Abstract
Human artificial chromosome (HAC) vectors are an important gene transfer system for expression and complementation studies. We describe a significant advance in HAC technology using infectious herpes simplex virus type 1 (HSV-1) amplicon vectors for delivery. This highly efficient method has allowed gene-expressing HACs to be established in glioma-, kidney- and lung-derived cells. We also developed an HSV-1 hypoxanthine phosphoribosyltransferase (HPRT) HAC vector, which generated functional HPRT-expressing HACs that complemented the genetic deficiency in human cells. The transduction efficiency of the HSV-1 HAC amplicons is several orders of magnitude higher than lipofection-mediated delivery. Studies on HAC stability between cell types showed important differences that have implications for HAC development and gene expression in human cells. This is the first report of establishing gene-expressing HACs in human cells by using an efficient, high-capacity viral vector and by identifying factors that are involved in cell-type-specific HAC instability. The work is a significant advance for HAC technology and the development of HAC gene expression systems in human cells.
Keywords: delivery, human artificial chromosome, HAC stability, herpes simplex virus type 1, HSV-1, human cells
Introduction
Human artificial chromosomes (HACs) are autonomous molecules that behave as normal chromosomes in human cells. Together with the endogenous chromosomes, HACs segregate during cell division and are maintained in the host cell (Larin & Mejía, 2002). De novo HACs are generated by introducing defined sequences such as human telomeres, α-satellite (alphoid) DNA and specific genomic fragments into human cells (Larin & Mejía, 2002; Basu & Willard, 2005; Grimes & Larin Monaco, 2005). Alphoid DNA, containing higher-order repeat sequences (Rudd & Willard, 2004), and a centromere protein B binding sequence (CENP-B box) are requirements for a functional centromere in a HAC (Ohzeki et al, 2002). Chromosome Y alphoid DNA lacks CENP-B boxes and is inefficient at HAC formation (Grimes et al, 2002; Mejía et al, 2002).
Previously, we generated a HAC gene expression vector in Escherichia coli (404 kb) containing 17 alphoid DNA and the entire hypoxanthine phosphoribosyltransferase (HPRT) gene locus for functional complementation studies (Mejía & Larin, 2000). The HAC vector was introduced by lipofection into human HPRT-deficient fibrosarcoma (HT1080) cells, and the HPRT HACs generated complemented the deficiency (Mejía et al, 2001). DNA analysis showed that the HACs were larger than the input DNA and contained alternating alphoid and HPRT DNA fragments, indicating that some amplification and recombination had occurred (Mejía et al, 2001). Similar HAC studies using the HPRT (Grimes et al, 2001) and guanosine triphosphate cyclohydrolase I genes (Ikeno et al, 2002) confirmed that HACs are useful gene transfer vectors that can accommodate large genomic loci including the regulatory elements for appropriate expression.
Methods for delivery of large DNA as HACs are inefficient, often resulting in DNA shearing and degradation (Marschall et al, 1999), which is a major obstacle in developing a HAC expression system in different cell types. Towards this aim, we developed a novel HAC delivery approach on the basis of the herpes simplex virus type 1 (HSV-1) amplicon vector system (Saeki et al, 2001). This high-capacity (∼150 kb) viral vector successfully delivers large genomic DNA (bacterial artificial chromosomes (BACs) and P1 artificial chromosomes (PACs)) intact as infectious amplicons into different cell types in the absence of contaminating viral genes (Wade-Martins et al, 2001, 2003). In this study, a novel and efficient HSV-1 HAC amplicon vector was developed for generating gene-expressing HACs in a range of human cells, including HT1080 (fibrosarcoma), G16-9 (glioma), MRC-5V2 (lung fibroblast), 293 (kidney), BeFA (primary keratinocyte) and MRC5 (primary cells, derived from fetal lung). The transduction efficiency in HT1080 cells monitored by green fluorescent protein (GFP) expression was several orders of magnitude higher than lipofection. Gene-expressing HACs were established for the first time in G16-9, MRC-5V2 and 293 cells, and the HSV-1 HPRT HACs successfully complemented the HPRT deficiency in HT1080 cells. The HACs generated in HT1080 and G16-9 cells bound centromere protein A (CENP-A) and were stable in the absence of selection for more than 3 months, indicating the maintenance of a functional centromere. In MRC-5V2 and 293 cells, HACs were rapidly lost in the presence or absence of selection. Further analysis of protein levels in these cells showed that overexpression of aurora B kinase (AIM-1) and topoisomerase II (Topo II) might have contributed to the instability. This is the first report of the generation of HACs in human cells using a highly efficient HSV-1 amplicon vector and identification of the possible factors involved in cell type-specific HAC instability. This work is an important advance for HAC technology and the development of HAC gene expression systems in human cells.
Results
HSV-1 human artificial chromosome vectors
Four HSV-1 HAC vectors were constructed—pHSV17α-1Neo (17α, 146 kb), pHSV17α-2Neo (17α, 65 kb), pHSV21αNeo (21α, 73 kb), pHSV21α HPRTNeo (21α, 75.7 kb)—and amplicons were prepared from each vector, as described (Fig 1A; supplementary information online).
Figure 1.
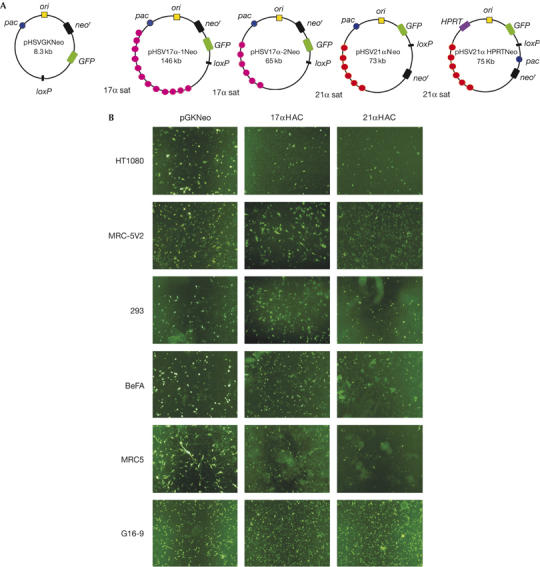
Green fluorescent protein expression in human cells after transduction with herpes simplex virus type 1 human artificial chromosome amplicons. (A) HSV-1 HAC vectors, including pHSVGKNeo, pHSV17α-1Neo, pHSV17α-2Neo, pHSV21αNeo and pHSV21α HPRTNeo. These vectors contain pac (for packaging and cleavage), oris (the origin of replication), the gene for GFP and the G418 resistance (neor) gene. pHSV21α HPRTNeo also contains an HPRT minigene. (B) GFP expression in different human cell lines 24 h after infection with amplicons at a multiplicity of infection of 10. G16-9 images are derived from titration experiments. GFP, green fluorescent protein; HAC, human artificial chromosome; HPRT, hypoxanthine phosphoribosyltransferase; HSV-1, herpes simplex virus type 1.
Infection of human cells
We infected 1 × 104 HT1080, MRC-5V2, G16-9, BeFA, 293 and MRC5 cells with the HSV-1 HAC amplicons. The transduction efficiency was estimated by GFP expression after 24 h (Table 1; Fig 1B). All the cells used were susceptible to infection with each amplicon, and efficiencies ranged from 2% to 100% depending on the amplicon used and the cell type, and was not always dose dependent. The number of GFP-positive cells at a multiplicity of infection (MOI) of 5 or 10 was usually higher than at MOI 1, although in some experiments it was similar at all MOIs (for example, pHSV21αNeo amplicons in HT1080 and MRC-5V2 cells). In general, the efficiency of transduction with HSV-1 HAC amplicons was at least 10,000-fold greater than introducing BACs by lipofection (Mejía et al, 2002).
Table 1.
Transduction efficiency (after 24 h) of herpes simplex virus type 1 human artificial chromosome amplicons monitored by green fluorescent protein expression
|
HT1080 |
HT1080 HPRT− |
MRC-5V2 |
293 |
BeFA |
MRC5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amplicon | MOI 1 (%) | MOI 10 (%) | MOI 1 (%) | MOI 5 (%) | MOI 1 (%) | MOI 10 (%) | MOI 1 (%) | MOI 10 (%) | MOI 1 (%) | MOI 10 (%) | MOI 1 (%) | MOI 10 (%) |
| pHSVGKNeo |
7 |
64 |
ND |
— |
20 |
40 |
3 |
40 |
2 |
30 |
5 |
40 |
| pHSV17α-1Neo |
3 |
60 |
ND |
— |
21 |
70 |
10 |
80 |
8 |
70 |
10 |
50 |
| pHSV17α-2Neo |
17 |
47 |
ND |
— |
55 |
100 |
8 |
41 |
ND |
— |
ND |
— |
| pHSV21αNeo |
16 |
20 |
ND |
— |
70 |
70 |
3 |
30 |
3 |
20 |
5 |
10 |
| pHSV21α HPRTNeo | 4.22 | 4.1 | 4.12 | 11 | ND | — | ND | — | ND | — | ND | — |
HPRT, hypoxanthine phosphoribosyltransferase; MOI, multiplicity of infection; ND, not determined.
Stable clone formation and FISH analysis
G418 selection was applied to the cell media, and colonies appeared in 14–21 days. The average efficiency of stable clones was estimated to be 10−2–10−4. By lipofection, stable clones were obtained (with all HSV-1 HACs) at a frequency of 10−5–10−6. Clones were screened by fluorescence in situ hybridization (FISH) with the 17α, 21α or pHSVGKNeo probes. HACs were detected in HT1080, HT1080 HPRT−, MRC-5V2, G16-9 and 293 cells (Fig 2). The percentage of HAC clones for each vector and the frequency of HACs in metaphase spreads are shown in Table 2. In HT1080, HACs were detected in 22% and 14% of clones for pHSV17α-1Neo and pHSV21αNeo, respectively, in 6–26% of metaphase spreads. For pHSV21α HPRTNeo, 94% of clones contained HACs in HT1080 in 15–80% of metaphase spreads, whereas in HT1080 HPRT− cells, 73% clones had HACs in 5–50% of metaphase spreads. In MRC-5V2, 90% of HAC clones were generated with pHSV17α-1Neo and 20% with pHSV21αNeo, in 5–40% spreads. In 293 cells, 67% of HAC clones were generated with pHSV17α-1Neo, with HACs in 10–13% spreads. In G16-9 cells, the frequency of HAC clones was 16% for pHSV17α-1Neo, 11% for pHSV21αNeo and 64% for pHSV17α-2Neo, with HACs in 5–65% of metaphase spreads. In some cell lines, DNA also integrated into the host chromosomes at a frequency similar to that observed in earlier work (Mejía et al, 2001, 2002). One HAC clone, 17α III.10 (derived from pHSV17α-1Neo in G16-9 cells), containing integrated DNA was successfully subcloned, generating 17α III.10.1 with only HACs in 85% of metaphases analysed. Another HAC clone, HF15 (HT1080 HPRT−), was subcloned generating HF15.1 with only HPRT-expressing HACs in 70% of metaphases analysed. The presence of the HPRT gene was confirmed by using FISH with the PAC 71G4 (Mejía et al, 2001), containing the entire HPRT genomic locus (supplementary Fig 4 online). In experiments using lipofection, HAC clones generated with pHSV17α-1Neo and pHSV21αNeo in HT1080 cells showed no significant difference in HAC frequency compared with HACs derived through HSV-1 delivery (data not shown).
Figure 2.
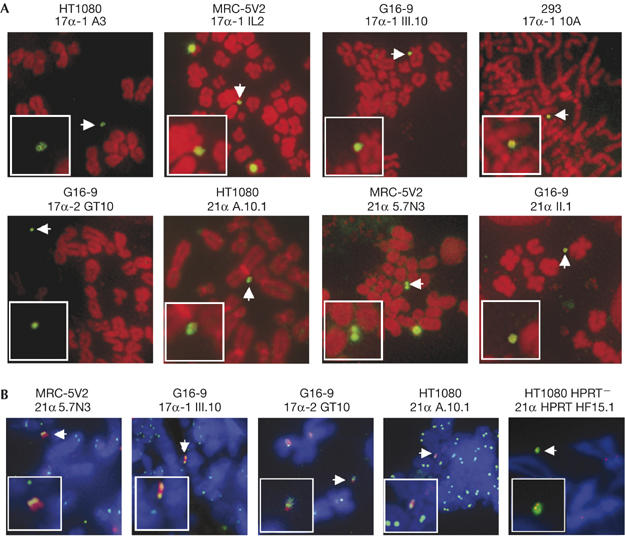
Analysis of human artificial chromosome clones by fluorescence in situ hybridization and immunofluorescence. (A) HAC clones generated in HT1080, MRC-5V2, G16-9 and 293 cells. Host chromosomes are shown in red, and the HACs were identified by using FISH (green, white arrow; see inset) using the 17α, 21α or pHSVGKNeo probe. (B) ACA and CENP-A staining of the HAC clones in HT1080, MRC-5V2 and G16-9. The HAC in 21α 5.7N3 (MRC-5V2) was identified by FISH with the 21α probe (red) and the signal colocalized with the ACA signal (green, white arrow; see inset). Chromosomes were counterstained with DAPI (blue). The HACs in clones 17α-1 III.10 (G16-9), 17α-2 GT10 (G16-9) and 21α A.10.1 (HT1080) were identified with the 17α or 21α probe (red, white arrow) and the signal colocalized with CENP-A signal (green, white arrow; see inset). Chromosomes were counterstained with DAPI (blue). The HAC in clone 21α HF15.1 (HT1080) was labelled by using FISH with the 21α probe (green, white arrow), and the signal was colocalized with CENP-A (red). ACA, anti-centromere autoimmune serum; CENP-A, centromere protein A; DAPI, 4,6-diamidino-2-phenylindole; FISH, fluorescence in situ hybridization; HAC, human artificial chromosome.
Table 2.
Fluorescence in situ hybridization analysis of human artificial chromosome clones
| Cell line | Amplicon | Clones analysed | HAC (percentage of spreads) | Other events |
|---|---|---|---|---|
| HT1080 |
pHSV17α-1Neo |
18 |
4 (6–26) |
14 |
| |
pHSV21αNeo |
21 |
3 (7–15)* |
18 |
| |
pHSV21αHPRTNeo |
17 |
16 (15–80)* |
1 |
| HT1080 HPRT− |
pHSV21αHPRTNeo |
26 |
19 (5–50)* |
7 |
| MRC-5V2 |
pHSV17α-1Neo |
10 |
9 (5–35%) |
1 |
| |
pHSV21αNeo |
20 |
4 (5–40%)* |
16 |
| G16-9 |
pHSV17α-1Neo |
30 |
5 (8–65%)* |
25 |
| |
pHSV17α-2Neo |
11 |
7 (5–40%)* |
4 |
| |
pHSV21αNeo |
9 |
1 (16%)* |
8 |
| 293 | pHSV17α-1Neo | 3 | 2 (10–13%) | 1 |
*These clones also contained integrations.
FISH, fluorescence in situ hybridization; HAC, human artificial chromosome; HPRT, hypoxanthine phosphoribosyltransferase.
Anti-centromere autoimmune serum and CENP-A detection
HAC clones were analysed by using immunoFISH on metaphase spreads with the 17α and 21α probes, CENP-A antibody or anti-centromere autoimmune serum (ACA; Fig 2). The 21α probe and ACA were colocalized on the HAC in 21α 5.7N3 (MRC-5V2; Fig 2B), and the ACA signal seemed to be localized to a discrete area, corresponding presumably to the centromere. CENP-A was bound to the HACs in clones 17α-1 III.10 (G16-9), 17α-2 GT10 (G16-9), 21α A.10.1 (HT1080) and 21α HPRT HF15.1 (HT1080 HPRT−; Fig 2B). The signal intensity of CENP-A on the HACs in both HT1080 and G16-9 cells was at a similar level to the signal at endogenous centromeres, suggesting that the HACs formed a functional centromere.
Mitotic stability
The mitotic stability of HACs derived from all amplicons in HT1080, G16-9, MRC-5V2 and 293 cells was investigated further. Cells were cultured in the absence of G418, and HAC frequencies were determined by using FISH at 0, 30, 60 and 90 days of culture. The HACs in 21α A10.1, 17α GT1 and HF15.1 were stable without G418, as the percentage of HACs present in metaphase spreads did not change from day 0 to day 60, only decreasing slightly in 21α A10.1 and HF15.1 at day 90 (Table 3). This indicates that the HACs segregated efficiently in >99% of mitoses. In comparison, the HACs derived from 17α or 21α in MRC-5V2 cells (for example, 21α 5.7N3) and those derived from 17α in 293 cells were lost in 30 days with or without G418. The data suggest that the HACs maintained a functional centromere in HT1080 and G16-9 cells but not in MRC-5V2 or 293 cells, and that other factors might be required for full function in these cells.
Table 3.
Stability of human artificial chromosomes in the absence of selection
| Clone |
HAC (%) after x days without selection |
Percentage of loss rate* | |||
|---|---|---|---|---|---|
| 0 days | 30 days | 60 days | 90 days | ||
| 21α A.10.1 (HT1080) |
40 |
40 |
40 |
37.5 |
0.07 |
| HF15.1 (HT1080 HPRT−) |
70 |
70 |
70 |
65 |
0.08 |
| 21α 57N3 (MRC-5V2) |
35 |
0 |
— |
— |
ND |
| 17α GT1 (G16-9) | 40 | 40 | 40 | 40 | 0 |
*Calculated by the formula Nn=N0x(1−R)n, where N0 is the number of metaphase chromosome spreads showing HACs in the cells cultured under selection, Nn is the number of HAC-containing metaphase chromosome spreads after n days of culture in the absence of selection and R is the daily rate of loss.
FISH, fluorescence in situ hybridization; HAC, human artificial chromosome; HPRT, hypoxanthine phosphoribosyltransferase; ND, not determined.
Gene expression studies
The GFP expression from the HACs in both 21α A.10.1 (HT1080) and 17α III.10.1 (G16-9) was monitored in cells with and without G418. The number of GFP-expressing cells remained unchanged from day 0 to day 90 in both HAC cell lines (supplementary Fig 5 online). The level of HPRT protein in the HAC clone HF15.1 (HT1080 HPRT−) was quantified by a western blot on cytoplasmic cell extracts with an HPRT antibody (Fig 3A). HT1080 HPRT− cells do not contain HPRT protein, whereas HT1080 cells have about ten times more HPRT protein than the MRC5 primary cells. The HF15.1 (HSV-1 HPRT HAC) cells have about 34 times the level of protein observed in HT1080 cells. After 40 days without selection, the amount of HPRT protein in HF15.1 remained the same (Fig 3A). After 90 days, there was only a 3% decrease in the level of HPRT protein (data not shown). The function of the HPRT gene was determined by measuring the plating efficiency of HF15.1 under HAT (hypoxanthine, aminopterin and thymidine) selection; 98% of the plated cells survived, whereas the parental HT1080 HPRT− cells died. Conversely, all the plated HF15.1 cells died under 6-thioguanine, which confirms that HAT resistance was conferred by a functional HPRT gene. Three months after the HF15.1 clone was initially isolated, it showed the same levels of 6-thioguanine sensitivity.
Figure 3.
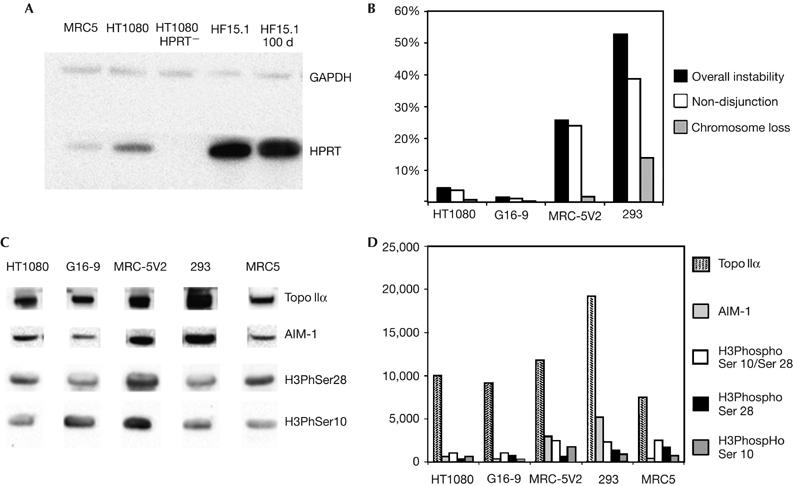
Gene expression and chromosomal instability analysis. (A) Western blot of cytoplasmic protein fractions from MRC5, HT1080, HT1080 HPRT− (10 μg per lane), HF15.1 and HF15.1 after 40 days without selection (1 μg per lane), reacted with an HPRT antibody. A GAPDH antibody was used as loading control. Clone HF15.1 contains about 34 times greater HPRT protein than HT1080 cells (wild type) from day 0 to day 100 (40 days without selection). (B) Cytokinesis block micronucleus assay and percentage of error-displaying bi-nucleated cells for each cell line. MRC-5V2 and 293 show a higher level of chromosomal instability (black column), mostly owing to non-disjunction events (white column). (C) Western blot of nuclear protein fractions from each cell type reacted with Topo IIα, AIM-1, H3PhosphoSer10 (H3PhSer10) and H3PhosphoSer28 (H3PhSer28) antibodies. (D) Quantification of western blot shown in (C). As H3PhosphoSer10 and H3PhosphoSer28 (mid-grey and black columns) are present only at mitosis, the quantification values were adjusted by taking into account the mitotic index of the different cell lines. The white column shows the total amount of H3PhosphoSer10 and H3PhosphoSer28. AIM-1, aurora B kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPRT, hypoxanthine phosphoribosyltransferase; Topo IIα, topoisomerase IIα.
Chromosomal instability analysis
Further work was undertaken to determine the HAC instability in MRC-5V2 and 293 cells. One factor affecting stability might be the presence of HSV elements incorporated into the HSV-1 HAC vectors influencing HAC behaviour through a transactivation mechanism in the specific cell type. An HPRT HAC lacking HSV elements (AG6-1; Mejía et al, 2001) was transferred from HT1080 into MRC-5V2 cells by microcell-mediated chromosome transfer (MMCT). Ten clones were isolated and analysed further. Two clones contained HACs similar to the parental AG6-1, but these HACs were lost in 30 days without G418. The remaining eight clones contained large HACs, which were grossly amplified and highly unstable because they underwent a breakage–fusion–bridge cycle (Shimizu et al, 2005). The results therefore show that the AG6-1 HAC was also unstable in MRC-5V2.
The overall chromosomal stability of HT1080, G16-9, MRC-5V2 and 293 cells was then investigated by the cytokinesis block micronucleus assay (supplementary information online). Actively growing cells treated with cytochalasin B (to stop cell division) were reacted with CENP-A antibody or hybridized by using FISH with 17α and 21α probes. Binucleated cells were scored for the presence of bridges, micronuclei and non-disjunction events (supplementary information online). The overall level of instability was low in HT1080 (5%) and G16-9 (1.5%), but high in MRC-5V2 (26%) and 293 (53%) cells, with non-disjunction events being the most frequent kind of aberration (Fig 3B).
We investigated whether the high chromosomal instability in MRC-5V2 and 293 cells was associated with specific protein levels. Possible candidates are the principal proteins involved in kinetochore/centromere function (CENP-A), chromatid cohesion maintenance and resolution (SMC3, Topo I and Topo II), recombination (Kin17), pericentromere-specific chromatin modifications (HP1, histone H3 trimethylated in lysine 9 (H3trimetK9)) and cell-cycle checkpoints (AIM-1; Amor et al, 2004). Western blots of nuclear protein fractions from HT1080, G16-9, MRC-5V2, 293 and MRC5 primary cells were reacted with antibodies for these proteins. No significant difference in protein levels was found between cell types for CENP-A, SMC3, Kin17, Topo I, HP1 and H3trimetK9. However, AIM-1 and Topo II were present in significantly higher amounts in MRC-5V2 and 293 cells (Fig 3C,D). Compared with HT1080 cells, the MRC-5V2 cells have 4.5 times more AIM-1 and 1.2 times more Topo II, and 293 cells have 7.75 times more AIM-1 and 2 times more Topo II. As AIM-1 is required for the phosphorylation of histone H3 in serine 10 and serine 28 before mitosis (Goto et al, 2002), the levels of these modifications were investigated on protein fractions prepared from colcemid-treated cells. The values obtained were adjusted to take into account the differences in mitotic index in each cell type. We observed, however, that overexpression of AIM-1 corresponded to an increase in phosphorylation of histone H3 in serine 10/28 only in MRC-5V2 and not in 293 cells (Fig 3C,D). As AIM-1 is also involved in the control of chromosome congression at metaphase (Hauf et al, 2003), we stained cells with anti-β-tubulin antibodies to detect the spindle, and reacted them with ACA serum to highlight the centromere for monitoring chromosome errors or delays at congression. Delays in chromosome congression were as follows: HT1080, 9%; G16-9, 6%; MRC-5V2, 30%; and 293 cells, 32%. Overall, the results indicated that AIM-1 overexpression in MRC-5V2 and 293 cells probably interferes with chromosome alignment, congression and segregation, and together with overexpression of Topo II probably has an important role in HAC instability.
Discussion
We successfully generated HSV-1 amplicons containing HAC vector DNA and infected several human cell types including human HT1080 cells at high efficiency, up to 104 times greater than lipofection (Mejía et al, 2001, 2002). The high transduction efficiencies were generally obtained at MOI 1 and 10, although an increase in MOI did not always correspond to an increase in efficiency. This might be important for gene therapy, as a smaller number of transducing particles can be as efficient as a large number.
HAC cell lines were established in HT1080, MRC-5V2, 293 and G16-9 cells after infection with amplicons. The GFP and HPRT genes were shown to be appropriately expressed from the HSV-1 HACs, and their expression levels did not change significantly during a period of 3 months. Also, the HPRT HACs complemented the genetic deficiency in HT1080 HPRT-deficient cells. The HACs in HT1080 and G16-9 were functional and stable for several months in the absence of selection. However, HACs were unstable in MRC-5V2 and 293 cells, both in the presence and in the absence of selection.
The possible causes for the instability were investigated further. We determined whether the HSV elements in the HACs were affected by a transactivation mechanism in the specific cell type, as MRC-5V2 and 293 cells have been transformed with viral sequences (simian virus 40 (SV40) large T antigen and adenovirus 5, respectively) unlike HT1080 and G16-9 cells (tumour derived). However, transfer of a stable HAC lacking HSV elements from HT1080 to MRC-5V2 cells by MMCT showed that the HAC was also unstable in the MRC-5V2 cells.
As HSV-1 amplicon DNA is packaged in a linear form and circularizes after infection, another possibility is that, in some cell types, the DNA remains linear and becomes unstable if there is a lack of telomerase activity. However, there is telomerase activity in 293 cells (Bryan et al, 1995) and SV40-transformed cells such as MRC-5V2 (Burger et al, 1998), and the presence of uncapped ends usually results in integrations, rather than total chromosome loss. Therefore, it is unlikely that this would have an important role.
The levels of several proteins involved in the cell cycle and centromere function were determined, and AIM-1 and Topo II were found to be overexpressed in MRC-5V2 and 293 cells compared with HT1080 and G16-9 cells. AIM-1 is involved in the checkpoint controlling the correct alignment of chromosomes on the metaphase spindle and in the congression of chromosomes at metaphase (Hauf et al, 2003). Topo II is associated with high levels of genome instability, mainly owing to centrosome amplification (Kronenwett et al, 2003). The results correlate with the high level of chromosomal instability, the large number of non-disjunction events and errors of congression observed in MRC-5V2 and 293 cells. The increased levels of AIM-1 in MRC-5V2 and 293 cells possibly interfere with the spindle checkpoint mechanism, and the cells proceed to anaphase when the chromosomes are not all correctly bi-orientated on the spindle. Overexpression of AIM-1 has been found in numerous types of cancer, in which it is usually associated with chromosomal instability (Ota et al, 2002). Overexpression of Topo II is often found in human cancer cells (Jiao et al, 2005), in which it might confer a selective advantage when cells become resistant to apoptosis. Hence, excess Topo II possibly adds to the overall chromosomal instability levels in MRC-5V2 and 293 cells.
In summary, the results indicate that the requirements for centromere function and HAC stability might be different in each cell type and depend on many factors including the genetic background, the proteins involved in centromere function and epigenetic factors, which influence chromosome segregation (Rudd et al, 2003). This will be important when designing new HAC vectors for gene expression studies in specific cell types. We have shown that HSV-1 is a key method for efficient HAC vector delivery and an invaluable tool for the generation of gene-expressing HACs in human cells. Gene delivery by HSV-1 HAC amplicons is a safe, alternative method to other viral vector systems (Check, 2003; Olschowka et al, 2003) and a promising application for gene therapy. Using the HSV-1 methodology, we have been able to identify possible factors that are important in HAC stability in human cultured cells, and this will be crucial for the development of expression studies in the relevant cell type. This work is a major step forward in the generation of gene-expressing HACs and a significant advance in the field of HAC technology.
Methods
HSV-1 amplicon preparation. Briefly, 1 × 106 Vero 2-2 cells were seeded in 6 cm dishes 24 h before transfection using LipofectAMINE Plus (Invitrogen, Paisley, UK). To each dish, the following DNA was added: 2 μg of fHSVΔpacΔ270 DNA, 1.8 μg of BAC/plasmid DNA and 200 ng of plasmid pEBHICP27. The cells were incubated at 37°C for 4 h, and then the medium was removed and replaced with DMEM containing 6% FCS. After 60 h, the cells were collected in DMEM, frozen on solid CO2 and sonicated. Amplicon preparations from nine dishes were pooled in a centrifuge tube onto a 25% sucrose cushion, and the tubes were spun at 20,000 r.p.m. (4°C) in a Beckman SW28 rotor for 3 h to concentrate the amplicons. The pellet was resuspended in 250 μl of DMEM containing 10% FCS and the titre of each amplicon preparation was estimated (supplementary information online).
Cell infection. For each human cell type, about 1–5 × 104 cells were plated in a 24-well dish. After 24 h, the confluent cells in each well were infected with HSV-1 amplicons, at an MOI ranging from 1 to 10, in 250 μl of DMEM and 10% FCS medium. After 24 h, the efficiency of transduction was determined and selection was applied (supplementary information online).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Supplementary Figure 4
Supplementary Figure 5
Acknowledgments
We thank H. Masumoto for the kind gift of BAC pWTR11.32 and helpful discussions, D. Melton for the kind gift of plasmid pGK-HPRT(RI), W. Earnshaw, F. Iborra and V. Buckle for the kind gift of antibodies, A. Velayos for advice and help with techniques and J. Mejía, M. Assenberg, A. Bristow and T. Anderson for discussions and technical contributions in the early stages of this work. R.W-.M. is a Wellcome Trust Research Career Development Fellow. This work was funded in part by the Dystrophic Epidermolysis Bullosa Research Association UK.
References
- Amor DJ, Kalitsis P, Sumer H, Choo KH (2004) Building the centromere: from foundation proteins to 3D organization. Trends Cell Biol 7: 359–368 [DOI] [PubMed] [Google Scholar]
- Basu J, Willard HF (2005) Artificial and engineered chromosomes: non-integrating vectors for gene therapy. Trends Mol Med 11: 251–258 [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR (1995) Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J 17: 4240–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger AM, Fiebig HH, Kuettel MR, Lautenberger JA, Kung HF, Rhim JS (1998) Effect of oncogene expression on telomerase activation and telomere length in human endothelial, fibroblast and prostate epithelial cells. Int J Oncol 5: 1043–1048 [DOI] [PubMed] [Google Scholar]
- Check E (2003) Cancer risk prompts US to curb gene therapy. Nature 422: 7. [DOI] [PubMed] [Google Scholar]
- Goto H, Yasui Y, Nigg EA, Inagaki M (2002) Aurora-B phosphorylates histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes Cells 7: 11–17 [DOI] [PubMed] [Google Scholar]
- Grimes BR, Larin Monaco Z (2005) Artificial and engineered chromosomes: developments and prospects for gene therapy. Chromosoma 114: 230–241 [DOI] [PubMed] [Google Scholar]
- Grimes BR, Schindelhauer D, McGill NI, Ross A, Ebersole TA, Cooke HJ (2001) Stable gene expression from a mammalian artificial chromosome. EMBO Rep 2: 910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes BR, Rhoades AA, Willard HF (2002) α-Satellite DNA and vector composition influence rates of human artificial chromosome formation. Mol Ther 5: 798–805 [DOI] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder LC, Peters JM (2003) The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161: 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno M, Inagaki H, Nagata K, Morita M, Ichinose H, Okazaki T (2002) Generation of human artificial chromosomes expressing naturally controlled guanosine triphosphate cyclohydrolase I gene. Genes Cells 7: 1021–1032 [DOI] [PubMed] [Google Scholar]
- Jiao W, Lin HM, Timmons J, Nagaich AK, Ng SW, Misteli T, Rane SG (2005) E2F-dependent repression of topoisomerase II regulates heterochromatin formation and apoptosis in cells with melanoma-prone mutation. Cancer Res 65: 4067–4077 [DOI] [PubMed] [Google Scholar]
- Kronenwett U, Castro J, Roblick UJ, Fujioka K, Ostring C, Faridmoghaddam F, Laytragoon-Lewin N, Tribukait B, Auer G (2003) Expression of cyclins A, E and topoisomerase II α correlates with centrosome amplification and genomic instability and influences the reliability of cytometric S-phase determination. BMC Cell Biol 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larin Z, Mejía JE (2002) Advances in human artificial chromosome technology. Trends Genet 18: 313–319 [DOI] [PubMed] [Google Scholar]
- Marschall P, Malik N, Larin Z (1999) Transfer of YACs up to 2.3 Mb intact into human cells with polyethylenimine. Gene Ther 6: 1634–1637 [DOI] [PubMed] [Google Scholar]
- Mejía JE, Larin Z (2000) The assembly of BACs by in vivo recombination. Genomics 70: 165–170 [DOI] [PubMed] [Google Scholar]
- Mejía JE, Willmott A, Levy E, Earnshaw WC, Larin Z (2001) Functional complementation of a genetic deficiency with human artificial chromosomes. Am J Hum Genet 69: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía JE, Alazami A, Willmott A, Marschall P, Levy E, Earnshaw WC, Larin Z (2002) Efficiency of de novo centromere formation in human artificial chromosomes. Genomics 79: 297–304 [DOI] [PubMed] [Google Scholar]
- Ohzeki J, Nakano M, Okada T, Masumoto H (2002) CENP-B box is required for de novo centromere chromatin assembly on human alphoid DNA. J Cell Biol 159: 765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, Bowers WJ, Hurley SD, Mastrangelo MA, Federoff HJ (2003) Helper-free HSV-1 amplicons elicit a markedly less robust innate immune response in the CNS. Mol Ther 7: 218–227 [DOI] [PubMed] [Google Scholar]
- Ota T, Suto S, Katayama H, Han ZB, Suzuki F, Maeda M, Tanino M, Terada Y, Tatsuka M (2002) Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora-B overexpression contributes to chromosome number instability. Cancer Res 62: 5168–5177 [PubMed] [Google Scholar]
- Rudd MK, Willard HF (2004) Analysis of the centromeric regions of the human genome assembly. Trends Genet 20: 529–533 [DOI] [PubMed] [Google Scholar]
- Rudd MK, Mays RW, Schwartz S, Willard HF (2003) Human artificial chromosomes with α satellite based de novo centromere show increased frequency of nondisjunction and anaphase lag. Mol Cell Biol 23: 7689–7697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y, Fraefel C, Ichikawa T, Breakefield XO, Chiocca EA (2001) Improved helper virus-free packaging system for HSV-1 amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol Ther 3: 591–601 [DOI] [PubMed] [Google Scholar]
- Shimizu N, Shingaki K, Kaneko-Sasaguri Y, Hashizume T, Kanda T (2005) When, where and how the bridge breaks: anaphase bridge breakage plays a crucial role in gene amplification and HSR generation. Exp Cell Res 302: 233–243 [DOI] [PubMed] [Google Scholar]
- Wade-Martins R, Smith ER, Tyminski E, Chiocca EA, Saeki Y (2001) An infectious transfer and expression system for genomic DNA loci in human and mouse cells. Nat Biotechnol 19: 1067–1070 [DOI] [PubMed] [Google Scholar]
- Wade-Martins R, Saeki Y, Chiocca EA (2003) Infectious delivery of a 135 kb LDLR genomic locus leads to regulated complementation of low density lipoprotein receptor deficiency in human cells. Mol Ther 7: 604–612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figure 4
Supplementary Figure 5


