Abstract
The Arabidopsis HOBBIT (HBT) gene encodes a homolog of the CDC27 anaphase-promoting complex/cyclosome subunit and is essential for postembryonic development. We induced loss-of-function clones by Cre/lox-mediated recombination of a single complementing HBT transgene in a background homozygous for the strong mutant allele hbt2311. Defects in cell division and cell expansion are the primary consequences of ubiquitous postembryonic HBT excision. In roots, both cell division and cell expansion are rapidly affected. In contrast, in leaf primordia, cell division and cell expansion halt after a lag phase, which results in different severities of defects in the proximodistal and mediolateral axes. Surprisingly, small clones reveal non-cell-autonomous rescue of hbt mutant cells, indicating a previously unrecognized compensation mechanism for reduced activity of an anaphase-promoting complex/cyclosome component critical for cell cycle progression.
Keywords: cell cycle, clonal analysis, growth control, root development
Multicellular organisms coordinate cell division and cell growth to generate organs of characteristic sizes. In plants, coordination of cell division and growth is especially important to regulate organ shape and size, because cells share semirigid walls and the growth of one cell affects the expansion of its neighbors. Mechanisms by which cell size and division rates are controlled across plant tissues, and how such interactions shape entire organs, remain largely unknown.
Control of cell division and cell expansion can be conveniently addressed in the continuously produced organs of the model plant Arabidopsis thaliana. In roots, dividing cells in the tip region (the meristem) maintain a specific size depending on their identity. A sharp increase in cell size occurs at the boundary of the more proximally located elongation zone. Roots have no fixed organ size but grow continuously, and cell production and cell expansion together determine their growth rate (1). In leaves, cell division and cell expansion are tightly coordinated along separate axes, and this process is required for attainment of their proper final shape and size (2, 3). Arabidopsis leaves display partial growth compensation; a reduction in leaf cell number results in an increase in cell volume (2, 4–6), and increased cell numbers caused by constitutive overexpression of several positive regulators of the cell cycle result in smaller cells (7, 8).
Progression through mitotic and endoreduplication cycles in eukaryotes is regulated by the periodic activity of cyclin-dependent kinases, which is in part achieved by E3 ligases such as the anaphase-promoting complex/cyclosome (APC/C) (9). The APC/C ubiquitinates proteins with destruction (D) or KEN box motifs, such as mitotic cyclins and proteins that regulate sister chromatid separation, and targets them for proteolysis (10, 11). Current evidence indicates that plant APC/C components have largely conserved functions (12–14). Ploidy levels in Arabidopsis leaves correlate with cell size (15) and, through its involvement in both mitotic cycles and endocycles, the APC/C and its regulators can influence both cell division and maximal cell size (16). Most APC/C components are expressed ubiquitously, and their mutation arrests the cell cycle early in plant development (13, 14). In contrast, the CDC27 homolog HOBBIT (HBT) is present in cycling cells and their expanding daughters only and is predominantly required after embryogenesis (17). Here, we describe the effects of postembryonic removal of a single complementing HBT gene copy in a strong hbt mutant background. HBT removal leads rapidly to defects in both cell division and cell expansion. We observe growth compensation in leaves that have to reach a set size, but not in roots that grow indeterminately. Surprisingly, HBT removal in small root or leaf sectors can be rescued by neighboring WT cells, which reveals non-cell-autonomous control of cell division and cell expansion within plant organs. Our data point to the existence of a regulatory mechanism in which low activity of critical cell cycle regulators is bypassed by factors from neighboring cells within and across plant tissue layers.
Results
Postembryonic Reduction of HOBBIT, an APC/C Subunit, Primarily Affects Cell Division and Cell Expansion in Roots.
We set out to investigate whether HBT has a primary function in cell division and cell expansion by monitoring the effect of acute HBT removal in GFP-marked cells. To this end, we analyzed mosaics for the strong hbt2311 allele generated by Cre/lox recombination induced by heat shock (HS) (Fig. 1A) (18) (see Methods). hbt2311 homozygotes containing a single WT copy of the HBT genomic region between lox sites in the pCB1-HBTg vector have WT appearance, indicating that this transgene is fully functional (Fig. 1B and data not shown).
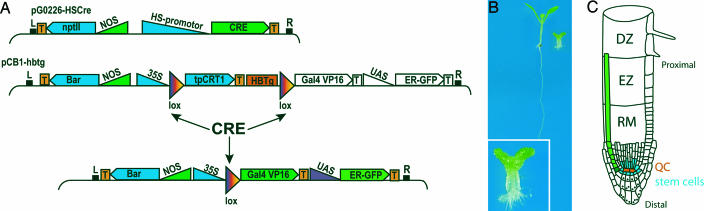
Fig. 1.
Genetic strategy for HBT clone induction. (A) DNA constructs for the induction of hbt2311 homozygous sectors. Colored boxes represent active genes; HBTg, complementing genomic fragment with HBT gene under its own promoter; tpCRT1, norfluorazone resistance; UAS, upstream activating sequence binding yeast; GAL4 transcription factor with VP16 activation domain; T, terminator; L and R, T-DNA borders; lox, recognition site for CRE recombinase. (B) hbt2311 homozygotes with one (Left) or zero (Right and Inset) pCB1-HTBg copies. (C) Root diagram highlighting the differentiation zone (DZ), elongation zone (EZ), and meristem (RM). QC, quiescent center. A single-cell clone that primarily affected the stem cell is marked in dark green, and its descendants are represented in light green. Black dots represent starch granules in differentiated columella cells.
In a first series of experiments, we removed the complementing HBT gene in entire organs. The root meristem harbors convenient markers for cell fate and cell differentiation (Fig. 1C). A set of stem cells surround quiescent center (QC) cells with low mitotic activity. Distally to the QC, a single layer of columella stem cells does not contain starch granules, which are characteristic of differentiated columella cells. Proximal stem cell daughters undergo a few rounds of cell division until they reach the meristem boundary, where they rapidly expand in the elongation zone and fully differentiate in the differentiation zone, thus contributing to root growth (19, 20).
A 60-min (HS) induces Cre/lox recombination of pCB1-HBTg in virtually all cells within the meristem, marking cells in which hbt2311 homozygosity has been unmasked with GFP from 8 h after HS onward (Fig. 2A). HS does not affect the development of plants containing pCB1-HBTg without the HS promoter HS::CRE or plants containing HS::CRE without pCB1-HBTg (data not shown). In plants harboring both transgene constructs, no differences from WT occur with respect to root growth, meristem length, and cell size until 2 days after HS (2 DAHS) when overall root growth ceases (Fig. 2 B–E and data not shown). Although cells within the meristem retain their normal lengths (non-HS, 17.1 ± 2.0 μm; HS, 16.1 ± 2.1 μm) 3 DAHS (n = 103), mature differentiated cells become progressively smaller from 2 DAHS onward (Fig. 2E), suggesting an additional requirement for HBT for rapid expansion of cells within the elongation zone. Flow cytometry revealed that the fraction of endoreduplicating nuclei (8C and 16C) is severely reduced in HS roots from 3 DAHS onward (19.0 ± 2.7%) compared with endoreduplicating nuclei of non-HS plants (35.8 ± 1.7%).
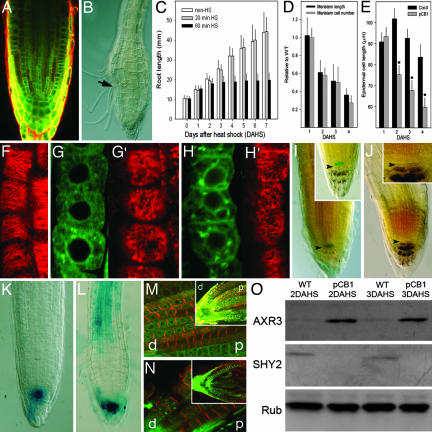
Fig. 2.
Unmasking the hbt2311 mutation by large-scale excision of the complementing HBT function. (A) Ubiquitous GFP expression after 60-min HS marks excision from 8 h after HS onward. (B) Meristem size is strongly reduced 2 DAHS, and the root differentiates 7 DAHS, with root hairs approaching the tip (arrow). (C–E) Time-course analysis of root growth after large-scale excision. Root length (C), root meristem (D), and mature differentiated epidermal cell size (E). (F–H′) Immunolocalization of microtubules (red channel) in WT (F) and hbt2311 cells (marked by GFP, green channel) at 1 (G and G′) and 3 (H and H′) DAHS. (I) QC46::GUS expression (blue) and columella stem cells (arrowhead) are unaffected by HBT removal 2 DAHS. Inset shows the WT root. (J) Columella stem cells remain undifferentiated (arrowhead and magnified in Inset) 4 DAHS when RM is almost totally consumed. (K and L) DR5::GUS expression monitors auxin response after large-scale excision of HBT at 2 (K) and 4 (L) DAHS. (M and N) PIN immunolocalization in hbt2311 clones 3 DAHS. PIN1 (M) and PIN2 (N) protein localization is represented in red, and hbt2311 cells are marked by GFP (green channel). p, proximal; d, distal. (O) Western blot analysis of AXR3 and SHY2 accumulation in hbt2311 clones. Rub, Rubisco (loading control).
Upon ubiquitous HBT removal, root hairs and differentiated vascular tissues approach the tip, and 7 DAHS the entire root differentiates (Fig. 2B). Epidermal cells in the tip region are much shorter and more swollen (Fig. 2B) than the WT cells, similar to the cells of strong hbt mutants (17). Before these visible shape changes, from 1 DAHS onward, cortical microtubules orient randomly (Fig. 2 G′ and H′). In contrast, the epidermal cells of control roots contain cortical microtubules arranged perpendicularly to the main growth axis (Fig. 2F).
The maintenance of cell division in the root meristem is under the control of patterning genes that cooperate to specify the QC, which in turn preserves the surrounding stem cells (21). We asked whether postembryonic removal of the HBT gene affected cell division by affecting QC and stem cell specification. QC identity marker QC46 is still expressed 2 DAHS (Fig. 2I), and columella stem cells remain present up to 4 DAHS (Fig. 2J, arrowhead and Inset), which clearly confirms that QC and stem cell identity are not primarily affected after HBT excision and that the observed growth arrest is due to impaired cell division and cell expansion within the root tip.
Strong hbt mutants show defects in auxin response (17). A subset of the patterning genes involved in stem cell maintenance, the PLETHORA genes, depend on proper auxin distribution and in turn influence auxin distribution through their requirement for transcription of PIN genes that encode auxin efflux facilitator components (22). To investigate whether cell division and cell expansion defects in hbt mutants are caused by defective auxin transport or signaling, we determined whether the position and magnitude of auxin response were affected after HBT removal. Auxin response, as indicated by the DR5::GUS reporter (23), retains its typical maximum around the stem cell region after HBT deletion 1 DAHS and becomes elevated in the elongation zone of roots only after 4 DAHS (Fig. 2 K and L). Among the PIN proteins, PIN1 mainly resides at the basal end of the vascular cells, whereas PIN2 in the meristem localizes apically in epidermal cells and predominantly basally in cortical cells (22). PIN1 and PIN2 protein expression and localization in roots do not significantly differ from the WT after HBT excision up to 3 DAHS (Fig. 2 M and N), when the meristem is largely consumed. These findings indicate that cell division and expansion defects caused by HBT removal are not primarily caused by altered perception or altered auxin distribution in the root meristem. However, in line with observations in strong hbt mutants (17), the auxin signal transduction protein AXR3 (24) is up-regulated from 2 DAHS onward, whereas the related AUX/IAA protein SHY2 (25) is down-regulated (Fig. 2O). Our observations corroborate that HBT influences the stability of auxin response regulators but indicate that this occurs well after cell division and cell expansion defects caused by HBT reduction.
HOBBIT Is Required for Cell Division and Cell Expansion in Leaves.
Leaves are lateral determinate organs that consist of an outer epidermal layer and inner photosynthetic mesophyll and vasculature. These tissues arise from three clonally distinct founder cell layers at the periphery of the shoot apical meristem (SAM), and they are patterned along adaxial–abaxial, proximodistal (PD), and mediolateral (ML) axes (Fig. 3A). Tight coordination of cell division and expansion along these axes during blade expansion is required for proper leaf development and strongly contributes to final leaf shape and form (2, 3, 26). To analyze the requirement for HBT in growth coordination among determinate organs, we investigated hbt2311 clones in leaves. We classified the clones with respect to the leaf tissues that display GFP expression. The extension of the clones in fully developed leaves reflects the layers in the SAM affected by HS and in which HBT excision took place (Fig. 3A′, classes I–VIII). Large hbt sectors were HS-induced (>40 min) in 9-day-old plants. Young leaf primordia (nos. 1 and 2) that already had emerged from the SAM at the time of the HS are fully exposed and express GFP in every cell from 1 DAHS onward. These primordia arrest their growth 3 DAHS (Fig. 3A′, class VIII; and data not shown), consistent with the overall defects in cell division and cell expansion observed in the hbt2311 mutants (J.M.P.-P., O.S. and B.S., unpublished data). Later leaf primordia reach maturity and contain smaller GFP-positive sectors, and GFP-marked clones extend along the entire PD axis and frequently (≈83%, n = 21) include the midvein (Fig. 3 A–E), indicating that cells within these primordia are less accessible for HS induction.
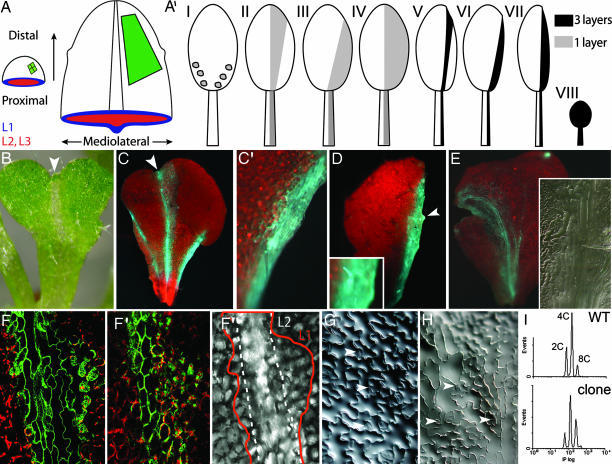
Fig. 3.
HBT mosaic analysis in leaves. (A) Schematic representation of a leaf primordium (Left) with three contributing layers, L1, L2, and L3, and a mature leaf (Right). Green sector depicts growth of a primordial clone that first predominantly grows along the PD axis and, only after lamina expansion, along the ML axis. (A′) Diagram depicting the different sectors obtained after HBT excision following HS. In the case of large clones, only one clone per leaf is depicted. Clones comprising three layers of the leaf are shown in black and display morphological defects whose severity depends on the extent of the clone; clones comprising only one leaf layer are represented in gray. (B–E) Large hbt2311 clones induced in leaf primordia. Clones can be recognized by their reduced chlorophyll content (B) and GFP signal (C–E, false blue). Clones show a mild growth defect in the PD axis (B and C, arrows) and a severe ML growth defect (C–E). (F–H) Cell morphology in three-layered leaf clones. Epidermal cells (F) and mesophyll cells (F′) within GFP-marked clones (shown in green) are elongated along the PD axis, and mesophyll cells lack chlorophyll (shown in red). (G and H) Epidermal cells within the clone are elongated following the PD leaf axis; arrowheads indicate stomatal precursors. (I) Ploidy measurements of sorted nuclei from non-HS (WT) and HS leaves with large clones (clone).
Consistent with a requirement of HBT for cell division, hbt2311 clones spanning all cell layers within the leaf (Fig. 3A′, classes V–VII) contain fewer but elongated cells along the PD axis (Fig. 3 C, C′, E, and F). Palisade mesophyll cells also expand along the PD leaf direction (Fig. 3F′). The indentation of the leaf margin at the distal edge of the clones (Fig. 3A′, class V) indicates that cell elongation is not capable of fully compensating growth in the PD axis (Fig. 3 B and C, arrows, and Table 1, which is published as supporting information on the PNAS web site). In the ML axis, however, clones have strongly reduced cell numbers and appear to lack growth compensation through cell expansion (Fig. 3 C–F and Table 1). Hydathodes (Fig. 3D, arrowhead) still differentiate in hbt2311 sectors, as do stomata and trichomes although their number is reduced compared with neighboring WT epidermis (Fig. 3 D Inset and H arrowheads). Epidermal pavement cells in large mutant sectors lack the jigsaw morphology characteristic of WT pavement cells (Fig. 3 G and H), and mesophyll cells lack chlorophyll (Fig. 3F′). Hence, different cell identities are acquired in hbt2311 sectors, but cell morphology and aspects of terminal cell differentiation are disrupted.
We compared the ploidy distribution of sorted nuclei from leaves (nos. 3 and 4) without HS and with large clones 7 DAHS. The 2C fraction in HS leaves decreases after HBT reduction, consistent with a reduction in the pool of dividing cells within affected leaf primordia (Fig. 3I). Interestingly, and in stark contrast to the root data, the nuclear ploidy level in leaves increases significantly after HBT removal (Fig. 3I), demonstrating that endoreduplication is stimulated, which may be associated with growth compensation in leaves.
Our results in leaves indicate that growth defects observed after removal of the HBT gene are primarily caused by (i) impaired cell division in the PD axis and (ii) defects in both cell division and cell elongation in the later expanded ML axis of the leaf blade. In hbt2311 HS leaves, only organ length (but not width) can partially be rescued by a compensatory cell elongation mechanism that operates only along the PD axis. These data confirm a dual requirement for HBT in cell division and cell expansion during leaf development and uncover HBT’s requirement for growth compensation along the ML axis of the leaves.
Non-Cell-Autonomous Rescue of HBT Loss-of-Function Clones.
To investigate the influence of neighboring WT cells on the removal of HBT in marked clones, we induced small root hbt2311 sectors by 20-min HS. Surprisingly, small hbt clones do not affect growth and cell size ≥3 DAHS within the clone nor in adjacent cells (Fig. 4 A and B and data not shown; n = 47), suggesting that HBT-deficient cells divide and elongate normally. In addition, all progeny from hbt2311 stem cell clones divide, elongate, and differentiate properly, as indicated by continuous files of GFP-positive cells derived from single stem cell clones that span the entire root meristem (Fig. 4 C and D) and the elongation zone (data not shown). Division of marked stem cell progeny is maintained up to 8 DAHS (n = 5). A histone 2B-YFP fusion protein reveals normal metaphase and anaphase figures within the small clones (Fig. 4 E and E′), which supports full rescue of cell division. As reported above, ubiquitous HBT removal in large clones leads to a rapid cessation of cell division between 24 and 48 h after HS, and hence the observed rescue in small stem cell clones cannot be explained by slow turnover of HBT protein. Therefore, HBT function within the clone appears to be compensated by a factor provided by neighboring WT cells.
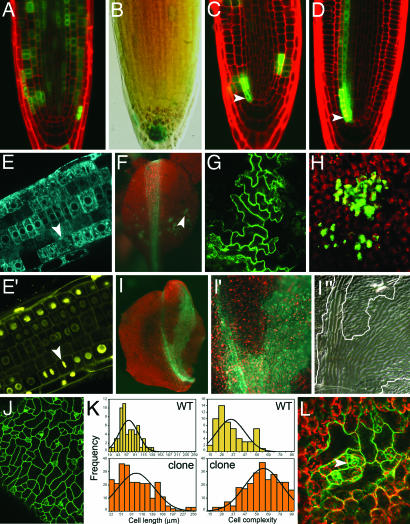
Fig. 4.
Non-cell-autonomous rescue in HBT loss-of-function clones. (A–E′) Non-cell-autonomous rescue in root clones. Small hbt2311 root clones (20-min HS) display no defects in cell division or expansion (A) and normal auxin responses (B). (C and D) hbt clones that primarily affect stem cell initials (arrowheads) divide and expand normally from 1 DAHS (C) onward (D, 3 DAHS). (E and E′) Cell divisions monitored by 35S::histone 2B-YFP expression in small clones 2 DAHS. GFP (E, false blue) marks HBT-excised cells; yellow channel (E′) marks histone 2B-YFP. Arrowheads in E and E′ point to metaphase figures. (F–K) Single-layer hbt2311 clones in leaves. WT shape of a leaf containing small, single-layered clones (F). Cell size and cell shape in epidermal (G) or mesophyll (H) cells within the clone. Green channel, GFP-marked cells; red channel, chlorophyll. In large clones induced only in the epidermis, defects in cell division are partially compensated by longitudinal cell expansion (I and I′), and defects in cell morphology are partially compensated at the clone boundaries (I′′). (J and K) Large epidermal clones display elongated cells with reduced jigsaw morphology. (L) Small hbt2311 clones affecting all layers within the leaf display aberrant cell morphologies. Arrowhead points to an undifferentiated stomatal precursor complex. Photographs were taken 21 days after sowing and 12 DAHS.
To assess whether the observed noncell autonomy of HBT function is dependent on developmental context (i.e., indeterminate vs. determinate growth) we analyzed leaf clones induced by 20-min HS. Several small hbt2311 clones restricted to up to five cell diameters affected only one cell layer of the leaf (Fig. 3A′, class I; and Fig. 4F, arrowhead), for example the epidermis (derived from the L1 layer of the leaf primordia, Fig. 3A) or the mesophyll (derived from L2 and L3). These small hbt2311 clones display similar cell sizes and shapes when compared with WT imprints (Fig. 4 G and H and data not shown). Like for small hbt2311 clones in the root, this growth restoration can be explained if neighboring HBT cells provide signal(s) that promote normal division and expansion of cells within the clone.
Clones in which hbt2311 epidermis is overlying WT mesophyll cells (Fig. 3A′, classes II–IV; and Fig. 4 I and J) posses epidermal pavement cells with lower complexity and more elongation along the PD leaf axis (Fig. 4 I′, I′′, and K). Although the width of clones and the number of epidermal cells in the ML direction of the clone is smaller than that of adjacent WT areas, it is significantly higher than in clones affecting all layers of the leaf, suggesting partial growth compensation in the ML direction in these types of clones. Because mutant epidermal cell widths are not significantly different in both types of clones (data not shown), this partial compensation in blade width appears to be due to increased cell proliferation along the ML axis. Whereas cell divisions in the epidermis are rescued by underlying mesophyll cells, WT morphology of the pavement cells within the clone is not rescued (Fig. 4I). However, GFP-expressing cells on the borders of the clone display more increased lobing compared with central cells (Fig. 4I), indicating nonautonomous rescue of cell morphology from adjacent HBT epidermal cells.
Surprisingly, the growth of small hbt2311 clones that span all layers within the leaf (Fig. 3A′, class V; and Fig. 4L) is not rescued. In addition, cell differentiation within these clones is affected, as suggested by the presence of immature meristemoid complexes in mature leaves at higher proportions than in the surrounding WT tissues (Fig. 4L arrowhead and data not shown). Together, our results indicate that epidermal cell divisions in hbt2311 clones are mainly rescued by the underlying WT mesophyll cells, suggesting that noncell autonomous factors act between leaf layers to promote cell division.
Discussion
Our results connect the role of the plant APC/C complex in mitotic and endoreduplication cycles to region-specific cell division and cell expansion control in roots and leaves and to mechanisms of organ size compensation. Furthermore, the noncell autonomy of clones lacking the HOBBIT CDC27 homolog reveals an intercellular control mechanism for coordinated cell cycle progression and cell differentiation during organ growth. Below, we discuss these two aspects separately.
Regional Control of Cell Division and Cell Expansion.
The embryonic role of HBT is restricted to a few specific cell divisions, but postembryonically HBT is essential to maintain cell identities, cell division activity, and organ development (17, 27). Our mosaic analysis of a strong allele supports a primary function of the HBT gene in cell division and cell expansion. Accordingly, the previously described accumulation of auxin signal transduction components and cell identity defects (17) occur significantly later in mutant clones than the rapidly emerging cell division and cell expansion defects. Weak mutant alleles also suggest that cell division and cell expansion control are primary functions of HBT activity, which is easy to reconcile with HBT’s function as an APC/C subunit (J.M.P.-P., O.S., and B.S., unpublished data).
HBT reduction in roots leads to a decrease in endoreduplication, whereas leaf clones reveal excessive endoreduplication. These data fit well with the contrast between the observed inhibition of cell expansion in roots and the larger cell sizes in leaf clones along the PD axis. The leaf clones reveal a polar compensation mechanism after reduction of mitotic activity that has been noted before (4, 28). To explain the differences in growth compensation response along the PD and ML leaf axes, we postulate the following scenario. During the early-occurring PD divisions, HBT levels decline. At first, decreasing HBT levels reduce mitotic activity, but these levels are still able to promote compensatory cell elongation, possibly associated with endoreduplication. This compensation is not perfect because all clones grow less than WT cells surrounding the leaf area on the PD axis. When later ML cell divisions give rise to the leaf blade, HBT levels have dropped and can sustain neither mitosis nor endoreduplication-driven compensatory cell expansion, which leads to dramatic loss of tissue in the ML axis.
In the root, our data suggest the following scenario: HBT activity is required for both cell division and cell expansion because it enables mitotic progression and endoreduplication, respectively. It is unknown at this point which factor triggers the switch from mitotic cycles to endocycles. One candidate is CycA2;3, which has been identified as a regulator of endocycles in Arabidopsis (29). HBT protein is degraded in the elongation zone because transcription is restricted to the G2/M phase of the cell cycle, which limits endoreduplication and sets a maximum cell size. This scenario is not fundamentally different in the leaf, where HBT is also required for cell division and expansion but where the protein may be more stable to allow (i) several rounds of cell division after HBT reduction and (ii) cell expansion during or after the cessation of cell division.
We note that cell expansion defects in hbt mutant cells lead not only to smaller cells but also to cell shape irregularities. Constitutive overexpression of a nondegradable form of cyclin B1 in tobacco leads to seedlings that resemble hbt mutants (30). Cells in these plants have a disorganized microtubular cytoskeleton strikingly similar to that of the hbt2311 clones described here. Therefore, the fast deregulation of cortical microtubule organization in the hbt2311 clones may be caused by stabilization of B cyclins in the absence of APC/C activity.
Non-Cell-Autonomous Rescue of APC/C Activity.
Perhaps the most striking observation from our studies is the ability of neighboring WT cells to rescue the removal of an essential APC/C component. In the context of the developing plant, non-cell-autonomous influences on cell division rates and patterns have been observed by using mosaics of organ identity factors (31–36), and nonautonomous control of cell division is exerted by stem cell organizing factors (18, 37–39) or ligands for receptor kinase signaling molecules (40). Although each of these factors may regulate, either directly or through the modulation of signal transduction pathways, core cell cycle components, it is surprising that such controls can compensate for the loss of a critical cell cycle factor.
What can be the molecular mechanism behind the non-cell-autonomous action of HBT described here? It is not likely a direct movement of the large HBT protein or the even larger APC/C complex, because protein trafficking is limited by the plasmodesmatal size exclusion limit that has been estimated to be between 40 and 60 kDa for Arabidopsis embryos (41). Might other cell cycle regulators, produced in cycling HBT cells as a consequence of ongoing cell division, move to hbt2311 neighboring cells? In Arabidopsis, at least one putative cyclin-dependent kinase inhibitor, Kip-related protein1 (KRP1) (6, 42) was recently shown to be capable of non-cell-autonomous action after ectopic expression (43). One possibility is that KRPs moving from actively dividing HBT cells counteract an excess of cyclin-dependent kinase/cyclin B activity in hbt2311 clones. Future research should reveal how this or other mechanisms can compensate for loss of APC/C activity and whether these mechanisms are used in physiologically relevant conditions such as during the coordination of organ growth.
Methods
Genetic Induction and Phenotypic Analysis of hbt2311 Clones.
A 9-kb rescuing HBT genomic fragment was cloned between two lox sites of pCB1 (18) to create the pCB1-HBTg vector and used to transform hbt2311 heterozygotes. Single-copy insertion lines were selected by Southern Blot analysis (data not shown). The CRE recombinase coding sequence under the control of an HS promoter (HS::CRE) was introduced by transformation in hbt2311 homozygotes rescued by one copy of the pCB1-HBTg vector. In this situation, a 37°C HS induces the expression of the CRE recombinase, which removes the HBT genomic region inserted between the two lox sites. The 35S promoter now recombines in front of the GAL4VP16 sequence, activating its expression which, in turn, drives UAS::GFP. As a result, cells in which the recombination occurs are marked with GFP and are genetically hbt2311/hbt2311 (Fig. 1A).
Flow Cytometry.
Thirty to forty root tips of 7-day-old seedlings were chopped in 500 μl of cold nuclear isolation buffer (45 mM MgCl2/30 mM sodium citrate/20 mM [4-morpholino]propanesulfonate, pH 7.0/0.1% [wt/vol] Triton X-100) (44) containing 2.5 μg/ml DAPI (Roche, Indianapolis, IN). The crude preparation of isolated nuclei was filtered (48 μm) and immediately analyzed on an ELITE ESP cytometer (Beckman–Coulter, Roissy, France) using UV excitation and gates to eliminate debris or doublets as described in ref. 45. DNA histograms corresponding to 5,000 isolated nuclei were drawn, and the frequency of ploidy levels was calculated by using WinMDI 2.8 software (Joe Troter, The Scripps Research Institute, La Jolla, CA; http://facs.scripps.edu/software.html).
Microscopy and Leaf Histology.
For whole-mount and starch granules visualization, seedlings were cleared and mounted in accordance with ref. 27. β-glucuronidase (GUS) staining and root length measurements were performed as described in ref. 27. The number of root meristematic cells was obtained by counting cortex cells that showed no signs of rapid elongation. Photomicrography of whole leaves and leaf sections was performed as described in ref. 46.
Images were captured with a Zeiss Axioskop microscope (Carl Zeiss, New York, NY) equipped with a Nikon DXM1200 digital camera (Nikon Instruments Europe, Badhoevedorp, The Netherlands) and processed digitally with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Western Blot Analysis and Immunolocalization.
Crude protein extract was obtained from root meristems of pCB1-HBTg seedlings after 60-min HS. Protein extraction, Western blot analysis, and staining with anti-AXR3 and anti-SHY2 were performed as described in ref. 17. The immunolocalization procedure was performed in accordance with ref. 22. Primary antibodies were used at the following concentrations: affinity-purified anti-PIN1, 1:300; affinity-purified anti-PIN2, 1:400. Fluorochrome-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used at the following concentrations: goat anti-rabbit FITC, 1:200; goat anti-mouse TRITC, 1:200.
Morphometric Analysis of Clones.
Tagged image file format (TIFF) files were converted to binary images after threshold adjustment by using the public-domain ImageJ 1.34s software (W. Rasband, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/). Individual cells within the clones were manually selected and their area, perimeter, and major and minor axes of the adjusted ellipse were measured. Cell complexity was estimated by using the circularity function [4π(area/perimeter2)], where a value of 1.0 indicates a perfect circle and, as it approaches 0.0, indicates an increasingly elongated polygon. Statistical analysis and histogram drawing were done with the SPSS 11.0.0 statistical package (SPSS, Chicago, IL).
Supplementary Material
Acknowledgments
We thank Marten Terlou for help in the morphometric analysis; Fritz Kindt, Ronald Leito, and Piet Brower for photography; Spencer Brown and Olivier Catrice for help with the FACS analyses; Mark Estelle (Indiana University, Bloomington, IN) and Klaus Palme (Institut für Biologie II, Freiburg, Germany) for the SHY2 and PIN antibodies, respectively; and Arp Schnittger, Viola Willemsen, and Jian Xu for critical reading of the manuscript. This work was supported by European Commission Marie Curie Fellowship HPMF-CT-1999-00013 (to O.S.) and by European Commission Research Training Network contract HPRN-CT-2002-00333 (to J.M.P.-P).
Abbreviations
- APC/C
anaphase-promoting complex/cyclosome
- QC
quiescent center
- HS
heat shock
- DAHS
days after HS
- PD
proximodistal
- ML
mediolateral.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Beemster G. T., Baskin T. I. Plant Physiol. 1998;116:1515–1526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukaya H. Curr. Opin. Plant Biol. 2003;6:57–62. doi: 10.1016/s1369526602000055. [DOI] [PubMed] [Google Scholar]
- 3.Tsukaya H. Int. J. Dev. Biol. 2005;49:547–555. doi: 10.1387/ijdb.041921ht. [DOI] [PubMed] [Google Scholar]
- 4.Hemerly A., Engler Jde A., Bergounioux C., Van Montagu M., Engler G., Inze D., Ferreira P. EMBO J. 1995;14:3925–3936. doi: 10.1002/j.1460-2075.1995.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizukami Y., Fischer R. L. Proc. Natl. Acad. Sci. USA. 2000;97:942–947. doi: 10.1073/pnas.97.2.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Veylder L., Beeckman T., Beemster G. T., Krols L., Terras F., Landrieu I., van der Schueren E., Maes S., Naudts M., Inze D. Plant Cell. 2001;13:1653–1668. doi: 10.1105/TPC.010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Veylder L., Beeckman T., Beemster G. T., de Almeida Englerqq J., Ormenese S., Maes S., Naudts M., van der Schueren E., Jacqmard A., Engler G., Inze D. EMBO J. 2002;21:1360–1368. doi: 10.1093/emboj/21.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewitte W., Riou-Khamlichi C., Scofield S., Healy J. M., Jacqmard A., Kilby N. J., Murray J. A. Plant Cell. 2003;15:79–92. doi: 10.1105/tpc.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed S. I. Nat. Rev. Mol. Cell Biol. 2003;4:855–864. doi: 10.1038/nrm1246. [DOI] [PubMed] [Google Scholar]
- 10.Harper J. W., Burton J. L., Solomon M. J. Genes Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- 11.Peters J. M. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 12.Capron A., Okresz L., Genschik P. Trends Plant Sci. 2003;8:83–89. doi: 10.1016/S1360-1385(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 13.Capron A., Serralbo O., Fülöp K., Frugier F., Parmentier Y., Dong A., Lecureuil A., Guerche P., Kondorosi E., Scheres B., Genschik P. Plant Cell. 2003;15:2370–2382. doi: 10.1105/tpc.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwee H. S., Sundaresan V. Plant J. 2003;36:853–866. doi: 10.1046/j.1365-313x.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- 15.Melaragno J. E., Mehrotra B., Coleman A. W. Plant Cell. 1993;5:1661–1668. doi: 10.1105/tpc.5.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cebolla A., Vinardell J. M., Kiss E., Olah B., Roudier F., Kondorosi A., Kondorosi E. EMBO J. 1999;18:4476–4484. doi: 10.1093/emboj/18.16.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blilou I., Frugier F., Folmer S., Serralbo O., Willemsen V., Wolkenfelt H., Eloy N. B., Ferreira P. C. G., Weisbeek P., Scheres B. Genes Dev. 2002;16:2566–2575. doi: 10.1101/gad.237302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidstra R., Welch D., Scheres B. Genes Dev. 2004;18:1964–1969. doi: 10.1101/gad.305504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benfey P. N., Scheres B. Curr. Biol. 2000;10:R813–R815. doi: 10.1016/s0960-9822(00)00814-9. [DOI] [PubMed] [Google Scholar]
- 20.Ueda M., Koshino-Kimura Y., Okada K. Curr. Opin. Plant Biol. 2005;8:71–76. doi: 10.1016/j.pbi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Aida M., Beis D., Heidstra R., Willemsen V., Blilou I., Galinha C., Nussaume L., Noh Y. S., Amasino R., Scheres B. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 23.Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P., Scheres B. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 24.Rouse D., Mackay P., Stirnberg P., Estelle M., Leyser O. Science. 1998;279:1371–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- 25.Tian Q., Reed J. W. Development (Cambridge, U.K.) 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- 26.Byrne M. E. Curr. Opin. Plant Biol. 2005;8:59–66. doi: 10.1016/j.pbi.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Willemsen V., Wolkenfelt H., de Vrieze G., Weisbeek P., Scheres B. Development (Cambridge, U.K.) 1998;12:521–531. doi: 10.1242/dev.125.3.521. [DOI] [PubMed] [Google Scholar]
- 28.Haber A. H. Am. J. Bot. 1962;49:583–589. [Google Scholar]
- 29.Imai K. K., Ohashi Y., Tsuge T., Yoshizumi T., Matsui M., Oka A., Aoyama T. Plant Cell. 2006;18:382–396. doi: 10.1105/tpc.105.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weingartner M., Criqui M. C., Mészàros T., Binavora P., Schmit A. C., Helfer A., Derevier A., Erhardt M., Bögre L., Genschik P. Plant Cell. 2004;16:643–657. doi: 10.1105/tpc.020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter R., Coen E. S. Development (Cambridge, U.K.) 1995;121:19–26. doi: 10.1242/dev.121.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Hantke S. S., Carpenter R., Coen E. S. Development (Cambridge, U.K.) 1995;121:27–35. doi: 10.1242/dev.121.1.27. [DOI] [PubMed] [Google Scholar]
- 33.Sieburth L. E., Drews G. N., Meyerowitz E. M. Development (Cambridge, U.K.) 1998;125:4303–4312. doi: 10.1242/dev.125.21.4303. [DOI] [PubMed] [Google Scholar]
- 34.Sessions A., Yanofsky M. F., Weigel D. Science. 2000;289:779–782. doi: 10.1126/science.289.5480.779. [DOI] [PubMed] [Google Scholar]
- 35.Jenik P. D., Irish V. F. Development (Cambridge, U.K.) 2001;128:13–23. doi: 10.1242/dev.128.1.13. [DOI] [PubMed] [Google Scholar]
- 36.Vincent C. A., Carpenter R., Coen E. S. Plant J. 2003;33:765–774. doi: 10.1046/j.1365-313x.2003.01666.x. [DOI] [PubMed] [Google Scholar]
- 37.Mayer K. F., Schoof H., Haecker A., Lenhard M., Jurgens G., Laux T. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 38.Schoof H., Lenhard M., Haecker A., Mayer K. F., Jurgens G., Laux T. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 39.Sabatini S., Heidstra R., Wildwater M., Scheres B. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fletcher J. C., Brand U., Running M. P., Simon R., Meyerowitz E. M. Science. 1999;283:1911–1914. doi: 10.1126/science.283.5409.1911. [DOI] [PubMed] [Google Scholar]
- 41.Kim I., Cho E., Crawford K., Hempel F. D., Zambryski P. C. Proc. Natl. Acad. Sci. USA. 2005;102:2227–2231. doi: 10.1073/pnas.0409193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnittger A., Weinl C., Bouyer D., Schobinger U., Hulskamp M. Plant Cell. 2003;15:303–315. doi: 10.1105/tpc.008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinl C., Marquardt S., Kuijt S. J., Nowack M. K., Jakoby M. J., Hulskamp M., Schnittger A. Plant Cell. 2005;17:1704–1722. doi: 10.1105/tpc.104.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galbraith D. W., Harkins K. R., Maddox J. M., Ayres N. M., Sharma D. P., Firoozabady E. Science. 1983;220:1049. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- 45.Coba de la Peña C. T., Brown S. C. In: Plant Cell Biology: A Practical Approach. 2nd Ed. Hawes C., Satiat-Jeunemaître B., editors. Oxford: Oxford Univ. Press; 2001. pp. 85–106. [Google Scholar]
- 46.Pérez-Pérez J. M., Ponce M. R., Micol J. L. Dev. Biol. 2002;242:161–173. doi: 10.1006/dbio.2001.0543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.