Abstract
In all cognitive tasks, general task-related processes operate throughout a given task on all items, whereas specific item-related processes operate differentially on individual items. In typical functional neuroimaging experiments, these two sets of processes have usually been confounded. Herein we report a combined positron emission tomography and event-related potential (ERP) experiment that was designed to distinguish between neural correlates of task-related and item-related processes of memory retrieval. Two retrieval tasks, episodic and semantic, were crossed with episodic (old/new) and semantic (living/nonliving) properties of individual items to yield evidence of regional brain activity associated with task-related processes, item-related processes, and their interaction. The results showed that episodic retrieval task was associated with increased blood flow in right prefrontal and posterior cingulate cortex, as well as with a sustained right-frontopolar-positive ERP, but that the semantic retrieval task was associated with left frontal and temporal lobe activity. Retrieval of old items was associated with increased blood flow in the left medial temporal lobe and with a brief late positive ERP component. The results provide converging hemodynamic and electrophysiological evidence for the distinction of task- and item-related processes, show that they map onto spatially and temporally distinct patterns of brain activity, and clarify the hemispheric encoding/retrieval asymmetry (HERA) model of prefrontal encoding and retrieval asymmetry.
Keywords: episodic memory, ecphory, mode, brain physiology
Memory involves three basic processes: encoding, storage, and retrieval. Recently, much research has examined the functional neuroanatomy of these processes with the techniques of positron emission tomography (PET) and functional magnetic resonance imaging. A consistent finding across many experiments is hemispheric encoding/retrieval asymmetry in the frontal lobes, or hemispheric encoding/retrieval asymmetry (HERA) (1, 2): encoding processes are more likely to be associated with activations in the left and retrieval processes are in the right frontal lobe (3–6). The question is, what does this pattern signify?
In all cognitive tasks, a basic distinction can be made between task-related and item-related processes. Task-related processes are evoked by the instructions to the subject as to what he or she must do. In episodic retrieval tasks, such as recall or recognition, these instructions call for the recovery of information that was stored at a particular time in a particular place. These instructions stay in force until the task ends. Item-related processes are evoked by the presentation of retrieval cues, such as test items in a recognition test. They vary with the properties of individual items and hence change item by item.
In light of this distinction, the HERA pattern may reflect (i) processes related to the retrieval task, including thinking back to the encoding episode; (ii) processes related to individual test items, including their successful recognition (ecphory); or (iii) a combination of, or interaction between, task- and item-related processes. Several previous experiments (7–11) have shown that thinking back to the encoding episode alone, even in the absence of ecphory, is sufficient to effect the activation of right frontal regions.
Although suggestive, these findings are not conclusive with respect to the distinction between task- and item-related processes, because of two problems. One is rooted in the fact that no previous experiment directly compared episodic and semantic retrieval with items held constant. The other has to do with the lack of data relating to the temporal course of task- and item-related processes. The distinction implies that task-related processes remain constant throughout the task whereas item-related processes shift with individual test items. The relevant temporal information can best be obtained by using the event-related potential (ERP) technique.
Many experiments have reported that successful recognition of old words correlates with a positive ERP wave (elicited between 500 and 800 msec after item presentation) termed the “late positive component” (LPC) (12, 13). The LPC is enhanced if high-level processing was performed at encoding (12), if subjects “remember” the encoding episode of an item rather than just “know” its recent presentation (14, 15), and if additional source information is available at retrieval (13). If old items are not relevant to the task or if they are not correctly recognized, a positive waveform between 300 msec and 500 msec is elicited but the LPC is not enhanced (16). All these findings are neutral concerning the separability of task-related and item-related processes, because ERPs are typically measured and analyzed in relation to single items rather than the entire task.
The purpose of the experiment reported here was to explore the nature of right frontal episodic retrieval activation of HERA from the vantage point of task- and item-related processes, in a design that was free from the two shortcomings just mentioned. We hypothesized that brain areas that mediate task-related processes (e.g., episodic retrieval mode) are spatially distinct from those mediating item-related processes, and continuously active throughout the duration of the task, while brain areas that mediate item-related processes are transiently active with each successive test item. We tested the first hypothesis with PET and the second with ERPs recorded by using the direct-coupled (DC) technique (17).
METHODS
In the design of each of the two parts (PET and ERPs) of the experiment, two tasks (episodic and semantic retrieval) were crossed with test items whose episodic (previously studied or not) and semantic (living or nonliving things) properties were systematically varied (Table 1). Task- and item-related brain activity of the same 11 (6 female) young (mean age 25 years) healthy subjects was measured in separate PET and ERP sessions. ERPs were averaged over sustained time periods of 10 sec to monitor the different memory tasks and over briefer periods of 2 sec to monitor the processing of different item types.
Table 1.
Experimental design and conditions
| New
|
Old
|
|||
|---|---|---|---|---|
| Liv | Nonliv | Liv | Nonliv | |
| Episodic retrieval | A | B | C | D |
| Semantic retrieval | E | F | G | H |
Each letter corresponds to one PET scan and ERP average waveform. Liv, words denoting living things; Nonliv, words denoting nonliving things; New, words presented for the first time; Old, words presented previously in the study list.
The stimulus materials consisted of two sets (one for PET and the other for ERPs) of 480 nouns (18) and 240 randomly generated letter strings, between four and nine (average 5.7) letters long. Half of the nouns represented “living” (e.g., rabbit) and half represented “nonliving” (e.g., sword) things. The stimuli were presented visually on a computer screen.
An experimental session in the ERP part consisted of a succession of encoding/retrieval blocks with a duration of approximately 3 min. During the encoding phase of each block, 20 words were presented (one every 2 sec, each displayed for 1,700 msec) and subjects made pleasant/unpleasant judgments on each word (which were not recorded). ERPs were acquired only during the retrieval phase which started 30 sec after the end of the encoding phase. Here, 60 test items consisting of 40 words of four different types and 20 random letter strings were presented. The four word types were (i) old words (presented previously in the encoding list) denoting living things, (ii) old words denoting nonliving things, (iii) new (presented for the first time in the experiment) words denoting living things, and (iv) new words denoting nonliving things. The 60 test items were presented in 15 short lists of four items; each list contained only words or only letter strings. Each four-item list was coupled with a task instruction that in the case of words was either episodic or semantic retrieval and in the case of letter strings a control task. Each task instruction was visible in the upper part of the monitor from 2 sec before the presentation of the first until 2 sec after the presentation of the fourth (last) item of each list. Subjects were told to follow the task instructions presented on the monitor and that episodic retrieval required a decision that the word was old (presented in the encoding-list) or new (had not occurred before in the experiment) and that semantic retrieval required a living or nonliving judgment. The control task required to make a random button press (two choices) to each random letter string. There was a break (4,400 msec) between successive lists to allow subjects to blink. List items appeared at a rate of one every 2 sec, were displayed for 300 msec, and replaced by a central fixation cross for 1,700 msec. Subjects responded by pressing one of two buttons with their right hand (mouse in the PET and response pad in the ERP). They used their index finger to indicate an old item in the episodic and a living item in the semantic task. They used their middle finger to indicate a new item in the episodic or a nonliving item in the semantic task. Responses were accepted within 300 to 1,900 msec after the onset of presentation of each item. Subjects were told that reaction time and accuracy were equally important.
Although visual stimulation parameters and task instructions were exactly the same in the PET and ERP parts of the experiment, there were differences in the distribution of task and item-types. In the ERP retrieval, the four-item lists were completely randomized as to the quantity and order of the tasks and items, and subjects performed a total of 12 encoding-retrieval blocks. In the PET study, one scan was acquired during retrieval in each of the eight conditions shown in Table 1. An encoding phase, in which the subject was presented 20 words for study, preceded each retrieval phase, in which the subject was presented 60 test items—40 words and 20 letter strings. The radioactive tracer was injected 10 sec after the onset of the retrieval task and approximately 20 sec before the start of scanning, which lasted for 60 sec. During the 60-sec scanning window, the subject was presented with a total of 20 test items: 17 test items (85%) from one of the eight conditions shown in Table 1 and 3 items (15%) from a condition in which items had the opposite value of the two manipulated dimensions (old/living with new/nonliving, old/nonliving with new/living, new/living with old/nonliving, or new/nonliving with old/living). The test items outside the 60-sec scanning window constituted a random sequence of the 40 remaining words and letter strings assigned to the condition. During each of the eight PET scans, subjects were given either episodic or semantic retrieval instructions, identical with those used in the ERP part. The order of the first four scans was randomized for each subject, and this random order was reversed for the second set of four scans. Finally, an additional, ninth scan was acquired in which the 60-sec scan window was filled with letter strings. However, because many subjects on their own remarked on the highly demanding nature of this control task, we omitted these data from the PET and ERP analyses.
Table 3.
PET results
| BA | x | y | z | Z score | Figure | ||
|---|---|---|---|---|---|---|---|
| Task-related activations | |||||||
| Episodic − semantic (ABCD − EFGH) | R postcingulate Ctx | 23 | 4 | −40 | 24 | 3.7 | a |
| R prefrontal Ctx | 10 | 22 | 56 | −4 | 3.6 | b | |
| Semantic − episodic (EFGH − ABCD) | L prefrontal Ctx | 45 | −38 | 30 | 8 | 4.0 | c |
| L temporal | 21 | −46 | −38 | −12 | 3.8 | d | |
| Item-related activations | |||||||
| Episodic retrieval | |||||||
| Old − new (CD − AB) | L medial temporal Ctx | 28 | −36 | 0 | −20 | 3.9 | e |
| L medial temporal Ctx | 36 | −26 | −32 | −16 | 3.8 | f | |
| Living − nonliving (AC − BD) | R putamen | 26 | 16 | −4 | 3.4 | ||
| R temporal Ctx | 22 | 54 | 6 | 0 | 3.3 | ||
| Semantic retrieval | |||||||
| Old − new (GH − EF) | R prefrontal Ctx | 45 | 46 | 32 | 8 | 3.6 | g |
| R prefrontal Ctx | 44 | 38 | 6 | 16 | 3.4 | h | |
| New − old (EF − GH) | R medial temporal | 28 | 16 | −14 | −28 | 3.9 | i |
| L anterior temporal Ctx | 38 | −38 | 4 | −16 | 3.9 | j | |
| L temporal Ctx | 37 | −36 | −54 | 8 | 3.6 | k | |
| R anterior cingulate Ctx | 24 | 4 | 32 | 0 | 3.6 | l | |
| Living − nonliving (EG − FH) | L anterior cingulate Ctx | 32 | −4 | 24 | 44 | 3.7 | |
| L frontal Ctx | 47 | −48 | 24 | 0 | 4.0 | ||
| Nonliving − living (FH − EG) | R cerebellar Ctx | 16 | −44 | −8 | 3.8 |
Uppercase letters refer to the scans listed in Table 1. Lowercase letters (right column) depict the location of activations in Figs. 1 and 2. Only subtractions that yielded significant activations are listed. The coordinates are from the atlas of Talairach and Tournoux (19), where x, y, and z correspond to the right-left, anterior-posterior, and superior-inferior dimensions, respectively. Ctx, cortex; R, right; L, left.
PET scans were obtained with a GEMS-Scanditronix PC2048- 15B head scanner using a bolus injection of 35 mCi of [15O]H2O (1 Ci = 37 GBq). The PET data were analyzed with the statistical parametric mapping technique (Wellcome Department of Cognitive Neurology, London, U.K.) implemented in matlab (Mathworks, Natick, MA). The analysis involved the following steps: the different images from each subject were realigned to the first image, using a rigid body transformation. These realigned images from each subject were then transformed into a standard space (19) by matching to a reference image that already conforms to the standard space. These images were then smoothed by using an isotropic Gaussian kernel with full-width/half-maximum of 15 mm. The effects of the conditions (cognitive tasks) on the regional cerebral blood flow at each voxel were then estimated with a general linear model wherein the changes in global counts are considered as a covariate (20, 21). The effects of each comparison are estimated with linear contrasts. These contrasts yield a t statistic for a given comparison at each voxel, which is usually expressed as a standardized Z score. An activation was considered significant if its peak had a Z > 3.1 (equivalent to P < 0.05 after correction for multiple comparisons), and its spatial extent (i.e., the number of voxels above the significance level) had a probability <0.05 (no correction necessary).
Electroencephalogram signals were recorded (Neuroscan Synamp DC-amplifiers; low-pass filter; 35 Hz; digitization rate, 200 Hz; interelectrode impedance <2 kΩ) from 35 Ag/AgCl scalp electrodes and 11 additional electrodes located near the eyes, on the mastoids and neck. All signals were referenced to Cz during acquisition and transformed to common average for analysis. Trials containing large artifacts (>600 μV) were rejected and remaining eye movement and blink artifacts were removed from the averaged ERP waveforms by using ocular source components (22, 23). List ERPs for the episodic and semantic retrieval tasks (only trials with at least three of four correct responses were averaged) extended from 500 msec before to 9,500 msec after the task instructions. Stimulus ERPs to each item type (using only correct trials) extended from 200 msec before to 1,500 msec after onset of presentation. The reaction times and accuracy for correct responses were assessed by using repeated measures ANOVAs. Statistical analyses on ERPs were performed by using ANOVA (with the Greenhouse–Geisser correction for inhomogeneity of variance) on mean amplitude measurements [from 300 msec to 500 msec (N400 time window) and 500 msec to 700 msec (LPC) for stimulus ERPs and from 4,000 to 9,500 msec for list ERPs] at those electrodes where the waves were maximally recorded. For the topographical effects ANOVAs were conducted on normalized data (24). Statistical analysis of peak latencies (LPC) were performed at the parietocentral electrode Pz.
A source analysis for the ERPs was conducted, guided by the anatomical localizations provided by the PET scans. Because PET images showed differences in blood flow between conditions (episodic versus semantic tasks, old versus new items), we related the corresponding subtractions of ERP waveforms. Source analysis was performed with brain electric source analysis (25).
RESULTS
Behavioral Results.
Reaction times and accuracy (Table 2) were similar across tasks. Reaction times showed a sequence effect in both tasks, being slowest for the first stimulus in each retrieval list [F(1, 10) = 27.0; P < 0.001]. For response accuracy a sequence effect (lowest accuracy for the first stimulus) was present only in the episodic task [task by sequence interaction F(1, 10) = 8.1; P < 0.05]. There were no significant item-related differences in reaction time and accuracy.
Table 2.
Task-related behavioral and electrophysiological data
| Item 1 | Item 2 | Item 3 | Item 4 | |
|---|---|---|---|---|
| Episodic retrieval | ||||
| RT | 957 (199) | 802 (189) | 803 (179) | 827 (171) |
| Rαcc | 84 (13) | 89 (11) | 88 (13) | 87 (14) |
| LPCamp | 4.9 (1.6) | 6.7 (1.8) | 6.8 (2.6) | 5.5 (2.1) |
| LPClat | 615 (85) | 588 (44) | 629 (56) | 646 (61) |
| Semantic retrieval | ||||
| RT | 952 (170) | 824 (182) | 834 (177) | 835 (166) |
| Racc | 83 (11) | 82 (11) | 86 (11) | 79 (12) |
| LPCamp | 4.2 (1.2) | 5.6 (1.7) | 4.8 (2.5) | 4.2 (2.2) |
| LPClat | 612 (99) | 591 (64) | 622 (64) | 661 (51) |
Data are the mean with standard deviations in parentheses. RT, reaction time in msec; Racc, response accuracy in percent; LPCamp, amplitude in μV; LPClat, peak latency in msec.
PET Results.
Cerebral sites showing significant task- and item-related activations are shown in Table 3 and displayed in Figs. 1 and 2 by superimposing statistical parametric mapping patterns on magnetic resonance imaging templates that were standardized into Talairach space. Task and item comparisons resulted in blood flow increases in different brain areas. Item comparisons yielded different activation patterns in the episodic and semantic retrieval task. The item-related old minus new difference in the left Brodmann area (BA) 28/36 (Fig. 2) showed a significant task by item interaction.
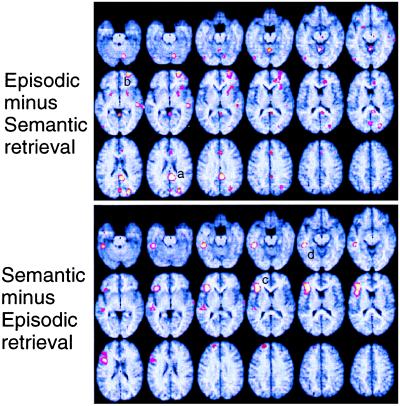
Figure 1.
Brain regions showing task-related blood flow differences in the PET study. (Upper) Regions with higher blood flow in the episodic than the semantic retrieval task are depicted. (Lower) Regions showing the opposite pattern are depicted. The horizontal slices are at intervals of 4 mm from −28 mm below the AC-PC line (top-left slice) to 40 mm above the anterior commissure–posterior commissure line (bottom-right slice). Rows: top, −28 to −8 mm; middle, −4 to 16 mm; bottom, 20 to 40 mm.
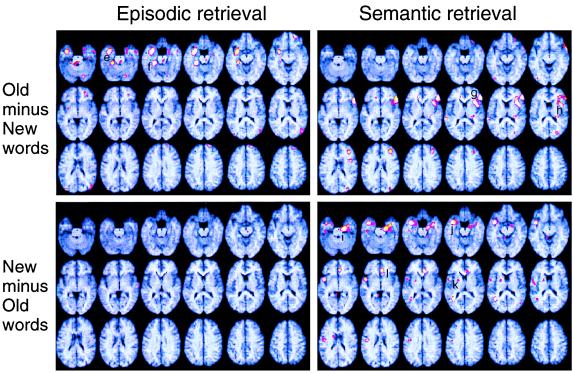
Figure 2.
Brain regions showing item-related blood flow differences in the PET study resulting from the subtractions of blood flow elicited by old and new items are depicted separately for episodic (left side) and semantic (right side) retrieval. See Fig. 1.
ERP Results.
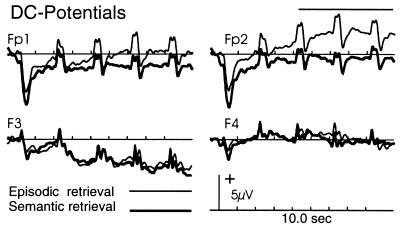
Compared with semantic retrieval, episodic retrieval was associated with a sustained positive DC shift over anterior frontal electrodes [main effect of task F(1, 10) = 5.2; P < 0.05] that was topographically (Fig. 3) maximal over right frontopolar electrodes [task × location (anterior and dorsolateral frontal electrodes) × laterality interaction F(1, 10) = 5.7; P < 0.05]. It commenced at the onset of the task cue, increased until the presentation of the second word, and stayed constant between the second and the fourth word. In both tasks, there was additionally a sustained negative left frontocentral DC shift. A source in the right frontal lobe accurately modeled the positive shift over the right frontal pole that resulted by subtracting the semantic task list ERP from the episodic (residual variance <10%). The small deflections on the DC shifts (caused by differences in the LPC between the two tasks) could be modeled by symmetrical dipoles in the medial temporal regions.
Figure 3.
Event-related potential wave forms elicited by lists presented during the episodic (thin lines) and semantic (bold lines) retrieval task. The displayed epoch of 10 sec encompasses the entire list, starting at 500 msec before the onset of task instruction and ending 1,500 msec after the presentation of the fourth (last) item. Traces: Fp1/Fp2, left and right frontopolar; F3/F4, left and right dorsolateral frontal electrodes.
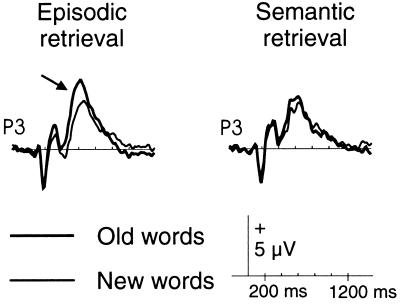
Peak latencies of the N400 and LPC occurred significantly F(1, 10) = 5.5; P < 0.05 and F(1, 10) = 11.8; P < 0.01, respectively) later for new than old items in both tasks (LPC latency: in the episodic task, 613 ± 62 msec vs. 664 ± 38 msec; in the semantic task, 608 ± 74 msec vs. 677 ± 94 msec). The LPC amplitude was more positive for old items, and this effect was much more prominent during the episodic task [item by task interaction F(1, 10) = 5.2; P < 0.05; Fig. 4]. No such interaction was found for the N400. Topographically, the LPC difference was largest over the left hemisphere at posterior electrodes [F(1, 10) = 6.7; P < 0.05) and could be reasonably well explained (residual variance <10%) on the basis of a source in the left medial temporal region.
Figure 4.
ERPs elicited by old (bold lines) and new (thin lines) items during episodic (Left) and semantic (Right) retrieval. The arrow depicts the LPC. P3, left parietal electrode.
DISCUSSION
The major finding of this experiment was a neuroanatomical dissociation between episodic-memory and semantic-memory retrieval that clearly reflected task-related but not item-related processes, by both the PET and ERP measures. As shown in Fig. 1, the PET contrast between episodic and semantic retrieval revealed hemodynamic changes (“activations”) in right prefrontal cortex (BA 10) and right posterior cingulate (BA 23). The PET contrast between semantic and episodic retrieval, on the other hand, revealed activations in left prefrontal cortex (BA 45/47) and left temporal lobe (BA 21). The ERP data for episodic retrieval (Fig. 3) revealed a sustained DC shift over four items of a test list that was maximal over right frontopolar electrodes, thus converging with the PET data. This convergence was also supported by source analysis of the ERP waveform. Although no distinctive ERP signal accompanied the left frontal semantic PET activation, it is conceivable that the left prefrontal activation occurred in a region that folds inwardly between two opposing cortical layers (Fig. 1). Electrical fields generated in this region would tend to cancel each other because of the opposing polarities of the two sides of the sulcus.
These frontal findings are in good agreement with the HERA model (1, 2, 26). More important, they strengthen the model by virtue of the converging PET and ERP data, and they clarify it by indicating that the HERA pattern is attributable to task-related rather than item-related processes. In much of previous work, task- and item-related processes have been confounded in that semantic retrieval in typical experiments has involved new items but episodic retrieval has involved previously encoded old items (27, 28). Other previous experiments have directly compared the neural correlates of retrieval of new and old items, but because these analyses have been limited to a single task, namely episodic retrieval (4, 7, 8), their results have not been fully informative about the relation between tasks and observed hemispheric asymmetries. In the present experiment, the clear frontal left/right contrast was observed between semantic and episodic retrieval tasks, under conditions in which all item-related variables were held constant, thus making possible a more precise characterization of HERA. The task-related involvement of right prefrontal cortical regions in episodic memory retrieval confirms earlier findings and suggestions (7–11) that at least some right frontal activations signal the establishment and maintenance of a neurocognitive set of episodic retrieval “attempt” or episodic retrieval “mode” (26).
The convergence of PET and ERP data in relating right BA 10 activity to task-related episodic retrieval processes was complemented by behavioral data. Accuracy and speed of episodic retrieval significantly improved from the first item in the list (at 2 sec) to the second item (at 4 sec), in parallel with an increase in the amplitude of the DC shift. The close relationship of the ERP to the onset and duration of the task, as well as the efficacy of performance, suggests that the task-related process represents episodic retrieval mode. The slightly sluggish time course of the DC signal is remarkably similar to that of the hemodynamic response of BA 10 as measured in a recent functional magnetic resonance imaging study of episodic retrieval (11) and suggests a certain degree of task-related “neurocognitive inertia” when the subject switches from the present-oriented (default) retrieval mode to one involving one’s personal past.
The other cerebral site at which episodic task-related processes showed higher activity than semantic processes was in the posterior cingulate cortex. Nothing much is known about this region of the brain and its relation to memory, although cases of “retrosplenial amnesia” have been described (29, 30), and the region has been noted in a few previous PET studies (31). This region is also intriguingly close to the posterior medial parietal area that Raichle (32) has identified as the metabolically most active cortical region in the default state of the brain.
We propose tentatively that the hemodynamic and electrophysiological changes in the frontal BA 10 (and perhaps the posterior BA 23 as well) represent a part of the neural signature of the episodic retrieval mode, a neurocognitive state of consciously (“autonoetically”) “thinking back to” the encoding episode. The somewhat sluggish onset of this state (“neurocognitive inertia”), revealed by the ERP data, suggests that it reflects an intentional “strategic” orientation in the present to the past. This interpretation is compatible with recent findings that show changes in BA 10 associated with shifts in retrieval strategy (10). It also fits previous results showing differential activity in BA 10 and the dorsolateral prefrontal cortex for old and new words (4, 33, 36) inasmuch as it suggests that the BA 10 activity reflects episodic retrieval mode. Finally, lesions disconnecting right BA 10 from the right temporal lobe can result in retrograde amnesia suggesting a failure to establish and maintain episodic retrieval mode (35).
A somewhat surprising finding was that of the old minus new activity in the right ventrolateral prefrontal cortex (BA 45) in the semantic retrieval condition only. However, there exists some suggestive electrophysiological (13, 15) and hemodynamic (5, 36) evidence that item-related right frontal activity may be elicited by the necessity of processing test items beyond a simple recognition judgment. These putative postretrieval processes involve specific areas in the dorsolateral (BA 46, BA 9) and ventrolateral (BA 25) prefrontal cortex (5). Most of our subjects mentioned after the experiment that they had become aware of the fact that many test words in the semantic (living/nonliving) task had been presented in the earlier encoding phase. The right frontal activation, therefore, may have reflected the inhibition of possible but task-inappropriate responses. Lesions in the right lateral frontal cortex can produce high rates of false recognition, suggesting a failure to inhibit information not relevant to the task on hand (37).
Interactive effects between task-related and item-related processing were seen in several areas of the brain. These included medial temporal lobe regions in which old words showed higher activation than new words, but during episodic retrieval only. The importance of the medial temporal lobes for memory processes is well documented by lesion studies (38, 39), but it has remained unclear to what extent their activity is determined by task instructions, item properties, or their interactions. Consistent with previous findings (28), the pattern of results seen in Fig. 2 suggests that medial temporal activity can reflect stimulus novelty, as well as stimulus familiarity. Novelty refers to higher blood flow for new as compared with old items (27, 34, 40), whereas familiarity (ecphory, successful retrieval) refers to the opposite pattern (8). Fig. 2 also shows that medial temporal novelty (right medial temporal, left temporal, and right anterior cingulate regions) and familiarity (left medial temporal) activations do not occur within the same task. Thus, although the fact that novelty and familiarity reflect item-related processes, medial temporal lobe activity seems to be determined by their interaction with task-related processes. It seems reasonable to argue that medial temporal lobe activity is not automatically driven by item properties but is determined by the relevance of these properties to the current task orientation, that is, the dominant neurocognitive set.
Besides depicting the interaction of medial temporal lobe activity with task-related processes, our converging PET and ERP data provide useful hints regarding the time course of activations associated with ecphory. Ecphory was associated with increased blood flow in left medial temporal regions (Fig. 2) and a more positive LPC primarily over left posterior electrodes peaking near 600 msec (Fig. 4). The source analysis in this experiment suggests that left medial temporal activity is indeed item-related, because it peaks before the old/new motor response is made. Nyberg et al. (41) found that left medial temporal activity increased as a function of the proportion of recognition hits during the PET scan and suggested that this activity is associated with the successful reactivation of memory representations. Others (9) too have suggested that medial temporal activity during episodic retrieval is specifically associated with conscious recollection of an item’s presentation in the encoding phase. The present combined PET and ERP findings of item-related activity during episodic retrieval are consistent with these ideas.
In summary, in this combined PET and ERP experiment, we separated neural correlates of cognitive activity into distinct task-related and item-related processes and their interactions. The results clarify the HERA model: They point to the involvement of right BA 10 in episodic retrieval by virtue of its functional role in the establishment and maintenance of the episodic retrieval mode. The ERP data suggested that BA 10 was active throughout the episodic retrieval task, whereas the left medial temporal lobe became intermittently active whenever an individual item appeared within the task. Thus it looks as if, when the brain is set in the episodic retrieval mode, left medial temporal lobe activity is higher for familiar than novel items, suggesting that it is more involved in ecphory than in novelty assessment.
Acknowledgments
We thank Dr. Shitij Kapur, Dr. Sylvain Houle, and Dr. Randy McIntosh for helpful comments. This study was supported by grants from the Bundesministerium für Bildung und Forschung (Grant BMWF/BEO 11 TW 10574), the Deutsche Forschungsgemeinschaft (Grant Du 280/1-1 to E.D.), and the Natural Sciences and Engineering Council of Canada.
ABBREVIATIONS
- PET
positron emission tomography
- ERP
event-related potential
- BA
Brodmann area
- HERA
hemispheric encoding/retrieval asymmetry
- LPC
late positive component of the ERP waveform
- DC
direct coupling
References
- 1.Tulving E, Kapur S, Craik F I, Moscovitch M, Houle S. Proc Natl Acad Sci USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyberg L, Cabeza R, Tulving E. Psychon Bull Rev. 1996;2:134–147. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 3.Buckner R L, Raichle M E, Miezin F M, Petersen S E. J Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S, Dolan R J. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher P C, Shallice T, Frith C D, Frackowiak R S, Dolan R J. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- 6.Grady C L. In: The Human Frontal Lobes. Miller B L, Cummings J L, editors. New York: Guilford; 1999. pp. 196–230. [Google Scholar]
- 7.Kapur S, Craik F I, Jones C, Brown G M, Houle S, Tulving E. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 8.Nyberg L, Tulving E, Habib R, Nilsson L-G, Kapur S, Houle S, Cabeza R, McIntosh A R. Neuroreport. 1995;7:249–252. [PubMed] [Google Scholar]
- 9.Schacter D L, Alpert N M, Savage C R, Rauch S L, Albert M S. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner A D, Desmond J E, Glover G H, Gabrieli J D. Brain. 1998;121:1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- 11.Buckner R L, Koutstaal W, Schacter D L, Dale A M, Rotte M, Rosen B R. Neuroimage. 1998;7:163–175. doi: 10.1006/nimg.1998.0328. [DOI] [PubMed] [Google Scholar]
- 12.Paller K A, Kutas M, McIsaac H K. Psychol Sci. 1995;6:107–111. [Google Scholar]
- 13.Wilding E L, Rugg M D. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- 14.Smith M E. J Cognit Neurosci. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Duzel E, Yonelinas A P, Mangun G R, Heinze H J, Tulving E. Proc Natl Acad Sci USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rugg M D, Mark R E, Walla P, Schloerscheidt A M, Birch C S, Allan K. Nature (London) 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- 17.Rockstroh B, Elbert T, Canavan A, Lutzenberger W, Birbaumer N. Slow Cortical Potentials and Behavior. Baltimore, MD: Urban and Schwarzenberg; 1989. [Google Scholar]
- 18.Baayen, R. L., Piepenbrock, R. & von Rijn, H. (1993) (Linguistic Database Consortium, University of Philadelphia).
- 19.Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- 20.Friston K J, Frith C D, Liddle P F, Frackowiak R S. J Comput Assisted Tomogr. 1991;15:634–639. doi: 10.1097/00004728-199107000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Friston K J. J Cereb Blood Flow Metab. 1995;15:361–370. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- 22.Berg P, Scherg M. Electroencephalogr Clin Neurophysiol. 1991;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 23.Lins O G, Picton T W, Berg P, Scherg M. Brain Topogr. 1993;6:65–78. doi: 10.1007/BF01234128. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy G, Wood C C. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 25.Scherg M, Berg P. Electroencephalogr Clin Neurophysiol Suppl. 1996;46:127–137. [PubMed] [Google Scholar]
- 26.Cabeza R, Nyberg L. J Cognit Neurosci. 1997;9:1–26. doi: 10.1162/jocn.1997.9.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Haxby J V, Ungerleider L G, Horwitz B, Maisog J M, Rapoport S I, Grady C L. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrieli J D E, Brewer J B, Desmond J E, Glover G H. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- 29.Verfaellie M, Bauer R M, Bowers D. Brain Cognit. 1991;15:10–25. doi: 10.1016/0278-2626(91)90012-w. [DOI] [PubMed] [Google Scholar]
- 30.Heilman K M, Bowers D, Watson R T, Day A, Valenstein E, Hammond E, Duara R. Neuropsychologia. 1990;28:161–169. doi: 10.1016/0028-3932(90)90098-9. [DOI] [PubMed] [Google Scholar]
- 31.Desgranges B, Baron J C, Eustache F. Neuroimage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- 32.Raichle M E. Philos Trans R Soc London B. 1998;353:1889–1901. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tulving E, Kapur S, Markowitsch H J, Craik F I, Habib R, Houle S. Proc Natl Acad Sci USA. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulving E, Markowitsch H J, Craik F E, Habib R, Houle S. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 35.Levine B, Black S E, Cabeza R, Sinden M, McIntosh A R, Toth J P, Tulving E, Stuss D T. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- 36.Rugg M D, Fletcher P C, Allan K, Frith C D, Frackowiak R S J, Dolan R J. Neuroimage. 1998;8:262–273. doi: 10.1006/nimg.1998.0363. [DOI] [PubMed] [Google Scholar]
- 37.Schacter D L, Curran T, Galluccio L, Milberg W P, Bates J F. Neuropsychologia. 1996;34:793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 38.Rempel-Clower N L, Zola S M, Squire L R, Amaral D G. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milner B, Squire L R, Kandel E R. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 40.Grady C L, McIntosh A R, Horwitz B, Maisog J M, Ungerleider L G, Mentis M J, Pietrini P, Schapiro M B, Haxby J V. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 41.Nyberg L, McIntosh A R, Houle S, Nilsson L G, Tulving E. Nature (London) 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]