid receptor gene expression in the neonatal rat. Developmental Neuroscience. 2003;25:26–33. doi: 10.1159/000071465. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Behaviorally functional opioid system in infant rats I: Evidence for olfactory and gustatory classical conditioning. Behavioral Neuroscience. 1986a;100:359–367. doi: 10.1037//0735-7044.100.3.359. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: Reversal of stress with maternal stimuli. Developmental Psychobiology. 1986b;19:385–398. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. Journal of Neuroscience. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucion AB, Pereira FM, Winkelman EC, Sanvitto GL, Anselmo-Franci JA. Neonatal handling reduces the number of cells in the locus coeruleus of rats. Behavioral Neuroscience. 2003;117:894–903. doi: 10.1037/0735-7044.117.5.894. [DOI] [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Sullivan RM, King SR. Serotonergic influence on olfactory learning in the neonate rat. Behavioral and Neural Biology. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adreno-cortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amota FR. Deficit in attachment behavior in mice lacking the μ-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Myers MM, Ali N, Weller A, Brunelli SA, Tu AY, Hofer MA, Shair HN. Brief maternal interaction increases number, amplitude, and bout size of isolation-induced ultrasonic vocalizations in infant rats (Rattus norvegicus) Journal of Comparative Psychology. 2004;118:95–102. doi: 10.1037/0735-7036.118.1.95. [DOI] [PubMed] [Google Scholar]
- Nelson E, Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behavioral Neuroscience. 1996;110:583–592. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Herman R, Conner P, Bishop T, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biological Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neuroscience and Biobehavioral Reviews. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S. Brain opioids and mother-infant social motivation. Acta Pediatrica Supplement. 1994;397:40–46. doi: 10.1111/j.1651-2227.1994.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Paschall GY, Davis M. Second-order olfactory-mediated fear-potentiated startle. Learning & Memory. 2002;9:395–401. doi: 10.1101/lm.50602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez-Nogueras M, Field TM, Hossain Z, Pickens J. Depressed mothers’ touching increases infants’ positive affect and attention in still-face interactions. Child Development. 1996;67:1780–1792. [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Becker LA, Smotherman WP. Endogenous mu opioid systems and suckling in the neonatal rat. Physiology & Behavior. 1998;65:591–599. doi: 10.1016/s0031-9384(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. Classical conditioning of responses to an artificial nipple in the rat fetus: Mu and kappa opioid systems. Developmental Psychobiology. 2000;37:59–72. doi: 10.1002/1098-2302(200009)37:2<59::aid-dev1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Randall CK, Kraemer PJ, Dose JM, Carbary TJ, Bardo MT. The biphasic effect of morphine on odor conditioning in neonatal rats. Developmental Psychobiology. 1992;25:355–364. doi: 10.1002/dev.420250506. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Arnold HM, Spear NE, Smotherman WP. Experience with milk and an artificial nipple promote conditioned opioid activity in the rat fetus. Developmental Psychobiology. 1993;26:375–387. doi: 10.1002/dev.420260702. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Stimulus contingencies that permit classical conditioning of opioid activity in the rat fetus. Behavioral Neuroscience. 1997;111:1086–1097. doi: 10.1037//0735-7044.111.5.1086. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Developmental Psychobiology. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiology & Behavior. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth, T. L., Wilson, D. A., & Sullivan, R. M. (2004). Neurobehavioral development of infant learning and memory: Implications for infant attachment. In P. J. B. Slater, J. S. Rosenblatt, T. J. Roper, & C. T. Snowden, et al. (Eds.), Advances in the Study of Behavior (Vol. 34, pp 103–133). San Diego: Academic Press.
- Schanberg SM, Field TM. Sensory deprivation stress and supplemental stimulation in the rat up and preterm human neonate. Child Development. 1987;58:1431–1447. [PubMed] [Google Scholar]
- Schanberg SM, Ingledue VF, Lee JY, Hannun YA, Bartolome JV. PKCα mediates maternal touch regulation of growth-related gene expression in infant rats. Neuropsychopharmacology. 2003;28:1026–1030. doi: 10.1038/sj.npp.1300125. [DOI] [PubMed] [Google Scholar]
- Shide DJ, Blass EM. Opioid mediation of odor preferences induced by sugar and fat in 6-day-old rats. Physiology & Behavior. 1991;50:961–966. doi: 10.1016/0031-9384(91)90422-k. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in pre-weanling rats. Developmental Psychobiology. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Campbell BA. Habituation of the forelimb-withdrawal response in neonatal rats. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Developing a sense of safety: The neurobiology of neonatal attachment. Annals of the New York Academy of Science. 2003;1008:122–131. doi: 10.1196/annals.1301.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcers in infancy: Classical conditioning using stroking or intra-oral infusions of milk as UCS. Developmental Psychobiology. 1988;21:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Role of the amygdala complex in early olfactory associative learning. Behavioral Neuroscience. 1993;107:254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Research Bulletin. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Taborsky-Barba S, Mendoze R, Itano A, Leon M, Cotman CW, Payne TF, Lott I. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers HJJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. Journal of Neuroscience. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers, A., Ohlsson, A., Lacy, J. B., & Horsley, A. (2000). Massage for promoting growth and development of preterm and/or low birth-weight infants. Cochrane Database of Systematic Reviews, 2, CD000390. [DOI] [PubMed]
- Weldon DA, Travis ML, Kennedy DA. Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behavioral Neuroscience. 1991;105:450–458. doi: 10.1037//0735-7044.105.3.450. [DOI] [PubMed] [Google Scholar]
- Weller A, Feldman R. Emotion regulation and touch in infants: The role of cholecystokinin and opioids. Peptides. 2003;24:779–788. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- White NR, Adox R, Reddy A, Barfield RJ. Regulation of rat maternal behavior by broadband pup vocalizations. Behavioral and Neural Biology. 1992;58:131–137. doi: 10.1016/0163-1047(92)90363-9. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. Journal of Neuroscience. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Kim JH, Davidson AN, Rosenthal AJ. Early deprivation alters the vocalization behavior of neonates directing maternal attention in a rat model of child neglect. Annals of the New York Academy of Science. 2003;1008:308–313. doi: 10.1196/annals.1301.039. [DOI] [PubMed] [Google Scholar]


