Abstract
IL-10-producing CD4+ type 1 regulatory T (Tr1) cells play a critical role in the maintenance of peripheral tolerance. Although immunosuppressive drugs, cytokines, costimulatory molecules, and immature dendritic cells are implicated in the induction of Tr1 cells, the signals that negatively regulate the generation and function of Tr1 cells have been elusive. We report that OX40 ligand (OX40L) completely inhibited the generation of IL-10-producing Tr1 cells from naïve and memory CD4+ T cells induced by the immunosuppressive drugs dexamethasone and vitamin D3. This unique function of OX40L was not shared by two costimulatory TNF family members, GITR ligand and 4-1BB ligand. OX40L strongly inhibited the generation of IL-10-producing Tr1 cells induced by two physiologic stimuli, the inducible costimulatory ligand and immature dendritic cells. In addition, OX40L strongly inhibited IL-10 production and suppressive function of differentiated IL-10-producing Tr1 cells. These two novel functions of OX40L shed light on the mechanism by which OX40/OX40L regulates immunity and tolerance.
Keywords: dendritic cell, dexamethasone, inducible costimulator ligand, TNF superfamily, vitamin D3
IL-10-producing CD4+ type 1 regulatory T (Tr1) cells were originally isolated from patients with severe combined immunodeficiency who had undergone successful HLA-mismatched bone marrow transplantation (1, 2). Subsequently, IL-10-producing Tr1 cells were generated from naïve CD4+ T cells during antigen-driven T cell immune responses (3, 4). IL-10-producing Tr1 cells are anergic in response to signaling through T cell receptor, CD28, and IL-2 receptors and can suppress antigen-driven proliferation of naïve CD4+ T cells in vivo and in vitro (5, 6). IL-10-producing Tr1 cells can also inhibit the development of autoimmune diseases (7) and limit the magnitude of immune responses to microbial pathogens (4, 8, 9).
The molecular mechanisms underlying the generation of IL-10-producing Tr1 cells are not well understood but have been extensively studied during the past decade. IL-10 and IFN-α have been described to induce the generation of IL-10-producing Tr1 cells from naïve CD4+ T cells activated through T cell receptor and CD28 (10). Most strikingly, a strategy to inhibit T helper (TH) 1 or TH2 differentiation by using anti-IL-12 and anti-IL-4 Abs, together with a combination of dexamethasone and the active form of vitamin D3 (Dex + Vit D3), was shown to induce the generation of large numbers of IL-10-producing Tr1 cells from naïve CD4+ T cells activated through T cell receptor and CD28 (7). In addition, immature dendritic cells (DCs) and DCs treated with IL-10 or IFN-α were shown to induce naïve CD4+ T cells to differentiate into IL-10-producing Tr1 cells (6, 11–13). Signaling through the inducible costimulator (ICOS) on CD4+ T cells by ICOS ligand (ICOSL) also promoted their differentiation into IL-10-producing Tr1 cells (14–16). However, little is known about the molecular signals that negatively regulate the generation of IL-10-producing Tr1 cells.
We recently reported that OX40 ligand (OX40L), a member of the TNF superfamily, strongly inhibits IL-10 production during TH2 responses (17). This observation stimulated us to investigate whether OX40L can suppress the generation of IL-10-producing Tr1 cells. Here we demonstrate a function of OX40L in the negative regulation of the generation and function of IL-10-producing Tr1 cells induced by the immunosuppressive agents dexamethasone and vitamin D3, ICOSL, or immature DCs. Our results revealed a general mechanism by which OX40L enhances immunity and breaks immunological tolerance.
Results
OX40L Inhibits the Generation of IL-10-Producing Tr1 Cells from CD4+ T Cells Induced by Dexamethasone and Vitamin D3.
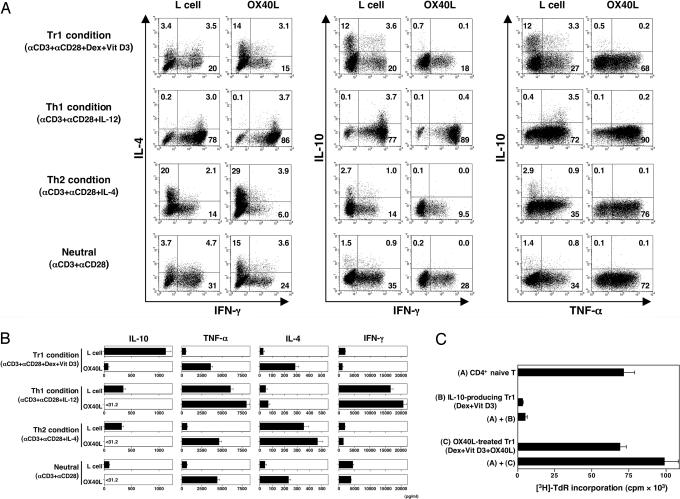
A previous study showed that a combination of the immunosuppressive drugs dexamethasone and vitamin D3 consistently induced the differentiation of naïve CD4+ T cells into IL-10-producing Tr1 cells (7). To investigate whether OX40L can inhibit the generation of IL-10-producing Tr1 cells, we cultured naïve CD4+ T cells with anti-CD3 plus anti-CD28 mAbs in the presence or absence of OX40L-transfected L cells in four different culture conditions for 7 days: Tr1 (Dex + Vit D3), TH1 (IL-12), TH2 (IL-4), or neutral (medium alone). IL-10 production by the primed T cells was analyzed by intracellular cytokine staining and ELISA. Between 2% and 4% of IL-10-producing T cells were generated from naïve CD4+ T cells cultured in neutral or TH1 or TH2 conditions (Fig. 1A). Most impressively, >15% of IL-10-producing T cells were generated in culture with Dex + Vit D3, which confirmed the results of the earlier study (7). Addition of OX40L completely blocked the generation of IL-10-producing T cells while promoting the generation of TNF-α-producing T cells in all culture conditions. These data were confirmed by ELISA (Fig. 1B).
Fig. 1.
OX40L inhibits the generation and function of IL-10-producing Tr1 cells from naïve CD4+ T cells induced by different polarizing signals. CD4+ naïve T cells were cultured with anti-CD3 and anti-CD28 mAbs in the presence of IL-2 on parental L cells or OX40L-L cells with the indicated recombinant cytokines or reagents for 7 days. (A) Cytokine production by CD4+ T cells was analyzed intracellularly by flow cytometry. Percentages of the respective cytokine-producing T cells are indicated. (B) Cytokine production was measured in supernatants after restimulation with anti-CD3 and anti-CD28 mAbs for 24 h by ELISA. Data are means ± SEM of four independent experiments. (C) Mixtures of indicated T cell populations were restimulated for 5 days by anti-CD3 and anti-CD28 mAbs. Suppressive function was assessed by [3H]thymidine incorporation. Error bars represent the SEM of triplicate wells. Data are representative of three independent experiments.
As previously reported (18), naïve CD4+ T cells primed with the Tr1 condition (Dex + Vit D3) were anergic and suppressed the proliferation of naïve CD4+ T cells in response to anti-CD3 plus anti-CD28 mAbs (Fig. 1C). We also showed that naïve CD4+ T cells primed with the same Tr1 condition in the presence of OX40L proliferated vigorously and failed to inhibit the proliferation of naïve CD4+ T cells in response to anti-CD3 plus anti-CD28 mAbs. These data suggested that OX40L blocks the generation of functional Tr1 cells from naïve CD4+ T cells induced by Dex + Vit D3.
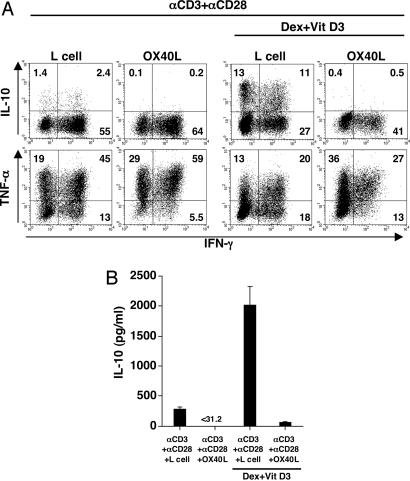
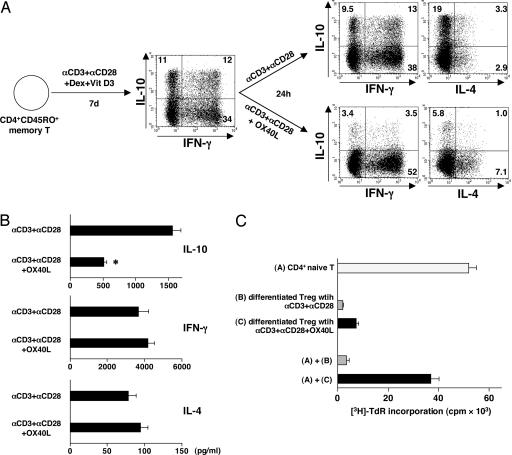
We next investigated whether IL-10-producing Tr1 cells can be generated from CD4+CD45RA−CD45RO+ memory T cells and whether OX40L can inhibit the generation of IL-10-producing Tr1 cells from CD4+ memory T cells. CD4+CD45RA−CD45RO+ memory T cells were cultured for 7 days with anti-CD3 plus anti-CD28 mAbs in the presence or absence of OX40L-transfected L cells in the Tr1 condition (Dex + Vit D3). Approximately 25% of IL-10-producing cells were generated from CD4+ memory T cells in culture with Dex + Vit D3 (Fig. 2A). Furthermore, addition of OX40L completely blocked the generation of IL-10-producing T cells and promoted the generation of TNF-α-producing cells from CD4+ memory T cells. These findings were confirmed by IL-10 ELISA analyses (Fig. 2B).
Fig. 2.
OX40L inhibits the generation of IL-10-producing Tr1 cells from memory CD4+ T cells under a condition with Dex + Vit D3. CD4+CD45RO+ memory T cells were cultured with anti-CD3 mAb, anti-CD28 mAb, and IL-2 on parental L cells or OX40L-L cells in the presence or absence of Dex + Vit D3 for 7 days. (A) Cytokine production by CD4+ T cells was analyzed intracellularly by flow cytometry. Percentages of the respective cytokine-producing T cells are indicated. (B) IL-10 production was measured in supernatants after restimulation with anti-CD3 and anti-CD28 mAbs for 24 h by ELISA. Data are means ± SEM of four independent experiments.
OX40L but Not Other TNF Family Members Inhibits the Generation of IL-10-Producing Tr1 Cells.
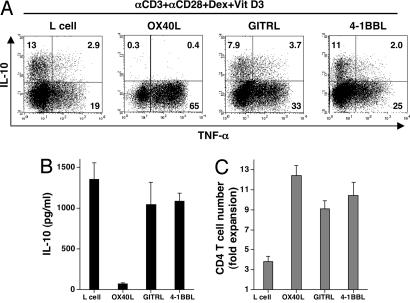
Within the TNF superfamily, OX40L, glucocorticoid-induced TNF receptor ligand (GITRL), and 4-1BB ligand (4-1BBL) have costimulatory function for T cells (19). To investigate whether OX40L is unique in the inhibition of IL-10-producing Tr1 cells, naïve CD4+ T cells were cultured with anti-CD3 plus anti-CD28 mAbs with Dex + Vit D3, with parental L cells or L cells transfected with OX40L, GITRL, or 4-1BBL for 7 days. Although OX40L, GITRL, and 4-1BBL all promoted the generation of TNF-α-producing cells, only OX40L inhibited the generation of IL-10-producing T cells (Fig. 3A and B). OX40L, GITRL, and 4-1BBL all promoted the expansion of T cells (Fig. 3C). These data suggested that, among the three members of the TNF superfamily known to costimulate T cells, OX40L has a novel and unique function in inhibiting the generation of IL-10-producing Tr1 cells.
Fig. 3.
OX40L but not GITRL or 4-1BBL inhibits the generation of IL-10-producing Tr1 cells. CD4+ naïve T cells were cultured with anti-CD3 mAb, anti-CD28 mAb, and IL-2 on parental L cells, OX40L-L cells, GITRL-L cells, or 4-1BBL-L cells in the presence of Dex + Vit D3 for 7 days. (A) IL-10 and TNF-α production by CD4+ T cells was analyzed intracellularly by flow cytometry. Percentages of the respective cytokine-producing T cells are indicated. (B) IL-10 production was measured in supernatants after restimulation with anti-CD3 and anti-CD28 mAbs for 24 h by ELISA. (C) The number of viable T cells was counted. In B and C, data are means ± SEM of four independent experiments.
OX40L Inhibits the Generation of IL-10-Producing Tr1 Cells Induced by ICOSL or Immature DCs.
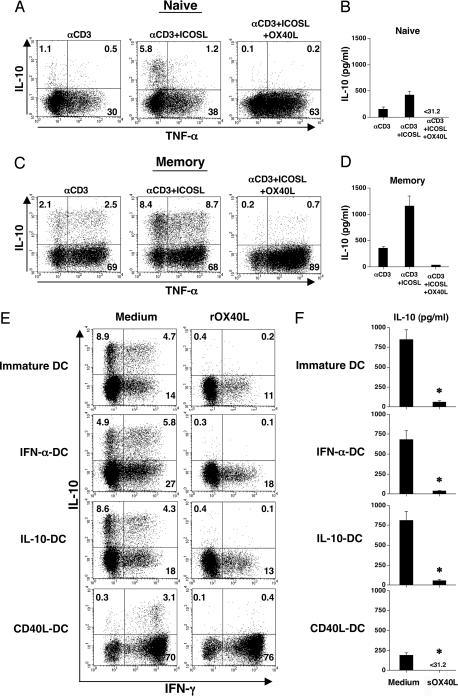
ICOS and CD28 represent the two positive costimulatory receptors within the CD28 family that are expressed on T cells. Signaling through ICOS by agonistic Abs or ICOSL has been shown to promote CD4+ T cell production of IL-10 (14, 16). To investigate whether OX40L can inhibit the ability of ICOS to induce IL-10 production by CD4+ T cells, naïve and memory CD4+ T cells were cultured with anti-CD3 mAb in the presence of ICOSL-transfected L cells in the presence or absence of OX40L for 7 days. We found that ICOSL significantly promoted the generation of IL-10-producing cells from both naïve (Fig. 4A and B) and memory (Fig. 4 C and D) CD4+ T cells. Addition of OX40L completely inhibited the generation of IL-10-producing cells from both naïve and memory CD4+ T cells while strongly promoting the generation of cells producing TNF-α.
Fig. 4.
OX40L inhibits the generation of IL-10-producing Tr1 cells from CD4+ T cells induced by ICOSL or immature DCs. (A–D) CD4+ naïve and memory T cells were cultured for 7 days on parental L cells, a mixture of ICOSL-L cells and L cells, or a mixture of ICOSL-L cells and OX40L-L cells, which were precoated with anti-CD3 mAb. (E and F) CD4+ naïve T cells were cocultured with immature DCs or DCs precultured with IFN-α, IL-10, or CD40L in round-bottom 96-well culture plates in the presence or absence of the soluble recombinant OX40L for 7 days. (A, C, and E) IL-10 and IFN-γ production by CD4+ T cells was analyzed intracellularly by flow cytometry. Percentages of the respective cytokine-producing T cells are indicated. (B, D, and F) IL-10 production was measured in supernatants after restimulation with anti-CD3 and anti-CD28 mAbs for 24 h by ELISA. Statistical significance was determined by using a paired Student t test. Data are means ± SEM of three independent experiments. sOX40L, soluble recombinant OX40L. ∗, P < 0.05.
As previous studies have demonstrated (6, 11–13), immature DCs or DCs treated with IFN-α or IL-10, but not mature DCs induced by CD40L, can induce naïve CD4+ T cells to differentiate into IL-10-producing Tr1 cells. We investigated whether OX40L could inhibit the generation of IL-10-producing Tr1 cells induced by DCs. Immature DCs or DCs treated with IL-10 or IFN-α induced the generation of >10% of IL-10-producing T cells from naïve CD4+ T cells (Fig. 4E). By contrast, DCs activated by CD40L induced a strong TH1 response accompanied by the generation of ≈3% IL-10-producing Tr1 cells. Addition of soluble recombinant OX40L into DC–T cell cultures completely inhibited the generation of IL-10-producing T cells induced by immature DCs and DCs treated with IL-10 and IFN-α. In addition, OX40L inhibited the generation of the residual number of IL-10-producing T cells induced by the CD40L-activated mature DCs. The ability of OX40L to inhibit the generation of IL-10-producing T cells induced by DCs was confirmed by ELISA data (Fig. 4F). These data demonstrated that OX40L could inhibit the generation of IL-10-producing Tr1 cells induced by the more physiological signals provided by ICOSL and immature DCs.
OX40L Inhibits IL-10 Production and Suppressive Function of Differentiated Tr1 Cells.
To investigate whether OX40L could inhibit the function of differentiated Tr1 cells, we first cultured the CD4+CD45RO+ memory T cells with anti-CD3 mAb, anti-CD28 mAb, IL-2, and Dex + Vit D3 for 7 days. These memory T cell cultures normally gave rise to >20% of IL-10-producing Tr1 cells (Figs. 2A and 5A), which was ≈2 fold higher than that generated from the CD4+CD45RA+ naïve T cells (Fig. 1A). After 7 days of culture, the already differentiated CD4+ memory T cells were restimulated with anti-CD3 and anti-CD28 mAbs in the presence or absence of OX40L. More than 20% of IL-10-producing T cells were still observed after restimulation with anti-CD3 and anti-CD28 mAbs for 24 h (Fig. 5A), which produced >1,500 pg/ml IL-10 (Fig. 5B). Restimulation with OX40L dramatically inhibited the ability of these 20% of Tr1 cells to produce IL-10, indicated by both intracellular IL-10 staining (Fig. 5A) and IL-10-specific ELISA (Fig. 5B). In addition, OX40L could strongly inhibit the suppressive function of differentiated Tr1 cells on the proliferation of naïve CD4+ T cells activated by anti-CD3 plus anti-CD28 mAbs (Fig. 5C).
Fig. 5.
OX40L inhibits IL-10 production and function of already differentiated Tr1 cells. CD4+CD45RO+ memory T cells were first cultured for 7 days with anti-CD3 mAb, anti-CD28 mAb, and IL-2 on parental L cells in the presence of Dex + Vit D3. Cells were washed and then restimulated for 24 h with anti-CD3 mAb and anti-CD28 mAb in the presence of parental L cells or OX40L-L cells. (A) Cytokine production by CD4+ T cells was analyzed intracellularly by flow cytometry. Percentages of the respective cytokine-producing T cells are indicated. (B) Cytokine production was measured in supernatants after restimulation with anti-CD3 and anti-CD28 mAbs for 24 h by ELISA. Data are means ± SEM of three independent experiments. Statistical significance was determined by using a paired Student t test. ∗, P < 0.05. (C) Indicated T cell population alone or mixtures of indicated T cell populations were restimulated for 5 days by anti-CD3 and anti-CD28 mAbs. Suppressive function was assessed by [3H]thymidine incorporation. Error bars represent the SEM of triplicate wells. Data are representative of three independent experiments.
Discussion
OX40/OX40L represents a pair of costimulatory molecules critical for T cell proliferation, survival, cytokine production, and memory cell generation (19). Early in vitro experiments demonstrated that signaling through OX40 on CD4+ T cells leads to TH2 but not TH1 development (20, 21). These results were supported by in vivo studies showing that blocking OX40/OX40L interaction prevents the induction and maintenance of TH2-mediated allergic immune responses (21–23). However, other studies revealed that blocking OX40/OX40L interaction ameliorates or prevents TH1-mediated diseases (24–26). Furthermore, administration of soluble OX40L or gene transfer of OX40L into tumors has been shown to strongly enhance antitumor immunity in mice (27, 28). OX40/OX40L may also play a role in promoting CD8 T cell-mediated immune responses (29). Taken together, these data suggest that OX40/OX40L may be critical in the global regulation of peripheral immunity versus tolerance. Our finding that OX40L completely inhibited the generation of IL-10-producing Tr1 cells induced by the immunosuppressive drugs dexamethasone and vitamin D3, ICOSL, or immature DCs, together with the finding that OX40L strongly inhibit IL-10 production from differentiated Tr1 cells highlights a novel mechanism by which OX40L promotes immunity and breaks tolerance during different forms of CD4- or CD8-mediated immune responses.
It has been proposed that Tr1 cells are critical for peripheral tolerance through the production of IL-10 in vivo, in particular in limiting tissue damage to the host during inflammatory immune responses against different classes of microbial pathogens, including intracellular pathogens that preferentially induce TH1 immune responses or some extracellular parasites that preferentially induce TH2 immune responses (4–9, 30). Indeed, the generation of IL-10-producing Tr1 cells appears to accompany both TH1 and TH2 immune responses in vivo and in vitro, as demonstrated by us here and by others (2, 10, 31). The ability of OX40L to inhibit the generation of IL-10-producing Tr1 cells during IL-12-induced TH1 responses or IL-4-induced TH2 responses suggests that OX40L may control the magnitude of TH1- or TH2-mediated immune responses. The ability of OX40L to inhibit the generation of IL-10-producing Tr1 cells appears to be a unique property of OX40L, because the two other TNF family members (GITRL and 4-1BBL) do not have this functional property.
Many molecules have been identified that promote the generation of IL-10-producing Tr1 cells, including IL-10, IFN-α, ICOSL, and immunosuppressive compounds such as dexamethasone and vitamin D3 (7, 10, 13, 16, 32). OX40L represents a potent inhibitor for the generation of IL-10-producing Tr1 cells from both naïve and memory CD4+ T cells. This novel property of OX40/OX40L may explain a recent report showing that OX40 signaling allows anergic autoreactive T cells to acquire effector cell functions (33). Interestingly, a recent study also showed that OX40 signaling blocks the inhibitory function of CD4+CD25+ naturally occurring regulatory T cells (34).
The molecular mechanisms by which OX40L inhibits the generation of IL-10-producing Tr1 cells are currently unknown. A recent study in the mouse system shows that OX40 signaling directly activates NFATc1, which is critical for the induction of IL-4 gene expression (35). It will be interesting to investigate whether OX40 signaling also induces NFATc1 activation and whether NFATc1 activation directly induces IL-10 gene expression in human T cells. Recent studies highlight the function of ERK, phosphatidylinositol 3-kinase, p38 MAPK, and Syk kinase in Toll-like receptor-mediated and lectin receptor-mediated IL-10 production in myeloid cells (36–40). The function of these signaling molecules in the regulation of IL-10 gene expression within T cells remains to be determined.
In conclusion, we discovered a novel function of OX40/OX40L in the negative regulation of the generation and function of IL-10-producing Tr1 cells from human naïve and memory TH cells during different type of immune responses. This discovery may highlight a novel mechanism by which OX40/OX40L breaks immunologic tolerance in autoimmune diseases, allergic diseases, and antitumor immune responses. Targeting OX40/OX40L may allow the development of treatments for human allergic and autoimmune diseases, infectious diseases, and cancer.
Materials and Methods
L Cell Lines.
Human GITRL-, OX40L-, 4-1BBL-, and ICOSL-expressing L cells were generated by retroviral-mediated transduction. Briefly, the full-length coding sequences for human GITRL (GenBank accession no. NM_005092), OX40L (GenBank accession no. NM_003326), 4-1BBL (GenBank accession no. NM_003811), and ICOSL (GenBank accession no. NM_015259) were amplified by RT-PCR with RNA prepared from herpes simplex virus 1-stimulated peripheral blood mononuclear cells. Subsequently, the cDNAs were cloned into the murine stem cell virus-based retroviral vector pMIGW2, and the resulting plasmids were verified by restriction enzyme digestion and DNA sequencing. To produce recombinant retroviruses, each vector was cotransfected with the packaging constructs pCL-gp (gag/pol) and pHCMV-VSVg (VSV glycoprotein envelope) in HEK293T cells, as described (41). Two days later, the virus-containing culture supernatants were harvested and used to infect CD32 L cells at a multiplicity of infection of 100. Under these conditions, >95% of cells were productively transduced.
Generation of Monocyte-Derived DCs and Analyses of Surface Markers.
The isolation of monocytes and the generation of DCs from monocytes were as described (42). Isolated CD14+ monocytes (purity >94%) were cultured in the presence of 100 ng/ml GM-CSF and 50 ng/ml IL-4 (both from R & D Systems, Minneapolis, MN) for 5 days. The resulting immature DCs were washed and cultured for 24 h with IFN-α (1,000 units/ml; PBL Biomedical Laboratories, Piscataway, NJ), IL-10 (10 ng/ml; R & D Systems), and irradiated CD40L-transfected L cells (DC/L cell ratio 4:1) to obtain mature DCs.
CD4+ T Cell Stimulation.
CD4+ naïve T cells and memory T cells (purity >99% for both) were isolated from peripheral blood mononuclear cells by using a CD4+ T cell isolation kit II from Miltenyi Biotec (Auburn, CA) followed by cell sorting (CD4+CD45RA+CD45RO− fraction as naïve T cells and CD4+CD45RA−CD45RO+ fraction as memory T cells). A total of 4 × 104 freshly purified allogeneic naïve CD4+ T cells were cocultured with immature DCs or cultured DCs (DC/T cell ratio 1:10) in round-bottom 96-well culture plates for 7 days in the presence or absence of soluble recombinant human OX40L (100 ng/ml; R & D Systems). Soluble recombinant OX40L was added at days 0 and 4. Purified CD4+ T cells were also cultured with IL-12 (10 ng/ml; R & D Systems), IL-4 (25 ng/ml), IL-10 (10 ng/ml), or a combination of dexamethasone (5 × 10−8 M; Life Technologies, St. Louis, MO) and 1α,25-dihydroxyvitamin D3 (1 × 10−7 M; Life Technologies) for 7 days in the presence of soluble anti-CD28 mAb (CD28.2, 1 μg/ml) and IL-2 (50 units/ml; R & D Systems) on the irradiated CD32/OX40L-L cells, CD32/GITRL-L cells, CD32/4-1BBL-L cells, or parental CD32-L cells, which had been precoated with anti-CD3 mAb (OKT3, 0.2 μg/ml) in 48-well culture plates (T cell/L cell ratio 2.5:1). In some experiments, CD4+ memory T cells were first cultured with Dex + Vit D3 on parental CD32-L cells as described above to differentiate into Tr1 cells, and the T cells were washed and then restimulated for another 24 h with parental CD32-L cells or CD32/OX40L-L cells precoated with anti-CD3 mAb and soluble anti-CD28 mAb. In some experiments CD4+ T cells were cultured for 7 days on the CD32-L cells, a mixture of CD32-L cells and CD32/ICOSL-L cells (ratio 1:1), or a mixture of CD32/ICOSL-L cells and CD32/OX40L-L cells (ratio 1:1) precoated with anti-CD3 mAb (0.2 μg/ml) in 48-well culture plates. We used RPMI medium 1640 and supplemented with 10% FCS, 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin G, and streptomycin for our cultures.
Analysis of T Cell Cytokine Production.
The cultured T cells were collected, washed, and then restimulated with plate-bound anti-CD3 mAb (5 μg/ml) and soluble anti-CD28 mAb (2 μg/ml) at a concentration of 106 cells per ml for 24 h. The levels of IL-4, IL-10, TNF-α, and IFN-γ in the supernatants were measured by ELISA (all kits from R & D Systems). For detection of intracellular cytokine production, the cultured T cells were restimulated with 50 ng/ml phorbol 12-myristate 13-acetate plus 2 μg/ml ionomycin for 6 h. Brefeldin A (10 μg/ml) was added during the last 2 h. The cells were stained with anti-IL-4-phycoerythrin or anti-TNF-α-phycoerythrin, anti-IFN-γ-FITC, and anti-IL-10-allophycocyanin (all from BD Biosciences, Sunnyvale, CA) by using the FIX and PERM kit from Caltag (Burlingame, CA).
T Cell Expansion and Suppressive Function Assay.
T cells were collected and resuspended in an EDTA-containing medium to dissociate the clusters. Viable cells were counted by trypan blue exclusion of the dead cells. For the proliferation and suppressive function assay, we used freshly isolated CD4+ naïve T cells and cultured T cells generated from autologous CD4+ naïve T cells with several conditions as described above. These cell types and their mixtures at a 1:1 ratio were then restimulated for 5 days with immobilized anti-CD3 mAb (5 μg/ml) and soluble anti-CD28 mAb (1 μg/ml), after which time cellular proliferation was assessed by [3H]thymidine incorporation.
Acknowledgments
We thank Karen Ramirez, Zhiwei He, and Eric Wieder for cell sorting and support. This project was supported by the M. D. Anderson Cancer Center Foundation.
Abbreviations
- DC
dendritic cell
- OX40L
OX40 ligand
- Tr1 cells
type 1 regulatory T cells
- ICOS
inducible costimulator
- ICOSL
ICOS ligand
- Dex + Vit D3
dexamethasone plus vitamin D3
- TH
T helper
- GITRL
glucocorticoid-induced TNF receptor ligand
- 4-1BBL
4-1BB ligand.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bacchetta R., Bigler M., Touraine J. L., Parkman R., Tovo P. A., Abrams J., de Waal Malefyt R., de Vries J. E., Roncarolo M. G. J. Exp. Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groux H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J. E., Roncarolo M. G. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 3.Asseman C., Powrie F. Gut. 1998;42:157–158. doi: 10.1136/gut.42.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuirk P., McCann C., Mills K. H. J. Exp. Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottrez F., Hurst S. D., Coffman R. L., Groux H. J. Immunol. 2000;165:4848–4853. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- 6.Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A. H. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrat F. J., Cua D. J., Boonstra A., Richards D. F., Crain C., Savelkoul H. F., de Waal-Malefyt R., Coffman R. L., Hawrylowicz C. M., O’Garra A. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boussiotis V. A., Tsai E. Y., Yunis E. J., Thim S., Delgado J. C., Dascher C. C., Berezovskaya A., Rousset D., Reynes J. M., Goldfeld A. E. J. Clin. Invest. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Garra A., Vieira P. L., Vieira P., Goldfeld A. E. J. Clin. Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levings M. K., Sangregorio R., Galbiati F., Squadrone S., de Waal Malefytqq R., Roncarolo M. G. J. Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 11.Levings M. K., Gregori S., Tresoldi E., Cazzaniga S., Bonini C., Roncarolo M. G. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 12.Ito T., Amakawa R., Inaba M., Ikehara S., Inaba K., Fukuhara S. J. Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 13.De Smedt T., Van Mechelen M., De Becker G., Urbain J., Leo O., Moser M. Eur. J. Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 14.Witsch E. J., Peiser M., Hutloff A., Buchner K., Dorner B. G., Jonuleit H., Mages H. W., Kroczek R. A. Eur. J. Immunol. 2002;32:2680–2686. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Akbari O., Freeman G. J., Meyer E. H., Greenfield E. A., Chang T. T., Sharpe A. H., Berry G., DeKruyff R. H., Umetsu D. T. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 16.Beier K. C., Hutloff A., Dittrich A. M., Heuck C., Rauch A., Buchner K., Ludewig B., Ochs H. D., Mages H. W., Kroczek R. A. Eur. J. Immunol. 2000;30:3707–3717. doi: 10.1002/1521-4141(200012)30:12<3707::AID-IMMU3707>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Ito T., Wang Y. H., Duramad O., Hori T., Delespesse G. J., Watanabe N., Qin F. X., Yao Z., Cao W., Liu Y. J. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira P. L., Christensen J. R., Minaee S., O’Neill E. J., Barrat F. J., Boonstra A., Barthlott T., Stockinger B., Wraith D. C., O’Garra A. J. Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 19.Watts T. H. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 20.Ohshima Y., Yang L. P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 21.Flynn S., Toellner K. M., Raykundalia C., Goodall M., Lane P. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiba H., Miyahira Y., Atsuta M., Takeda K., Nohara C., Futagawa T., Matsuda H., Aoki T., Yagita H., Okumura K. J. Exp. Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jember A. G., Zuberi R., Liu F. T., Croft M. J. Exp. Med. 2001;193:387–392. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Orozco N., Chen Z., Poirot L., Hyatt E., Chen A., Kanagawa O., Sharpe A., Mathis D., Benoist C. J. Immunol. 2003;171:6954–6960. doi: 10.4049/jimmunol.171.12.6954. [DOI] [PubMed] [Google Scholar]
- 25.Ndhlovu L. C., Ishii N., Murata K., Sato T., Sugamura K. J. Immunol. 2001;167:2991–2999. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 26.Pakala S. V., Bansal-Pakala P., Halteman B. S., Croft M. Eur. J. Immunol. 2004;34:3039–3046. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 27.Ali S. A., Ahmad M., Lynam J., McLean C. S., Entwisle C., Loudon P., Choolun E., McArdle S. E., Li G., Mian S., Rees R. C. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Andarini S., Kikuchi T., Nukiwa M., Pradono P., Suzuki T., Ohkouchi S., Inoue A., Maemondo M., Ishii N., Saijo Y., et al. Cancer Res. 2004;64:3281–3287. doi: 10.1158/0008-5472.can-03-3911. [DOI] [PubMed] [Google Scholar]
- 29.Serghides L., Bukczynski J., Wen T., Wang C., Routy J. P., Boulassel M. R., Sekaly R. P., Ostrowski M., Bernard N. F., Watts T. H. J. Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 30.Hawrylowicz C. M., O’Garra A. Nat. Rev. Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 31.Stock P., Akbari O., Berry G., Freeman G. J., Dekruyff R. H., Umetsu D. T. Nat. Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 32.Oral H. B., Kotenko S. V., Yilmaz M., Mani O., Zumkehr J., Blaser K., Akdis C. A., Akdis M. Eur. J. Immunol. 2005;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 33.Lathrop S. K., Huddleston C. A., Dullforce P. A., Montfort M. J., Weinberg A. D., Parker D. C. J. Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 34.Valzasina B., Guiducci C., Dislich H., Killeen N., Weinberg A. D., Colombo M. P. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 35.So T., Song J., Sugie K., Altman A., Croft M. Proc. Natl. Acad. Sci. USA. 2006;103:3740–3745. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loscher C. E., Draper E., Leavy O., Kelleher D., Mills K. H., Roche H. M. J. Immunol. 2005;175:4990–4998. doi: 10.4049/jimmunol.175.8.4990. [DOI] [PubMed] [Google Scholar]
- 37.Dillon S., Agrawal S., Banerjee K., Letterio J., Denning T. L., Oswald-Richter K., Kasprowicz D. J., Kellar K., Pare J., van Dyke T., et al. J. Clin. Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian C., Jiang X., An H., Yu Y., Guo Z., Liu S., Xu H., Cao X. Blood. 2006 doi: 10.1182/blood-2006-03-005595. in press. [DOI] [PubMed] [Google Scholar]
- 39.Caparros E., Munoz P., Sierra-Filardi E., Serrano-Gomez D., Puig-Kroger A., Rodriguez-Fernandez J. L., Mellado M., Sancho J., Zubiaur M., Corbi A. L. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 40.Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Qin X. F., An D. S., Chen I. S., Baltimore D. Proc. Natl. Acad. Sci. USA. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilliet M., Liu Y. J. J. Exp. Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]