Abstract
Reelin regulates telencephalic and cerebellar lamination during mammalian development and is expressed in several structures of the adult brain; however, only traces of reelin were believed to be in peripheral tissues. Because reelin structurally resembles extracellular matrix proteins, and because many of these proteins are expressed in blood, we hypothesized that reelin also might be detectable in the circulation. Reelin (420 kDa) and two reelin-like immunoreactive bands (310 and 160 kDa) are expressed in serum and platelet-poor plasma of rats, mice, and humans, but these three bands were not detectable in serum of homozygous reeler (rl/rl) mice. Reelin plasma levels in heterozygous (rl/+) mice were half of those in wild-type littermates. Western blotting and immunocytochemistry using antireelin mAbs indicated that reelin-like immunoreactivity was expressed in a subset of chromaffin cells within the rat adrenal medulla and in a subset of cells coexpressing α-melanocyte-stimulating hormone within the pituitary pars intermedia. However, surgical removal of adrenal or pituitary failed to decrease the amount of reelin (420-kDa band) expressed in serum. Adult liver expressed one-third of the reelin mRNA concentration expressed in adult mouse cerebral cortex. Full-length reelin protein was detectable in liver extracts in situ; acutely isolated liver cells also secreted full-length reelin in vitro. Liver appears to be a prime candidate to produce and maintain the circulating reelin pool. It now becomes relevant to ask whether circulating reelin has a physiologic role on one or more peripheral target tissues.
Keywords: disabled-1, reeler, serum
Homozygous null mutant reeler (rl/rl) mice exhibit developmental abnormalities in the neuronal layering of cerebral cortex and in the formation of cerebellum (1, 2). Recently, reelin was identified as the gene product that is mutated or partially deleted in rl/rl mouse strains (3–8). Reelin is a 420-kDa glycoprotein structurally resembling extracellular matrix proteins, which, in developing cerebral cortex, is secreted by Cajal–Retzius cells located in the marginal zone and which regulates migration of cortical plate neurons. Reelin must be secreted to exert its biological effects (6, 8), and current findings suggest that reelin signals in a paracrine, extrasynaptic manner on neighboring cells that express DAB1 (disabled-1). DAB1 is a cytosolic adapter protein that is a critical target of reelin signaling in developing neurons (9–13). The very low density lipoprotein receptor and apoE receptor 2 bind reelin and have been proposed to play a putative role in the phosphorylation of DAB1 in embryonic neurons (14, 15).
Reelin is expressed in several neuronal subtypes expressed in the adult brain, including glutamatergic cerebellar granule neurons and specific GABAergic interneurons of cerebral cortex and hippocampus (16–19); conversely, the DAB1 protein is expressed in adjacent neurons (19, 20), i.e., in cerebellar Purkinje cells and cortical pyramidal neurons. Heterozygous (rl/+) mice that express reelin at 50% of wild-type levels have grossly normal brains but exhibit a progressive loss during aging of a neuronal target of reelin action, Purkinje cells (21). The rl/+ mice also exhibit behavioral abnormalities (increased neophobia and reduced prepulse inhibition of the startle response), which first become apparent after puberty and become progressively worse with age (22). These studies suggest that reelin plays an important role throughout adulthood. Furthermore, studies of postmortem human brain have shown that reelin mRNA and protein levels are reduced ≈50% in patients with schizophrenia and bipolar disorder (ref. 20 and A.G., J. Davis, Y. Dwivedi, F.I., F. Pandey, C. Pesold, R. Sharma, D. P. Uzunov, and E.C., unpublished results). These changes have been observed in all brain regions examined so far (frontal cortex, temporal cortex, hippocampus, caudate, and cerebellum); they do not appear to be the result of antipsychotic drug exposure, and the changes are not seen in postmortem brains of patients with unipolar depression (ref. 20 and A.G. et al., unpublished results).
Several laboratories have reported that reelin mRNA is expressed in a variety of peripheral tissues in the adult mouse (3, 5, 23, 24). However, there is little agreement across laboratories regarding which peripheral tissues are positive for reelin, and even those tissues described as positive were said to express reelin mRNA only at trace levels and not to express detectable reelin protein (3, 5, 23, 24). The rl/rl mice do tend to be smaller in size and show changes in immune function and fertility, but these changes have been presumed to be secondary to ataxia and poor feeding, because the embryogenesis of peripheral organs appears normal (24). For these reasons, the expression of peripheral reelin previously has been negated or ascribed little or no significance. However, the present paper suggests that blood-borne reelin may have a physiologic role in the periphery.
Methods
Antibodies.
Monoclonal antireelin antibodies G10 and 142 were a generous gift of Andre Goffinet, University of Namur, Belgium (ref. 25; also see ref. 20). Affinity-purified rabbit anti-DAB1 was a generous gift of Brian Howell (National Institutes of Health, Bethesda, MD) (9). Polyclonal antibody (IC-1 AFP-173P) was a gift of R. Kineman (originally from National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health); this antibody reacts with a number of proopiomelanocortin gene peptides including α-melanocyte-stimulating hormone (α-MSH) and ACTH, but within the intermediate lobe its staining pattern is indicative of α-MSH-containing cells (26). mAb against choline acetyltransferase was purchased from Chemicon.
Immunoblotting.
Serum (20 μl) was diluted with water (1 ml), precipitated with a 7- to 10-fold excess of methanol (−20° overnight), dissolved into SDS/PAGE sample buffer (100 μl) under reducing conditions, and boiled (3 min). Tissues were Dounce-homogenized in 50 mM ice-cold Tris/150 mM NaCl/5 mM EDTA, pH 7.6, containing 10 mM N-ethylmaleimide, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 0.2 mM PMSF, and centrifuged to remove particulate matter before precipitating with methanol (27). The Bradford protein assay used BSA as a standard. In some cases, Triton X-100 (1%) was present in the extraction buffer.
Samples were separated on 6.5% resolving gels (with 2.5% stacking gel), run in parallel with Bio-Rad high-molecular-weight prestained standards, and transferred to poly(vinylidene difluoride) membranes as described (27). Estimates of molecular mass were made in both 6.5% gels and 5–15% gradient gels, and reduced mouse laminin as well as Bio-Rad standards were employed as size standards. Rat cerebellar granule cell-conditioned medium was run routinely as a calibration standard. Recombinant reelin also was expressed in 293T cells, using a full-length reelin cDNA clone (generously provided by Gabriella D'Arcangelo, St. Jude Children's Hospital, Memphis, TN) transfected into 293T cells (gift of Brian Howell) via Lipofectamine 2000 (GIBCO), and collecting the conditioned medium (obtained in serum-free DMEM) for 2 days. In some cases, Reln H fusion protein synthesized in Escherichia coli (25) was employed as a reference standard (comprising amino acids 164–496 of reelin; Reln H cDNA was a gift of A. Goffinet). Reln H protein binds antibody G10 with the same affinity as native reelin-like immunoreactive bands (35).
Blots were blocked in 1% nonfat dry milk for 1 hr (all steps at room temperature), incubated in primary antibody for 2 hr, rinsed, incubated in peroxidase-conjugated secondary antibody for 1.5–2 hr, rinsed, incubated in enhanced chemiluminescence reagent (Amersham), and exposed to film (Hyperfilm ECL; Amersham) for 4–12 min. Antibody G10 ascites was employed at 1:5,000–1:10,000; 142 was employed at 1:400; and anti-DAB1 was employed at 1:5,000. Anti-mouse IgG (adsorbed against human serum proteins; Sigma A-6782) was used at 1:1,500, and anti-rabbit IgG (adsorbed against human IgG; Sigma A-0545) was used at 1:5,000 (for DAB1). No bands were observed when the primary antibody was omitted, other than endogenous mouse IgG when mouse tissues were probed.
Immunocytochemistry at the LM Level.
Adult rats (≈200 g) were perfused extensively with cold PBS (≈0.3 ml/g body weight) followed by 4% p-formaldehyde (≈0.75 ml/g body weight) in 0.1 M phosphate buffer, pH 7.4. Brains, adrenal glands, and pituitaries were trimmed and postfixed for 2 hr or overnight at 4°. Sections were cut on a vibratome (40 microns) and stored at 4° in 0.1 M phosphate buffer containing 0.1% sodium azide. For immunolabeling, sections were placed in Netwells (Costar) with constant rotation.
Endogenous peroxidase activity was suppressed by incubation with 0.3% hydrogen peroxide/10% methanol/PBS for 30 min. Because reelin may be present in the animal sera usually employed for blocking sections, we tested a variety of procedures: (i) blocking with 3% goat serum/0.1% Triton/PBS and incubating primary antibody in the same solution; (ii) blocking and incubating primary antibody in 1% nonfat dry milk only; and (iii) blocking 3% goat serum/0.1% Triton and incubating primary antibody in 1% nonfat dry milk. All procedures gave similar results. In some cases an avidin/biotin blocking kit (Vector Laboratories) was used to reduce background.
Primary antibodies were applied overnight at 4° (G10, 1:1,000–1:10,000; 142, 1:10; anti-DAB1, 1:5,000; anti-MSH, 1:10,000–1:25,000) and rinsed, and secondary antibodies were applied for at least 2 hr at room temperature (biotinylated anti-mouse IgG 1:1,500 or anti-rabbit IgG 1:5,000, both adsorbed to remove cross-reactivity with rat serum proteins), rinsed, incubated in avidin-biotin-peroxidase conjugate (Vectastain Elite, 1:7; Vector Laboratories), rinsed, and reacted with 0.3 mg/ml 3,3′-diaminobenzidine/hydrogen peroxide (4 μl of 30% stock per 50 ml) for 15 min. For fluorescent detection of reelin, avidin-Cy3 conjugate (1:500) was added in place of avidin-biotin-peroxidase, and sections were coverslipped by using Fluoromount G (Southern Biotechnology Associates) to prevent fading.
As a positive control, sections of cerebral cortex were routinely processed in parallel with other tissues and gave the expected patterns of cellular reactivity as reported previously by our group (18–20). As a negative control, the primary antibody was omitted. Confocal microscopy was carried out by using both Leica and Zeiss LSM 510 systems. Specimens double-labeled for reelin and α-MSH immunoreactivity were detected by using avidin-Cy3 and anti-rabbit IgG-fluorescein, respectively, and multitracker scanning (channels were scanned sequentially to avoid bleed-over from one channel to another).
Immunocytochemistry at the Electron Microscopy Level.
For preembedding immunocytochemistry using diaminobenzidine detection, selected vibratome sections were treated as described (28), using material that had been perfused in 4% paraformaldehyde/0.1% glutaraldehyde. Sections were viewed, without poststaining, in a Zeiss 902 electron microscope.
Animal Surgery.
Female Sprague–Dawley rats (100 g) were subjected to a variety of organ removals by the supplier, Harlan–Sprague–Dawley, and shipped after recovery from surgery (n = 4 in each group): sham adrenalectomy, adrenalectomy, selective removal of the adrenal medulla bilaterally, sham hypophysectomy, and hypophysectomy. As well, three rats had both spleen and thymus removed. Serum was collected by tail bleed at 6 and 14 days after surgery and examined for reelin by Western blotting. At 7 days after surgery, the four adrenalectomized rats received s.c. implants of slow-release corticosterone pellets (25 mg, designed to release over 3 weeks; Innovative Research of America). Some of the experiments were repeated with a second cohort of 200-g male rats, with similar results.
Results
Reelin Expression in Blood.
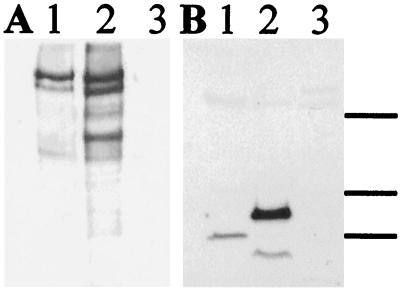
Using mAbs G10 and 142, specific for distinct epitopes of the N-terminal domain of reelin (25), a reelin-immunoreactive band with an apparent molecular mass of 420 kDa was observed in Western blots of adult rat, mouse, and human serum (Fig. 1 A–C). This band was readily detected in 1 μl of unfractionated serum per lane (Fig. 1) and also was immunoprecipitated with G10 antibody (not shown). The 420-kDa band comigrated with recombinant full-length reelin expressed in 293T cells. In addition, G10 and 142 antibodies recognized two reelin-like immunoreactive bands migrating at 310 and 160 kDa, whose relative abundance differed between serum and neural tissue. Whereas the 160-kDa band was the most abundant band in brain (Fig. 1E) and in cerebellar granule cell-conditioned medium (Fig. 6), only traces of the 160-kDa band were observed in serum of adults (Fig. 1 A–C). Both 310- and 160-kDa bands have been reported to arise from reelin by action of a specific metalloprotease (29). The reelin-like immunoreactive bands were similar in serum vs. platelet-poor plasma, but were not detectable in isolated platelets; this indicates that the profile of reelin-like bands was not generated during the process of blood clotting, that the majority of reelin is not incorporated into clots, and that platelets are not a source of circulating reelin.
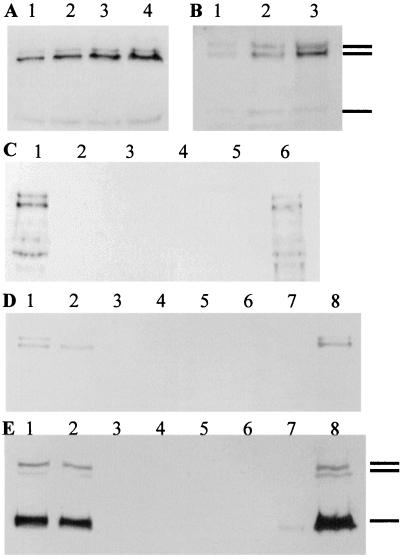
Figure 1.
Reelin-like immunoreactivity in serum, adrenal, and brain extracts of various species. (A) Serum from an adult rat (loaded at 1, 2, 3, and 4 μl per lane) was Western-blotted with G10 antibody. (B) Serum from an adult human (loaded at 1, 2, and 3 μl per lane) was blotted with antibody 142. (C–E) No reelin-like immunoreactivity is detectable in serum, adrenal glands, or brain extracts of rl/rl mice. (C) Adult mice (4–6 months old). Lanes: 1, wild-type +/+ mouse; 2, blank; 3 and 4, rl/rl mice; 5, blank; 6, wild-type mouse. (D) Three-week-old mice were sacrificed and perfused with saline, and adrenal extracts were prepared. Lanes: 1 and 2, rl/+ mice; 3, blank; 4–6, rl/rl mice; 7, blank; 8, rl/+ mouse. Protein was adjusted to ≈60 μg in lanes 1–6 and 140 μg in lane 8. (E) Brain extracts of the same mice shown in D. Protein was adjusted to ≈70 μg in lanes 1–2 and ≈140 μg in lanes 3–8. Marks indicate 420-, 310-, and 160-kDa bands.
Figure 6.
Reelin-immunoreactive bands in liver in situ and in vitro. (A) Adult rat liver extracted and Western-blotted by using antireelin antibody G10. Lane 1: calibration standard (cerebellar granule cell-conditioned medium). Lane 2: liver extract. (B) Adult rat liver was dissociated into single cells by perfusion with collagenase, followed by sieving to remove tissue clumps. Cells were plated in collagen-coated dishes at ≈80% confluence, allowed to recover in full feeding medium containing serum for 4 hr, rinsed five times in minimal serum-free medium (Williams' E medium with BSA, glutamine, and antibiotics), and then incubated with this medium with changes immediately, after 5 hr, and overnight. Conditioned medium was harvested, spun to remove cellular debris, and assayed for reelin by Western blotting. Lanes: 1 and 2, conditioned medium collected overnight from duplicate culture wells shows that reelin was released primarily as the full-length, 420-kDa reelin gene product; 3 and 4, same as lanes 1 and 2 but incubated in the presence of dexamethasone (2 μM); 5, calibration standard (cerebellar granule cell-conditioned medium). As a control, no reelin-like immunoreactivity was observed in the conditioned medium incubated with liver cells momentarily, and more reelin was observed after overnight incubation than after 5 hr, indicating that the release of reelin was time-dependent (not shown). Extracts of the liver cells examined after overnight incubation also contained a predominant, 420-kDa reelin band that was decreased in the presence of dexamethasone (not shown).
Most important, none of the G10-immunoreactive bands were observed in serum samples from rl/rl mice (Fig. 1C). In fact, rl/+ mice expressed about half the content of 420-kDa reelin in plasma as did wild-type littermates [4.2 ± 1.0 fmol/ml Reln H equivalents (see Methods) vs. 9.5 ± 1.6 fmol/ml; n = 4 adult male mice in each group, significant at P = 0.05]. Therefore, we conclude that the bands recognized by antireelin antibodies consist of authentic reelin (420 kDa) and metabolites or processed forms of authentic reelin (310 and 160 kDa). Reelin was detected in the serum of rats at all ages examined, from the day of birth (P0) to maturity (P88).
To estimate the relative abundance of reelin-related bands in the plasma vs. the brain, samples of both tissues were Western-blotted in parallel with calibrated amounts of Reln H fusion protein. As shown in Table 1, the steady-state plasma content of 420-kDa full-length reelin was about 4% of that observed in an equal volume of adult frontal cortex, whereas the plasma content of the major 310-kDa reelin-immunoreactive band was 52% of that in frontal cortex. It is difficult to assess the relative amounts of functional reelin in the two sources because it is unknown which reelin-related band(s) are biologically active and because much of the reelin extracted from brain may be sequestered (i.e., present within neurons or bound to extracellular matrix). However, the qualitative data (Fig. 1) and estimated quantitation (Table 1) suggest that reelin found in the blood is unlikely to arise simply as an impurity because of brain reelin leaking into the peripheral circulation. As well, the steady-state amount of reelin expressed in the circulation appears to be adequate to exert a putative function in the periphery.
Table 1.
Estimation of reelin-like immunoreactivity in plasma (fmol/ml) vs. brain (fmol/g wet weight)
| Tissue | 420 kDa | 310 kDa |
|---|---|---|
| Plasma | 9 ± 3 | 57 ± 16 |
| Brain | 255 ± 25 | 110 ± 25 |
Frontoparietal cortex and plasma from three wild-type B6C3F mice (2 months old) were Western-blotted by using G10 antibody, using Reln H fusion protein (25) as reference standard and expressing reelin-like immunoreactivity as Reln H protein equivalents.
Reelin Immunoreactivity in Adrenal and Pituitary Glands.
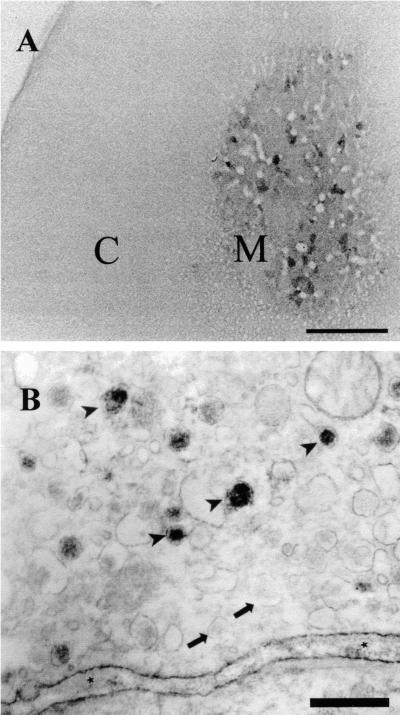
Because reelin has been associated with neural tissue, we examined a number of neurosecretory glands for reelin expression. In vibratome sections of adult rat adrenal gland, using antireelin antibodies G10 and 142, reelin-like immunoreactivity was observed in clusters of 2–10 chromaffin cells scattered throughout the adrenal medulla; adrenal cortex was negative (Fig. 2A). Using the confocal microscope, one could appreciate that reelin-like immunostaining in chromaffin cells had a granular appearance within the cytoplasm and excluded the nucleus (Fig. 3A). This pattern of immunostaining was not observed when the primary antibody was omitted. The data extend previous evidence by Ikeda and Terashima that adrenal chromaffin cells express reelin mRNA during fetal development (24). At the electron microscopy level, reelin-like immunostaining was observed in the cytoplasm in a subset of the chromaffin cells (Fig. 2B). There was a strong apparent association with some but not all chromaffin granules; the immunoreactivity appeared to permeate the chromaffin granules (Fig. 2B), suggesting that reelin is packaged within the granules and not simply adhering to the outside. By Western blotting, G10-immunoreactive bands were readily observed in adrenal extracts of adult rats and in 3-week-old rl/+ mice but were not detectable in rl/rl littermates (Figs. 1D and 4A). Reelin also was detected in highly enriched cultures of bovine chromaffin cells purified by differential absorption to the substratum (30) by Western blotting and immunocytochemistry using antibody 142 (not shown).
Figure 2.
Adult rat adrenal gland reacted with antireelin antibody G10. (A) Low-power photo. C, adrenal cortex; M, adrenal medulla. Scattered clusters of chromaffin cells are positive, whereas the cortex is negative. (Bar = 0.4 mm.) (B) Immunoelectron microscopy of chromaffin cells. Dense reelin-like immunoreactivity is observed in a subset of chromaffin granules. Arrowheads show reelin-positive granules; arrows show reelin-negative granules. Asterisks indicate reelin-like immunoreactivity within the extracellular space. (Bar = 0.5 μm.)
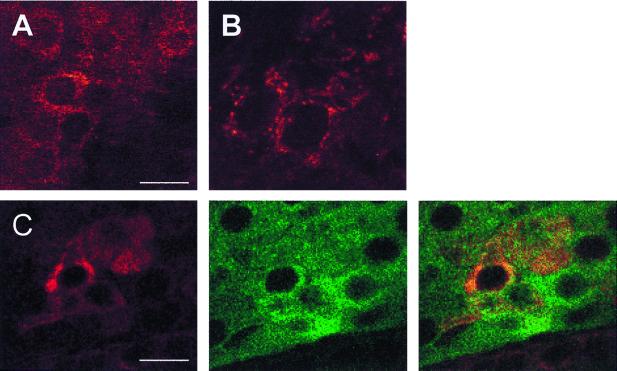
Figure 3.
Reelin-like immunostaining in adult rat adrenal medulla and pituitary pars intermedia viewed by confocal microscopy. (A) Adrenal chromaffin cells, stained with antireelin antibody 142. (Bar = 20 μm.) (B) Pituitary pars intermedia, stained with antireelin antibody G10. (C) Double labeling of pituitary pars intermedia cells for reelin and α-MSH. (Left) Antibody G10. (Bar = 10 μm.) (Center) Anti-MSH. (Right) Overlay shows that a subset of the α-MSH-positive cells express reelin.
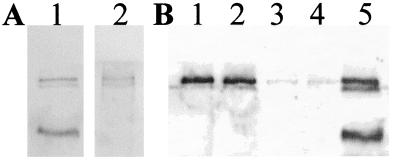
Figure 4.
Immunoblotting of adult rat adrenal and pituitary glands. Rats were perfused extensively with saline, and then glands from several animals were pooled and extracted. An equal amount of protein (50 μg) was loaded on each lane. (A) Western blotted with antireelin antibody G10. (B) Same blot reblotted with anti-DAB1 antibody. Lanes: 1, adrenal gland; 2, intermediate-posterior pituitary dissected as a unit; 3, anterior pituitary. Marks indicate the position of molecular mass standards at 207, 121, and 81 kDa.
Reelin-like immunoreactivity was abundant within certain cells of the pituitary pars intermedia but was undetectable within the anterior or posterior lobes (Fig. 5A and data not shown). As in the adrenal medulla, the pars intermedia reelin-positive cells tended to reside in small clusters, and the reelin immunostaining had a granular appearance within the cytoplasm, which excluded the nucleus (Fig. 3B). Using an antibody recognizing α-MSH as a marker for hormone-secreting cells, we double-labeled pituitary sections with antibody G10, and by laser confocal microscopy we found that the reelin-positive cells represented a subset (≈20% of the total) of the α-MSH-positive cells within the pars intermedia (Fig. 3C). For Western blotting analyses of the rat pituitary, the gland was dissected into two parts—anterior lobe vs. the intermediate/posterior lobes—and each was assayed separately. Reelin-like immunoreactive bands that comigrated with brain reelin were abundant in the preparation including the intermediate lobe (Fig. 4A).
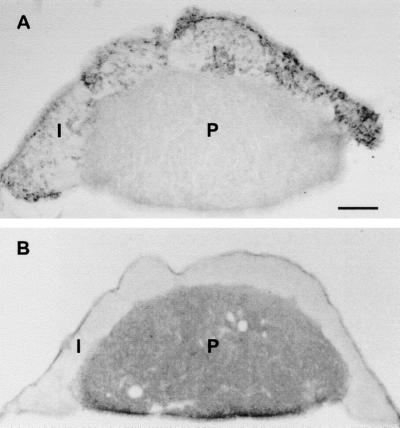
Figure 5.
Low-power photo of pituitary (intermediate and posterior lobes). (A) Reacted with antireelin antibody G10. Scattered clusters of cells are observed throughout the intermediate lobe (I), whereas the posterior lobe (P) is negative. (B) Reacted with anti-DAB1 antibody. The intermediate lobe is negative, whereas strong immunoreactivity is observed throughout the posterior lobe. (Bar = 0.2 mm.)
In adult rats, adrenalectomy, adrenal demedulloectomy, hypophysectomy, or splenectomy/thymectomy failed to decrease full-length reelin in the serum relative to sham-operated controls, as assessed by immunoblotting at 6 or 14 days after surgery. The 310-kDa, reelin-derived fragment was reduced significantly after adrenal and pituitary removal, an effect that was reversed by supplementing rats with slow-release corticosterone pellets.
Liver Expression of Reelin mRNA and Reelin Protein.
Not only does the liver synthesize most of the proteins found in the blood, but both fetal and/or adult liver can express detectable amounts of reelin mRNA in mouse and human (5, 23, 24, 31). To measure directly the abundance of full-length reelin mRNA in liver, a competitive reverse transcription–PCR assay with internal standard was performed (primers: forward, base pairs 9211–9234; reverse, base pairs 9549–9572) (20, 22). Liver from adult mice (n = 3) expressed 238 ± 13 attomol/μg total RNA, whereas the cerebral cortex (frontal pole) of the same mice assayed in parallel expressed 683 ± 46 attomol/μg total RNA. In contrast, spleen and thymus mRNA levels were very low, less than 3 attomol/μg total RNA. As a control to ensure that the assay was measuring authentic reelin mRNA, under the same conditions, no reelin mRNA was detected in the liver of rl/rl mouse.
Reelin protein also was detectable in extracts of adult rat liver (Fig. 6A); it is unlikely that this simply reflects reelin-related bands expressed in the blood that is trapped within the tissue, because the most abundant band seen in liver was 420-kDa full-length reelin (cf. Fig. 1). As well, when liver cells were acutely dissociated, rinsed extensively, and placed in serum-free medium overnight, reelin was detectable both in the cells and in the conditioned medium, where it appeared primarily as the 420-kDa full-length product (Fig. 6B). Dexamethasone (2 μM) greatly inhibited the accumulation of reelin in both the conditioned medium (Fig. 6B) and the liver cell extracts, suggesting that reelin production and/or turnover in the liver can be modulated by hormonal influences. This was not a toxic side effect, because dexamethasone treatment caused a marked increase in the accumulation of fibronectin in the conditioned medium and did not alter β-actin levels in the liver cell extracts (not shown). When well perfused rat liver was examined by immunocytochemistry, little reelin-like immunoreactivity was observed except for a zone surrounding the sinusoids, indicating that reelin is not stored appreciably by cells within the liver.
DAB1-Like Immunoreactivity in Peripheral Tissues That Contain Reelin.
To learn whether DAB1 was expressed in peripheral tissues shown to contain reelin, we carried out immunocytochemistry and Western blotting by using an affinity-purified antibody specific for DAB1. A few strongly DAB1-positive cell bodies and processes were noted within the rat adrenal medulla that had the distinctive cytologic appearance of parasympathetic ganglion cells (not shown). This is consistent with previous evidence that peripheral neurons and peripheral nerves express DAB1 during earlier, embryonic stages (9). Double-labeling studies showed that some cells were positive for both DAB1 and choline acetyltransferase, confirming that these are cholinergic. Some of the chromaffin cells also appeared to be faintly labeled with DAB1 antibody. The adrenal cortex failed to show any DAB1-like immunoreactivity. Western blotting of adrenal extracts revealed a single, prominent, DAB1-immunoreactive band at 80 kDa (Fig. 4B). In the rat pituitary, strong, diffuse DAB1-like immunoreactivity was observed throughout the posterior lobe (Fig. 5B), whereas the intermediate and anterior lobes were negative. By Western blotting, DAB1-like immunoreactive bands at ≈100 and 60 kDa were observed in extracts that included intermediate lobe but were not detectable in the anterior pituitary (Fig. 4B). DAB1 protein was not detectable in adult rat liver, either by immunocytochemistry or Western blotting.
Discussion
A circulating pool of reelin is readily detectable in adult mice, rats, and humans. This pool is not released from platelets and is unlikely to reflect a leakage of brain reelin into the periphery. Reelin protein is expressed in specific peripheral cell types of adult mammals, including chromaffin cells within the adrenal medulla and α-MSH-secreting cells within the pituitary pars intermedia. However, these tissues do not contribute substantially to the pool of circulating reelin. Rather, the liver synthesizes and secretes reelin very likely in amounts that may be important in maintaining blood reelin levels, although it cannot be excluded that other peripheral tissues may also secrete reelin. These findings stimulate investigation of the potential roles that circulating reelin may play in extra-central nervous system tissues.
In developing and adult brain, reelin-expressing cells are observed to be adjacent to DAB1-immunopositive cells that represent putative targets for reelin action. In the adrenal medulla, DAB1-like immunoreactivity was observed in parasympathetic ganglion cells that are known to make synaptic contacts with chromaffin cells. In the pituitary, reelin was expressed in a subset of intermediate lobe cells, whereas DAB1-like immunoreactivity was expressed by cells of the adjacent posterior lobe. The intermediate and posterior lobes are not synaptically connected; rather, cells from one lobe are thought to secrete products into the portal circulation that affect the other (e.g., ref. 32). These findings raise the possibility that DAB1 may be a molecular target for reelin action postnatally in peripheral neurons and in the posterior pituitary. In contrast, DAB1 protein was not detected within the liver, which is consistent with the suggestion that liver-derived reelin is secreted into the blood for action on distal target(s). Further work is needed to understand how reelin synthesis and release are regulated physiologically in the liver and to identify potential peripheral targets for reelin action.
The presence of reelin in blood, an accessible bodily fluid, makes it feasible to study reelin gene expression and function in living humans. For example, our laboratory recently has described the existence of polymorphisms in the human reelin gene in pedigrees of schizophrenic patients (33, 34). Examining circulating reelin and its metabolites in people bearing reelin polymorphisms may help us to learn whether these different reelin genotypes are associated with alterations in reelin synthesis, processing, or steady-state levels. If so, then measurements of circulating reelin are likely to be relevant in making important decisions related to preventive treatment of individuals potentially at risk of psychosis.
Acknowledgments
We thank Drs. Jim Artwohl, Carolyn Bruzdzinski, Mei Ling Chen, Yogesh Dwivedi, Lech Kiedrowski, Rhonda Kineman, andNicholas Kriho (all at University of Illinois, Chicago) for expert advice and invaluable assistance. Dr. Gabriella D'Arcangelo (St. Jude Children's Hospital, Memphis, TN) generously provided full-length reelin cDNA clones. Finally, we are indebted to Drs. Andre Goffinet (University of Namur, Belgium) and Brian Howell (National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD) for reading the manuscript and offering insightful, critical suggestions. This work is supported by National Institutes of Health Grant NS 28931.
Abbreviation
- α-MSH
α-melanocyte-stimulating hormone
References
- 1.Rakic P, Caviness V S. Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 2.Lambert de Rouvroit C, Goffinet A M. Adv Anat Embryol Cell Biol. 1998;150:1–108. [PubMed] [Google Scholar]
- 3.D'Arcangelo G, Miao G G, Chen S-C, Soares H D, Morgan J I, Curran T. Nature (London) 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 5.Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, et al. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- 6.D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curran T, D'Arcangelo G. Brain Res Rev. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- 8.De Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet A M, Miyata T, Ogawa M, Mikoshiba K. Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- 9.Howell B W, Gertler F B, Cooper J A. EMBO J. 1997;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell B W, Hawkes R, Soriano P, Cooper J A. Nature (London) 1997;389:733–736. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 11.Sheldon M, Rice D S, D'Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell B W, Cooper J A, Goldowitz D, Curran T. Nature (London) 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 12.Howell B W, Herrick T M, Cooper J A. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice D S, Sheldon M, D'Arcangelo G, Nakajima K, Goldowitz D, Curran T. Development. 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- 14.D'Arcangelo G, Homayouni R, Keshvara L, Rice D S, Sheldon M, Curran T. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 15.Hiesberger T, Trommsdorff M, Howell B W, Goffinet A, Mumby M C, Cooper J A, Herz J. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 16.Schiffmann S N, Bernier B, Goffinet A M. Eur J Neurosci. 1997;9:1055–1071. doi: 10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 17.Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesold C, Impagnatiello F, Pisu M G, Costa E, Guidotti A, Caruncho H J. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pesold C, Liu W S, Guidotti A, Costa E, Caruncho H J. Proc Natl Acad Sci USA. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H, Pisu M G, Uzunov D P, Smalheiser N R, Davis J M, Pandey G N, et al. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadj-Sahraoui N, Frederic F, Delhaye-Bouchaud N, Mariani J. J Neurogenet. 1996;11:45–58. doi: 10.3109/01677069609107062. [DOI] [PubMed] [Google Scholar]
- 22.Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. NeuroReport. 1999;10:1–6. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- 23.DeSilva U, D'Arcangelo G, Braden V V, Chen J, Miao G G, Curran T, Green E D. Genome Res. 1997;7:157–164. doi: 10.1101/gr.7.2.157. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Terashima T. Dev Dyn. 1997;210:157–172. doi: 10.1002/(SICI)1097-0177(199710)210:2<157::AID-AJA8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.De Bergeyck V, Naerhuyzen B, Goffinet A M, Lambert de Rouvroit C. J Neurosci Methods. 1998;82:17–24. doi: 10.1016/s0165-0270(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 26.Eipper B A, Mains R E. Endocrine Rev. 1980;1:1–7. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Smalheiser N R, Schwartz N B. Proc Natl Acad Sci USA. 1987;84:6457–6461. doi: 10.1073/pnas.84.18.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H-Y, Kriho V, Lieska N, Pappas G D. J Comp Neurol. 1996;371:461–468. doi: 10.1002/(SICI)1096-9861(19960729)371:3<461::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Lambert de Rouvroit C, de Bergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet A. Exp Neurol. 1999;156:214–217. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- 30.Michalewicz P, Laurito C E, Pappas G D, Lu Y, Yeomans D C. Cell Transplant. 1999;8:103–109. doi: 10.1177/096368979900800103. [DOI] [PubMed] [Google Scholar]
- 31.Lambert de Rouvroit C, Bernier B, Royaux I, de Bergeyck V, Goffinet A M. Exp Neurol. 1999;156:229–238. doi: 10.1006/exnr.1999.7019. [DOI] [PubMed] [Google Scholar]
- 32.Knepel W, Meyer D K. J Physiol (London) 1983;341:507–515. doi: 10.1113/jphysiol.1983.sp014820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uzunov DP, Impagnatiello F, Sharma R, Guidotti A, Costa E. Soc Neurosci Abstr. 1998;24:525. [Google Scholar]
- 34.Uzunov DP, Grayson DR, Sharma R, Guidotti A, Costa E. Soc Neurosci Abstr. 1999;25:1293. [Google Scholar]
- 35.Lacor, P. N., Grayson, D. R., Auta, J., Costa, E. & Guidotti, A. (2000) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]