Abstract
In order to identify good animal models for investigating therapeutic and preventive strategies for pancreatic cancer, we analyzed pancreatic lesions from several transgenic models and made a series of novel findings. Female MT-tgfα mice of the MT100 line developed pancreatic proliferation, acinar-ductal metaplasia, multilocular cystic neoplasms, ductal adenocarcinomas and prominent fibrosis, while the lesions in males were less severe. MT-tgfα-ES transgenic lines of both sexes developed slowly progressing lesions that were similar to what was seen in MT100 males. In both MT100 and MT-tgfα-ES lines, TGFα transgene was expressed mainly in proliferating ductal cells. Ela-myc transgenic mice with a mixed C57BL/6, SJL and FVB genetic background developed pancreatic tumors at 2–7 months of age, and half of the tumors were ductal adenocarcinomas, similar to what was reported originally by Sandgren et al [1]. However, in 20% of the mice, the tumors metastasized to the liver. MT100/Ela-myc and MT-tgfα-ES/Ela-myc double transgenic mice developed not only acinar carcinomas and mixed carcinomas as previously reported but also various ductal-originated lesions, including multilocular cystic neoplasms and ductal adenocarcinomas. The double transgenic tumors were more malignant and metastasized to the liver at a higher frequency (33%) compared with the Ela-myc tumors. Sequencing of the coding region of p16ink4, k-ras and Rb cDNA in small numbers of pancreatic tumors did not identify mutations. The short latency for tumor development, the variety of tumor morphology and the liver metastases seen in Ela-myc and MT-tgfα/Ela-myc mice make these animals good models for investigating new therapeutic and preventive strategies for pancreatic cancer.
Background
Pancreatic cancer is the fourth leading cause of cancer death in the United States and many other western countries [2,3]. The five-year survival rate is less than 5% in most countries [4]. About 75% of human pancreatic cancer is ductal adenocarcinomas, whereas acinar cell carcinomas and other histological types are less common [5]. An important morphologic feature is that pancreatic ductal adenocarcinomas are frequently associated with prominent fibrosis and multiple cysts [4,6,7]. The cell origin of ductal adenocarcinomas is still under debate [8]. Studies with several experimental animal models suggest that it may derive from metaplasia (transdifferentiation) of acinar cells or even islet endocrine cells [9,10]. In human cases, however, hyperplastic and dysplastic epithelial lesions of the pancreatic ducts have been observed frequently in association with ductal adenocarcinomas [5]. Several studies have suggested a strong association of severely dysplastic ductal lesions with invasive carcinomas [11-14]. More convincingly, a series of pancreatic intraepithelial neoplasia (PanINs) have been developed at a think tank sponsored by National Cancer Institute of the United States as precursors to invasive pancreatic cancer [15]. These lines of evidence suggest that ductal cells may be the origin of ductal adenocarcinomas.
Concomitant expression of epidermal growth factor receptor (EGFR) with its ligand, such as EGF, transforming growth factor α (TGFα) or amphiregulin has been associated with decreased patient survival in pancreatic cancer [16]. In one report, strong TGFα immunoreactivity was found in 95% of pancreatic tumors, whereas EGF immunoreactivity was observed in only 12% of the tumors [17]. Similarly, carcinogen-induced pancreatic cancer in the hamster and rat expressed only TGFα and EGFR, but not EGF [18,19]. Data from these human and animal studies are in line with a generally accepted notion that TGFα is the preferred trophic factor over other EGFR ligands for normal ductal cells and cancer cells in the pancreas [20-23]. Several lines of transgenic mice have been established to study the effects of TGFα on pancreatic carcinogenesis, of which the tgfα transgenic mouse using elastase-1 gene promoter (Ela-tgfα) has been studied extensively [10,24]. The pancreas of these Ela-tgfα mice develops not only pronounced fibrosis but also obvious ductal metaplasia of acinar cells because the Ela-promoter targets the transgene mainly to the acinar cells [24,25]. These acinar-derived ductal cells show progressive proliferation and dysplasia. At one year of age or older, about 25–30% of the animals develop pancreatic tumors, but the majority of them are acinar cell carcinomas. Several other tgfα transgenic mouse lines were established using metallothionin-1 gene promoter (MT-tgfα) [26], but these mice were much less studied for pancreatic lesions, although they were frequently used for studies of carcinogenesis of the mammary gland [27,28] and liver [29].
Overexpression of c-myc mRNA [30] and protein [31] has been found in about 50% and 43.5% of human pancreatic ductal adenocarcinomas, respectively, with about 32.3% of the samples bearing c-myc gene amplification [31]. Another genetic analysis of 31 human pancreatic cancer cell lines also showed that 54% of the cell lines analyzed had c-myc gene amplification [32]. These data suggest that overexpression or amplification of c-myc may play an important role in the development or progression of human pancreatic cancer [33], although there are still relatively few immunohistochemical data to verify whether c-Myc protein levels are also increased correspondingly. Animal studies have revealed that pancreastic cancer induced by chemical carcinogens in the rat also manifests increased c-myc expression [34,35]. A more direct and convincing evidence for a critical role of c-myc in pancreatic carcinogenesis comes from transgenic mice. Mice carrying c-myc transgene under Ela-promoter develop pancreatic cancer with 100% penetrance at an early (2–7 months) age [1]. One-half of the pancreatic tumors are acinar cell carcinomas, while the remaining one-half are ductal adenocarcinomas or mixed ductal and acinar carcinomas [1]. Although this Ela-myc mouse is the first, and seemingly the only, single-transgene model that gives rise to frank pancreatic tumors with ductal elements in the shortest latency period compared with other single-transgene models [1,36], the pathological characterization of the pancreatic lesions from this model has not yet been described in detail.
Besides the Ela-myc mice, several other transgenic mouse models have also been generated for study of exocrine pancreatic cancers [37-42]. However, because currently no gene promoter/enhancer has been known to be specific for pancreatic ductal cell [40], all these transgenic mouse models share a common deficiency: If the transgene is specifically targeted to the pancreas, such as when driven by Ela-promoter, it is dominantly expressed in the acinar cells. Conversely, if the transgene is driven by a promoter specific for ductal cells, its expression is not pancreas specific and is usually at low levels. For instance, the MT-promoter targets the transgene to the mammary gland, liver and pancreas while cytokeratin 19 gene promoter targets the transgene to the stomach, pancreas and probably other organs as well [43]; both promoters are much weaker than the Ela-promoter. Most of the currently existing transgenic mouse models of pancreatic carcinogenesis produce only acinar-ductal metaplasia and ductal proliferation, with zero or very low penetrance of developing frank pancreatic tumors, unless the transgenic mice are concomitantly deficient in certain tumor suppressor genes such as Ink4/Arf or p53 [43-49]. For instance, mice with both Cre-mediated k-ras mutation and Ink4a/Arf gene deficiency develop metastatic pancreatic ductal adenocarcinomas [44]. This animal model requires that a mouse concomitantly bears at least four transgene alleles, i.e. Pdx1-Cre, LSL-KrasG12D and homozygous Ink4a/Arflox/lox, and thus involves extensive animal breeding and genotyping. Crossing two heterozygous breeders (e.g. Ela-myc male × Ela-myc female) may increase the frequency of pups that are transgene carriers. However, pups bred by this procedure may not be used for testing new therapeutic or preventive methods, because it will produce a mixture of homozygous and heterozygous pups. It is technically difficult, if not impossible, to distinguish homozygosites from heterozygotes for a large number of pups, and homozygous carriers of transgene may show different sensitivity to the tested agents compared with heterozygotes. This concern becomes an issue when the animals are used for testing therapeutic or preventive agents, although it may not be an issue for study of carcinogenic mechanisms.
Before a gene promoter specific for pancreatic ductal cells is identified, the best choice may still be to use mice expressing double oncogenes driven individually by a pancreas-specific promoter such as Ela-promoter and by a ductal cell dominant promoter such as MT-promoter. We used this strategy to study pancreatic carcinogenesis by crossing MT-tgfα and Ela-myc mice to create MT-tgfα/Ela-myc mice, considering that the Ela-myc transgene might also be expressed in the ductal cells, as reflected by the appearance of various ductal lesions in the Ela-myc pancreas, and thus might synergize with the MT-tgfα transgene to induce ductal cell carcinogenesis. This report summarizes the novel findings from these single and double transgenic mice.
Materials and methods
The MT100 line of MT-tgfα transgenic mouse with FVB/N genetic background [26] was originally purchased from Jackson Laboratories and maintained at our laboratory in FVB/N background. In addition, we also received one male MT-tgfα transgenic mouse [50] and one male Ela-myc transgenic mouse [1], all in C57BL/6 × SJL background, from Dr. Eric Sandgren at University of Wisconsin-Madison. In this study the MT-tgfα mouse from Dr. Eric Sandgren is defined with the initial of his name as MT-tgfα-ES line, in order to distinguish it from the MT100 line. The FVB mice used for breeding were purchased from Jackson Laboratories. The breeding procedure for each single or double transgenic line is described accordingly in the result section.
Paraffin blocks of 15 cases of human pancreatic ductal adenocarcinomas were retrieved from the Pathology Tissue Repository of Harper University Hospital at Wayne State University, under a protocol approved by the human investigate committee of the University. Criteria for case selection were histologically proved ductal adenocarcinomas and no major treatment before surgical removal of the tumor. Serial sections in 5 μm thickness were prepared from each tissue block and were immunohistochemically stained for TGFα or c-Myc. Sections from mouse pancreatic tissue were prepared in the same way. The primary TGFα antibody was Ab-2 from Oncogene Research Products, San Diego, CA and was used at 1:150 dilution. The c-Myc antibodies were 9E10 monoclonal (from Sigma, St Louis, MO) used at 1:150 dilution and C19 polyclonal (from Santa Cruz Biotech. Inc, Santa Cruz, CA) used at 1:80 dilution. An Avidin-biotin-complex (ABC) method was used for the staining, as described previously [51,52]. A normal rabbit IgG and a normal mouse IgG were used to replace the primary antibodies in the mock staining.
Mouse pancreatic tumor tissues that were kept frozen at -80°C were used for extraction of total cellular RNAs using the RNeasy kit from Qiagen (Valencia, CA). The extracted total RNAs were immediately converted into cDNAs using the TaqMan Reverse Transcription kit from Applied Biosystems (Branchburg, NJ). The cDNAs were then used as PCR templates for the amplification of p16ink4a, k-ras and Rb. For p16ink4a, the primer pair is p16-L2 (TCACAGTGAGGCCGCCGCTGAG)/p16-R592 (AGCTCTGCTCTTGGGATTGG) that covers the whole coding sequence. For k-ras, the primer pair is KRAS-L140 (TGAGGCGCGGCGGCTCCG)/KRAS-R878 (CTGACAGTTTGCACGAACAGAAG) that also spans the whole coding sequence. For Rb1, the coding sequence was amplified as three fragments using the following overlapping primer pairs: Rb-L57 (CGCGCCTCCCTCGGCTGCT)/Rb-R1200 (GAGTGTGTGGAGTAACCACG), Rb-L926 (GTGTAATATAGATGAGGTGAA)/Rb-R2071 (AGTGTATTTAGTCGGAGATAT), and Rb-L2011 (CCTCCCTTGCCCTGTTTTAC)/Rb-R2919 (CCATGAGCCAGGAGTCTGGT). The amplified PCR products were subsequently subjected to DNA sequencing analysis by the Sequencing Core Facilities of Wayne State University. When DNA sequencing revealed nucleotide alterations that lead to amino acid changes, the above whole procedure was repeated starting from amplifying PCR fragments using the cDNAs. This would rule out the possibility that the PCR process introduced the alterations randomly.
Results
Features of pancreatic lesions in MT-tgfα transgenic mice
During our original study of mammary gland tumorigenesis involving the MT100 line of MT-tgfα transgenic mice (FVB/N background), we accidentally found that the female mice had a much shorter life span than their male littermates. While males survived well and were still very healthy at 14 months of age (older mice were not monitored systematically), most females died during 6–8 months of age, showing progressively decreased activity about one month before death. Because the original report of this transgenic line described pathologic alterations in the liver and pancreas as well [26], we performed autopsies of the dead animals and also sacrificed some transgenic females (in total of 20 animals) at the time when they were less active or moribund (at the age of 6–8 months). We also sacrificed another 20 transgenic females at 2–3 months of age as controls of young age and 8 animals at different time points during 3–5 months of age. Female wild type littermates at 2–3 months (5 animals) and 7 months (4 animals) of age were also included as normal controls. The liver and pancreas were examined macroscopically and histologically.
The pancreas of female MT100 mice at 2–3 months appeared as a solid organ whereas the pancreas of the wild type littermates appeared as loose tissue. Histological observation showed progressive death of acinar cells, while ductal cells proliferated progressively to form lesions that resembled mouse pancreatic intraductal neoplasia (mouse PanINs) described in the literature [43,45,46], but these lesions are collectively coined as "ductal proliferation" herein (fig 1A), since it is currently unclear whether such lesions in the mouse are also cancer precursors as PanINs in humans. Progressive fibrosis was associated with the continuing acinar cell death and ductal proliferation (fig 1A). Acinar-ductal metaplasia was observed, although it seemed to be much less evident than what was described for Ela-tgfα mice [10,24,25].
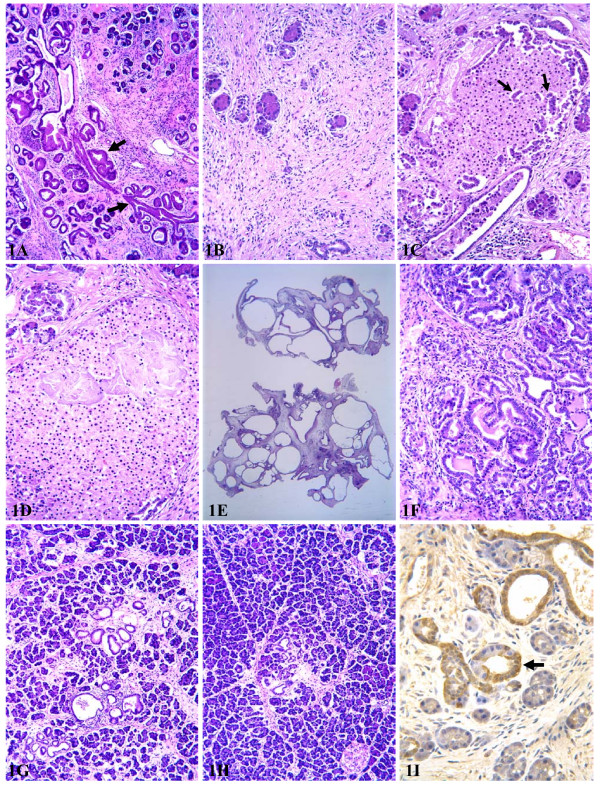
Figure 1.
Histological alterations of the pancreas from MT-tgfα transgenic mice. A: a representative area of the pancreas from a 3-month-old female MT100 mouse, showing formation of fibrosis, proliferating ductal lesions (arrow) that manifest intraductal mucinous changes. B: a pancreas from an 8-month-old female MT100 mouse showing prominent fibrosis and severe loss of acini, with features of chronic pancreatitis. C: hyperplastic ducts (arrows) in an islet resembling ductuloinsular body in humans, from a 6-month-old female MT100 mouse. D: Large necrotic areas seen in an islet, from a 6-month-old female MT100 mouse. E: Low magnification of multiocular cystic neoplasms from an 8-month-old female MT100 mouse. F: Well differentiated ductal adenocarcinomas with chronic pancreatitis, from a 6-month-old female MT100 mouse. G: A representative area of the pancreas from a 9-month-old female MT-tgfα-ES mouse, showing early formation of small cysts and fibrosis. H: A representative area of the pancreas from a 9-month-old male MT-tgfα-ES mouse, which was a littermate of the female shown in G. I: Immunohistochemical staining of TGFα in a 3-month-old female MT100 mouse, noting that within the same acinar-ductal loop, only the ductal cells, but not the acinar cells, are positive.
In 15 of the 20 MT100 females at age of 6–8 months, the pancreas became much smaller, roughly about 10% to 20% of the size of the pancreas at age of 2–3 months. At age of 6–8 months, the pancreas lost about 90% of its acini and basically consisted of only fibrous tissue and proliferating ducts, with features of chronic pancreatitis (fig 1B). Thus, the severe loss of acini is likely the cause of death, and the female mice might be a good animal model of chronic pancreatitis. Interestingly, proliferating ducts also appeared in islets (arrow in fig 1C), resembling the ductuloinsular body in humans. This trait indicates that in this transgenic line, either the islets might contain certain stem cells with multiple potential that could proliferate to form ductal lesions or some endocrine cells might be capable of undergoing ductal metaplasia and forming ductal lesions, as suggested by certain animal models [8,9]. Areas of cell death (likely necrosis) were also observed frequently in islets (fig 1D). In the other five older females the pancreas showed the opposite changes macroscopically, i.e. enlargement, due to the fact that proliferating ducts formed multiple cysts; some cysts were filled with liquid and developed multilocular cystic neoplasms (fig 1E) that were as large as 1.5 cm3. Areas of well differentiated ductal adenocarcinomas (fig 1F) were observed in two of the 20 older mice studied.
We also sacrificed 30 and 20 male MT100 mice at ages of 12–14 months and 2–3 months, respectively, and an additional 15 male transgenic mice in between ages. We also sacrificed five 3-month-old and five 13-month-old male wild type littermates as normal controls. Male MT100 mice at age of 2–3 months started to show death of acinar cells, ductal metaplasia of acinar cells, proliferation of ductal cells, and formation of fibrosis, but these alterations were not as obvious as seen in the age-matched females. Opposite to what was seen in female MT100 mice, the pancreas of males became larger with increased age and body weight. Thus, the pancreas of the males at age of 12–14 months was much larger than the pancreas of males at 2–3 months of age and showed pronounced fibrosis and ductal proliferation and metaplasia, although these lesions were still much less severe compared with the pancreas of females at 6–8 months of age. No multilocular cystic neoplasms and ductal adenocarcinomas were observed in male mice.
We also crossed a male MT-tgfα-ES line (C57BL/6 × SJL background) with a female FVB mouse. The F1 transgene carriers were crossed with F1 wild type mice to produce F2 animals and some F2 mice were crossed together to produce F3 mice. Both F1 and F2 MT-tgfα-ES carriers did not show obvious sex difference in survival since all 30 animals of both sexes survived well over 14 months of age (older mice were not monitored systematically). In addition, we also sacrificed another 37 F2 and F3 mice (18 males and 19 females) at different time points during 2–10 months of age. Histologically, the pancreas of these mice manifested ductal metaplasia of acinar cells, proliferation of ductal cells, formation of small cysts, and fibrosis, similar to what was reported originally by Sandgren et al. for the MT-tgfα-ES line with C57BL/6 × SJL genetic background [50]. However, these alterations not only occurred much later (after 4 months of age) but also progressed much slower compared with age- and sex-matched MT100 mice. Female predilection could be discerned after 9 months of age (fig 1Gvs 1H), but even at 14 months of age the sex difference was still not as pronounced as in MT100 mice at age of 6–8 months, because the lesions in female MT-tgfα-ES mice were much less severe. No multilocular cystic neoplasm or ductal adenocarcinoma was discerned in MT-tgfα-ES mice, but mice older than 14 months were not examined. Unlike what was reported by Sandgren et al. [53], we did not observe any macroscopic liver tumor in our MT-tgfα-ES mice with mixed C57BL/6, SJL and FVB background.
Immunohistochemistry showed a preferential staining of TGFα in ductal cells in both MT100 and MT-tgfα-ES lines (fig. 1I). The staining was strong in most proliferating ductal cells but was weak or undetectable in acinar cells. Even within the same acinar-ductal loop (arrow in fig. 1I), the ductal cells showed intense positive staining while the acinar cells were negative.
Features of pancreatic lesions from Ela-myc transgenic mice
We crossed one male Ela-myc mouse (C57BL/6 × SJL background) with a female FVB mouse and then crossed F1 pups together to produce F2 transgene carriers. Some F2 animals were also crossed together to produce F3 mice. Sixty F1, F2 and F3 Ela-myc mice of both sexes showed enlarged abdomen, a sign of bearing large pancreatic tumor, at ages between 2–7 months as originally reported by Sandrgen et al. [1]. The animals were sacrificed at this period of age when the tumor burden reached the ethical limit or the animals became weak and showed decreased activity. Most tumors weighed 3–4 grams (fig 2A). Peritoneal metastatic tumor seeds were found in 41 (68%) animals. Although Sandgren et al. did not mention liver metastasis in their original report of this transgenic line with C57BL/6 × SJL background [1], in 12 (20%) mice we observed macroscopic liver metastasis (fig 2B), which was confirmed histologically to be of pancreatic origin (fig 2C). All the peritoneal and liver metastases occurred at advanced stage, i.e. 4–7 months of age. Microscopic metastasis in the liver was not examined systematically but likely existed in some of the animals that did not show macroscopic metastasis. No macroscopic metastasis in the lung or other organs was found.
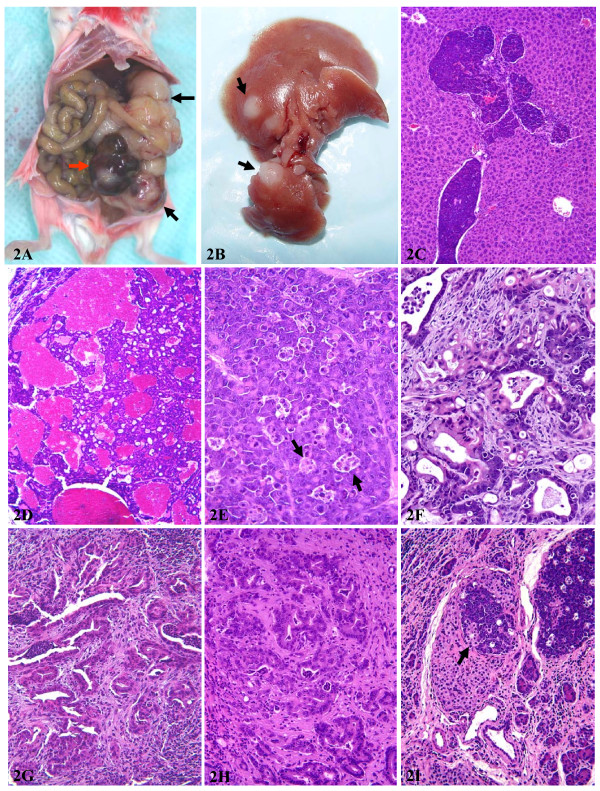
Figure 2.
Alterations of the pancreas from Ela-myc transgenic mice. A: a photo showing a huge nodular pancreatic tumor (arrows). Note that one tumor nodule is in red color while other tumor nodules are in white color. B: liver metastases (arrows) of a pancreatic tumor. C: histological examination confirming that the liver tumors are pancreatic origin (acinar cell carcinoma). D: a typical histology of acinar cell carcinoma that shows red color macroscopically. E: a typical histology of the acinar cell carcinoma that shows white color macroscopically. Note that there are many apoptotic cells that are organized in clusters, coined as "death cell islands" (arrows). F: a typical area of mixed acinar and ductal adenocarcinomas. G: a pancreatic ductal adenocarcinoma. Note that the tumor contains abundant stroma. H: another typical ductal adenocarcinoma. I: an acinar cell carcinoma within an islet (arrow).
At the time of sacrifice, the tumor from each animal manifested multiple nodules, presumably a reflection of multiple original tumors (fig 2A). Some tumor nodules were fish-meat-like white, a typical sign of solid cancer in humans, while some other tumor nodules were a deep red color due to hemorrhage within the tumor (fig 1A). Histologically, about one-half of the tumors were pure acinar cell carcinomas (fig 2D and 2E), while the other one-half were mixed ductal and acinar carcinomas that could be manifested either as mixed cell carcinomas (fig 2F) or as patches of ductal adenocarcinomas (fig 2G and 2H) and other patches of acinar cell carcinomas, similar to what was described in Sandgren et al's original report [1]. While acinar tumors could be either white or red in color, all ductal adenocarcinomas were white in color. Interestingly, some acinar tumors also appeared in endocrine islets (arrow in fig 2I). Acinar cell carcinomas manifested many apoptotic cells (fig 2E) and large areas of necrosis. The apoptotic cells were not randomly distributed but, instead, were usually organized in clusters that were coined "dead cell islands" in the description of apoptotic cells in the mammary tumors of MMTV-c-myc transgenic mice [28]. Acinar cell tumors contained little stroma (fig 2E), but invasive growth into the adjacent stroma was observed at advanced stages. Unlike acinar cell tumors, ductal tumor cells were usually disseminated in the dense stromal tissue, (fig 2F and 2G), similar to desmoplasia observed in human pancreatic ductal adenocarcinomas. Some ductal areas had some acidophilic cells reminiscent of oncocytic changes. Apoptotic cells and large necrotic areas were much less frequently observed in ductal tumors, compared with acinar tumors.
We also sacrificed 10 mice at 2 months of age that had not yet shown enlarged abdomen and found that 4 of the animals already had small cancer nodules (1–6 mm in diameter). Two of these 4 mice had only one tumor nodule in the head of the pancreas while the other two mice had two tumor nodules, one at the head and the other at the tail of the pancreas. It is likely that the head of pancreas is more susceptible for the tumor development, although the c-myc transgene can also induce tumors in other parts of the pancreas. This phenomenon was not mentioned in the original report by Sandgren et al [1] and thus whether it also occurred in the original Ela-myc mice with C57BL/6 × SJL background is unclear.
Features of pancreatic lesions in MT-tgfα/Ela-myc double transgenic mice
We crossed Ela-myc mice with MT100 mice bred during the above-described procedure and obtained 21 double transgenic animals with mixed C57BL/6, SJL and FVB background. The majority (16; 76%) of these mice were sacrificed at 3–5 months of age, but the others were sacrificed as early as 2 months or as late as 6 months of age, because they became weak or the tumor burden reached the ethical limit. It seemed that the double transgenic tumors developed earlier or grew faster than the Ela-myc tumors, but the difference was not statistically significant in this small number of tumors.
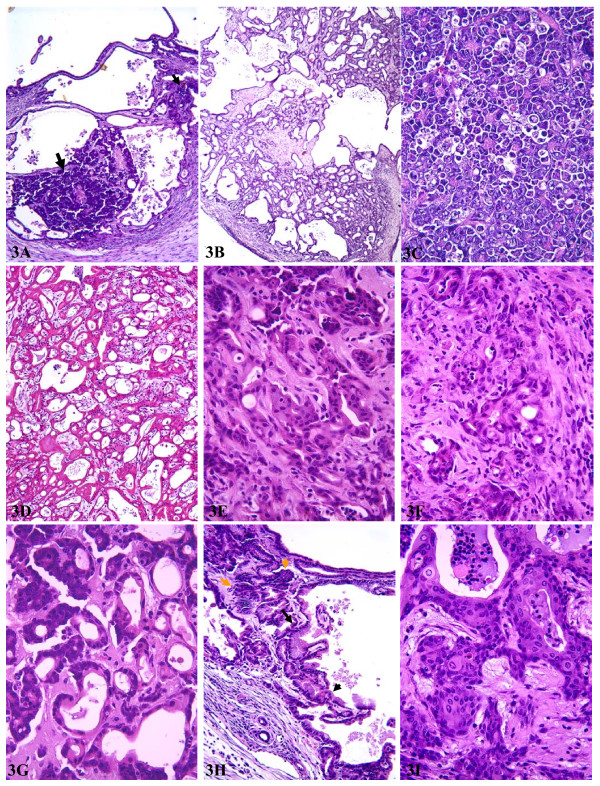
Macroscopically, the tumors looked similar to those from Ela-myc mice. However, usually part of the white color tumors, but not the red color ones, manifested cystic features. Although Sandgen et al. did not observe ductal elements in their Ela-tgfα/Ela-myc and MT-100/Ela-myc double transgenic mice [53], we found that the pancreas of our double transgenic mice manifested a combination of lesions seen individually in the MT-tgfα mice and the Ela-myc mice, including proliferating ducts (fig 3A), multilocular cystic neoplasms (fig 3A and 3B), acinar cell carcinomas (fig 3C), various ductal adenocarcinomas (fig 3D, 3E and 3F) and mixed acinar and ductal carcinomas (fig 3G). Dysplastic ductal lesions were also observed and usually mixed with acinar tumor cells (fig 3H). The ductal lesions, such as multilocular cystic neoplasms and ductal adenocarcinomas, seemed to be more prominent in the female mice than in males. Some tumor cells manifested certain squamous differentiation (fig 3I). Fibrosis was also observed (fig 3B) but was much less severe compared with age- and sex-matched mice of MT100 line. Similar to what was seen in Ela-myc mice, one-half of the tumors were acinar cell carcinomas and another one-half were ductal tumors or mixed ductal and acinar cell tumors. In general, double transgenic tumors of either acinar or ductal cell origin were more malignant, i.e. less differentiated, than the tumors from Ela-myc mice, as previously reported by Sandrgen et al. [53]. Acinar cell tumors contained little stroma, similar to Ela-myc acinar tumors, whereas ductal tumors were abundant with stromal tissue, somewhat resembling pancreatic ductal adenocarcinomas in humans. Necrotic areas and apoptotic cells appeared in both acinar tumors and ductal tumors but were more frequent in acinar tumors.
Figure 3.
Histological alterations of the pancreas from MT-tgfα/Ela-myc double transgenic mice. A: multilocular cystic neoplasms mixed with acinar tumor cells (arrows). B: a large benign multilocular cystic neoplasm. C: a typical acinar cell carcinoma. D: one type of ductal adenocarcinoma. E: another type of ductal adenocarinoma. F: a much less differentiated ductal adenocarcinoma with feature of desmoplasia. G: a mixed acinar and ductal adenocarcinoma. H: an area showing feature of mouse PanIN3 or ductal adenocarcinomas (dark arrows) with acinar tumor cells (yellow arrows) in the surrounding. I: a tumor area showing squamous differentiation.
We observed macroscopic liver metastasis in 7 of 21 (33%) double transgenic animals. This rate is significantly (χ2 test, p < 0.05) higher than the metastatic rate (20%) in Ela-myc mice. Microscopic metastasis was not systematically examined but likely existed in some of those animals that did not show macroscopic metastasis. Peritoneal tumor seeds were also frequently found, as seen in Ela-myc mice. No macroscopic metastasis in the lung or other organs was found.
We also crossed some Ela-myc mice with the MT-tgfα-ES line and got 9 double transgenic pups. The pancreatic lesions of these mice were in general similar to the MT100/Ela-myc mice described above, but less multilocular cystic neoplasms were observed compared with MT100/Ela-myc mice. Two of these mice appeared to have macroscopic liver metastasis.
Expression of c-Myc and TGFα in human ducal pancreatic adenocarcinomas
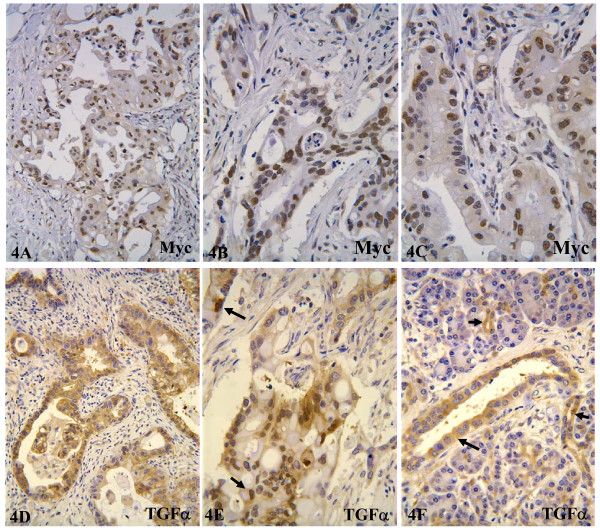
So far there are still relatively few publications on immunohistochemical data of c-Myc in human pancreatic cancer, and some of the early studies might be limited due to the lack of optimal c-Myc antibodies for immunohistochemistry on paraffin-embedded tissue sections. Therefore, we conducted immunohistochemical staining on a small number of human samples of ductal adenocarcinoma to confirm the relevance of c-Myc oncoprotein to human pancreatic cancer. Immunohistochemical staining was carried out with one monoclonal and one polyclonal antibody. The two antibodies gave rise to very similar staining, and only those tissue areas that showed staining with both antibodies were considered positive. We found that 13 of 15 cases showed moderate to strong staining in about 20–70% of the tumor cells. The staining was mainly localized to the nucleus (fig 4A, 4B and 4C), although many tumor cells also showed weak cytoplasmic staining. Fibroblasts in the stroma were negative. "Normal" pancreatic tissue and pancreatitis tissue adjacent to the tumors were also negative. The mock staining using normal rabbit IgG to replace c-Myc antibodies did no give rise to any staining, confirming the signal specificity of the antibodies.
Figure 4.
Immunohistochemical staining for c-Myc and TGFα in human pancreatic ductal adenocarcinomas. 4A, 4B and 4C: c-Myc staining showing that most tumor cells manifest positive nuclear staining. 4D and 4E: TGFα staining showing most tumor cells are positive for TGFα. Note that the staining is mainly localized in the cytoplasm of most tumor cells, but it is also localized in the nucleus (arrows) of some tumor cells. 4F: a "normal" area of pancreatic tissue adjacent to cancer, showing that ductal cells (arrows), but not acinar cells, are positive for TGFα.
The staining for TGFα revealed that TGFα was also highly expressed in the 13 cases that were positive for the c-Myc but negative in the 2 cases that failed to show c-Myc staining. It is likely that these two cases might not have been properly fixed or might have had other unknown defects. In these 13 cases, about 30–70% of the tumor cells were moderately to strongly positive for TGFα. In most cancer cells, the staining was localized to the cytoplasm (fig. 4D and 4E), but nuclear staining was also observed in some cancer cells (arrows in fig. 1E), suggesting that TGFα might have transcriptional activity, like EGF [54]. In the adjacent "normal" or pancreatitis tissues that might have certain proliferating potential, many ductal cells, but not acinar cells, were also positive for TGFα (arrows in fig 4F). These results not only dovetail with the thought that TGFα is important for the growth and development of the pancreas but also indicate that the positivity of TGFα may be indicative of ductal cell origin of the cancer. Fibroblasts in the tumor stroma were negative.
Sequencing of p16ink4a, k-ras and Rb cDNA
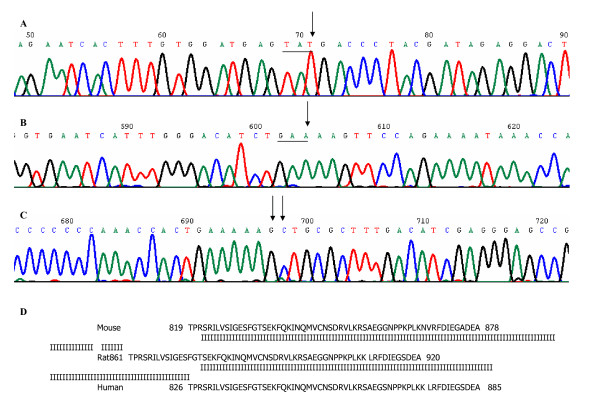
Eleven (8 Ela-myc and 3 MT-tgfα/Ela-myc) tumors were sequenced for the entire coding region of p16ink4a cDNA. None of them showed any nucleotide alteration when compared with the NCBI gene bank sequence (accession#: NM_009877). Twelve (9 Ela-myc and 3 MT-tgfα/Ela-myc) tumors were sequenced for the entire coding region of the k-ras cDNA. Eleven of them did not show any nucleotide alterations when compared with the NCBI gene bank sequence (accession#: NM_021284), while one sample had a single nucleotide change (TAC→TAT) at codon 32, which does not change amino acid sequence (fig. 1, panel A) and thus is considered a polymorphism.
Five Ela-myc tumors were sequenced for the coding region of the Rb gene. All of them showed one nucleotide difference at codons 836 (GAG→GAA), 867 (AAC→AAG) and 868 (GTG→CTG) (panel B and C of fig 1), when compared with the NCBI gene bank sequences (accession# NM_009029 and M26391). Whereas the nucleotide difference at codon 836 did not change the amino acid sequence, the difference at codons 867 and 868 caused changes of two consecutive amino acids, i.e. an asparagine (N) was changed to a lysine (K) at codon 867 and a valine (V) to a leucine (L) at codon 868. Comparison of the Rb protein sequences among mouse, rat and human revealed that the domain that harbors the mouse codons 867 and 868 were highly conserved among these three species. Rb cDNA in both rat and human has the lysine (K) and leucine (L) at the positions corresponding to codons 867 and 868 in the mouse Rb cDNA, as shown in panel D of Figure 1. Moreover, the mouse genome sequence (access # NC-000080) deposited more recently in the NCBI data base also showed that the mouse Rb genomic DNA sequence at these three codons was the same as our sequencing data. We thus considered that the mouse Rb cDNA sequences at NCBI gene bank might be wrong. To further clarify this issue, we sequenced the cDNA of a cell line derived from one Ela-myc pancreatic tumor (Ela-myc-1) that was described in our recent publication [55], as well as the NMuMG cells, a normal mouse mammary epithelial cell line purchased from ATCC. We also sequenced cDNA from a normal mouse kidney tissue. The sequencing data from all these cell lines and kidney tissue showed the same nucleotide sequence as Ela-myc tumor tissue. Therefore, it is likely that the Rb gene in Ela-myc tumors was not mutated but, instead, the mouse Rb cDNA sequences at the NCBI gene bank (accession# NM_009029 and M26391) may have mistakes at these three nucleotides.
Discussion
Although the MT100 and MT-tgfα-ES lines of MT-tgfα mice as well as the Ela-myc mice have been established for over a decade, so far there are only several published studies using these transgenic mice to address pancreatic carcinogenesis, and detailed characterization of the pancreatic lesions from these mice is still lacking. During a study of mammary carcinogenesis of MT100 mice, we accidentally found, for the first time, the obvious sex differences in the pancreatic lesions of this transgenic line. Bardeesy et al. observed multiple cystic lesions with similarly low penetrance in MT42, another MT-tgfα transgenic line, but only in the mice that were concomitantly deficient in one allele of Ink4a and/or p53 gene, not in the MT42 mice with wild type of any of these two genes [56]. The reason for this discrepancy could be due to the facts that their mice were from another transgenic line and had different genetic background, and that the gender of the mice (25 animals) in their study was not described. It is known that in humans various types of cystic neoplasms of the pancreas have malignant potential and show female predominance [57-60]. It deserves further studies whether our observation of female predilection of multilocular cystic neoplasms in the MT100 mice is etiologically or mechanistically relevant to such benign neoplasms in the human pancreas, although the multilocular cystic neoplasms in the mice resemble, histologically, the cystic lesions observed in the human pancreas, as have shown by Bardeesy et al. [56]. In our MT100 mice, these well-demarcated multiolcular cystic lesions had loose mesenchyme in the septae, closely resembling ovarian like stroma of human mucinous cystic neoplasm. Moreover, there were luteal type cells, further supporting this hypothesis.
In both MT100 and MT-tgfα-ES lines, TGFα is found expressed preferentially in the proliferating ductal lesions, not in the acinar cells or stromal tissue. This expression pattern is different from the dominant expression of the transgenes in the acinar cells of Ela-promoter driven transgenic mice. This difference in the expression patterns may partly explain why the transdifferentiation from acinar cells to ductal cells was not as evident in our MT100 mice as described by Schmid et al. for the Ela-tgfα mice [10,24,25]. Because Ela-tgfα and MT-tgfα preferentially target to different cell types, the underlying mechanisms for the formation of fibrosis and the death of acinar cells in these two different transgenic lines may be mechanistically different. It is possible that in MT100 mice, constitutive overexpression of the tgfα transgene in the ductal cells causes, perceivably via epithelial-stromal interaction, continuous growth of connective tissue to form fibrosis, which in turn leads to continuous death of acinar cells. It needs to be further explored why and how ductal-derived TGFα induces progressive death of acinar cells and growth of stroma in MT100 mice. On the other hand, MT-tgfα/Ela-myc double transgenic mice showed much less pronounced fibrosis in the pancreas, compared with MT-tgfα mice. An explanation is that overexpressed c-Myc suppresses TGFα induced formation of fibrosis, although TGFα facilitates c-Myc induced pancreatic carcinogenesis by inducing more malignant phenotypes of the tumors. A similarly less evident fibrosis was also observed in the mammary tumors from MT-tgfα/MMTV-myc double transgenic mice than in the fibrous mammary gland of MT-tgfα mice [28]. The molecular mechanism behind this phenomenon is currently unknown.
The finding of liver metastasis of the pancreatic tumors in Ela-myc mice is a surprise, since it was not described by Sandgren et al. in their original report of this transgenic line [1]. The reason for this different observation is unclear. We intend to consider that the mixed C57BL/6, SJL and FVB genetic background of our animals renders the pancreatic tumor a higher metastatic ability. However, the changed genetic background does not seem to alter the latency for the tumor development, since our animals develop pancreatic tumors at the same age as reported by Sandgren et al. [1]. Despite the unexplained discrepancy, this finding makes the Ela-myc mouse the first single-transgene model that yields a high metastatic rate. Endogenous expression of mutant k-ras in a transgenic model has been shown to cause ductal proliferation that occasionally progresses to frank tumors with invasive and metastatic potential [46]. Another two transgenic mouse lines using Ela-promoter to target k-ras mutant or Ela-SV49TAg to the pancreas develop only mouse PanINs [1,43,45,47] but not frank tumors of exocrine origin. Development of frank, metastatic tumors requires combination of expression of k-ras mutant or Ela-SV49TAg with the deficiency of the Ink4a/Arf gene [44,46,47]. The k-ras mutant/Ink4a-/- model [44] gives rise to metastatic ductal adenocarcinomas and is thus very useful for mechanistic research of pancreatic carcinogenesis. However, it requires that a mouse concomitantly bears four transgene alleles, i.e. Pdx1-Cre, LSL-KrasG12D and homozygous Ink4a/Arflox/lox. This model, and another one that involves expression of k-ras mutant and p53 knockout, requires extensive animal breeding and genotyping, which greatly limits its use for the purpose of studying new therapeutic or preventive methods or agents. Therefore, several characteristics of Ela-myc mouse, i.e. high frequency of ductal adenocarcinomas, high metastatic rate, short latency of carcinogenesis, and the easiness of breeding and genotyping make it a very useful animal model for studying pancreatic carcinogenesis and testing new therapeutic and preventive methods or agents.
Although Sandgren et al. did not observe ductal elements in the pancreatic tumors from their Ela-tgfα/Ela-myc or MT-tgfα/Ela-myc double transgenic mice [53], we found that proliferating ductal lesions, multilocular cystic neoplasms and ductal adenocarcinomas occurred frequently in the pancreas of our MT-tgfα/Ela-myc mice. Sandgren et al. considered that the reason for the lack of ductal elements could be due to the earlier development of more malignant tumors with reduced life span of these animals [53]. Reduction in the life span seems to be less evident in our double transgenic animals since it does not reach the statistically significant level when compared with the Ela-myc mice. In addition, we also found that MT-tgfα/Ela-myc double transgenic tumors metastasize to the liver at a higher frequency than the Ela-myc pancreatic tumors, although the liver metastasis of the double transgenic tumors was, again, not observed in the study of Sandgren et al. [53]. Differences in the genetic background of the mice may be one of the explanations for the different observations. This seemingly banal caveat is supported by the facts that Sandgren et al. found primary liver tumors in their MT-tgfα mice at a high frequency (16 of 27 animals) and in their wild type mice at a low frequency (1 of 20 animals) during 26–104 weeks of age [53], whereas we did not find any primary liver tumors in any of our MT-tgfα mice or their wild type littermates up to 12 months of age.
k-ras, Rb and p16ink4a are the genes showing mutations or inactivation at high frequencies in human pancreatic cancer. Surprisingly, although the Ela-myc and MT-tgfα/Ela-myc tumors were highly malignant and have metastatic ability, they did not show mutations in these genes, at least not at high frequencies. Schaeffer et al had also sequenced the k-ras in Ela-myc tumors and did not find mutation [61].
In summary, this study documents a series of novel findings in several single and double transgenic mouse models established previously by other investigators. Female, but not male, MT-tgfα (MT100 line) mice developed multilocular cystic neoplasms and ductal adenocarcinomas at low penetrance. Ela-myc mice with mixed genetic background develop pancreatic cancers that metastasize to the liver. MT-tgfα/Ela-myc dual transgenic mice develop a variety of pancreatic lesions including multilocular cystic neoplasms and ductal adenocarcinomas that are not reported previously. Moreover, the double transgenic tumors metastasize to the liver at a higher frequency than the Ela-myc pancreatic tumors. The early formation and the variety of the tumor morphology, as well as the potent metastatic ability seen in the Ela-myc and MT-tgfα/Ela-myc transgenic mice, make them good animal models for testing new therapeutic and preventive regimens or agents for the management of pancreatic cancer.
Abbreviations
Ela-: elastase-1 gene promoter
MT-: metallothionin-1 gene promoter
MMTV: mouse mammary tumor virus long terminal repeat
PanINs: pancreatic intraductal neoplasia
TGFa: transforming growth factor alpha
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
DJL is the principle investigator of this study who summarized the data and drafted this manuscript. YW is a postdoctoral fellow who helped with the collection of animal experiment data. JW is a research assistant who performed sequencing of Rb, p16 and k-ras. NVA is a pathologist who helped with characterization of pathologic alterations of the mouse and human pancreatic lesions. DG is a pathologist who helped with characterization of pathologic alterations of the mouse and human pancreatic lesions. FK is a pathologist who helped with characterization of pathologic alterations of the mouse and human pancreatic lesions. FHS is an expert in pancreatic cancer who contributed valuable opinions in the interpretation of the data and help drafting the manuscript. All authors read and approved the final manuscript.
Figure 5.
Sequencing data of k-ras and Rb cDNA. Panel A, chromatogram showing nucleotide change of k-ras; panel B and C, chromatograms showing nucleotide changes of Rb; panel D, Comparison of part of the amino acid sequences of the Rb proteins among mouse, rat and human. The single-letter amino acid sequence of the mouse, rat and human Rb protein is represented. A vertical bar represents amino acid identity between any two of the three species.
Acknowledgments
Acknowledgements
We would like to thank Dr. Eric Sandrgen at University of Wisconsin-Madison for generously providing us the original Ela-myc and MT-tgfα-ES mice and for reading the manuscript. This work was supported by NIH grant RO1 CA100864 to Dr. D.J. Liao and by a Pardee Foundation grant to Dr. D.J. Liao on pancreatic cancer research.
Contributor Information
Dezhong Joshua Liao, Email: dliao@med.wayne.edu.
Yong Wang, Email: kingwyong@sohu.com.
Jiusheng Wu, Email: wuj@karmanos.org.
Nazmi Volkan Adsay, Email: adsayv@med.wayne.edu.
David Grignon, Email: dgrignon@med.wayne.edu.
Fayyaz Khanani, Email: fkhanani@med.wayne.edu.
Fazlul H Sarkar, Email: fsarkar@med.wayne.edu.
References
- Sandgren EP, Quaife CJ, Paulovich AG, Palmiter RD, Brinster RL. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc Natl Acad Sci USA. 1991;88:93–97. doi: 10.1073/pnas.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol. 2004;34:238–244. doi: 10.1093/jjco/hyh045. [DOI] [PubMed] [Google Scholar]
- Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI, Abrams RA, Laheru D, Jaffee EM, Hidalgo M, Yeo CJ. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- Cubilla AL, Fitzgerald PJ. Classification of pancreatic cancer (nonendocrine) Mayo Clin Proc. 1979;54:449–458. [PubMed] [Google Scholar]
- Kloppel G, Lingenthal G, von BM, Kern HF. Histological and fine structural features of pancreatic ductal adenocarcinomas in relation to growth and prognosis: studies in xenografted tumours and clinico-histopathological correlation in a series of 75 cases. Histopathology. 1985;9:841–856. doi: 10.1111/j.1365-2559.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- Pour PM, Pandey KK, Batra SK. What is the origin of pancreatic adenocarcinoma? Mol Cancer. 2003;2:13. doi: 10.1186/1476-4598-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour PM, Standop J, Batra SK. Are islet cells the gatekeepers of the pancreas? Pancreatology. 2002;2:440–448. doi: 10.1159/000064718. [DOI] [PubMed] [Google Scholar]
- Schmid RM. Acinar-to-ductal metaplasia in pancreatic cancer development. J Clin Invest. 2002;109:1403–1404. doi: 10.1172/JCI200215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Konishi Y. Precancerous conditions for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7:575–579. doi: 10.1007/s005340070006. [DOI] [PubMed] [Google Scholar]
- Luttges J, Kloppel G. Precancerous conditions of pancreatic carcinoma. J Hepatobiliary Pancreat Surg. 2000;7:568–574. doi: 10.1007/s005340070005. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV, bores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Wilentz RE, Maitra A. Identification and analysis of precursors to invasive pancreatic cancer. Methods Mol Med. 2004;103:1–14. doi: 10.1385/1-59259-780-7:001. [DOI] [PubMed] [Google Scholar]
- Kleeff J, Friess H, Berberat PO, Martignoni ME, Z'graggen K, Buchler MW. Pancreatic cancer – new aspects of molecular biology research. Swiss Surg. 2000;6:231–234. doi: 10.1024/1023-9332.6.5.231. [DOI] [PubMed] [Google Scholar]
- Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR. Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol. 1991;163:111–116. doi: 10.1002/path.1711630206. [DOI] [PubMed] [Google Scholar]
- Visser CJ, Bruggink AH, Korc M, Kobrin MS, de Weger RA, Seifert-Bock I, van Blokland WT, van Garderen-Hoetmer A, Woutersen RA. Overexpression of transforming growth factor-alpha and epidermal growth factor receptor, but not epidermal growth factor, in exocrine pancreatic tumours in hamsters. Carcinogenesis. 1996;17:779–785. doi: 10.1093/carcin/17.4.779. [DOI] [PubMed] [Google Scholar]
- Visser CJ, Woutersen RA, Bruggink AH, van Garderen-Hoetmer A, Seifert-Bock I, Tilanus MG, de Weger RA. Transforming growth factor-alpha and epidermal growth factor expression in the exocrine pancreas of azaserine-treated rats: modulation by cholecystokinin or a low fat, high fiber (caloric restricted) diet. Carcinogenesis. 1995;16:2075–2082. doi: 10.1093/carcin/16.9.2075. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-X. [DOI] [PubMed] [Google Scholar]
- Giraud AS. X. Trefoil peptide and EGF receptor/ligand transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2000;278:G501–G506. doi: 10.1152/ajpgi.2000.278.4.G501. [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, Pascall JC, Brown KD. Tissue distribution of mRNA for heparin-binding epidermal growth factor. Biochem J. 1992;287:681–684. doi: 10.1042/bj2870681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan TJ, James PS, Pascall JC, Brown KD. Molecular cloning and tissue distribution of pig transforming growth factor alpha. Biochem J. 1993;296:837–842. doi: 10.1042/bj2960837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RM, Kloppel G, Adler G, Wagner M. Acinar-ductal-carcinoma sequence in transforming growth factor-alpha transgenic mice. Ann N Y Acad Sci. 1999;880:219–230. doi: 10.1111/j.1749-6632.1999.tb09526.x. [DOI] [PubMed] [Google Scholar]
- Greten FR, Wagner M, Weber CK, Zechner U, Adler G, Schmid RM. TGF alpha transgenic mice. A model of pancreatic cancer development. Pancreatology. 2001;1:363–368. doi: 10.1159/000055835. [DOI] [PubMed] [Google Scholar]
- Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-Q. [DOI] [PubMed] [Google Scholar]
- Liao DJ, Natarajan G, Deming SL, Jamerson MH, Johnson M, Chepko G, Dickson RB. Cell cycle basis for the onset and progression of c-Myc-induced, TGFalpha-enhanced mouse mammary gland carcinogenesis. Oncogene. 2000;19:1307–1317. doi: 10.1038/sj.onc.1203430. [DOI] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. c-Myc in breast cancer. Endocr Relat Cancer. 2000;7:143–164. doi: 10.1677/erc.0.0070143. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Factor VM, Snyderwine EG. Transgenic mouse models in carcinogenesis research and testing. Toxicol Lett. 2000;112–113:553–555. doi: 10.1016/S0378-4274(99)00224-6. [DOI] [PubMed] [Google Scholar]
- Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–469. doi: 10.1038/modpathol.3880547. [DOI] [PubMed] [Google Scholar]
- Mahlamaki EH, Barlund M, Tanner M, Gorunova L, Hoglund M, Karhu R, Kallioniemi A. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002;35:353–358. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- Brackett DJ, Smith BJ, Lerner MR, Hanas JS, Postier RG. Gene activity associated with cancers treated by surgical oncologists. J Okla State Med Assoc. 2003;96:485–494. [PubMed] [Google Scholar]
- Silverman JA, Kuhlmann ET, Zurlo J, Yager JD, Longnecker DS. Expression of c-myc, c-raf-1, and c-Ki-ras in azaserine-induced pancreatic carcinomas and growing pancreas in rats. Mol Carcinog. 1990;3:379–386. doi: 10.1002/mc.2940030610. [DOI] [PubMed] [Google Scholar]
- Calvo EL, Dusetti NJ, Cadenas MB, Dagorn JC, Iovanna JL. Changes in gene expression during pancreatic regeneration: activation of c-myc and H-ras oncogenes in the rat pancreas. Pancreas. 1991;6:150–156. doi: 10.1097/00006676-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Corominas JM, Malats N, Pereira JA, Dufresne M, Real FX, Navarro P. Tissue plasminogen activator in murine exocrine pancreas cancer: selective expression in ductal tumors and contribution to cancer progression. Am J Pathol. 2004;165:1129–1139. doi: 10.1016/S0002-9440(10)63374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy AM. Transgenic models of pancreatic cancer. Int J Gastrointest Cancer. 2003;33:71–78. doi: 10.1385/IJGC:33:1:71. [DOI] [PubMed] [Google Scholar]
- Wei D, Xiong HQ, Abbruzzese JL, Xie K. Experimental animal models of pancreatic carcinogenesis and metastasis. Int J Gastrointest Cancer. 2003;33:43–60. doi: 10.1385/IJGC:33:1:43. [DOI] [PubMed] [Google Scholar]
- Leach SD. Mouse models of pancreatic cancer: the fur is finally flying! Cancer Cell. 2004;5:7–11. doi: 10.1016/S1535-6108(03)00337-4. [DOI] [PubMed] [Google Scholar]
- Grippo PJ, Sandgren EP. Modeling pancreatic cancer in animals to address specific hypotheses. Methods Mol Med. 2004;103:217–244. doi: 10.1385/1-59259-780-7:217. [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Pinkert CA, Ornitz DM, Palmiter RD, Brinster RL. Pancreatic neoplasia induced by ras expression in acinar cells of transgenic mice. Cell. 1987;48:1023–1034. doi: 10.1016/0092-8674(87)90710-0. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Hammer RE, Messing A, Palmiter RD, Brinster RL. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987;238:188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, Grippo P, Stoffers DA, Silberg DG, Rustgi AK. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003;63:2005–2009. [PubMed] [Google Scholar]
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63:2016–2019. [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- Lewis BC, Klimstra DS, Varmus HE. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 2003;17:3127–3138. doi: 10.1101/gad.1140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. A new mouse model of pancreatic cancer: PTEN gets its Akt together. Cancer Cell. 2005;8:171–172. doi: 10.1016/j.ccr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-P. [DOI] [PubMed] [Google Scholar]
- Blancato J, Singh B, Liu A, Liao DJ, Dickson RB. Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses. Br J Cancer. 2004;90:1612–1619. doi: 10.1038/sj.bjc.6601703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. Cell death in MMTV-c-myc transgenic mouse mammary tumors may not be typical apoptosis. Lab Invest. 2003;83:1437–1449. doi: 10.1097/01.LAB.0000090153.13977.AE. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Luetteke NC, Qiu TH, Palmiter RD, Brinster RL, Lee DC. Transforming growth factor alpha dramatically enhances oncogene-induced carcinogenesis in transgenic mouse pancreas and liver. Mol Cell Biol. 1993;13:320–330. doi: 10.1128/mcb.13.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- Biliran H, Jr, Wang Y, Banerjee S, Xu H, Heng H, Thakur A, Bollig A, Sarkar FH, Liao JD. Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin Cancer Res. 2005;11:6075–6086. doi: 10.1158/1078-0432.CCR-04-2419. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Morgan J, Sinha M, Signoretti S, Srivastava S, Loda M, Merlino G, DePinho RA. Obligate roles for p16(Ink4a) and p19(Arf)-p53 in the suppression of murine pancreatic neoplasia. Mol Cell Biol. 2002;22:635–643. doi: 10.1128/MCB.22.2.635-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573–580. doi: 10.1093/ajcp/69.6.573. [DOI] [PubMed] [Google Scholar]
- Thompson LD, Becker RC, Przygodzki RM, Adair CF, Heffess CS. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1–16. doi: 10.1097/00000478-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Wilentz RE, bores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, Hruban RH. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327. doi: 10.1097/00000478-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F, Mariuzzi GM, Kloppel G. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Schaeffer BK, Terhune PG, Longnecker DS. Pancreatic carcinomas of acinar and mixed acinar/ductal phenotypes in Ela-1-myc transgenic mice do not contain c-K-ras mutations. Am J Pathol. 1994;145:696–701. [PMC free article] [PubMed] [Google Scholar]