Abstract
The transcription factor NF-κB, a central regulator of immunity, is subject to regulation by redox changes. We now report that cysteine-179 of the inhibitory κB kinase (IKK) β-subunit of the IKK signalosome is a central target for oxidative inactivation by means of S-glutathionylation. S-glutathionylation of IKK-β Cys-179 is reversed by glutaredoxin (GRX), which restores kinase activity. Conversely, GRX1 knockdown sensitizes cells to oxidative inactivation of IKK-β and dampens TNF-α-induced IKK and NF-κB activation. Primary tracheal epithelial cells from Glrx1-deficient mice display reduced NF-κB DNA binding, RelA nuclear translocation, and MIP-2 (macrophage inflammatory protein 2) and keratinocyte-derived chemokine production in response to LPS. Collectively, these findings demonstrate the physiological relevance of the S-glutathionylation–GRX redox module in controlling the magnitude of activation of the NF-κB pathway.
Keywords: H2O2, TNF, sulfenic acid
In recent years, inflammatory diseases have been associated with oxidative stress. For instance, lung pathologies such as asthma are accompanied by elevated amounts of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), that are produced both by infiltrating inflammatory cells and resident cells that express nonphagocytic NADPH oxidases (1). Although ROS damage macromolecules, there is mounting evidence that they can also serve as regulators of cellular processes.
Among the most susceptible oxidant-sensitive targets are protein thiol groups, which can be reversibly oxidized to sulfenic acid (−SOH) or irreversibly oxidized to sulfinic (−SO2H) and sulfonic (−SO3H) acid, although sulfiredoxin recently has been found to specifically reduce the sulfinic acid moiety in peroxiredoxins (2–4). Reversible oxidations are believed to protect proteins from irreversible oxidation but may also modulate protein function. The sulfenic acid moiety is very unstable and readily reacts with other thiols to form intra- or intermolecular disulfides. For example, the reaction with glutathione (GSH) to form S-glutathionylated proteins is an important posttranslational modification that affects the function of proteins such as H-ras (5), actin (6), and HIV-1 protease (7). Furthermore, both the transcription factors NF-κB and AP-1 (activator protein 1) demonstrate reduced DNA-binding activity in vitro after S-glutathionylation of their respective p50 (8) and c-Jun subunits (9).
S-glutathionylation is regulated by glutaredoxins (GRXs) or thioltransferases, members of the thiol-disulfide oxidoreductase family that contain a thioredoxin fold (10). To date, two mammalian GRX enzymes have been characterized. GRX1 is a cytosolic protein, whereas alternative splicing of the primary RNA transcript controls subcellular trafficking of GRX2 to mitochondria and the nucleus (11–13). GRXs catalyze the reversible reduction of protein-glutathionyl mixed disulfides to free sulfhydryl groups through a monothiol mechanism (14, 15). In this reaction, GRX itself is S-glutathionylated at Cys-22, and the reduced state of GRX is restored by GSH coupled to glutathione disulfide (GSSG) reductase (15). GRX2 can also be reduced by the thioredoxin/thioredoxin reductase system (12). In contrast to bacterial GRX, mammalian GRX display substrate specificity toward S-glutathionylated proteins (14, 16) and could therefore play a unique role in redox signaling.
NF-κB is a transcription factor that is constituted by homo- or heterodimers of the Rel protein family with a pivotal role in inflammation, cell survival, and proliferation. In unstimulated cells, NF-κB is maintained in a latent form in the cytoplasm by means of sequestration by inhibitory κB (IκB) proteins. NF-κB activating stimuli, such as cytokines, viruses, and LPS, induce the degradation of IκBs by the proteasome, unmasking the nuclear localization signal of NF-κB, resulting in its nuclear translocation, binding to NF-κB motifs, and gene transcription. The enzyme complex that is responsible for phosphorylation of IκBs on specific serine residues is IκB kinase (IKK), a large, 700- to 900-kDa complex consisting of at least two catalytic subunits: IKK-α and IKK-β (17, 18). Knockout studies have revealed that IKK-β is responsible for the proinflammatory cytokine-induced activation of NF-κB (19). IKK-α, on the other hand, is crucial for B cell maturation and p100 processing, as well as activating NF-κB-dependent gene transcription by phosphorylating histone H3 (20, 21). IKK-γ is the regulatory subunit (22). The mechanisms that activate the IKK complex are not completely understood, but it is known that activation requires phosphorylation of Ser-177 and Ser-181 in the activation loop of IKK-β (23).
Although IKK is essential for NF-κB activation in response to most stimuli, IKK is also subject to negative regulation to prevent activation of NF-κB. For example, arsenite (24), cyclopentenone prostaglandins (25), S-nitrosothiols (26), and some antiinflammatory drugs (27, 28) have been reported to inhibit IKK-β by means of targeting a critical cysteine residue, resulting in a failure to activate NF-κB. Thereby, covalent modification of Cys-179 provides a powerful mode by which antiinflammatory agents repress NF-κB. It has not been established whether the inactivation of IKK-β after oxidation of Cys-179 is reversible, permitting rapid regeneration of IKK-β activity and propagation of inflammatory signals.
Previously, we established that H2O2 inhibits TNF-stimulated IKK-β activity by means of oxidation of IKK-β (29). The aim of the present study was to investigate the mode of oxidation and identify the critical target that is responsible for the inhibitory effects of H2O2 on IKK-β. We also sought to determine whether H2O2-induced oxidative inactivation is reversible and to elucidate the redox systems that restore IKK activity and examine their impact on cytokine-induced NF-κB activation.
Results
Inhibition of IKK Activity Through Reversible Oxidation of Cys-179 of the IKK-β Subunit.
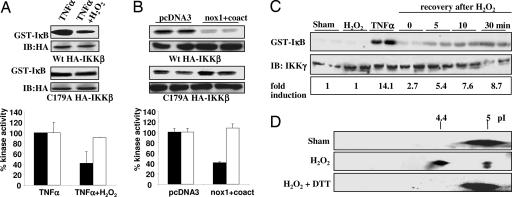
Because it has been established that S-nitrosothiols, arsenite, and cyclopentenone prostaglandins inhibit IKK activity through oxidation of Cys-179 of the β-subunit, we first investigated whether inhibition by H2O2 also occurs by means of oxidation of this residue. H2O2 added exogenously (Fig. 1A), or produced endogenously by expression of nox1 plus its coactivators, p41 and p51 (Fig. 1B), markedly inhibited IKK-β activity induced by TNF-α (Calbiochem, La Jolla, CA) in cells expressing WT HA-IKK-β. In contrast, a construct containing a Cys-179-Ala mutant version of HA-IKK-β was largely refractory to inhibition by H2O2, indicating that H2O2 inhibits IKK-β through oxidation of Cys-179.
Fig. 1.
Inhibition of IKK activity through reversible oxidation of Cys-179 of IKK-β. (A) C10 cells transfected with WT (filled bars) or Cys-179-Ala (open bars) HA-IKK-β expression vectors were exposed to 10 ng/ml TNF-α alone or in combination with 200 μM H2O2 for 5 min, and IKK activity was assessed. (B) C10 cells transfected with WT (filled bars) or Cys-179-Ala (open bars) HA-IKK-β expression vectors in combination with pcDNA3 or expression vectors of Nox1 plus its coactivators, p41 and p51, were stimulated with 10 ng/ml TNF-α for 5 min before assessment of IKK-β activity. (A and B) Blots labeled “IB:HA” are Western blots for HA, and graphs show quantification by phosphoimage analysis. (C) C10 cells were treated with 200 μM H2O2 for 5 min, washed, and supplied with fresh media. (Top) At different time points thereafter, cells were stimulated with 10 ng/ml TNF-α for 5 min and assayed for IKK-β activity. (Middle) Western blot for IKK-γ. (Bottom) Quantification by phosphoimage analysis. (D) C10 cells were transfected with WT HA-IKK-β and exposed to 200 μM H2O2 for 5 min, and oxidation of IKK-β was assessed by two-dimensional gel electrophoresis. To determine the reversibility of oxidation, cells were incubated with 10 mM DTT for 30 min after treatment with H2O2.
To determine whether inhibition of IKK-β by H2O2 is reversible, cells were treated with H2O2 for 5 min, washed, and, at different times thereafter, stimulated with TNF-α. The ability of TNF-α to activate IKK-β started to recover as soon as 5 min after removal of H2O2, with an ≈61% recovery of maximal TNF-α-stimulated activity (Fig. 1C). Two-dimensional gel electrophoresis demonstrated that H2O2 induced a shift in the apparent pI of WT HA-IKK-β from 5 to 4.4, which was fully reversed by DTT (Fig. 1D), consistent with reversible oxidation of IKK-β.
S-Glutathionylation of IKK-β by Means of the Formation of a Sulfenic Acid Intermediate.
To investigate how Cys-179 of IKK-β is modified under oxidizing conditions, we used a synthetic 15-aa peptide corresponding to the primary sequence of mouse IKK-β containing Cys-179 (IKK-β 173–187; Fig. 6A, which is published as supporting information on the PNAS web site). Analysis by electrospray ionization-liquid chromatography MS revealed S-glutathionylation of the peptide in the presence of H2O2 and GSH, indicated by a mass increase of 305 Da, which could be fully reversed by incubation with DTT (Table 1, which is published as supporting information on the PNAS web site) and prevented by pretreatment with the sulfhydryl-specific alkylating agent N-ethylmaleimide (data not shown). These findings indicate that GSH incorporation occurred at Cys-179, which was confirmed by MS/MS (data not shown). Incubation of the IKK-β 173–187 peptide with GSSG similarly resulted in S-glutathionylation (Fig. 6B and Table 1). Despite not being able to directly detect sulfenic acid in IKK-β 173–187 after exposure to H2O2 alone, presumably because it further reacted with a second peptide to form a disulfide, H2O2-mediated S-glutathionylation of IKK-β 173–187 (Table 1 and Fig. 6C) or immunoprecipitated WT HA-IKK-β (Fig. 6D) was prevented in the presence of the sulfenic acid trapping agent dimedone. Quantitative assessment of cellular levels of GSH, GSSG, or S-glutathionylated proteins (PSSG) in response to 200 μM H2O2 (Table 2, which is published as supporting information on the PNAS web site) revealed transient decreases in GSH, which were accompanied by 3-fold increases in PSSG. In contrast, cellular levels of GSSG increased only modestly, suggesting that protein S-glutathionylation likely occurred by protein sulfenic acid formation rather than by intermediate formation of GSSG.
S-Glutathionylation of Cys-179 of IKK-β Corresponds to Inactivation by H2O2.
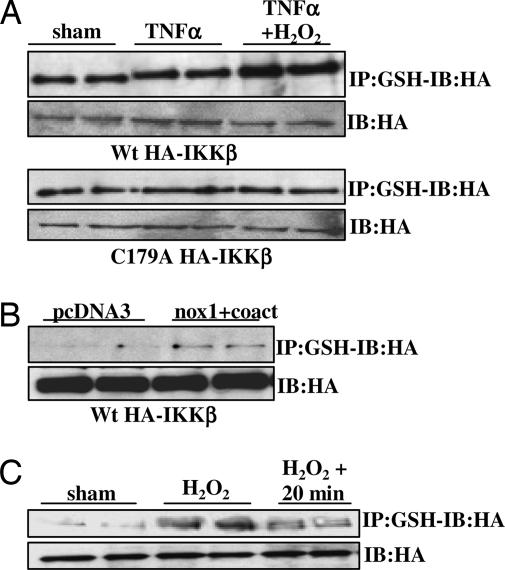
To assess whether S-glutathionylation of Cys-179 is responsible for the observed reversible inhibition of IKK in H2O2-exposed cells, we used two methods to demonstrate S-glutathionylation of IKK-β. First, S-glutathionylated proteins were precipitated from extracts of cells expressing either WT or Cys-179-Ala HA-IKK-β by using an antibody directed against GSH (Virogen, Watertown, MA). As is demonstrated in Fig. 2A, H2O2 caused an increase in S-glutathionylation of WT IKK-β but not the Cys-179-Ala mutant; in contrast, TNF-α itself had no effect on S-glutathionylation of IKK-β, nor did it influence S-glutathionylation of IKK-β by H2O2. Alternatively, cells were preloaded with biotinylated glutathione ethyl ester (Bio-GEE) before H2O2 treatment. Immunoprecipitation of HA-tagged IKK-β followed by blotting with streptavidin-HRP demonstrated enhanced binding of Bio-GEE to WT IKK-β, but not the Cys-179-Ala mutant, in response to H2O2 treatment (Fig. 7, which is published as supporting information on the PNAS web site). As a control, an H2O2-treated sample was resolved under reducing conditions (DTT), which resulted in a loss of biotin reactivity (Fig. 7), consistent with the reversibility of S-glutathionylation (Table 1). Furthermore, endogenous generation of H2O2 by overexpression of nox1 plus its coactivators also induced S-glutathionylation of IKK-β (Fig. 2B), corresponding to the repression of IKK activity seen in Fig. 1B. Lastly, in agreement with the partial recovery of the kinase activity of IKK-β 20 min after removal of H2O2 (Fig. 1C), S-glutathionylation of IKK-β was markedly diminished by that time (Fig. 2C). Collectively, these data demonstrate that S-glutathionylation of Cys-179 of IKK-β is associated with the reversible inhibition of kinase activity by H2O2.
Fig. 2.
S-glutathionylation of Cys-179 of IKK-β corresponds to inactivation by H2O2. (A) C10 cells transfected with WT or Cys-179-Ala HA-IKK-β expression vectors were exposed to 10 ng/ml TNF-α with or without 200 μM H2O2 for 5 min. S-glutathionylated proteins were immunoprecipitated by using an antibody against GSH, followed by detection of HA-IKK-β by Western blotting. (B) C10 cells were transfected with WT HA-IKK-β plus Nox1, p41, and p51 expression vectors, and S-glutathionylation was investigated as in A. (C) C10 cells transfected with WT HA-IKK-β expression vector were treated with 200 μM H2O2 for 5 min. Cells were washed, supplied with fresh media, and harvested after 20 min. S-glutathionylation of IKK-β was investigated as in A. Blots labeled “IB:HA” are control Western blots for HA.
GRX1 Modulates the Inhibitory Effects of H2O2 on IKK-β and NF-κB.
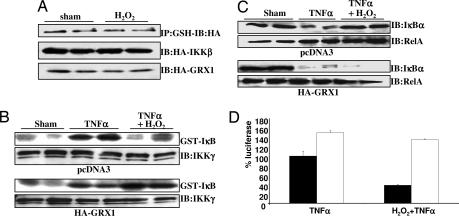
Our findings demonstrate that cells recover IKK-β activity rapidly upon removal of H2O2 (Fig. 1C), corresponding to the reversal of S-glutathionylation (Fig. 2D). S-glutathionylation may therefore constitute an important regulatory switch, allowing rapid regeneration of IKK enzymatic activity through GRX-dependent catalysis. To directly test this hypothesis, we overexpressed cytosolic GRX1 (Fig. 8, which is published as supporting information on the PNAS web site), which abolished the increase in H2O2-induced S-glutathionylation of IKK-β (Fig. 3A) and allowed activation of IKK enzymatic activity by TNF-α in the presence of H2O2 (Fig. 3B). Consequently, overexpression of GRX1 resulted in marked TNF-α-induced degradation of IκB-α (Fig. 3C) and recovery of NF-κB transcriptional activity (Fig. 3D) in the presence of H2O2. These data demonstrate that S-glutathionylation of Cys-179 of IKK-β by H2O2 is indeed responsible for inhibition of IKK-β activity and that inhibitory effects of H2O2 can be overcome by GRX1 overexpression, allowing activation of IKK-β and NF-κB in the presence of H2O2.
Fig. 3.
GRX1 modulates the inhibitory effects of H2O2 on IKK-β and NF-κB. (A Top) Cells were treated with 200 μM H2O2 for 5 min, and S-glutathionylation of IKK-β was assessed as in Fig. 2A. (Middle and Bottom) Control Western blots for HA-IKK-β and HA-GRX1, respectively. (B and C) C10 cells overexpressing HA-GRX1 were exposed to agents as before and evaluated after 5 min for IKK activity (B) or after 15 min for IκB-α levels (C). The level of RelA was measured as a loading control. (D) Cells were cotransfected with 6x κB-tk-luc reporter vector and pcDNA3 (filled bars) or HA-GRX1 (open bars) expression vectors and exposed to 200 μM H2O2 for 5 min before treatment with 10 ng/ml TNF-α for 6 h. Luciferase units were corrected for the amount of protein and expressed as the percentage of pcDNA3-transfected, TNF-α-stimulated luciferase activity.
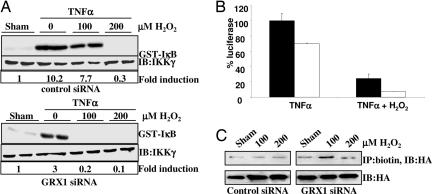
Based on our observations that GRX1 activity is present in C10 cells (Fig. 8), we used siRNA to knock down expression of GRX1. Results shown in Fig. 9A, which is published as supporting information on the PNAS web site, demonstrate that GRX1 siRNA resulted in the specific knockdown of GRX1 mRNA by 60%, whereas GRX2 mRNA expression was unaffected. GRX1 protein levels and activity were inhibited to the same extent by siRNA (Fig. 9B). Importantly, siRNA knockdown of GRX1 greatly sensitized cells for inhibition of IKK-β by H2O2 (Fig. 4A), leading to enhanced repression of NF-κB transcriptional activity (Fig. 4B). Moreover, the ability of TNF-α to activate IKK-β and NF-κB transcriptional activity was also markedly attenuated after knockdown of GRX1. Concomitantly, knockdown of GRX1 increased the basal level of S-glutathionylation of IKK-β and enhanced the sensitivity of IKK-β to H2O2-induced S-glutathionylation (Fig. 4C). Together, these results indicate that GRX1 expression levels play an important role in controlling the magnitude of activation of the NF-κB pathway through regulation of S-glutathionylation of IKK-β.
Fig. 4.
GRX1 knockdown dampens IKK-β and NF-κB activation. (A) C10 cells were transfected with control or GRX1 siRNA and treated with TNF-α in the presence or absence of H2O2 and analyzed as described before. (Upper) IKK activity. (Lower) IKK-γ Western blots. “Fold induction” shows fold increases in IKK activity over sham controls, based on phosphoimage analysis. (B) C10 cells stably transfected with 6x κB-tk-luc were transfected with control siRNA (filled bars) or siRNA for GRX1 (open bars) and treated with 200 μM H2O2 and 10 ng/ml TNF-α for 6 h. Luciferase units were corrected for the amount of protein and expressed as the percentage of control siRNA TNF-α-stimulated luciferase activity. (C) C10 cells were transfected with HA-IKK-β and control or GRX1 siRNA. Cells were loaded with Bio-GEE and treated with H2O2. Biotinylated proteins were immunoprecipitated by using a biotin antibody, followed by detection of IKK-β by Western blotting for HA. Blots labeled “IB:HA” are control Western blots for HA.
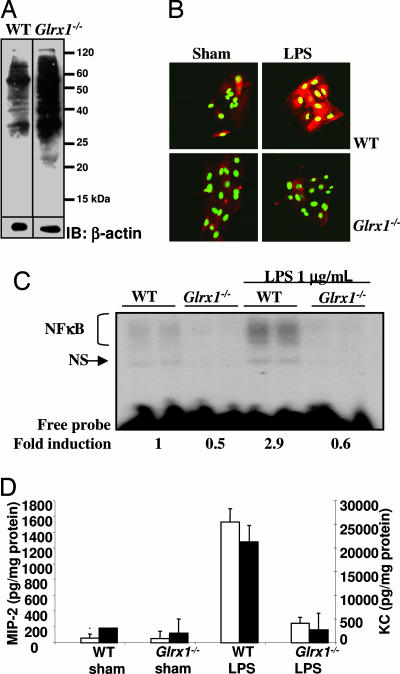
Airway Epithelial Cells from Glrx1-Deficient Mice Display Attenuated Activation of NF-κB, MIP-2, and Keratinocyte-Derived Chemokine Production in Response to LPS.
To further address the physiological relevance of GRX1-controlled IKK and NF-κB activation, we cultured primary tracheal epithelial cells (MTE) from WT or Glrx1−/− mice and assessed the magnitude of activation of NF-κB by LPS. Compared with WT cells, Glrx1−/− cells have elevated levels of S-glutathionylated proteins (Fig. 5A). Importantly, MTE cells derived from Glrx1−/− mice stimulated with LPS displayed no apparent nuclear translocation of RelA, whereas marked RelA nuclear presence was observed in WT cells exposed to LPS (Fig. 5B). Similarly, NF-κB DNA-binding activity in response to LPS was also absent in Glrx−/− MTE cells, in contrast to WT cells, which show the expected increases in DNA-binding activity in response to LPS (Fig. 5C). Lastly, Glrx1-deficient MTE cells produced markedly reduced levels of the chemokines MIP-2 (macrophage inflammatory protein 2) and keratinocyte-derived chemokine after treatment with LPS when compared with MTE cells isolated from WT mice (Fig. 5D), illustrating the importance of GRX1 in the regulation of expression of proinflammatory mediators in association with its control over activation of the NF-κB pathway.
Fig. 5.
Attenuation of NF-κB activation and chemokine production by primary airway epithelial cells from Glrx1−/− mice in response to LPS. GRX1 activity in WT cells was 37.4 units (undetectable in Glrx1−/− cells). (A) Primary tracheal epithelial cells (MTE) were loaded with 1.5 mM Bio-GEE for 1 h, lysates were resolved by nondenaturing SDS/PAGE, and blots were reacted with streptavidin-HRP. IB:β-actin is a loading control. (B) WT or Glrx1−/− MTE cells were treated with 1 μg/ml LPS for 4 h for evaluation of RelA nuclear translocation. Red, RelA immunoreactivity; green, nuclear Sytox green counterstain. (C) WT or Glrx1−/− MTE cells were treated with 1 μg/ml LPS for 6 h, and NF-κB DNA binding was assessed by EMSA. NS, nonspecific binding. (D) WT or Glrx1−/− MTE cells were treated with 1 μg/ml LPS for 24 h, and concentrations of keratinocyte-derived chemokine (KC) (filled bars) and MIP-2 (open bars) were assessed by ELISA on culture media and corrected for protein content.
Discussion
Oxidation of Cys-179 as a Repressive Mechanism for IKK Activation.
We have demonstrated in this study that Cys-179 of the β-subunit of the IKK complex is a direct target for reversible S-glutathionylation and that S-glutathionylation is responsible for the repression of kinase activity by H2O2. Cys-179 has previously been demonstrated to be redox sensitive and to be oxidized by arsenite (24) and S-nitrosothiols (26), among others. Cys-179 is strategically located in the activation loop between Ser-177 and Ser-181, which are required to be phosphorylated for IKK enzymatic activity. It is conceivable that oxidation of Cys-179 could interfere with phosphorylation of neighboring Ser residues, but evidence in the previous studies argues against this hypothesis (24, 26). Alternatively, oxidation of Cys-179 could prevent the binding of substrate or accessory proteins to the complex or promote dephosphorylation of the neighboring Ser residues. Although additional investigation is needed to unravel the exact mechanism of oxidant inhibition of IKK activity, multiple studies have now underscored the importance of Cys-179 in the redox regulation of the IKK complex and consequent NF-κB activation.
Redox Regulation of the NF-κB Activation Pathway at Multiple Levels.
It has been well established that NF-κB is a redox-sensitive transcription factor. For example, S-glutathionylation of Cys-62 of the p50 subunit is known to prevent binding of the transcription factor to κB sites in the promoter regions of genes (8, 34). Recent work from our laboratory has demonstrated that the prerequisite NF-κB-activating enzyme IKK-β is also regulated by oxidants (29), providing a second mode of redox control that occurs proximal to DNA binding. The observation that IKK activity remains partially repressed despite reversibility of its proper oxidation (Fig. 1 C and D) suggests that H2O2 may also interfere with the activation pathway upstream of the kinase complex. In support of this possibility, our laboratory has demonstrated that H2O2 alters the recruitment of adapter proteins to TNF receptor 1, leading to repression of IKK and increased JNK activation in response to TNF-α (35).
S-Glutathionylation of IKK-β by Means of a Sulfenic Acid Intermediate.
The biochemical mechanisms by which proteins are S-glutathionylated involve either intermediate formation of protein sulfenic acid moieties that subsequently react with GSH or accumulation of GSSG, which then reacts with susceptible protein Cys residues by means of a thiol exchange mechanism. Studies with the IKK-β peptide and the intact IKK complex (Fig. 6 and Table 1) indicate that H2O2-mediated S-glutathionylation of IKK-β occurs through a sulfenic acid intermediate, because it was prevented by a sulfenic acid reactive compound, dimedone. Although GSSG is capable of glutathionylating the IKK-β peptide IKK (Fig. 6B), in vitro kinase assays indicate that the observed levels of GSSG found in H2O2-treated cells (Table 2) are insufficient to inactivate IKK (data not shown). Indeed, the redox potential of most Cys residues is such that the ratio of GSSG versus GSH in cells would need to change ≈100-fold to induce S-glutathionylation through a thiol exchange mechanism (10), which we did not observe, perhaps in part because of the buffering capacity of GSSG reductase. These considerations suggest that thiol exchange is probably not a significant mechanism for S-glutathionylation of IKK-β in vivo and favor direct intermediate oxidation of Cys-179 to a sulfenic acid.
GRX1-Controlled NF-κB Activation.
S-glutathionylation is considered a redox-dependent posttranslational modification with potential relevance to signal transduction. The existence of antioxidant enzymes that serve the unique role of specifically reducing GSH mixed disulfides emphasizes the importance of S-glutathionylation in modulating protein function. Our findings demonstrate that modulation of GRX1 levels, through both overexpression and knockdown by RNA interference, influences S-glutathionylation and consequently impinges on IKK and NF-κB activation after H2O2 or TNF-α exposure. The strongly attenuated responses of primary airway epithelial cells from Glrx1−/− mice to LPS with regard to NF-κB DNA binding, RelA nuclear translocation, and chemokine production (Fig. 5) further support a role for GRX1 in regulating inflammation by controlling the magnitude of activation of the NF-κB pathway.
Collectively, the present data demonstrate that S-glutathionylation is a physiologically relevant mechanism for controlling the magnitude of activation of the NF-κB pathway. We propose that GRX1-dependent reversal of S-glutathionylation of IKK-β constitutes a protective mechanism that modulates the extent and timing of activation of NF-κB in response to redox changes by protecting IKK-β from irreversible inactivation (36) and allowing for rapid regeneration of enzymatic activity (Fig. 10, which is published as supporting information on the PNAS web site). As emerging studies document the relevance of H2O2 as a second messenger (37) and unravel the intricacies of redox control of biological processes, our findings suggest broad implications in diverse (patho)physiological conditions, many of which have been causally linked to the activation of NF-κB and oxidative stress.
Materials and Methods
Cell Culture and Reagents.
A line of spontaneously transformed mouse alveolar type II epithelial cells (C10) was propagated in CMRL-1066 tissue culture medium containing 50 units/ml penicillin/50 μg/ml streptomycin (P/S), 2 mM l-glutamine, and 10% FBS (all from GIBCO-BRL, Carlsbad, CA). One hour before adding the test agents, cells were switched to phenol red-free DMEM/F12 containing P/S and 0.5% FBS. Primary tracheal epithelial cells were isolated from homozygous GRX1 knockout (Glrx1−/−) mice in a C57BL/6 and 129SV hybrid background and genetically matched WT mice according to the methods of Wu and Smith (30), with minor modifications (31). The knockout mice were generated by deleting exons 1 and 2 of the Glrx1 gene, resulting in abolishment of GRX1 expression in all tissues (Y.-S.H., unpublished data). For experiments, primary cells were plated on collagen I-coated culture dishes or glass slides. The Institutional Animal Care and Use Committee of the University of Vermont granted approval for all procedures. Reagents were purchased from Sigma (St. Louis, MO) unless stated otherwise. Experiments were repeated at least three times.
Kinase Assays.
C10 cells were exposed to test agents, transferred to ice, washed twice with PBS, and lysed in buffer containing 50 mM Hepes, 150 mM NaCl, 1 mM EDTA, 2 mM MgCl2, 10 mM Na3VO4, 1 mM PMSF, 0.1% Nonidet P-40, 10 μg/ml leupeptin, 1% aprotenin, 250 μΜ DTT, and 100 μM NaF. The IKK complex was immunoprecipitated from 200 μg of protein extract with an IKK-γ (Santa Cruz Biotechnology, Santa Cruz, CA) or HA (Upstate, Charlottesville, VA) antibody by using protein G agarose beads. Precipitates were washed once with lysis buffer and twice with kinase buffer (20 mM Hepes/20 mM β-glycerophosphate/1 mM MnCl2/5 mM MgCl2/2 mM NaF/250 μΜ DTT). Kinase reactions were performed with 1 μg of GST-IκB-α as a substrate (provided by R. Ten, Mayo Clinic, Rochester, MN) and 5 μCi (1 Ci = 37 GBq) of [γ-32P]adenosine triphosphate at 30°C for 30 min. Reactions were stopped by addition of 2× Laemmli sample buffer. Samples were boiled and separated on 15% polyacrylamide gel; gels were dried and examined by autoradiography. Results were quantified by phosphoimaging.
Western Blotting.
Cell lysates were resolved on polyacrylamide gels and transferred to nitrocellulose, and levels of IκB-α, IKK-γ, IKK-β, RelA, and HA were detected according to the following immunoblotting protocol. Membranes blocked in TBS/5% milk were washed two times in TBS containing 0.05% Tween 20 (TBS-Tween) and incubated with primary antibodies for 1 h at room temperature (RT). Membranes were washed three times in TBS-Tween and incubated with a peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at RT. After three washes with TBS-Tween, conjugated peroxidase was detected by chemiluminescence (Amersham Pharmacia Biosciences, Piscataway, NJ) according to the manufacturer’s instructions.
Two-Dimensional Gel Electrophoresis.
At the indicated times, cells were washed twice with 10 mM Tris and 25 mM sorbitol (pH 7.0) and lysed in sample buffer containing 9 M urea, 2% CHAPS, 1 mM EDTA, 4 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 0.8% ampholytes (Amersham Pharmacia Biosciences), 0.007% bromophenol blue, and 10 mM iodoacetamide. Lysates were cleared by centrifugation and applied to an immobilized pH gradient (pH 3–10, 13 cm; Amersham Pharmacia Biosciences). Strips were rehydrated with sample for 10 h at 50 V, and isoelectric focusing was performed for a total of 13,299 Vh at a maximum of 8,000 V. Each strip was equilibrated two times for 15 min in buffer containing 6 M urea, 30% glycerol, 50 mM Tris·HCl (pH 6.8), 0.007% bromophenol blue, 2% SDS, and 65 mM DTT. The second dimension was resolved by SDS/10% PAGE, proteins were transferred to nitrocellulose, and immunoblotting was performed as described above.
GRX1 Vector Construction and Transfection.
The expression vectors of HA-tagged WT and Cys-179-Ala mutant IKK-β (HA-IKK-β) were a gift from M. Karin (University of California at San Diego, La Jolla, CA). The expression vectors of nox1, p41 nox, and p51 nox were a gift from J. D. Lambeth (Emory University, Atlanta, GA), and HA-tagged mouse GRX1 expression plasmid was made as described in ref. 31. Plasmids were transfected into cells by using Lipofectamine plus (Invitrogen, Carlsbad, CA) according to the manufacturer’s directions. Two hours after transfection with nox1 and 24 h after transfection with other plasmids, test reagents were added. Control and GRX1 siRNA were purchased from Ambion (Austin, TX) and transfected into cells at a concentration of 20 nM by using siPORTamine, and test agents were added 48 h later.
Bio-GEE Preparation and Assessment of S-Glutathionylation.
Bio-GEE was prepared as described in ref. 32. Cells were loaded with 1.5 mM Bio-GEE for 1 h before the addition of test agents and lysed in buffer containing 25 mM Hepes (pH 7.7), 0.1 mM EDTA, 0.01 mM neocuproine, 0.5% CHAPS, and 20 mM N-ethylmaleimide. After resolution by SDS/PAGE, proteins were transferred to nitrocellulose, and membranes were blocked in TBS containing 5% BSA at RT. After two washes in TBS-Tween, membranes were incubated with streptavidin-peroxidase for 4 h at RT to detect biotinylated proteins. Alternatively, biotinylated proteins were immunoprecipitated by using an antibody directed against biotin, followed by detection of HA-IKK-β by immunoblotting.
NF-κB Luciferase Reporter Assay.
A stable C10 cell line harboring a 6x κB-tk-luc reporter gene (29) was transfected with plasmids or siRNA. Luciferase units were corrected for the total amount of protein.
EMSA.
To assay DNA-binding activity of NF-κB complexes, binding to a radiolabeled double-stranded oligonucleotide containing a NF-κB consensus sequence was analyzed (Promega, Madison, WI). Nuclear extracts and gel-shift assays were prepared as described in ref. 33.
RelA Immunostaining.
Cells were fixed with 4% paraformaldehyde for 30 min at RT and permeabilized for 20 min by using 0.2% Triton X-100. After blocking with 1% BSA for 30 min, cells were incubated with an antibody directed against RelA (Santa Cruz Biotechnology) for 1 h, followed by a 1-h incubation with an Alexa Fluor 568-conjugated secondary antibody. Nuclei were counterstained with Sytox green (Molecular Probes, Eugene, OR). Sections were scanned by using a BX50 upright microscope (Olympus, Center Valley, PA) configured to an MRX 1000 confocal scanning laser microscope system (Bio-Rad, Hercules, CA).
ELISA.
MIP-2 and keratinocyte-derived chemokine concentrations were determined in cell culture media by using a DuoSet ELISA kit (R & D Systems, Minneapolis, MN). Values were normalized to a standard curve, corrected for protein content, and expressed as pg/mg of protein.
Supplementary Material
Acknowledgments
We thank Dr. Alan Howe (University of Vermont) for assistance with two-dimensional gel electrophoresis. This work was supported by National Institutes of Health Grants R01 HL60014 and HL60812, Public Health Service Grants P20 RL15557 (National Center for Research Resources/Centers of Biomedical Research Excellence) and P01 HL074295, and a grant from the Dutch Asthma Foundation.
Abbreviations
- IκB
inhibitory κB
- IKK
IκB kinase
- GRX
glutaredoxin
- Bio-GEE
biotinylated glutathione ethyl ester
- GSH
glutathione
- GSSG
glutathione disulfide
- RT
room temperature.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lambeth J. D. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 2.Budanov A. V., Sablina A. A., Feinstein E., Koonin E. V., Chumakov P. M. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 3.Woo H. A., Jeong W., Chang T. S., Park K. J., Park S. J., Yang J. S., Rhee S. G. J. Biol. Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 4.Chang T. S., Jeong W., Woo H. A., Lee S. M., Park S., Rhee S. G. J. Biol. Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 5.Mallis R. J., Buss J. E., Thomas J. A. Biochem. J. 2001;355:145–153. doi: 10.1042/0264-6021:3550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Boja E. S., Tan W., Tekle E., Fales H. M., English S., Mieyal J. J., Chock P. B. J. Biol. Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 7.Davis D. A., Dorsey K., Wingfield P. T., Stahl S. J., Kaufman J., Fales H. M., Levine R. L. Biochemistry. 1996;35:2482–2488. doi: 10.1021/bi951525k. [DOI] [PubMed] [Google Scholar]
- 8.Pineda-Molina E., Klatt P., Vazquez J., Marina A., Garcia de Lacoba M., Perez-Sala D., Lamas S. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 9.Klatt P., Molina E. P., De Lacoba M. G., Padilla C. A., Martinez-Galesteo E., Barcena J. A., Lamas S. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 10.Shelton M. D., Chock P. B., Mieyal J. J. Antioxid. Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 11.Su D., Gladyshev V. N. Biochemistry. 2004;43:12177–12188. doi: 10.1021/bi048478t. [DOI] [PubMed] [Google Scholar]
- 12.Gladyshev V. N., Liu A., Novoselov S. V., Krysan K., Sun Q. A., Kryukov V. M., Kryukov G. V., Lou M. F. J. Biol. Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg M., Johansson C., Chandra J., Enoksson M., Jacobsson G., Ljung J., Johansson M., Holmgren A. J. Biol. Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 14.Gravina S. A., Mieyal J. J. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Jao S., Nanduri S., Starke D. W., Mieyal J. J., Qin J. Biochemistry. 1998;37:17145–17156. doi: 10.1021/bi9806504. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes A. P., Holmgren A. Antioxid. Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 17.DiDonato J. A., Hayakawa M., Rothwarf D. M., Zandi E., Karin M. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 18.Mercurio F., Zhu H., Murray B. W., Shevchenko A., Bennett B. L., Li J., Young D. B., Barbosa M., Mann M., Manning A., Rao A. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 19.May M. J., Ghosh S. Science. 1999;284:271–273. doi: 10.1126/science.284.5412.271. [DOI] [PubMed] [Google Scholar]
- 20.Anest V., Hanson J. L., Cogswell P. C., Steinbrecher K. A., Strahl B. D., Baldwin A. S. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y., Verma U. N., Prajapati S., Kwak Y. T., Gaynor R. B. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 22.Rothwarf D. M., Zandi E., Natoli G., Karin M. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 23.Delhase M., Hayakawa M., Chen Y., Karin M. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 24.Kapahi P., Takahashi T., Natoli G., Adams S. R., Chen Y., Tsien R. Y., Karin M. J. Biol. Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 25.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M. G. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 26.Reynaert N. L., Ckless K., Korn S. H., Vos N., Guala A. S., Wouters E. F., van der Vliet A., Janssen-Heininger Y. M. Proc. Natl. Acad. Sci. USA. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon K. I., Byun M. S., Jue D. M. Exp. Mol. Med. 2003;35:61–66. doi: 10.1038/emm.2003.9. [DOI] [PubMed] [Google Scholar]
- 28.Kwok B. H., Koh B., Ndubuisi M. I., Elofsson M., Crews C. M. Chem. Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 29.Korn S. H., Wouters E. F., Vos N., Janssen-Heininger Y. M. J. Biol. Chem. 2001;276:35693–35700. doi: 10.1074/jbc.M104321200. [DOI] [PubMed] [Google Scholar]
- 30.Wu R., Smith D. In Vitro. 1982;18:800–812. doi: 10.1007/BF02796504. [DOI] [PubMed] [Google Scholar]
- 31.Reynaert N. L., Ckless K., Guala A. S., Wouters E. F., van der Vliet A., Janssen-Heininger Y. M. Biochim. Biophys. Acta. 2006;1760:380–387. doi: 10.1016/j.bbagen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Demasi M., Silva G. M., Netto L. E. J. Biol. Chem. 2003;278:679–685. doi: 10.1074/jbc.M209282200. [DOI] [PubMed] [Google Scholar]
- 33.Janssen Y. M., Driscoll K. E., Howard B., Quinlan T. R., Treadwell M., Barchowsky A., Mossman B. T. Am. J. Pathol. 1997;151:389–401. [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews J. R., Botting C. H., Panico M., Morris H. R., Hay R. T. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantano C., Shrivastava P., McElhinney B., Janssen-Heininger Y. J. Biol. Chem. 2003;278:44091–44096. doi: 10.1074/jbc.M308487200. [DOI] [PubMed] [Google Scholar]
- 36.Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schoneich C., Cohen R. A. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 37.Finkel T. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.