Abstract
Oxidative stress plays a dominant role in the pathogenesis of diabetes mellitus. Bcl-2 gene has close connection with antioxidant stress destruction in many diseases including diabetes. Carvedilol, an adrenoceptor blocker, also has antioxidant properties. To study the effect of carvedilol on the antioxidant status in diabetic hearts, we investigated carvedilol-administrated healthy and streptozotocin-induced diabetic rats. After small and large dosage carvedilol-administered for 5 weeks, hemodynamic parameters, the levels of malondialdehyde, activities of antioxidant enzymes and expression of Bcl-2 mRNA in the cardiac tissues were measured. The diabetic rats not only had cardiac disfunction, weaker activities of antioxidant enzymes, but also showed lower expression of Bcl-2. Carvedilol treatment increased activities of antioxidant enzymes and expression of Bcl-2 in healthy rats as well as diabetic rats. These results indicated that carvedilol partly improves cardiac function via its antioxidant properties in diabetic rats.
Keywords: Carvedilol, Diabetes, Oxidative stress, Bcl-2
INTRODUCTION
Diabetes is the largest morbidity of patients with heart failure and adversely affects outcomes of cardiovascular diseases (Beller, 2001). Oxidative stress has been associated with the pathogenesis of chronic diabetic complications including cardiomyopathy (Cai and Kang, 2003). The ability of antioxidants to inhibit these injuries has raised the possibility of newer therapeutic treatment for diabetic heart diseases. Recently, Bcl-2 gene has been focused because it was involved in preventing oxidant-induced cell death and in decreasing radical oxygen production (Jang and Surh, 2003).
Carvedilol is a nonselective beta-adrenoceptor and selective alpha1-adrenoceptor blocker. So far, it has been widely used in the treatment of heart failure, hypertension with or without diabetes. It was demonstrated that carvedilol has antioxidant and free radical scavenger properties. In physicochemical, biochemical, and cellular assays carvedilol inhibited the formation of reactive oxygen radicals and lipid peroxidation, scavenged oxygen free radicals, and prevented the depletion of endogenous anti-oxidants (Noguchi et al., 2000). It has been suggested that carvedilol may provide greater benefit than traditional β-blockers in chronic heart failure because of its antioxidant actions that synergize with its nonspecific β- and α-blocking effects.
Whether carvedilol can improve cardiac function in diabetic animal model and this protection via antioxidant pathway is not clear. Therefore, in the present study we investigated the effect of carvedilol on cardiac function, activities of oxidant and antioxidant enzymes and Bcl-2 mRNA expression in the diabetic rat hearts.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (180~220 g) were housed at constant temperature of (22±2) °C, with a 12 h light/dark cycle, and given free access to food or water. All procedures were approved by the Ethics Committee for the Use of Experimental Animals in Zhejiang University.
Chemicals and assay kits
Streptozotocin (STZ) was obtained from Sigma Chemical Co. (St. Louis, MO). Carvedilol troche was a gift from Roche. Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) assay kits were obtained from Nanjing Jiancheng Bioengineering Company (Nanjing, China).
Experimental groups
Diabetes was induced by a single dose of STZ (60 mg/kg) as previously described (Rosen et al., 1984). Age-matched control healthy rats received vehicle only. About 72 h after STZ treatment, blood samples were taken from the caudal vein, and glucose levels were measured with a glucometer (One Touch II glucometer). Rats with glucose levels >15 mmol/L were considered diabetic. Three days after injection of STZ or vehicle, the rats were randomly divided into the following six groups: (1) untreated diabetic rats (D, n=8); (2) diabetic rats treated with small dose of carvedilol (DS, 1 mg/(kg·d), n=9); (3) diabetic rats treated with large dose of carvedilol (DL, 10 mg/(kg·d), n=9); (4) control healthy rats (C, n=9); (5) healthy rats treated with small dose of carvedilol (CS, 1 mg/(kg·d), n=7); (6) healthy rats treated with large dose of carvedilol (CL, 10 mg/(kg·d), n=8).
Hemodynamic parameters and heart disposition
Hemodynamic studies were performed at 5th week. The rats were anesthetized with 10% hydrated chloral (350 mg/kg), the right carotid artery was cannulated with a BD Angiocatheter (20GA 1.1 mm×48 mm, Italy) for recording of arterial pressure. The aortic catheter was then advanced into the left ventricle for recording of left ventricular systolic pressure (LVSP), left ventricular end diastolic pressure (LVEDP), maximal rate of rise/fall left ventricle pressure development and decline (±dP/dt max). All pressure data were recorded on MedLab data acquisition system (Nanjing MedEase Co., Nanjing, China) (Tao et al., 2004). All rats were sacrificed after their hearts were carefully removed from the thorax under anesthesia. The specimens were harvested and stored at −70 °C.
Biochemical procedure
The frozen heart tissue samples were weighed and homogenized (Ultra Turrax T25, Germany) (1:10, w/v) in 50 mmol/L phosphate buffer (pH 7.4) and kept in an ice-bath. The homogenate and supernatant were frozen at −20 °C in aliquots until used for biochemical assays. The protein content of the supernatant was determined using the Lowry method. MDA level, activities of SOD, CAT and GSH-Px were measured strictly according to the instructions of test kits, respectively.
Malondialdehyde (MDA) levels were estimated by the thiobarbituric acid (TBA) method, the spectrophotometric measurement of the color generated by the reaction of TBA with MDA. The final solution absorbance was measured by using a spectrophotometer at 532 nm. The concentration of MDA was calculated according to the standard sample 1,1,3,3-tetraethorypropane and expressed as nmol per gram protein.
Total (Cu-Zn and Mn) superoxide dismutase (SOD) activity was determined according to the kits. The principle of the method is based on the inhibition of nitrite production generated from hydroxylamine oxidant by the xanthine-xanthine oxidase system as a superoxide generator. The final solution absorbance was measured by using a spectrophotometer at 550 nm. Total SOD activity was calculated according to the formula in the kits and expressed as units per gram protein.
Catalase (CAT) decomposed H2O2 directly under certain conditions. The principle of the assay is based on the change due to hydrogen peroxide reduction in absorbance at 240 nm. By measuring the absorbance changes after one minute, CAT activity was calculated according the formula in the kits and expressed as units per gram protein.
Glutathione peroxidase (GSH-Px) catalyzes the reaction between hydrogen peroxide (H2O2) and glutathione. GSH-Px activity was measured by determining the reduction of glutathione. However, H2O2 could react with GSH under nonenzymatic condition. Deducting this part from total reduction, GSH was measured by a spectrophotometer which monitors the change in absorbance at 412 nm. Activity was given in units per gram protein. All samples were assayed in duplicate.
Reversal transcription polymerase chain reaction (RT-PCR)
Cardiac tissue was pulverized to powder. Total RNA was extracted using Trizol (life technologies). RNA quality and integrity was assured by gel visualization and spectrophotometric analysis (OD 260/280) and quantified at 260 nm. Experiment was performed using a light cycle rapid thermal cycle (Roche Diagnostics). The RT reaction was amplified using TaqDNA polymerase and primers to murine Bcl-2 cDNA (sense: 5′-TCCATTATAAGCTGTCACAG-3′; antisense: 5′-GAAGAGTTCCTCCACCAC-3′). The PCR profile was set at 94 °C melting, 55 °C annealing, and 72 °C extension for 1 min, and semi-quantitation was optimized to 35 cycles. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) transcript abundance was used as an endogenous control to which Bcl-2 transcript abundance was normalized.
Statistical analysis
Data were presented as means±standard deviation (SD). Student’s t-test was used for evaluating the statistical significance of differences in means. A P value of <0.05 was considered to be statistically significant.
RESULTS
Weight and blood glucose level
After 5 weeks, animals injected with STZ had lost weight compared with normal controls, and carvedilol treatment alleviated the weight loss significantly in diabetes (P<0.05 vs D). There was no significant change in healthy rats treated with carvedilol (P>0.05 vs C).
Three days after injection of STZ, the average blood glucose levels were >20 mmol/L in the diabetic group and remained substantially constant during the experimental period of 5 weeks. In the diabetic rats, blood glucose levels neither recovered to normal, nor deteriorated after 5 weeks of treatment with small or large doses of carvedilol, and remained at high level throughout the study period. Level of blood glucose had no significant change in healthy rats treated with carvedilol (P>0.05 vs C) (Table 1).
Table 1.
Changes in blood glucose level and body weight at the initial and the 5th week of experiment (Huang et al., 2005)
| Groups | Body weight (g) |
Blood glucose (mmol/L) |
||
| Initial | At the 5th week | Initial | At the 5th week | |
| Normal control | 209±11 | 363±56c | 4.75±0.54c | 4.94±0.48c |
| Control+carvedilol (1 mg/kg) | 210±15 | 342±50 | 4.92±0.50 | 4.62±0.50 |
| Control+carvedilol (10 mg/kg) | 209±13 | 378±66 | 4.64±0.49 | 5.47±0.49 |
| Untreated diabetic rat | 224±13 | 167±12b | 20.20±1.58b | 20.59±2.62b |
| Diabetic rat+carvedilol (1 mg/kg) | 214±17 | 247±66b,c | 20.27±1.66b | 19.47±1.93b |
| Diabetic rat+carvedilol (10 mg/kg) | 208±10 | 255±60b,c | 20.05±1.75b | 20.20±1.97b |
P<0.01 vs control group
P<0.05 vs untreated diabetic group
Hemodynamics
Left ventricular systolic pressure (LVSP), left ventricular developed pressure (LVDP), and ±dP/dt max were significantly lower in untreated diabetes (D), and left ventricular end diastolic pressure (LVEDP) was substantially elevated compared with normal control rats (P<0.01). Left ventricular systolic pressure (LVSP), ±dP/dt max in diabetic rats treated with small and large dosage of carvedilol were significantly elevated, whereas their LVEDP values were slightly lowered but no significance (P>0.05 vs D). Nevertheless, there were significant differences compared with control untreated carvedilol (P<0.05). Heart rate (HR) in untreated diabetic rats was significantly decreased (P<0.05). Carvedilol treatment reduced HR slightly without significance (P>0.05 vs D) (Table 2). There was reduced HR in healthy control treated with carvedilol, and this fall was dose-related (Table 2).
Table 2.
Homodynamic parameters in normal control (C), untreated diabetic rat (D), normal rat treated with small dose of carvedilol (1 mg/kg) (CS), normal rat treated with large dose of carvedilol (10 mg/kg) (CL), diabetic rat receiving small dose of carvedilol (DS), diabetic rat receiving large dose of carvedilol (DL)
| C | CS | CL | D | DS | DL | |
| HR (bpm) | 426±45 | 390±40 | 348±46a | 362±49a | 347±44a | 326±38a,b |
| LVSP (mmHg) | 98±14 | 94±12 | 92±14 | 84±15a | 94±11b | 96±19b |
| LVEDP (mmHg) | 5±1 | 5±1 | 6±2 | 12±2a | 10±2a | 9±2a,b |
| LVDP (mmHg) | 82±14 | 80±13 | 82±14 | 49±9a | 52±11a | 62±12a,b,c |
| +dP/dtmax | 4890±690 | 4760±670 | 4590±600 | 3236±590a | 3409±578a | 3835±730a,b |
| −dP/dtmax | 4698±646 | 4700±606 | 4490±640 | 3014±685a | 3333±642a | 3711±730a,b |
P<0.05 vs C
P<0.05 vs D
P<0.05 DS vs DL
Antioxidant system evaluation in cardiac muscle
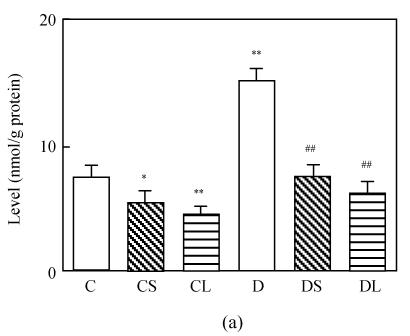
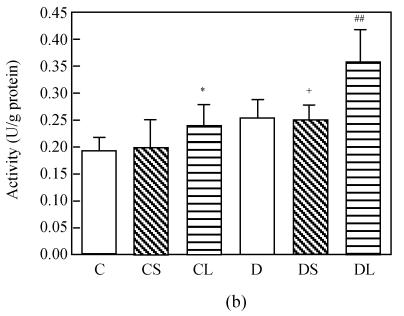
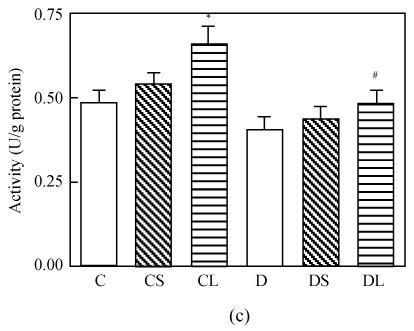
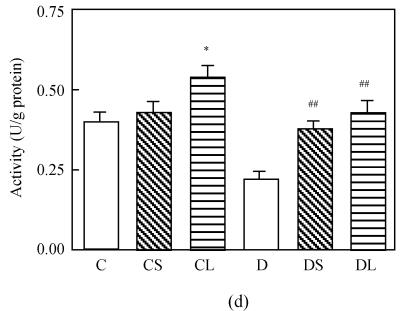
The level of MDA in the heart was increased markedly in untreated diabetic rats compared with the rats in the control group (P<0.0001, Fig.1a). Treatment with carvedilol reduced the level of MDA in both healthy groups and diabetic groups. SOD and GSH-Px activities were lower in diabetic untreated rats significantly (P<0.0001 vs C, Figs.1c and 1d). Treatment with carvedilol increased activities of CAT, SOD and GSH-Px in healthy control and diabetic rats significantly (P<0.05 vs C, Figs.1b, 1c and 1d). Moreover, large dosage of carvedilol administration enhanced this rise (P<0.05 vs C).
Fig. 1.
Malondialdehyde (MDA) levels (a), activities of CAT (b), activities of GSH-Px (c) and activities of SOD (d) in the heart tissue of normal controls (C), normal rat treated with small dose of carvedilol (1 mg/kg) (CS), normal rat treated with large dose of carvedilol (10 mg/kg) (CL), untreated diabetic rat (D), diabetic rat treated with small dose of carvedilol (DS), diabetic rat treated with large dose of carvedilol (DL)
Each bar represents the mean±SD; * P<0.05 vs C; ** P<0.01 vs C; # P<0.05 vs D; ## P<0.01 vs D; + P<0.05 DS vs DL
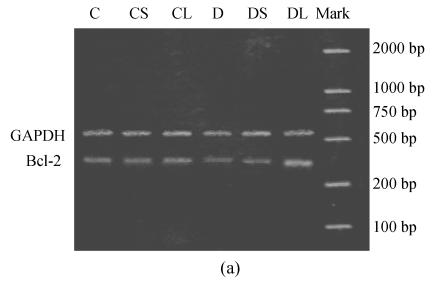
RT-PCR assay
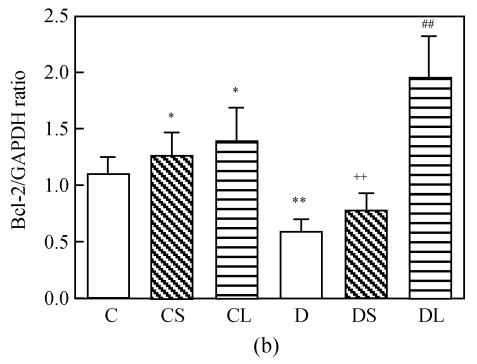
Expression of Bcl-2 mRNA was compared between untreated and carvedilol-treated cardiac tissue. Administration of carvedilol significantly increased the expression of Bcl-2 mRNA in healthy groups and diabetic groups. The expression of Bcl-2 mRNA was moderate in untreated healthy cardiac tissue, but very slow in untreated diabetic cardiomyocytes. The expression was significantly upregulated in carvedilol-treated healthy and diabetic groups compared with untreated groups. And these increases were dose-related change (Fig.2). The levels of mRNA encoding for GAPDH were not found to be significantly different between each group.
Fig. 2.
Semiquantitative RT-PCR analysis of Bcl-2 mRNAs in the heart tissue of normal controls (C), normal rat treated with small dose of carvedilol (CS), normal rat treated with large dose of carvedilol (CL), untreated diabetic rat (D), diabetic rat treated with small dose of carvedilol (DS), diabetic rat treated with large dose of carvedilol (DL). (a) Expression of Bcl-2 mRNAs; (b) Semiquantitative RT-PCR analysis of Bcl-2 mRNAs
Each bar represents the mean±SD; n=5 for each group; * P<0.05 vs C; ** P<0.01 vs C; ## P<0.01 vs D; ++ P<0.01 DS vs DL
DISCUSSION
Carvedilol, a third generation β-blocker, did not have unwanted effects on glucose and lipid metabolism, which is not like the first and second generation β-blockers (Giugliano et al., 1997). Our results showed that carvedilol treatment did not affect blood glucose level and body weight (Fig.1). Carvedilol was shown to have antioxidant actions in healthy volunteers treated with small and moderate doses (6.25~25 mg/d) (Dandona et al., 2000). We selected corresponsive small and large doses in healthy and diabetic rats (1 mg/(kg·d) and 10 mg/(kg·d)).
Oxidative stress often causes cell death via apoptosis that is regulated by a plenty of functional genes and their protein products. Bcl-2, which is an integral mitochondrial membrane protein, blocks apoptosis induced by a wide array of death signals (Jang and Surh, 2003). Bcl-2 protein itself has antioxidant ability. Bcl-2 family proteins could prevent cytochrome c from entering the cytosol or by binding directly with cytochrome c to reduce generation of free radicals (Shimizu et al., 1995). Furthermore, Bcl-2-overexpressing cells exhibit elevated expression of antioxidant enzymes and higher levels of cellular GSH compared with the control cells transfected with the vector alone (Amstad et al., 2001).
Carvedilol has been investigated to inhibit cardiomyocyte apoptosis following ischemia and reperfusion, which was related to the increased Bcl-2 mRNA expression (Zeng et al., 2003). Evidently, it is via antioxidant pathways that Bcl-2 prevents apoptosis. Spallarossa et al.(2004) have observed that carvedilol pre-treatment blunted both the decrease of Bcl-2 and the increase of Bax-alpha mRNA expression of cardiomyocytes induced by doxorubicin, lead to reduced free radical release and apoptosis.
Our results displayed that the expression of Bcl-2 was significantly upregulated in carvedilol-treated diabetic hearts compared with untreated diabetic groups, as well as in control treated groups. This upregulation was more obviously in large carvedilol-treated groups. In addition, this upregulation was concomitant with antioxidant enzymes positive changes both control and diabetes treated with carvedilol.
Increased oxidative stress and altered antioxidant pool have been implicated in both clinical and experimental type 1 diabetes (Vincent et al., 2002). We found that the level of MDA in the cardiac tissue of diabetic rats significantly increased compared with the control rats. MDA, a routine index of lipid peroxidation, increased in diabetes mellitus, which implies that hyperglycemia induces peroxidative reactions in lipids and suggests increased oxidative stress in STZ-induced diabetes (Pieper et al., 1995). It revealed an alleviated oxidative status in healthy and treated diabetes in which carvedilol administration reduced MDA level markedly.
SOD catalyzes the conversion of superoxide radicals to H2O2, which protects the cell against the toxic effects of superoxide radicals. GSH-Px is responsible both for metabolizing lipid peroxides and for the decomposition of H2O2. We found that the SOD and GSH-Px activity in the cardiac tissue of diabetic rats significantly decreased compared with the control rats. This implied weak antioxidant status in diabetes, with difficulty to transform superoxide radicals to H2O2, if it could, hardly discomposed H2O2. However, surprisingly, there was increased CAT activity in diabetic untreated rats in the study. If diabetic antioxidant system was impaired, catalase, an important hydroxyl free radicals clearer, should reveal weak activity. But reviewing data, we found that antioxidant enzymes activity varied in the different organs and different diabetic phases including, particularly correlative with Bcl-2 protein. In general, lower expression of Bcl-2 mRNA, even no expression, like in Bcl-2-knockout, led to perturbations of oxidative and antioxidant status instead of pure infirmness. For example, catalase revealed significantly higher levels in brain of 8 d and lower levels at 30 d in Bcl-2-knockout mice compared to wild type, whereas higher levels in liver and kidneys from 8 to 30 d in Bcl-2-knockout mice were found (Hochman et al., 2000). In this study, we observed lower expression of Bcl-2 mRNA in diabetic hearts. Thus higher activities of catalase may be due to lower expression of Bcl-2 mRNA and perturbations of oxidative and antioxidant status.
GSH-Px, CAT and SOD activities in the cardiac tissue were elevated concomitantly with higher expression of Bcl-2 mRNA by carvedilol treatment in both control and diabetic rats. In addition, the rise of activity was more obvious in large dose carvedilol group. We knew from the above finding that Bcl-2-overexpressing cells elevated expression of antioxidant enzymes and led to higher levels of cellular GSH (Amstad et al., 2001). In addition, application of many antioxidants and methods to lighten oxidative injury like hypothermia reinforced Bcl-2 expression concomitantly with increased antioxidant enzymes activity (Jung et al., 2005; Slikker et al., 2001). Although the intrinsic mechanism of positive change was not clear, it is recognized that Bcl-2 high expression is correlated with antioxidant enzymes activity and antioxidant system.
Our results also showed that administration of carvedilol to diabetic rats improved cardiac systolic and diastolic ability partly based on the increased activity of anti-oxidative enzymes. LVSP, LVDP, LVEF, and +dP/dt max were elevated, close to normal, whereas reduced LVEDP and −dP/dt max indicated diastolic improved function. Similarly, it was reported that administration of antioxidants including carvedilol resulted in a decrease in the oxidative stress level together with amelioration of cardiac function (Nakamura et al., 2002; Qin et al., 2003). In healthy treated groups, carvedilol did not exhibit positive effect on the cardiac function although it upregulated expression of Bcl-2 mRNA and increased activities of antioxidant enzymes. It is difficult logically to display the curative effect on the healthy condition.
CONCLUSION
In sum, oxidative stress was involved in the early diabetic cardiac dysfunction that not only led to reduce activities of antioxidant enzymes but also subdued cardiac dysfunction. Carvedilol treatment upregulated expression of Bcl-2 mRNA, increased activities of antioxidant enzymes and improved cardiac function resultantly. Our study indicated that carvedilol was beneficial to hearts of early diabetic rats due partly at least to its antioxidant property.
References
- 1.Amstad PA, Liu H, Ichimiya M, Berezesky IK, Trump BF, Buhimschi IA, Gutierrez PL. BCL-2 is involved in preventing oxidant-induced cell death and in decreasing oxygen radical production. Redox Rep. 2001;6(6):351–362. doi: 10.1179/135100001101536535. [DOI] [PubMed] [Google Scholar]
- 2.Beller GA. Coronary heart disease in the first 30 years of the 21st century: challenges and opportunities. . Circulation. 2001;103(20):2428–2435. doi: 10.1161/01.cir.103.20.2428. The 33rd annual James B. Herrick lecture of the council on clinical cardiology of the American Heart Association. [DOI] [PubMed] [Google Scholar]
- 3.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3(3):219–228. doi: 10.1385/CT:3:3:219. [DOI] [PubMed] [Google Scholar]
- 4.Dandona P, Karne R, Ghanim H, Hamouda W, Aljada A, Magsino CHJr. Carvedilol inhibits reactive oxygen species generation by leukocytes and oxidative damage to amino acids. Circulation. 2000;101(2):122–124. doi: 10.1161/01.cir.101.2.122. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano D, Acampora R, Marfella R, de Rosa N, Ziccardi P, Ragone R, de Angelis L, D’Onofrio F. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med. 1997;126(12):955–959. doi: 10.7326/0003-4819-126-12-199706150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hochman A, Liang H, Offen D, Melamed E, Sternin H. Developmental changes in antioxidant enzymes and oxidative damage in kidneys, liver and brain of bcl-2 knockout mice. Cell Mol Biol. 2000;46(1):41–52. [PubMed] [Google Scholar]
- 7.Huang H, Shang J, Pan XH, et al. Carvedilol Protects Early Diabetic Rat Hearts through Reducing Oxidative Stress; Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference; Shanghai. 2005. p. 339. [DOI] [PubMed] [Google Scholar]
- 8.Jang JH, Surh YJ. Potentiation of cellular antioxidant capacity by Bcl-2: implications for its antiapoptotic function. Biochem Pharmacol. 2003;66(8):1371–1379. doi: 10.1016/S0006-2952(03)00487-8. [DOI] [PubMed] [Google Scholar]
- 9.Jung CH, Seog HM, Choi IW, Choi HD, Cho HY. Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98(3):245–250. doi: 10.1016/j.jep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Kusano K, Nakamura Y, Kakishita M, Ohta K, Nagase S, Yamamoto M, Miyaji K, Saito H, Morita H, et al. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002;105(24):2867–2871. doi: 10.1161/01.CIR.0000018605.14470.DD. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi N, Nishino K, Niki E. Antioxidant action of the antihypertensive drug, carvedilol, against lipid peroxidation. Biochem Pharmacol. 2000;59(9):1069–1076. doi: 10.1016/S0006-2952(99)00417-7. [DOI] [PubMed] [Google Scholar]
- 12.Pieper GM, Jordan M, Dondlinger LA, Adams MB, Roza AM. Peroxidative stress in diabetic blood vessels. Reversal by pancreatic islet transplantation. Diabetes. 1995;44(8):884–889. doi: 10.2337/diab.44.8.884. [DOI] [PubMed] [Google Scholar]
- 13.Qin F, Shite J, Mao W, Liang CS. Selegiline attenuates cardiac oxidative stress and apoptosis in heart failure: association with improvement of cardiac function. Eur J Pharmacol. 2003;461(2-3):149–158. doi: 10.1016/S0014-2999(03)01306-2. [DOI] [PubMed] [Google Scholar]
- 14.Rosen P, Rosen R, Hohl C, Reinauer H, Klaus W. Reduced transcoronary exchange and prostaglandin synthesis in diabetes rat heart. Am J Physiol. 1984;247(4 Pt 2):H563–H569. doi: 10.1152/ajpheart.1984.247.4.H563. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374(6525):811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 16.Slikker W, Desai VG, Duhart H, Feuers R, Imam SZ. Hypothermia enhances bcl-2 expression and protects against oxidative stress-induced cell death in Chinese hamster ovary cells. Free Radic Biol Med. 2001;31(3):405–411. doi: 10.1016/S0891-5849(01)00593-7. [DOI] [PubMed] [Google Scholar]
- 17.Spallarossa P, Garibaldi S, Altieri P, Fabbi P, Manca V, Nasti S, Rossettin P, Ghigliotti G, Ballestrero A, Patrone F, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37(4):837–846. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Tao ZW, Huang YW, Xia Q, Xu QW. Early association of electrocardiogram alteration with infarct size and cardiac function after myocardial infarction. J Zhejiang Univ Sci. 2004;5(4):494–498. doi: 10.1631/jzus.2004.0494. [DOI] [PubMed] [Google Scholar]
- 19.Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959(4):368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 20.Zeng H, Liu X, Zhao H. Effects of carvedilol on cardiomyocyte apoptosis and gene expression in vivo after ischemia-reperfusion in rats. J Huazhong Univ Sci Technol Med Sci. 2003;23(2):127–130. doi: 10.1007/BF02859934. [DOI] [PubMed] [Google Scholar]