Abstract
The well regulated activities of microglia and T cells specific to central nervous system (CNS) antigens can contribute to the protection of CNS neural cells and their renewal from adult neural stem/progenitor cells (aNPCs). Here we report that T cell-based vaccination of mice with a myelin-derived peptide, when combined with transplantation of aNPCs into the cerebrospinal fluid (CSF), synergistically promoted functional recovery after spinal cord injury. The synergistic effect was correlated with modulation of the nature and intensity of the local T cell and microglial response, expression of brain-derived neurotrophic factor and noggin protein, and appearance of newly formed neurons from endogenous precursor-cell pools. These results substantiate the contention that the local immune response plays a crucial role in recruitment of aNPCs to the lesion site, and suggest that similar immunological manipulations might also serve as a therapeutic means for controlled migration of stem/progenitor cells to other acutely injured CNS sites.
Keywords: microglia, neurogenesis, T cells, protective autoimmunity, neurodegeneration
Attempts to promote recovery of the central nervous system (CNS) after acute injuries have focused on two main goals: the stimulation of axonal regrowth (regeneration) and the arrest of self-perpetuating degeneration (neuroprotection). Recently, a third goal has been added, namely the generation of new neurons and glia that will repopulate the site of injury and functionally integrate into the surviving neural tissue. Studies have highlighted the role of immune cells in the postinjury recovery of the CNS. Experiments with rodent models have shown that implantation of specifically activated blood-borne macrophages (1, 2) or dendritic cells (3, 4) promotes recovery from spinal cord injury (SCI). Further studies showed that T cells that recognize CNS antigens can promote recovery from CNS insults, provided that their activity (in terms of onset, intensity, and duration) is well controlled (5–8). The beneficial effect of such T cells has been attributed to their dialogue with microglia (9–11). Microglia activated by T cell-derived cytokines can protect neurons and induce adult neural stem/progenitor cells (aNPCs) to differentiate into neurons and oligodendrocytes (11). We recently showed that immune cells help to maintain neurogenesis in adult germinal centers of the CNS even under nonpathological conditions (12).
In the present study, we postulated that combining an immune-based therapy with transplantation of syngeneic aNPCs would promote functional recovery after SCI, and that this dual procedure would be more effective than either of the therapies administered separately.
Results
Adult Neural Progenitor Cells Require Local Immune Activity to Promote Motor Recovery.
We first investigated whether a T cell-based vaccination creates favorable conditions for postinjury treatment with aNPCs. Four groups of mice were examined: (i) C57BL/6J mice that were vaccinated, immediately after SCI, with peptide 35-55 of the encephalitogenic protein, myelin oligodendrocyte glycoprotein (pMOG 35-55), emulsified in complete Freund’s adjuvant (CFA) containing 0.5 mg/ml Mycobacterium tuberculosis (MOG-CFA), and 7 days later received aNPCs via the intracerebroventricular (i.c.v.) route (MOG-CFA/aNPC); (ii) mice that were vaccinated with the same MOG-CFA but were not transplanted with aNPCs and instead were injected i.c.v. with PBS (MOG-CFA/PBS); (iii) mice that were injected with PBS and transplanted i.c.v. with aNPCs (PBS/aNPC); and (iv) mice that were injected with PBS at the time of vaccination and 7 days later were injected i.c.v. with PBS instead of aNPCs (PBS/PBS).
To assess behavioral outcome after SCI, we used the Basso mouse scale (BMS) (13), in which a score of 0 indicates complete paralysis of the hind limbs and 9 denotes full mobility. In mice treated with PBS/aNPC, recovery was not better than in control mice treated with PBS/PBS (their BMS scores at the last time point measured were 1.5 ± 0.4 and 1.5 ± 0.27, respectively; all values are means ± SEM). Mice treated with MOG-CFA/PBS achieved a significantly higher BMS score (2.71 ± 0.5) than that of PBS/aNPC or PBS/PBS mice (F = 11.78, P = 0.023, and F = 9.79. P = 0.0047, respectively, repeated measures ANOVA; Fig. 1A). However, the BMS score of MOG-CFA/aNPC mice (4.21 ± 0.45) was significantly higher than those of all other tested groups (when compared to MOG-CFA/PBS, F = 4.83, P = 0.0038). A BMS score of 4.21 indicates extensive movement of the ankle and plantar placement of the paw (some of these mice obtained scores as high as 5 and showed occasional coordination and plantar steps), whereas a score of 1.5 indicates slight to extensive ankle movement. These results demonstrate synergistic interaction between the transplanted aNPCs in vaccinated mice and the elicited immune response. Because transplantation of aNPCs in the absence of vaccination did not improve recovery from SCI, this control group (PBS/aNPC) was not included in our subsequent experiments.
Fig. 1.
A myelin-specific T cell response operates synergistically with transplanted aNPCs in promoting functional recovery from SCI. The figure depicts recovery of motor function after SCI in C57BL/6J mice. (A) Mice were vaccinated with MOG-CFA (CFA containing 0.5 mg/ml M. tuberculosis), or injected with PBS. Seven days after SCI, aNPCs were transplanted into the lateral ventricles (MOG-CFA/aNPC or PBS/aNPC). The lateral ventricles of mice in similarly injured and vaccinated control groups were injected with PBS (MOG-CFA/PBS or PBS/PBS). Values of the BMS rating scale are presented (n = 7 in all groups except for the PBS/aNPC group, where n = 6; P < 0.001; F = 15.5, repeated measures ANOVA). (B) Mice were vaccinated with peptide 45D emulsified in CFA with or without transplanted aNPCs. Similarly injured and vaccinated control groups, instead of being transplanted with aNPCs, were injected with PBS (n = 7 for 45D-CFA/aNPCs- and 45D-CFA/PBS-treated mice and n = 8 for PBS/PBS-treated mice; P < 0.001; F = 16.1, repeated measures ANOVA). (C) Injury and aNPC transplantation were as in A, but vaccination was carried out 7 days before SCI and the mice were vaccinated with OVA-CFA (n = 8 for OVA-CFA/PBS and OVA-CFA/aNPCs and n = 7 for PBS/PBS-treated mice; intergroup differences are not significant). (D) Mice were vaccinated immediately after SCI with MOG peptide emulsified in CFA containing 2.5 mg/ml M. tuberculosis with (n = 9) or without (n = 9) transplanted aNPCs. A third group was spinally injured and received only PBS (n = 6; P < 0.001; F = 25.03; repeated measures ANOVA). (E) Mice were vaccinated 1 week before SCI as in D with (n = 9) or without (n = 7) transplanted aNPCs. A control group received only PBS (n = 7; P = 0.026; F = 4.4; repeated-measures ANOVA). Results for all groups in all experiments are means ± SEM (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; analysis by Tukey’s procedure, indicating significant post hoc differences relative to the PBS/PBS-treated group in A–D or relative to the MOG-CFA/PBS-treated group in E).
For therapeutic purposes, weak agonists of encephalitogenic peptides can be used for vaccination without the risk of inducing experimental autoimmune encephalomyelitis (5, 14, 15). Here we used the pMOG 35-55-derived altered peptide ligand (peptide 45D) (15). We vaccinated the mice with this peptide (emulsified in CFA containing 2.5 mg/ml M. tuberculosis) 7 days before the injury (15). The aNPCs were transplanted i.c.v. 7 days after the injury, as in the experiment described in Fig. 1A. The BMS score obtained by these 45D-CFA/aNPC-treated mice was significantly higher than that of PBS/PBS-treated mice (4.11 ± 0.27 compared to 1.94 ± 0.22; Fig. 1B). Vaccination with 45D-CFA without aNPCs transplantation (45D-CFA/PBS) resulted in only a slight increase in motor recovery (2.57 ± 0.24) relative to the PBS/PBS-treated control.
To determine whether specificity to CNS antigens is required for the observed synergistic effect of vaccination and aNPC transplantation, mice were vaccinated with the non-self protein ovalbumin (OVA) emulsified in CFA containing 2.5 mg/ml M. tuberculosis, and 7 days later were injected i.c.v. with aNPCs or with PBS (control). Vaccination with OVA-CFA resulted in a slight, insignificant increase in motor recovery, which was not further increased by transplantation of aNPCs (BMS score was 2.43 ± 1.78 for OVA-CFA/aNPC-treated mice compared to 2.2 ± 0.68 for OVA-CFA/PBS-treated mice and 1.5 ± 0.29 for PBS/PBS-treated controls, Fig. 1C), suggesting that specificity to CNS antigens is a prerequisite for a beneficial and synergistic effect of aNPCs on functional recovery.
The role of immune activation in the injured CNS, and specifically in the spinal cord, has long been a matter of debate (16–18). Some studies showed that neuroprotection can be achieved by a T cell-based vaccination, provided that the ensuing immune response is of moderate intensity and can be self-limited (15, 19). As expected on the basis of previous results (15, 20), mice that were vaccinated immediately after SCI, with the same MOG peptide 35-55 emulsified in CFA containing a high percentage (2.5 mg/ml) of M. tuberculosis (a dosage of CFA five times higher than that used in the experiment presented in Fig. 1A), did not recover better than the PBS/PBS-treated group (their BMS scores were 1.0 ± 0.27 and 1.58 ± 0.42, respectively; Fig. 1D). Importantly, however, when the same vaccination protocol was combined with transplanted aNPCs (MOG-CFA/aNPC), it resulted in significantly better recovery (BMS scores 3.72 ± 0.35; Fig. 1D) than that obtained with either PBS/PBS or MOG-CFA/PBS treatment. Therefore, we considered the possibility that aNPCs, which home to the site of damage in vaccinated mice, contribute to the local control of the very immune response that facilitates their recruitment.
We also examined whether aNPCs can contribute to functional recovery even when the local immune activity is excessive. Overwhelming immune activity with a detrimental effect on recovery was achieved, as previously reported (15), in mice that were vaccinated, 7 days before SCI, with MOG peptide 35-55 emulsified in CFA containing 2.5 mg/ml M. tuberculosis. A group of similarly vaccinated mice was also transplanted with aNPCs. Comparison of the two groups showed that, even under conditions in which motor recovery from SCI was worse after vaccination with MOG-CFA than after injection with PBS (their BMS scores were 0.35 ± 0.2 and 1.94 ± 0.32, respectively), vaccinated mice transplanted with aNPCs recovered significantly better than any other tested group (BMS score = 2.68 ± 0.51; Fig. 1E). Thus, the injected aNPCs were able to offset the negative effects of the destructive immune activity, thereby making a further contribution to recovery. Scores achieved by the individual mice in all tested paradigms, 1 month after contusion, are shown in Fig. 6, which is published as supporting information on the PNAS web site.
We repeated the experiment shown in Fig. 1A, using aNPCs labeled with GFP, to follow their homing to the injured site. On day 14 after injection, GFP-labeled cells could be detected in the lateral ventricles on the injected side, as well as in the third ventricle (Fig. 2A). Staining with anti-GFP antibodies revealed GFP-labeled cells in the parenchyma of the injured spinal cords of mice treated with MOG-CFA/aNPC, but not in aNPC-injected mice that were not vaccinated (Fig. 2B). The injected GFP-labeled aNPCs were seen in the vaccinated mice as early as 7 days after SCI, where they were found to be accumulating laterally in the spinal cord parenchyma adjacent to the meninges (Fig. 2B) and surrounding the epicenter of the lesion (Fig. 2C). Transplanted GFP-labeled cells could be detected in the area of the lesion as late as 60 days after SCI, the last time point examined (Fig. 2D). To follow the fate of the transplanted aNPCs, we stained sections of the spinal cord with antibodies to GFP and antibodies to neuronal (doublecortin; DCX) or glial marker [glial fibrillary acidic protein (GFAP) for astrocytes, RIP for oligodenrocytes]. No GFP-labeled cells were found to express markers of neuronal or glial cell differentiation (data not shown), even in spinal cord sections examined as late as 60 days after the insult, suggesting that local differentiation of the injected aNPCs, if it occurred at all, was a very rare event.
Fig. 2.
GFP-labeled aNPCs in the spinal cord parenchyma of MOG-vaccinated mice. (A) GFP-labeled cells (green) lining the ventricle wall adjacent to the hippocampus, 14 days after transplantation of aNPCs into the lateral ventricles. (Scale bar, 50 μm.) Longitudinal sections of spinal cords, excised 7 or 60 days after transplantation, were stained with anti-GFP antibody (red) and counterstained with Hoechst (blue) for detection of nuclei. Seven days after transplantation, GFP-labeled cells were detected laterally near the meninges (B) and in areas adjacent to the lesion site (C). (Scale bar, 20 μm.) (D) GFP-labeled cells adjacent to the lesion site 60 days after transplantation of aNPCs. (Scale bar, 10 μm.)
Tissue Repair Correlates with T Cell Accumulation.
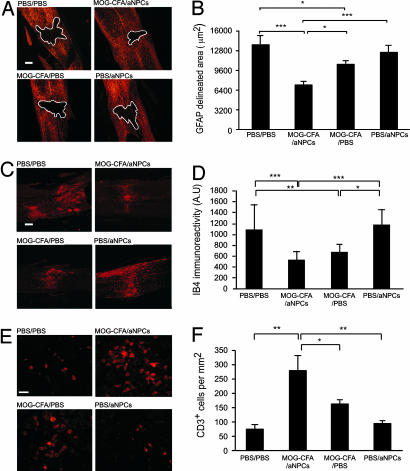
To determine the effect of the various treatments on the size of the injury site, we stained serial longitudinal sections of the spinal cord (at least three sections per spinal cord) with anti-GFAP antibodies (21). The part marked by high-density GFAP-labeling defined the margin of the lesion site, and we measured the unstained (lesioned) area that was surrounded by the dense GFAP staining. As early as 7 days after transplantation of aNPCs, the lesion was significantly smaller, on average, in mice treated with MOG-CFA/aNPCs than in MOG-CFA/PBS-treated, PBS/PBS-treated, or PBS/aNPC-treated mice (Fig. 3A and B).
Fig. 3.
Local changes in immune activity correlate with tissue preservation. On the day of SCI, mice were vaccinated with MOG peptide emulsified in CFA containing 0.5 mg/ml M. tuberculosis. Seven days later, the vaccinated and control mice were either transplanted with aNPCs or injected with PBS. Their spinal cords were excised 1 week after cell transplantation. (A) Representative micrographs showing GFAP staining of spinal cords from mice treated with MOG-CFA/aNPC, MOG-CFA/PBS, PBS/aNPC, or PBS/PBS are shown. (B) Quantification of the area delineated by GFAP staining. (C) Representative micrographs of IB4-stained areas. (D) Quantification of IB4 immunoreactivity. (E) Representative micrographs of CD3 staining, identifying infiltrating T cells in the area surrounding the site of injury. (F) Quantification of CD3+ cells in the area surrounding the site of injury (n = 4 for MOG-CFA/aNPC and n = 3 for MOG-CFA/PBS, PBS/aNPC, and PBS/PBS; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA). Data are means ± SEM. (Scale bar, 100 μm in A and C; 20 μm in E.)
Next we examined whether the observed differences in the extent of recovery could be correlated with local immunological changes beyond those shown to occur after vaccination alone (9, 19). Tissues were excised 7 days after cell transplantation (14 days after the injury), and spinal cord sections were stained for markers of T cells (CD3) and of activated microglia/macrophages (IB4). All sections were also stained with Hoechst dye as a nuclear marker (not shown). Significantly fewer microglia/macrophages (Fig. 3 C and D) but significantly more T cells (Fig. 3 E and F) were seen in mice treated with MOG-CFA/aNPC (the dual-treatment protocol) than in the other experimental groups. This finding was in line with our earlier observations that T cell-based vaccination changes the course of the postinjury local immune response (19).
Staining for brain-derived neurotrophic factor (BDNF) revealed significantly more BDNF immunoreactivity in the spinal cords of mice treated with MOG-CFA/aNPC than in the other groups (Fig. 4A). Double staining for BDNF and IB4 showed that microglia in the injured spinal cord produced BDNF (Fig. 4A).
Fig. 4.
Local microglial production of BDNF and noggin. Quantification of BDNF (A) and noggin (B) immunoreactivity (Upper) (n = 4 for MOG-CFA/aNPC and n = 3 for MOG-CFA/PBS, PBS/aNPC, and PBS/PBS; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA). (Lower) Both BDNF and noggin are colocalized to IB4+ cells. (Scale bar, 20 μm.)
Noggin, an inhibitor of bone morphogenetic protein, was recently shown to induce neuronal differentiation from aNPCs in the injured spinal cord (22). This protein is also needed for maintenance of a neurogenic environment in the subventricular zone (23). Relative to PBS/PBS-treated mice, noggin immunoreactivity was significantly increased in MOG-CFA/aNPC treated mice, but was unaffected by MOG vaccination alone and was slightly decreased by aNPC transplantation alone (Fig. 4B). Like BDNF, noggin was also localized to IB4+ cells (Fig. 4B). This latter finding is in line with the recent demonstration that immune cells can produce noggin within the inflamed CNS (24). Studies by our group have shown that T cell-based vaccination after SCI induces expression of MHC-II by microglia at the margins of the lesion site (10, 11), and produces relatively large amounts of insulin-like growth factor (IGF-I), a growth factor known to be important for neurogenesis (25) (O. Butovsky and M.S., unpublished results). In the present study, MHC-II expression by IB4+ cells was observed mainly at the margins of the lesion site (Fig. 7, which is published as supporting information on the PNAS web site).
Local Differentiation of Endogenous Stem/Progenitor Cells.
The above results raised an important question: does the combined treatment create conditions favorable for neuronal differentiation of endogenous aNPCs? To examine this possibility, we repeated the experiment described in Fig. 1A. Starting on day 7 after aNPC transplantation (i.e., 14 days after SCI), we injected the cell-proliferation marker 5-bromo-2-deoxyuridine (BrdU) twice daily for 3 days. Staining for BrdU and the early differentiation marker DCX, 7 days after the last BrdU injection, disclosed significantly more new neurons in the spinal cords of mice that had received the dual treatment (Fig. 5A). It should be noted that BrdU could be incorporated into damaged cells in the injured brain (26), and thus it is possible that some of the BrdU-labeled cells found in the injured spinal cord were not proliferating. However, in our analysis of endogenous neurogenesis, we counted only BrdU+ cells that were also labeled with DCX, a protein that is specifically expressed by newly formed neurons. Fig. 5B depicts BrdU+/DCX+ cells in the vicinity of the injured site. Most BrdU+ cells appeared inside the lesion, whereas DCX+ cells were found at the margins of the lesion (Fig. 8, which is published as supporting information on the PNAS web site). Staining for both BrdU and GFP or for both DCX indicated GFP revealed virtually no double-positive cells, indicating that the DCX+ cells in the injured spinal cord had originated from endogenous NPC reservoirs, rather than from the injected GFP+-aNPCs.
Fig. 5.
Increased neurogenesis from endogenous progenitors in the spinal cords of dual-treated mice. The numbers of BrdU/DCX double-positive cells in the vicinity of the site of injury are increased after dual treatment with vaccination and aNPC transplantation. SCI and aNPC transplantation were as in Fig. 1. Seven days after aNPC transplantation, mice were injected with BrdU twice daily for 3 days. Longitudinal sections of spinal cords excised 14 days after cell transplantation and 1 month after contusive SCI were stained for BrdU and DCX. (A) Quantification of BrdU+/DCX+ cells in the injured spinal cord (n = 7 for MOG-CFA/aNPC and n = 5 for MOG-CFA/PBS, PBS/aNPC, and PBS/PBS, pooled data from two independent experiments; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, ANOVA). (B) Representative images showing newly formed neuronal cells stained for BrdU (green) and DCX (red). (Scale bar, 10 μm.)
The observation that, after an injury, neurogenesis from endogenous aNPCs was up-regulated in the normally nonneurogenic region of the spinal cords of mice treated with MOG-CFA/aNPC prompted us to examine whether the same conditions would promote neurogenesis in uninjured areas of the CNS where neurogenesis occurs constitutively. Analysis of brain sections from the same spinally injured mice (on day 14 after aNPC transplantation) revealed a marked increase in the numbers of BrdU+/DCX+ cells in the hippocampal dentate gyrus on the aNPC-injected side (Fig. 9A, which is published as supporting information on the PNAS web site). This effect was more robust in vaccinated than in nonvaccinated mice, and correlated with the increasing number of surviving GFP+ cells located along the brain ventricle wall. As in the spinal cord, none of the GFP+ cells expressed DCX. These findings further implied that the transplanted aNPCs, together with an induced CNS-specific T cell response, created conditions that could promote neurogenesis from endogenous progenitor reservoirs in neurogenic areas. This increase in neurogenesis, as in the case of activity-induced neurogenesis (12), was correlated with the appearance of activated microglia (IB4+ and MHC-II+) in the subgranular zone of the dentate gyrus and along the ventricle wall, in close proximity to the injected GFP-labeled NPCs (Fig. 9B). Some of the IB4-labeled cells found in the subgranular zone and ventricle wall of mice treated with MOG-CFA/aNPC produced IGF-I (25) (Fig. 9C). Taken together, these results implied that similar mechanisms, which underlie the cross talk between immune cells and aNPCs, act in both normally neurogenic and injury induced neurogenic areas of the CNS.
Discussion
This study demonstrates synergy between a T cell-based vaccination in mice and transplantation of aNPCs into the lateral ventricles. The synergistic effect appears to be an outcome of two successive stages in the postinjury process of recovery. In the first stage, myelin-specific T cells induce the transplanted aNPCs to migrate to the site of injury, while also activating the local immune cells (microglia) at the injured site to adopt a neuroprotective phenotype. In the second stage, the immune modulation that results from local interaction of the aNPCs with T cells and microglia leads to better tissue preservation, increased neurogenesis from endogenous precursors, and improved functional recovery.
The vaccination protocol used in this study evokes a T cell response with a T helper 1 (Th1/Th0) bias, which was previously shown to exert a neuroprotective effect after acute CNS insults (10, 27, 28). The vaccination with encephalitogenic peptides was used here for proof of concept only; we do not advocate such vaccination for therapeutic purposes, because it carries the risk of inducing, in individuals susceptible to autoimmune diseases, an overwhelming inflammatory response that is detrimental for recovery (20). Instead, as in previous studies (5, 29), we demonstrated that a weak agonist of an encephalitogenic antigen can effectively replace the encephalitogenic peptide. The timing of aNPC transplantation (7 days after injury) was chosen on the basis of a previous finding that the local immune response reaches a peak 7 days after injury (5, 29).
Injury or damage to neural tissues can induce recruitment, proliferation, and differentiation of both exogenously delivered and endogenous aNPCs in the adult CNS (30–32). In the present study, aNPCs were injected into the CSF at a site remote from the lesion. The fact that GFP-labeled cells in the spinal cords were detected only in dual-treated mice indicates that migration of aNPCs to the site of the lesion requires local myelin-specific T cell response. This contention might be in line with studies showing that exogenously delivered aNPCs home to sites of T cell associated inflammation in the CNS (24, 33, 34).
Differentiation of aNPCs (similar to those used in this study) to neurons and oligodenrocytes can be induced in vitro by microglia preactivated by T cell derived cytokines (11). That finding led us to anticipate that a T cell-based vaccination would promote not only aNPC migration but also their local differentiation. However, our analysis revealed that, although local differentiation of endogenous aNPCs took place, differentiation of the transplanted aNPCs, if it occurred at all, was very rare. This finding raised the possibility that the transplanted aNPCs contribute to recovery in their nondifferentiated form. The presence of undifferentiated (systemically injected) aNPCs in perivascular areas of the inflamed CNS was recently demonstrated in a mouse model of experimental autoimmune encephalomyelitis (24).
There are several possible ways, other than local differentiation, in which the transplanted aNPCs could contribute to functional recovery. Adult NPCs can locally generate growth factors that promote neuronal survival or axonal outgrowth (35, 36). In addition, the fact that the adverse effects on recovery induced by a robust vaccination protocol could be reversed by transplanted aNPCs suggests that the transplanted aNPCs might participate in local immune modulation. This finding is in line with a recent report showing that aNPCs contribute to neuroprotection by acting as immune modulators (24). The contention that CNS repair needs a well controlled immune response is supported by the improved motor recovery observed in dual-treated mice relative to the other experimental groups and its correlation with increased numbers of CD3+ T cells in the vicinity of the lesion site. This finding suggests that the transplanted aNPCs acted locally to recruit more T cells, or alternatively to sustain their prolonged activity. In the injured CNS parenchyma, T cells can provide growth factors (37, 38) or induce growth factor production from resident microglia (10, 39). Upon activation by moderate amounts of Th1 cell-derived cytokines (such as IFN-γ) or Th2-derived cytokines (interleukin-4) the microglia acquire a phenotype that supports neuronal survival and cell renewal from aNPCs (10, 11), in part via production of IGF-1 and down-regulation of tumor necrosis factor-α production. The present results suggested that the immune cells (T cells and microglia) and the aNPCs provide an infrastructure for tissue repair by modulating each other’s activity. Our findings implied that the neuroprotective microglia found in the spinal cord of the dual-treated mice (Fig. 4) are indeed phenotypically distinct from those found in the other experimental groups. Previous studies have shown that a T cell-based neuroprotective response correlates with early activation and early shut-down of microglial activity in the injured optic nerve (19) and spinal cord (O. Butovsky and M.S., unpublished results). It is possible that the decrease in numbers of microglia found in the spinal cords of vaccinated and dual-treated mice (Fig. 3) reflects such a change in the kinetics of the local immune response.
The increased expression of noggin and BDNF by microglia at injured sites in the dual-treated mice suggested that the local interaction between immune cells and aNPCs might create ectopic neurogenic compartments. Thus, the exogenously applied aNPCs might contribute to neuronal cell renewal by creating a neurogenic environment for endogenous precursors. Notably, some increase in neurogenesis from endogenous progenitors could be seen in the CFA-MOG/PBS-treated group relative to PBS/PBS-treated controls. These results suggest that neurogenesis is supported not only by noggin and BDNF but also by other factors that are induced by myelin-specific T cells in the absence of exogenous aNPC. In line with our findings, neurogenesis from endogenous aNPCs in the spinal cord was found to be induced in a model of experimental autoimmune encephalomyelitis (40) and augmented by immunological treatment with dendritic cells after injury (4).
The functional contribution of the newly formed neurons (BrdU+/DCX+) found in the spinal cord of dual-treated mice has yet to be established. Some of the new neurons might eventually become integrated into the local circuitry and function there as interneurons. Examination of the hippocampal dentate gyrus and adjacent ventricle walls revealed more GFP-labeled cells lining the ventricle wall in vaccinated mice than in nonvaccinated mice, suggesting that vaccination, in addition to promoting migration of aNPCs, also positively affects aNPC survival. The relatively large numbers of newly formed neurons found in the dentate gyrus of dual-treated mice further supports the concept that the local interaction between immune cells and aNPCs contributes to the formation of a neurogenic environment.
Stem cells of allogeneic or xenogeneic origin are currently being considered as potential treatments for neurodegenerative conditions of the CNS. Such treatments are likely to necessitate the use of immune suppression. Our present results imply that, at least at the subacute phase, the use of immune suppression should be considered with extreme caution, as it might diminish the potentially beneficial effects of the stem cells.
Materials and Methods
Animals.
Inbred adult wild-type C57BL/J6 mice were supplied by the Animal Breeding Center of The Weizmann Institute of Science. All animals were handled according to the regulations formulated by the Institutional Animal Care and Use Committee (IACUC).
Antigens.
Two peptides, synthesized in the Synthesis Unit at the Weizmann Institute, were used in this study: MEVGWYRSPFSRVVHLYRNGK (pMOG 35-55) and an altered MOG peptide MEVGWYRSPFDRVVHLYRNGK (45D), an analog of pMOG 35-55 in which aspartic acid is substituted for serine (41). OVA was purchased from Sigma-Aldrich (Rehovot, Israel).
Neural Progenitor Cell Culture.
GFP-expressing aNPCs were obtained as described (33).
Vaccination.
Adult mice were vaccinated with pMOG 35-55, 45D, or OVA (all 100 μg), each emulsified in an equal volume of CFA (Difco, Franklin Lakes, NJ) containing Mycobacterium tuberculosis (0.5 or 2.5 mg/ml; Difco). The emulsion (total volume, 0.15 ml) was injected s.c. at one site in the flank. Control mice were injected with PBS.
Spinal Cord Injury.
Mice were anesthetized, their spinal cords were exposed by laminectomy at T12, and a force of 200 kdyn was placed for 1 s on the laminectomized cord by using the Infinite Horizon spinal cord impactor (Precision Systems, Lexington, KY), a device shown to inflict a well calibrated contusive injury of the spinal cord.
Assessment of Functional Recovery from Spinal Cord Contusion.
Recovery was scored by the BMS open-field locomotor rating scale, which was developed specifically for mice, and allows achievement of scores ranging from 0 (complete paralysis) to 9 (normal mobility) (13). For additional information about the assessment procedure see Supporting Text, which is published as supporting information on the PNAS web site.
Quantification.
The GFAP-delineated area and intensity per unit surface area of BDNF, noggin, and IB4 staining, were measured by using Image-Pro Plus 4.5 software (Media Cybernetics). CD3+ cells and BrdU+/DCX+ cells were quantified by manual counting of four microscopic fields (each 0.16 mm2 in area) surrounding the epicenter of the lesion. Values shown represent the average of measurements from three to four spinal cord sections per mouse.
Supplementary Material
Acknowledgments
We thank S. R. Smith and R. Halper for editing the manuscript and Sharon Ovadia for animal maintenance. M.S. is the incumbent of the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. The work was supported by Proneuron Biotechnologies, Ltd. (Weizmann Science Park, Ness-Ziona, Israel), the Irwin Green Alzheimer’s Research Fund, and the Mario Negri Institure for Pharmacological Research–Weizmann Institute of Science Exchange Program.
Abbreviations
- SCI
spinal cord injury
- aNPCs
adult neural stem/progenitor cells
- CFA
complete Freund’s adjuvant
- MOG
myelin oligodendrocyte glycoprotein
- BMS
Basso mouse scale
- OVA
ovalbumin
- DCX
doublecortin
- GFAP
glial fibrillary acidic protein
- BDNF
brain-derived neurotrophic factor.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rapalino O., Lazarov-Spiegler O., Agranov E., Velan G. J., Yoles E., Fraidakis M., Solomon A., Gepstein R., Katz A., Belkin M., et al. Nat. Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 2.Bomstein Y., Marder J. B., Vitner K., Smirnov I., Lisaey G., Butovsky O., Fulga V., Yoles E. J. Neuroimmunol. 2003;142:10–16. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 3.Hauben E., Gothilf A., Cohen A., Butovsky O., Nevo U., Smirnov I., Yoles E., Akselrod S., Schwartz M. J. Neurosci. 2003;23:8808–8819. doi: 10.1523/JNEUROSCI.23-25-08808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikami Y., Okano H., Sakaguchi M., Nakamura M., Shimazaki T., Okano H. J., Kawakami Y., Toyama Y., Toda M. J. Neurosci. Res. 2004;76:453–465. doi: 10.1002/jnr.20086. [DOI] [PubMed] [Google Scholar]
- 5.Hauben E., Butovsky O., Nevo U., Yoles E., Moalem G., Agranov G., Mor F., Leibowitz-Amit R., Pevsner E., Akselrod S., et al. J. Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moalem G., Leibowitz-Amit R., Yoles E., Mor F., Cohen I. R., Schwartz M. Nat. Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 7.Yoles E., Hauben E., Palgi O., Agranov E., Gothilf A., Cohen A., Kuchroo V., Cohen I. R., Weiner H., Schwartz M. J. Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofstetter H. H., Sewell D. L., Liu F., Sandor M., Forsthuber T., Lehmann P. V., Fabry Z. J. Neuroimmunol. 2003;134:25–34. doi: 10.1016/s0165-5728(02)00358-2. [DOI] [PubMed] [Google Scholar]
- 9.Butovsky O., Hauben E., Schwartz M. Faseb J. 2001;15:1065–1067. doi: 10.1096/fj.00-0550fje. [DOI] [PubMed] [Google Scholar]
- 10.Butovsky O., Talpalar A. E., Ben-Yaakov K., Schwartz M. Mol. Cell. Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A. E., Pluchino S., Martino G., Schwartz M. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Ziv Y., Ron N., Butovsky O., Landa G., Sudai E., Greenberg N., Cohen H., Kipnis J., Schwartz M. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 13.Engesser-Cesar C., Anderson A. J., Basso D. M., Edgerton V. R., Cotman C. W. J. Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- 14.Brocke S., Gijbels K., Allegretta M., Ferber I., Piercy C., Blankenstein T., Martin R., Utz U., Karin N., Mitchell D., et al. Nature. 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 15.Hauben E., Agranov E., Gothilf A., Nevo U., Cohen A., Smirnov I., Steinman L., Schwartz M. J. Clin. Invest. 2001;108:591–599. doi: 10.1172/JCI12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones T. B., Hart R. P., Popovich P. G. J. Neurosci. 2005;25:6576–6583. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcondes M. C., Furtado G. C., Wensky A., Curotto de Lafaille M. A., Fox H. S., Lafaille J. J. Am. J. Pathol. 2005;166:1749–1760. doi: 10.1016/S0002-9440(10)62485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz M., Hauben E. Prog. Brain Res. 2002;137:401–406. doi: 10.1016/s0079-6123(02)37031-6. [DOI] [PubMed] [Google Scholar]
- 19.Shaked I., Porat Z., Gersner R., Kipnis J., Schwartz M. J. Neuroimmunol. 2004;146:84–93. doi: 10.1016/j.jneuroim.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 20.Jones T. B., Ankeny D. P., Guan Z., McGaughy V., Fisher L. C., Basso D. M., Popovich P. G. J. Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaugrund E., Duvdevani R., Lavie V., Solomon A., Schwartz M. Exp. Neurol. 1992;118:105–115. doi: 10.1016/0014-4886(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 22.Setoguchi T., Nakashima K., Takizawa T., Yanagisawa M., Ochiai W., Okabe M., Yone K., Komiya S., Taga T. Exp. Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Buylla A., Lim D. A. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 24.Pluchino S., Zanotti L., Rossi B., Brambilla E., Ottoboni L., Salani G., Martinello M., Cattalini A., Bergami A., Furlan R., et al. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 25.Aberg M. A., Aberg N. D., Hedbacker H., Oscarsson J., Eriksson P. S. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuan C. Y., Schloemer A. J., Lu A., Burns K. A., Weng W. L., Williams M. T., Strauss K. I., Vorhees C. V., Flavell R. A., Davis R. J., et al. J. Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kipnis J., Mizrahi T., Yoles E., Ben-Nun A., Schwartz M., Ben-Nur A. J. Neuroimmunol. 2002;130:78–85. doi: 10.1016/s0165-5728(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 28.Shaked I., Tchoresh D., Gersner R., Meiri G., Mordechai S., Xiao X., Hart R. P., Schwartz M. J. Neurochem. 2005;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- 29.Hauben E., Gothilf A., Cohen A., Butousky O., Nevo U., Smirnov I., Yoles E., Akselrod S., Schwartz M. J. Neurosci. 2003;25:8808–8819. doi: 10.1523/JNEUROSCI.23-25-08808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., Nakafuku M. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 31.Imitola J., Raddassi K., Park K. I., Mueller F. J., Nieto M., Teng Y. D., Frenkel D., Li J., Sidman R. L., Walsh C. A., Snyder E. Y., Khoury S. J. Proc. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacko D. M., Das A. V., Zhao X., James J., Bhattacharya S., Ahmad I. Vision Res. 2003;43:937–946. doi: 10.1016/s0042-6989(02)00688-0. [DOI] [PubMed] [Google Scholar]
- 33.Pluchino S., Quattrini A., Brambilla E., Gritti A., Salani G., Dina G., Galli R., Del Carro U., Amadio S., Bergami A., et al. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 34.Belmadani A., Tran P. B., Ren D., Miller R. J. J. Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu P., Jones L. L., Tuszynski M. H. Exp. Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Llado J., Haenggeli C., Maragakis N. J., Snyder E. Y., Rothstein J. D. Mol. Cell. Neurosci. 2004;27:322–331. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Hohlfeld R., Kerschensteiner M., Stadelmann C., Lassmann H., Wekerle H. J. Neuroimmunol. 2000;107:161–166. doi: 10.1016/s0165-5728(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 38.Moalem G., Gdalyahu A., Shani Y., Otten U., Lazarovici P., Cohen I. R., Schwartz M. J. Autoimmun. 2000;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 39.Elkabes S., DiCicco-Bloom E. M., Black I. B. J. Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danilov A. I., Covacu R., Moe M. C., Langmoen I. A., Johansson C. B., Olsson T., Brundin L. Eur. J. Neurosci. 2006;23:394–400. doi: 10.1111/j.1460-9568.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 41.Ford M. L., Evavold B. O. Eur. J. Immunol. 2004;34:388–397. doi: 10.1002/eji.200324502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.