Abstract
Apicomplexan parasites are the cause of numerous important human diseases including malaria and AIDS-associated opportunistic infections. Drug treatment for these diseases is not satisfactory and is threatened by resistance. The discovery of the apicoplast, a chloroplast-like organelle, presents drug targets unique to these parasites. The apicoplast-localized fatty acid synthesis (FAS II) pathway, a metabolic process fundamentally divergent from the analogous FAS I pathway in humans, represents one such target. However, the specific biological roles of apicoplast FAS II remain elusive. Furthermore, the parasite genome encodes additional and potentially redundant pathways for the synthesis of fatty acids. We have constructed a conditional null mutant of acyl carrier protein, a central component of the FAS II pathway in Toxoplasma gondii. Loss of FAS II severely compromises parasite growth in culture. We show FAS II to be required for the activation of pyruvate dehydrogenase, an important source of the metabolic precursor acetyl-CoA. Interestingly, acyl carrier protein knockout also leads to defects in apicoplast biogenesis and a consequent loss of the organelle. Most importantly, in vivo knockdown of apicoplast FAS II in a mouse model results in cure from a lethal challenge infection. In conclusion, our study demonstrates a direct link between apicoplast FAS II functions and parasite survival and pathogenesis. Our genetic model also offers a platform to dissect the integration of the apicoplast into parasite metabolism, especially its postulated interaction with the mitochondrion.
Keywords: apicomplexa, fatty acid biosynthesis, lipoic acid, Plasmodium, plastid
Chemotherapeutic treatment of human infections with apicomplexan parasites faces many challenges including limited efficacy, side effects, and widespread drug resistance. New drugs with novel mechanisms of action are therefore urgently needed. The discovery of a parasite chloroplast, the apicoplast (1, 2), provides an exciting opportunity to identify new drug targets. Genomic analysis revealed apicoplast pathways for the synthesis of fatty acids, isoprenoids, and heme in Plasmodium falciparum and Toxoplasma gondii (3). These pathways are especially attractive targets because they are of cyanobacterial origin and differ from the host (4). Apicoplast prokaryotic type II fatty acid synthesis (FAS II) has received particular attention (5). This pathway differs in structure, kinetics, and inhibitor susceptibility from the eukaryotic FAS I pathway found in the mammalian host (6, 7). FAS II inhibitors have been shown to affect parasite growth (8–10); however, the specificity of some of these inhibitors has been questioned (11). Furthermore, it appears that, in addition to FAS II, apicomplexans also harbor fatty acyl-elongases (http://toxodb.org, TgTwinScan_3930, 2967, and 6237) and, in the case of Toxoplasma, a FAS I pathway (12) (TgTwinScan_0460). These multiple pathways potentially could provide redundant sources of fatty acids to the parasite and might therefore render FAS II ineffective as a drug target. Here we use the genetic model apicomplexan T. gondii to rigorously evaluate apicoplast FAS II as a drug target and to establish its role in parasite biology.
Results and Discussion
Isolation of a Conditional Null Mutant for the FAS II Acyl Carrier Protein (ACP)
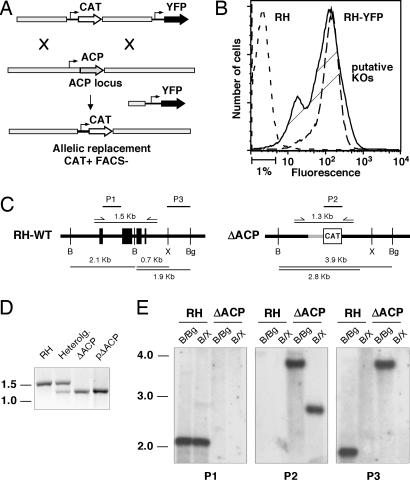
ACP is a central component of the apicoplast FAS II pathway. The protein is encoded by a single-copy gene in the T. gondii nuclear genome and posttranslationally imported into the apicoplast (13). To isolate a conditional T. gondii mutant for this gene an ectopic minigene copy was introduced into the TAti tet-transactivator line by stable transformation (14). The ectopic copy was placed under the control of the tetracycline regulatable promoter 7tetOSag4 (14) and tagged with a c-myc epitope (see Materials and Methods for details). In this background (ACP/ACPi) the native ACP locus was targeted by double homologous recombination. More than 400 clones from multiple independent transfection experiments were screened by PCR; however, all clones were found to harbor nonhomologous insertions of the targeting plasmid instead of allelic replacements (data not shown). To overcome this high background of nonhomologous recombination a yellow fluorescent protein (YFP) expression cassette was introduced into the targeting plasmid to enable counterselection by cell sorting (Fig. 1A and B). By using this new double-marker strategy loss of the wild-type PCR product was observed in 2 of 25 clones. Deletion of the ACP coding sequence and allelic replacement with the chloramphenicol acetyl-transferase marker were confirmed by Southern blot analysis with three independent probes (see Fig. 1 C–E for details).
Fig. 1.
Gene targeting of the ACP locus using a positive/negative selection scheme to enrich homologous recombinants. (A) Schematic representation of the two-marker targeting plasmid and positive/negative selection for homologous recombination at the ACP locus. (B) FACS profiles of the nonfluorescent wild type (RH, short-dashed line), a YFP-expressing clone derived from this strain (long-dashed line), and a population of stable drug-resistant parasites after transfection with the double-marker targeting plasmid (solid line, putative KOs; the indicated 1% least fluorescent of parasites were sorted and cloned). Two of 25 of these clones showed successful targeting of the locus (compared with >400 clones unsuccessfully screened by using a single-marker approach). (C) Allelic replacement of the native ACP gene by chloramphenicol acetyl transferase (CAT) gene yields altered restriction profile after digestion with restriction enzymes BamHI, BglII, and XhoI. (D) PCR detection of endogenous (1.5 kb) and inducible (1.3 kb) ACP genes. Heterolog., clone harboring a heterologous insertion of the targeting plasmid in addition to the native locus. The pKO targeting plasmid and RH genomic DNA serve as control for KO insert and native ACP, respectively. (E) Southern blot analysis of BamHI/BglII and BamHI/XhoI digests of RH and ΔACP/ACPi genomic DNA with probes P1 (ACP intron, hybridizes to native ACP but not to the ectopic minigene copy), P2 (CAT coding sequence), and P3 (3′ noncoding region present in both loci). All autoradiographs show the same membrane, which was stripped and reprobed.
Expression of ACP Can Be Tightly Controlled in Parent and Mutant Strains.
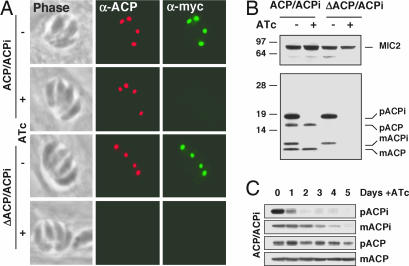
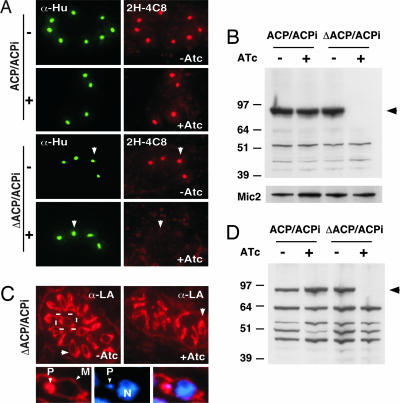
The deletion as well as the parental strain were analyzed for ACP expression by using specific antibodies against ACP (13) or the c-myc epitope tag. As shown in Fig. 2A both antibodies label the apicoplast in immunofluorescence experiments in the parental strain (ACP/ACPi) and the knockout strain (ΔACP/ACPi). Upon anhydrous tetracycline (ATc) treatment reactivity toward the c-myc antibody is lost in both strains. The apicoplast in the ACP/ACPi strain retains labeling by using the ACP antibody during ATc treatment because of the presence of the native locus. In contrast, ATc treatment of the mutant strain abolishes ACP labeling completely. These results were confirmed by Western blot analysis using the ACP antibody (Fig. 2B) (an antibody against the microneme protein MIC2 was used to control loading). Note that nuclear encoded apicoplast proteins are synthesized as precursor proteins (p), which are proteolytically processed to the smaller, mature form (m) after posttranslational targeting to the apicoplast (13, 15), and that ACPi shows reduced mobility due to the presence of the epitope tag. We carefully analyzed the kinetics of ATc-mediated ACP knock-down (Fig. 2C). The ACPi precursor protein showed a dramatic reduction within 24 h, suggesting a fast effect of ATc on transcription. Mature ACPi appears to be a stable protein and has to be diluted by growth; after 5 days ACPi is no longer detectable (native ACP was used as loading control). To ensure full ACP knockdown all biochemical experiments described below were conducted with cells treated with ATc for at least 5 days.
Fig. 2.
Regulation of ACP expression by ATc treatment. (A) ACP/ACPi and ΔACP/ACPi parasites were treated with ATc for 5 days and fixed and stained with antibodies to ACP and c-myc (same exposure time for all micrographs). (B) Western blot analysis of the same set of samples using ACP antibody. p, precursor; m, mature protein; MIC2, loading control. (C) ACPi parasites were grown in the presence of ATc, and samples were collected daily for 5 days and analyzed by Western blot by using the ACP antibody (native ACP serves as loading control). Note that the precursor protein disappears within a day, suggesting fast suppression of transcription, whereas the mature protein is stable and has to be diluted by growth.
Apicoplast FAS II Is Essential for Parasite Growth and Virulence.
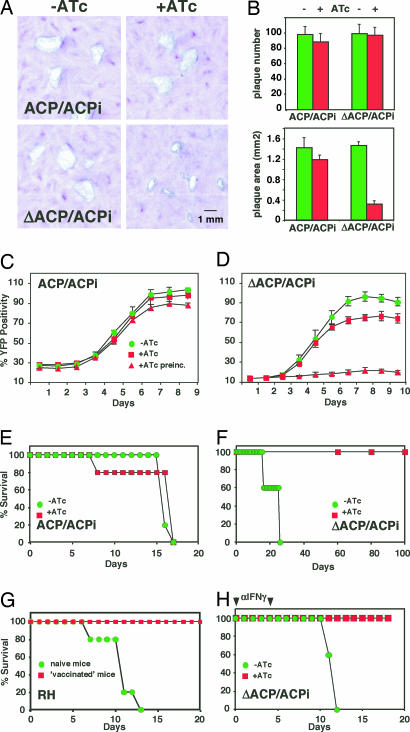
We next examined the effect of ACP depletion on parasite growth using plaque assays. ΔACP/ACPi formed markedly smaller plaques under ATc [Fig. 3A and B; area was measured for 50 plaques per flask (n = 3; P < 0.0001, Student's t test)]. To measure growth dynamically, a YFP-YFP transgene was introduced by transfection and cell sorting, and the new lines were analyzed by using a real-time fluorescence assay (16). ΔACP/ACPi parasites incubated in the presence of ATc initially grow at a rate similar to untreated parasite, but growth levels off after 6 days (Fig. 3D). Parasites preincubated with ATc for 6 days (second passage, red triangles) showed severe growth inhibition (no viable parasites could be detected by plaque assay after a third passage under ATc; data not shown). No significant growth effects of ATc treatment were observed in the ACPi parental strain.
Fig. 3.
Growth and virulence of mutant and parental strain. (A) Growth of ACP/ACPi and ΔACP/ACPi parasites incubated in the presence and absence of ATc was scored by plaque assay. (B) Plaque number and area were quantified in three independent flasks. (C and D) ATc dependence of growth was also measured by following fluorescence in YFP-YFP-expressing lines derived from parent and mutant (second-passage parasites were cultured for 6 days under ATc before inoculation of multiwell plates). C57BL/6 mice were infected with parent (E) and mutant (F) parasites (note slight delay in killing by the mutant when compared with the parental parasites). Mice received 0.2 mg/ml ATc (red) or placebo (green) in the drinking water, and survival was followed for 100 days. (G) Surviving mice from F were rechallenged with 10,000 RH wild-type parasites (vaccinated parasites) in parallel to a group of naïve control mice. (H) Mice were immunosuppressed by injection of 0.5 mg of an IFNγ-specific antibody on days 0 and 5 (arrows), infected with ΔACP/ACPi, and treated as described for F.
To establish whether FAS II knock-down affects the ability of these parasites to cause disease, mice were infected with 1,000 ΔACP/ACPi or ACP/ACPi tachyzoites (n = 10) and treated with ATc or placebo in the drinking water. In mice challenged with the parental strain large numbers of parasites were evident in the peritoneum, and all mice succumbed to infection irrespective of ATc treatment (Fig. 3E). In contrast, whereas mice infected with the ΔACP/ACPi strain and given placebo were susceptible to infection, those receiving ATc showed no signs of disease and survived this challenge (Fig. 3F). Infection with the mutant combined with ATc treatment effectively vaccinated mice; these mice were refractory to a subsequent lethal challenge with 10,000 wild-type RH parasites (Fig. 3G; compare to naïve control). IFNγ is the key mediator for the control of T. gondii (17), and neutralization of this cytokine by antibody treatment resulted in a faster course of disease (18) (Fig. 3, compare H with E). Importantly, mice infected with mutant parasites and receiving ATc were again fully protected. Together, these data suggest that apicoplast FAS is essential for robust growth and pathogenesis regardless of the host's immune status.
Loss of Apicoplast FAS II Does Not Affect [14C]Acetate Incorporation into Fatty Acids.
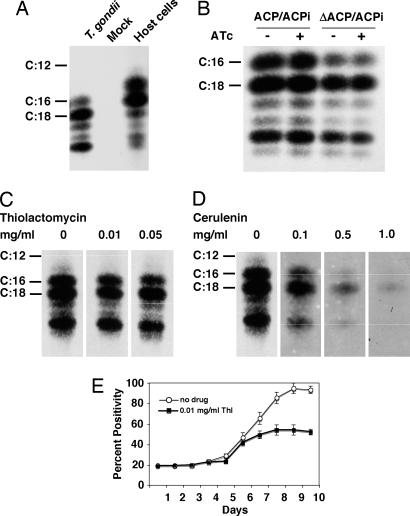
FAS proceeds through sequential addition of two carbon units derived from acetyl-CoA. A recent study (19) described [14C]acetate labeling of extracellular T. gondii tachyzoites, which resulted in radiolabeling of numerous parasite lipids. The authors interpreted [14C]acetate labeling as the product of bulk de novo FAS by the apicoplast FAS II system (19). We therefore monitored [14C]acetate incorporation into fatty acids in mutant and parent cells. Interestingly, C18 and longer-chain fatty acids were the most abundant products in T. gondii (Fig. 4A). In control experiments [14C]acetate labeling of host cells resulted in the production of shorter-chain-length fatty acids (especially palmitate, C16) as typical for de novo FAS (Fig. 4A). Surprisingly, ATc treatment of the mutant strain showed no effect on FAS as measured by [14C]acetate incorporation (Fig. 3B). These observations suggest that acetate incorporation into fatty acids in T. gondii results from the action of FAS I and/or fatty acid elongases (12, 20) rather than de novo synthesis by FAS II. To test this hypothesis, experiments were repeated in the presence of cerulenin, which inhibits FAS I and II, and thiolactomycin, a FAS II-specific inhibitor (9, 21, 22). Acetate incorporation was sensitive to cerulenin but resistant to thiolactomycin, confirming that FAS I is responsible for the conversion of acetate into fatty acids (Fig. 4 C and D). This result is not due to resistance of apicoplast FAS II to thiolactomycin; in control experiments parasite growth was highly sensitive to treatment with 0.01 mg/ml thiolactomycin. Growth inhibition showed delayed kinetics as observed for the genetic FAS II knockdown and previously described for clindamycin, a drug targeting apicoplast transcription (23). Acetate incorporation into fatty acids requires the activity of acetyl-CoA synthetase for precursor synthesis. The putative acetyl-CoA synthetase encoded in the T. gondii genome (TgTwinScan_3199) appears to lack an apicoplast-targeting motif, which suggests that the apicoplast cannot use acetate. Instead, acetyl-CoA must be generated by the action of pyruvate dehydrogenase (PDH) (3, 24).
Fig. 4.
14C lack of apicoplast FAS II does not affect acetate incorporation but blocks lipoylation of apicoplast PDH. (A) Extracellular tachyzoites were metabolically labeled with [14C]acetate. Lipids were extracted and hydrolyzed, and the resulting fatty acids were methylated. Radiolabeled fatty acid methyl esters were analyzed by reversed-phase high-performance thin-layer chromatography (methyl esters of radiolabeled fatty acid of known chain length were run in parallel as standards). Mock infection or direct labeling of host cell cultures served as control for potential host cell contamination. (B) ΔACP/ACPi and ACP/ACPi parasites were grown in the presence or absence of ATc for 6 days followed before [14C]acetate labeling. (C and D) Parasite acetate incorporation into fatty acids was resistant to the FAS II inhibitor thiolactomycin (C) but sensitive to the general FAS inhibitor cerulenin (D). (E) Growth of the ΔACP/ACPi-YFP-YFP strain was measured in the presence of 0.01 mg/ml thiolactomycin (filled squares) in comparison with untreated controls (open circles). Thiolactomycin showed strong growth inhibition, and [14C]acetate incorporation was resistant to a 5-fold-higher drug concentration (C).
FAS II Is Required for Lipoylation of the Apicoplast PDH Complex.
In addition to producing fatty acids, the FAS II pathway could also provide precursors for lipoic acid synthesis (25, 26). Lipoic acid is an essential cofactor for oxidative decarboxylases and is involved in the response to oxidative stress. PDH is the only lipoylated protein in the apicoplast (3). In immunofluorescence experiments the apicoplast is specifically labeled by an antibody recognizing lipoylated PDH E2 (2H-4C8) (27) (Fig. 5A). ATc treatment of the mutant but not the parent strain completely abolished this labeling in immunofluorescence and Western blot analysis (Fig. 5 B and C). This observation demonstrates that FAS II is essential for the lipoylation pathway and confirms that ATc treatment results in effective suppression of the FAS II pathway. Several mitochondrial enzymes also require lipoylation (28), and the mitochondrial lipoylation pathways appear to be distinct from those in the apicoplast (25, 26). A polyclonal serum that reacts with all lipoylated proteins detects multiple protein bands in Western blots and labels the plastid as well as the mitochondrion (Fig. 5 C and D). Whereas ATc treatment again abolished labeling of the apicoplast and PDH (black arrowhead), mitochondrial labeling remained unaffected. This finding demonstrates that mitochondrial lipoylation does not depend on apicoplast-produced lipoic acid but likely salvages lipoic acid from the host.
Fig. 5.
Loss of apicoplast FAS II abolishes lipoylation of apicoplast but not mitochondrial enzymes. (A and B) Immunofluorescence (A) and Western blot (B) analysis of parent and mutant cells with antibody to lipoylated PDH-E2 (2H-4C8). ATc treatment of the mutant abolishes PDH labeling (arrow). An antibody to the T. gondii apicoplast histone-like protein (Hu protein; S. Vaishnava and B.S., unpublished observations) served as apicoplast marker, and an antibody to MIC2 served as loading control. (C and D) Immunofluorescence (C) and Western blot (D) analysis using a polyclonal serum reactive to all lipoylated enzymes localized to the plastid (P) and the mitochondrion (M). Note loss of PDH labeling (black arrowhead) and persistence of mitochondrial labeling (white arrowhead).
Lack of FAS II Causes Severe Defects in Apicoplast Biogenesis.
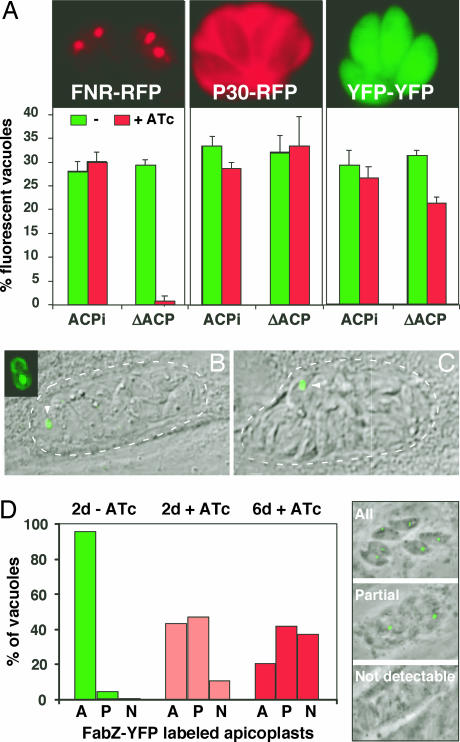
Lack of FAS II function has been shown to cause defects in the biogenesis of chloroplasts and bacteria (29, 30). To evaluate apicoplast morphology under FAS II knock-down conditions we transiently transfected ATc-treated parasites with the apicoplast marker ferredoxin NADPH reductase (FNR)-RFP (31). Whereas the untreated mutant showed typical plastid labeling in 30% of the cells (the usual transfection efficiency), few fluorescent plastids could be detected in treated mutants (Fig. 6A). No difference in plastid labeling was observed in the parent strain under drug. Control experiments using markers for the cytoplasm and the secretory pathway showed no morphological changes upon ATc treatment, indicating that this effect is specific to the apicoplast (Fig. 6A). A stable parasite line expressing the apicoplast marker FabZ-YFP (13) was constructed in the mutant background. Upon ATc treatment we frequently observed a single or a few large plastid(s) per vacuole, reminiscent of a previously described apicoplast division mutant, which led to unequal segregation of the organelle (Fig. 6 B and C) (32). Quantification of plastid numbers over time after ATc treatment in this strain revealed a progressive loss (Fig. 6D) (similar results were obtained in immunofluorescence assays detecting apicoplast HU protein; data not shown). To control for organellar specificity the FabZ-expressing mutant line was transiently transfected with a TY-tagged version of the T. gondii superdioxide dismutase 2 gene (a kind gift of D. Soldati, University of Geneva, Geneva, Switzerland). The protein encoded by this gene is dually targeted to the apicoplast as well as the mitochondrion. Prolonged ATc pretreatment again abolished apicoplast labeling; however, mitochondrial labeling appeared unchanged (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 6.
Depletion of ACP leads to defects in apicoplast morphology and biogenesis. (A) ACP/ACPi and ΔACP/ACPi cells were grown for 6 days in the presence or absence of ATc and then transiently transfected with plasmids, resulting in the expression of FNR-RFP (apicoplast), P30-RFP (dense granules and parasitophorous vacuole), and YFP-YFP (cytoplasm) before infection of coverslip cultures. After 24 h parasites were scored for fluorescent protein expression. (B and C) A ΔACP/ACPi line stably expressing the apicoplast marker FabZ-YFP was treated with ATc for 3 days and imaged in vivo. Vacuoles with a single or a few large apicoplasts were frequently observed (arrowhead; parasitophorous vacuoles are indicated by dotted lines). (D) The same line was treated with ATc for 0, 2, and 6 days, and plastid morphology was scored by using the three categories indicated (A, all parasites in a vacuole show a fluorescent apicoplast; P, one or few apicoplasts per vacuole; N, no apicoplast labeling detected).
Conclusions
Contrary to a previous report (19) our analyses do not support bulk FAS (as measured by [14C]acetate incorporation) as the main function of the apicoplast FAS II in T. gondii. These observations are consistent with the view that T. gondii relies heavily on the host for its lipid needs (33, 34). However, as discussed here the apicoplast likely lacks the enzyme required for acetate activation, preventing us from directly measuring FAS II activity. Further work is needed to formally demonstrate or refute bulk de novo FAS by the apicoplast FAS II system. However, we show that FAS II is required for the lipoylation of PDH. This finding is consistent with a recent pharmacological study (35). Interestingly, providing octanoic acid–ACP as a precursor for lipoylation is also viewed as a main function of the FAS II pathway in the mitochondrion of green plants (36). Blocking lipoylation should render the apicoplast PDH inactive (37). The fact that this complex is the only PDH found in apicomplexans (24) might affect pathways dependent on acetyl-CoA in addition to those in the apicoplast. Whether the apicoplast exchanges acetyl-CoA with other compartments (especially with the mitochondrion) remains to be determined. Our studies also indicate that a major function of FAS II is in apicoplast maintenance and biogenesis, likely because of a requirement for the synthesis of lipids required for the growth and division of plastid membranes (29, 30) or organellar protein import (38). Together these studies establish that the apicoplast FAS II pathway is essential for parasite survival in vitro and in vivo and represents a viable target for new antiapicomplexan drugs.
Materials and Methods
Plasmid Construction.
A plasmid for regulatable ACP expression was engineered through sequential modification of ptubYFP-YFP/sagCAT (16). First the ACP coding region was introduced, replacing the first YFP cassette (BglII/AvrII). Next the regulatable promoter was amplified by PCR from plasmid p7tetOS4LacZCAT (39) and cloned in place of the tubulin promoter (PpuMI/BglII). Then the second YFP cassette was replaced with a myc epitope tag cassette derived from pgra1-PCNA-c-myc-dhfr/HXGPRT (AvrII/NotI, along with the 3′ UTR). Last, the CAT marker was replaced with a DHFR-TS pyrimethamine resistance cassette from TSc3ABP (40) by PpuM I–XbaI/ApaI digestion and end filling to generate ptet07sag4-ACPmyc/DHFR-TS.
In the targeting plasmid pTgACP-KO-CAT the marker gene was flanked with 1.0 kb of the 5′ and 3.1 kb of the 3′ ACP flanking sequences amplified from genomic DNA. A tubYFP cassette was amplified from plasmid ptubYFP-YFP (5′-ATGCATATGATGCATGTCCCGCGTTCGTGA-3′ and 5′-TCTGCGGCCGCTTACTTGTACAGCTCGTCCATGCCG-3′) and introduced downstream of ACP 3′ flanking sequences (NdeI/NotI). Plasmids, tubYFP-YFP/sagCAT (16), tubP30-RFP/sagCAT (41), and tubFNR-RFP/sagCAT (31) were described previously. ptub-FabZ-YFP/sagCAT was generated by exchanging ACP in ptub-ACPYFP/sagCAT with the FabZ coding sequence (13) amplified from T. gondii cDNA by PCR.
Parasite Culture and Selections.
T. gondii tachyzoites were maintained in human foreskin fibroblasts or hTERT cells. To generate a strain with a regulatable ectopic copy of ACP (ACP/ACPi) the TAti strain (14) was transfected with plasmid ptet07sag4-ACPmyc/DHFR-TS and selected under 1 μM pyrimethamine. A stable clone showing tight transgene regulation (data not shown) was electroporated in the presence of a fragment of pTgACP-KO-CAT (bacterial sequences were removed by NotI/KpnI digestion and gel purification). Parasites were cultured in the presence of 20 μM chloramphenicol for three passages and then sorted for YFP expression by using a MoFlo high-speed cell sorter (Dako, Fort Collins, CO). The 1% least fluorescent cells (510/540 nm) were directly sorted into 384-well plates seeded with human foreskin fibroblast cells, expanded, and tested for gene replacement by PCR and Southern blotting (see Fig. 1 for details). Expression of the ectopic copy of ACP was suppressed by culture in the presence of 1 μg/ml ATc (IBA, St. Louis, MO). Stable transgenics of mutant and parental strain transfected with YFP-YFP and FabZ-YFP plasmids where isolated by three successive rounds or cell sorting before cloning.
In Vitro Growth Assays.
Growth of parasites in cultured cells was scored 10 days after infection by plaque assay, as previously described (42). High-resolution scans were obtained for three independent flasks, and the longest and shortest diameter of 50 plaques per flask was measured by image analysis (plaque area was calculated by using the ellipsoid formula πab/4). ΔACP/ACPi-YFP and ACP/ACPi-YFP parasites were cultured in optical-bottom 96-well plates as indicated, and growth was measured after fluorescence as described (16).
Virulence Assay in Mice.
Parasites were harvested from freshly lysed human foreskin fibroblast cultures, and 1,000 tachyzoites (in 0.2 ml of PBS) were injected i.p. into 6- to 8-week-old C57BL/6 mice. Groups of five mice received sterile drinking water supplemented with 0.1% sucrose. In indicated groups the drinking water was further supplemented with 0.2 mg/ml ATc. Mice that survived infection were rechallenged with 10,000 RH strain tachyzoites. In some experiments the level of IFNγ was reduced by injecting each mouse with 500 μg of an anti-IFNγ antibody on days 0 and 5 of the experiment. This protocol has previously been shown to result in significant immunosuppression and increased susceptibility to severe toxoplasmosis (18).
Metabolic Labeling and Fatty Acid Analysis.
A total of 108 tachyzoites (pretreated with ATc as indicated) were incubated with 10 μCi (1 Ci = 37 GBq) of Na-[14C]acetate (Movarek, Brea, CA) in 1 ml of DMEM for 4 h at 37°C and 5% CO2. Thiolactomycin (a generous gift of J. Ondeyka, Merck Research Laboratories, Rahway, NJ), cerulenin, or vehicle (ethanol) was added as indicated. Total lipids were extracted with chloroform/methanol (2:1) and subjected to Folch washing as described (43). The extract was dried under nitrogen, and total lipids were taken up in anhydrous methanol containing 0.2% sulfuric acid and 0.1% benzene and heated to 70°C for 4 h as previously described (44). The resulting fatty acid methyl esters were extracted with hexane and analyzed on reversed-phase C18 high-performance thin-layer chromatography plates developed in methanol/chloroform/water (75:25:5) and exposed to film. Incubation of free parasites with [14C]pyruvate or [14C]malonic acid did not result in the detection of radiolabeled fatty acids.
Immunostaining of Parasites and Proteins.
Immunofluorescence assays were performed as described (41) by using primary antibodies at 1:1,000 dilution and secondary antibodies at 1:300 dilution. Endogenous and inducible ACP was detected with anti-ACP polyclonal rabbit serum (13) and anti-c-myc (9E10) monoclonal antibodies. Lipoylated PDC-E2 was detected by using monoclonal antibody 2H-4C8 (27) (a kind gift of Eric Gershwin, University of California, San Diego, CA) and polyclonal anti-LA antibodies (Calbiochem, San Diego, CA). For Western blot analysis the following antibodies and dilutions were used: anti-ACP and monoclonal anti-Mic2 antibodies at 1:10,000 (generously provided by Jean-Francois Dubremetz, University of Montpellier, Montpellier, France), anti-2H-4C8 and anti-LA antibodies at 1:5,000, and all HRP-conjugated secondary antibodies at 1:3,000. Parasites transfected with YFP-YFP, FNR-RFP, P30-GFP, and FabZ-YFP were visualized by in vivo fluorescence microscopy without additional staining.
Supplementary Material
Acknowledgments
We thank J. Nelson for help with cell sorting; R. Donald (Merck Research Laboratories), J. Ondeyka, G. McFadden (University of Melbourne, Melbourne, Australia), D. Soldati, J. F. Dubremetz, and E. Gershwin for antibodies, drugs, and plasmids; P. Englund, M. Crawford, and M. J. Gubbels for discussion; and G. van Dooren and L. Hedstrom for editorial comments. Preliminary genomic and/or cDNA sequence data were accessed via http://toxodb.org. Genomic data were provided by The Institute for Genomic Research (supported by National Institutes of Health Grant AI05093) and by the Sanger Center (supported by The Wellcome Trust). EST sequences were generated by Washington University (St. Louis, MO) (supported by National Institutes of Health Grant AI045806-01A1). This work was funded in part by National Institutes of Health Grants AI64671 (to B.S.) and AI42334 (to C.A.H.).
Abbreviations
- ACP
acyl carrier protein
- ATc
anhydrous tetracycline
- FAS
fatty acid synthesis
- FNR
ferredoxin NADPH reductase
- YFP
yellow fluorescent protein
- PDH
pyruvate dehydrogenase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kohler S., Delwiche C. F., Denny P. W., Tilney L. G., Webster P., Wilson R. J., Palmer J. D., Roos D. S. Science. 1997;275:1485–1489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 2.McFadden G. I., Reith M. E., Munholland J., Lang-Unnasch N. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 3.Ralph S. A., Van Dooren G. G., Waller R. F., Crawford M. J., Fraunholz M. J., Foth B. J., Tonkin C. J., Roos D. S., McFadden G. I. Nat. Rev. Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 4.McFadden G. I., Roos D. S. Trends Microbiol. 1999;7:328–333. doi: 10.1016/s0966-842x(99)01547-4. [DOI] [PubMed] [Google Scholar]
- 5.Surolia A., Ramya T. N., Ramya V., Surolia N. Biochem. J. 2004;383:401–412. doi: 10.1042/BJ20041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier T., Jenni S., Ban N. Science. 2006;311:1258–1262. doi: 10.1126/science.1123248. [DOI] [PubMed] [Google Scholar]
- 7.Heath R. J., White S. W., Rock C. O. Prog. Lipid Res. 2001;40:467–497. doi: 10.1016/s0163-7827(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 8.Surolia N., Surolia A. Nat. Med. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- 9.Waller R. F., Ralph S. A., Reed M. B., Su V., Douglas J. D., Minnikin D. E., Cowman A. F., Besra G. S., McFadden G. I. Antimicrob. Agents Chemother. 2003;47:297–301. doi: 10.1128/AAC.47.1.297-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLeod R., Muench S. P., Rafferty J. B., Kyle D. E., Mui E. J., Kirisits M. J., Mack D. G., Roberts C. W., Samuel B. U., Lyons R. E., et al. Int. J. Parasitol. 2001;31:109–113. doi: 10.1016/s0020-7519(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 11.Paul K. S., Bacchi C. J., Englund P. T. Eukaryot. Cell. 2004;3:855–861. doi: 10.1128/EC.3.4.855-861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu G. J. Eukaryot. Microbiol. 2004;51:381–388. doi: 10.1111/j.1550-7408.2004.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 13.Waller R. F., Keeling P. J., Donald R. G., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S., McFadden G. I. Proc. Natl. Acad. Sci. USA. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meissner M., Schluter D., Soldati D. Science. 2002;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 15.DeRocher A., Hagen C. B., Froehlich J. E., Feagin J. E., Parsons M. J. Cell Sci. 2000;113:3969–3977. doi: 10.1242/jcs.113.22.3969. [DOI] [PubMed] [Google Scholar]
- 16.Gubbels M. J., Li C., Striepen B. Antimicrob. Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 18.Hunter C. A., Abrams J. S., Beaman M. H., Remington J. S. Infect. Immun. 1993;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisanz C., Bastien O., Grando D., Jouhet J., Marechal E., Cesbron-Delauw M. F. Biochem. J. 2006;394:197–205. doi: 10.1042/BJ20050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu G., Li Y., Cai X., Millership J. J., Marchewka M. J., Keithly J. S. Mol. Biochem. Parasitol. 2004;134:127–135. doi: 10.1016/j.molbiopara.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Magnuson K., Jackowski S., Rock C. O., Cronan J. E., Jr. Microbiol. Rev. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omura S. Bacteriol. Rev. 1976;40:681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fichera M. E., Roos D. S. Nature. 1997;390:407–409. doi: 10.1038/37132. [DOI] [PubMed] [Google Scholar]
- 24.Foth B. J., Stimmler L. M., Handman E., Crabb B. S., Hodder A. N., McFadden G. I. Mol. Microbiol. 2005;55:39–53. doi: 10.1111/j.1365-2958.2004.04407.x. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen-Zieger N., Schachtner J., Seeber F. FEBS Lett. 2003;547:80–86. doi: 10.1016/s0014-5793(03)00673-2. [DOI] [PubMed] [Google Scholar]
- 26.Wrenger C., Muller S. Mol. Microbiol. 2004;53:103–113. doi: 10.1111/j.1365-2958.2004.04112.x. [DOI] [PubMed] [Google Scholar]
- 27.Migliaccio C., Nishio A., Van de Water J., Ansari A. A., Leung P. S., Nakanuma Y., Coppel R. L., Gershwin M. E. J. Immunol. 1998;161:5157–5163. [PubMed] [Google Scholar]
- 28.Salcedo E., Sims P. F., Hyde J. E. Trends Parasitol. 2005;21:406–411. doi: 10.1016/j.pt.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnowsky F., Fuchs K., Jeschek C., Hogenauer G. J. Bacteriol. 1989;171:6555–6565. doi: 10.1128/jb.171.12.6555-6565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mou Z., He Y., Dai Y., Liu X., Li J. Plant Cell. 2000;12:405–418. doi: 10.1105/tpc.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Striepen B., Crawford M. J., Shaw M. K., Tilney L. G., Seeber F., Roos D. S. J. Cell Biol. 2000;151:1423–1434. doi: 10.1083/jcb.151.7.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C. Y., Shaw M. K., Pletcher C. H., Striepen B., Tilney L. G., Roos D. S. EMBO J. 2001;20:330–339. doi: 10.1093/emboj/20.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charron A. J., Sibley L. D. J. Cell Sci. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- 34.Coppens I. Cell. Microbiol. 2006;8:1–9. doi: 10.1111/j.1462-5822.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 35.Crawford M. J., Thomsen-Zieger N., Ray M., Schachtner J., Roos D. S., Seeber F. EMBO J. 2006;25:3214–3222. doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gueguen V., Macherel D., Jaquinod M., Douce R., Bourguignon J. J. Biol. Chem. 2000;275:5016–5025. doi: 10.1074/jbc.275.7.5016. [DOI] [PubMed] [Google Scholar]
- 37.Radke G. A., Ono K., Ravindran S., Roche T. E. Biochem. Biophys. Res. Commun. 1993;190:982–991. doi: 10.1006/bbrc.1993.1146. [DOI] [PubMed] [Google Scholar]
- 38.Chen L.-J., Li H.-m. Plant J. 1998;16:33–39. doi: 10.1046/j.1365-313x.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- 39.Meissner M., Brecht S., Bujard H., Soldati D. Nucleic Acids Res. 2001;29:E115. doi: 10.1093/nar/29.22.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Striepen B., White M. W., Li C., Guerini M. N., Malik S. B., Logsdon J. M., Jr., Liu C., Abrahamsen M. S. Proc. Natl. Acad. Sci. USA. 2002;99:6304–6309. doi: 10.1073/pnas.092525699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Striepen B., Soldati D., Garcia-Reguet N., Dubremetz J. F., Roos D. S. Mol. Biochem. Parasitol. 2001;113:45–54. doi: 10.1016/s0166-6851(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 42.Pfefferkorn E. R., Pfefferkorn L. C. Exp. Parasitol. 1976;39:365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- 43.Folch J., Lees M., Sloane Stanley G. H. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 44.Fosbrooke A. S., Tamir I. Clin. Chim. Acta. 1968;20:517–522. doi: 10.1016/0009-8981(68)90311-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.