Abstract
Hypertension is one of the most prevalent diseases in the developed and developing countries. Based on the long historical association and the provocative findings of blood pressure effects at low level of lead exposure a study was carried out to determine if an association existed between low blood lead concentration and hypertension. In this study the effects of low-level exposure to lead on blood pressure were examined among 244 adults using atomic absorption spectrometer. For quality assurance purpose certified reference materials i.e., Animal blood A-13, Bovine liver 1577 and cotton cellulose V-9 from IAEA (International Atomic Energy Agency) and NIST (National Institute of Standard Technology) were analyzed under identical experimental conditions. The mean age of hypertensive adults was 52 years (range 43~66). The mean values of systolic and diastolic blood pressure were (209±11.7) (range 170~250) and (117±3.9) (range 105~140) mmHg respectively. Blood lead concentration ranged from 78~201 µg/L with a mean of 139 µg/L and 165~497 µg/L with a mean of 255 µg/L in normal and hypertensive adults respectively. Increase in systolic blood pressure was significantly predictive with increase in blood lead levels. Body mass index (BMI) and lipid profile including total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol and triglyceride correlated with blood pressure.
Keywords: Hypertension, Lead exposure, Biochemical parameters, Body mass index
INTRODUCTION
Blood-Pb levels are taken as representative of dose/exposure, as all clinical symptoms resulting from the toxic effects of Pb are manifested mainly in blood. Lead exposure of children correlates with decreased IQ, symptoms of hyper kinesis or minimal brain dysfunction, poor learning, or defects in specific neuromotor tasks (Landgrin et al., 1980). Large number of observations over the years has associated lead exposure with human diseases (McMichael and Johnson, 1982). Cardiovascular system has been a focus of investigations on the toxic effects of lead (Batuman et al., 1983). With moderate to heavy exposure to lead, there are studies reporting cerebrovascular diseases and nephritis resulting in increased mortality (Pocock et al., 1984). Moreover, toxic effects of lead on the kidney have been associated with the development of hypertension and its complications (Harlan, 1998). The issue of toxicity to the cardiovascular system at relatively low levels of blood-lead exposure remained problematic until recently.
The etiology of hypertension is also unknown in about 90% of cases. Numerous epidemiological studies and investigations using experimental animals have dealt with the putative relationship between blood-Pb and hypertension (Bargman, 1985). The results to date have been quite controversial, thus the issue appears to be unresolved at present so the pathogenesis of hypertension is still open to question. The effects of heavy metals, especially lead, is considered among contributing factors of hypertension (Bargman, 1985). However, recent study has revealed the effect of lead on arterial blood pressure at levels not causing clinical symptoms of renal impairment (Muntner et al., 2003). This study focuses more on epidemiological, clinical and toxicological data. The epidemiological evidence is consistent with low-level exposure to lead causing an elevation in blood pressure (McMichael and Johnson, 1982).
Based on the long historical association and the provocative findings of blood pressure effects at low levels of lead exposure, a study was carried out and the data were analyzed to determine if an association exists between relatively low blood lead levels and blood pressure. The purpose of the study was three fold, first to find out whether any relationship exists between blood Pb levels and hypertension, second to relate the findings with clinical biochemical parameters and third to see whether there is any effect of skin fold thickness or body mass index (BMI) on the development of hypertension.
Determination of trace metals in human whole blood has been reported by inductively coupled plasma mass spectrometry (Nixon, 1996), anodic stripping voltametry (Brainina et al., 1996), neutron activation analysis (Xilei et al., 1988) and atomic absorption spectrophotometry (Shang and Hang, 1997). Atomic absorption spectrophotometry (AAS) is one of the preferred techniques due to its rapidness, specificity and comparatively low cost.
EXPERIMENTAL DETAILS
Instruments
All the measurements were made with Hitachi model Z-8000 polarized Zeeman atomic absorption spectrophotometer (AAS), coupled with a microprocessor-based data-handling facility and a printer. Electrothermal atomic absorption spectrometer (ETAAS) mode of AAS was used for the analysis of lead (Pb). Hollow cathode lamp of Pb was used as radiation sources. Argon was used as an inert purging gas at a rate of 100 ml/min during drying, charring and cleaning stages and its flow was interrupted during atomization stage. The absorption signals during atomization step were recorded as peak heights.
Vitalab selectra and photometric system was used for the determination of biochemical parameters such as cholesterol and triglyceride in whole blood. Ten microlitres of blood sample was mixed with 100-µl of reagents with the absorbance measured against reagent blank within 60 min (Rifai et al., 1999).
Reagents
Stock solutions of 1000 mg/L of Pb was prepared by dissolving appropriate amount of spec pure metal (Johnson Matthey Chemicals Ltd.) in minimum amount of nitric acid and the volume was made up to 100 ml with water. For the preparation of calibration curve fresh working standards were made by appropriate dilution of the stock solution in 0.02 mol/L HNO3 immediately before use. Glassware was cleaned by overnight soaking in HNO3 (1:1) followed by repeated rinsing with water. Distilled and deionized water was used throughout this work.
Sampling and sample preparation
Sampling was done under the supervision of trained medical staff. Before taking the sample detailed information on the volunteers was recorded in the designed questionnaire for this study. Questionnaires are usually used to gather data on health and other factors. Any unusual finding regarding the subject’s habit or health was also recorded. Blood samples of both healthy and diseased subjects were drawn by vein puncture under contamination-controlled conditions (Behne, 1981).
Care was taken to avoid haemolysis of blood samples, disposable syringes (Monovetten Sarstedt, Numbrecht, FRG) were used and slow transfer to a 4 ml VACUETTES (Grainer labortechnik), dipotassium EDTA was added as an anticoagulant. The EDTA salt was also analyzed for its lead contents and found to be below the detection limit. Minimum time was taken for transport of sample whereas storage was mostly avoided and when required the samples were refrigerated at −4 °C prior to analysis. Almost all samples were analyzed within 8 h after collection. The medical staff of Federal Government Services Hospital and Shifa International Hospital, Islamabad helped in collecting the venous blood samples, during 2003~2004 for both normal and hypertensive adults.
Digestion procedure of blood samples/certified reference materials (CRMs)
About 0.5 ml of blood sample was taken in triplicate in 100 ml digestion flask fitted with 30 cm long air condenser and 5.0 ml distilled HNO3 was added to the sample. The contents were heated at 80 °C for 30 min. After cooling 1.5 ml of concentrated perchloric acid (70%) was added and heated again at 250 °C with occasional shaking till white fumes evolved. The clear solution obtained was cooled and transferred into a 10 ml measuring flask and the volume was made up with deionized water for subsequent measurement of Pb. A blank was also prepared under similar conditions. All certified reference materials were digested using the same procedure. This digestion of whole blood is mandatory for the deprotenization of blood and for the reproducibility of results because if blood is analyzed simply by dilution with 0.02 mol/L HNO3, then some charred material is left behind in the graphite tube after each analysis, which affects the reproducibility of results.
Lead was determined after digesting the liquid or freeze-dried blood. Ten microlitres sample was injected into the graphite tube with the help of micropipette. The heating programme i.e. drying, ashing, atomizing and cleaning was initiated. All the samples (blank, standards, sample blank and samples) were analyzed at least in triplicate and average reading was used for subsequent calculations. The value of the blank was subtracted from the sample.
RESULTS AND DISCUSSION
The digested blood samples were analyzed by ETAAS under the optimized instrumental conditions as given in Table 1. The criteria for the optimization of instrumental conditions were the conditions that produced maximum stable signal with low background. Limits of detection (LOD) and sensitivities for lead in the blood sample were determined by using the optimized conditions after acid digestion. The determined values of LOD and sensitivity were found to be 0.1 and 0.5 μg/L respectively. The detection limit of Pb found by the present method is low enough to determine the concentration of metal in the whole blood samples of healthy subjects as well as hypertensive adults with adequate precision and accuracy.
Table 1.
Optimized instrumental conditions used for ETAAS determination of Pb in whole blood
| Parameters | Value | |
| Lamp current (mA) | 7.5 | |
| Slit width (nm) | 1.3 | |
| Wavelength (nm) | 283.3 | |
| Sample volume (µl) | 10 | |
| Cuvettes | Cup type | |
| Drying 1 | Temperature (°C) | 60~100 |
| Time (s) | 60 | |
| Drying 2 | Temperature (°C) | 100~130 |
| Time (s) | 30 | |
| Ashing | Temperature (°C) | 800~800 |
| Time (s) | 40 | |
| Atomizing | Temperature (°C) | 2200~2200 |
| Time (s) | 7 | |
| Cleaning | Temperature (°C) | 3000~3000 |
| Time (s) | 3 | |
As the certified reference material of human whole blood was not available, freeze dried animal blood A-13 from International Atomic Energy Agency (IAEA) Bovine liver 1577 and Cotton cellulose V-9 from National Institute of Standard Technology (NIST) were used for quality assurance of the analytical method employed. Table 2 depicts the determined values of Pb by ETAAS in certified reference materials along with their reported/certified values. Our determined values for reference materials are in good agreement with the certified values with good precision.
Table 2.
Determined blood-Pb concentration (ng/g) in certified reference materials by EAAS
| No. | Certified reference materials | Concentration of lead (Pb) |
|
| Certified values±SD | Our values±SD | ||
| 1 | Cotton cellulose V-9 | 0.25±0.002 | 0.27±0.003 |
| 2 | Animal blood A-13 | 180±39 | 200±16 |
| 3 | Bovine liver 1577 | 135±1 | 142±2 |
The 215 whole blood samples of normal healthy adults from Rawalpindi/Islamabad including 118 males and 97 females, of 22~57 years of age as volunteers were analysed using the optimized instrumental parameters. The results are summarized in Table 3 in the form of determined concentration range, mean and median along with standard deviation. Examination data in Table 3 indicates that the determined concentration range of Pb varies with age. The concentration range of Pb in healthy subjects was 78~201 μg/L (mean=139 μg/L, median=141 μg/L). The determined median values are close to the mean concentration indicating the Gaussians distribution of our dataset. The output of this analysis indicates positive correlation of age with Pb levels in whole blood. A similar study carried out in India concludes that blood lead concentration increases with age (Bhardwaj et al., 1991). The positive correlation of blood lead with variation in age is depicted pictorially in Fig.1 clearly showing that the blood lead is generally high in successively older age groups.
Table 3.
Blood lead levels (µg/L) in male and female normal subjects of different age groups
| N (sex) | Age range (years) | Concentration range (µg/L) | Mean (µg/L) | SD (µg/L) | Median(µg/L) |
| 215 (M+F) | 22~54 | 78~201 | 139 | 34 | 141 |
| 118 (M) | 22~54 | 93~201 | 155 | 32 | 165 |
| 97 (F) | 22~54 | 78~191 | 121 | 29 | 119 |
| 48 (M) | 22~40 | 93~156 | 119 | 21 | 118 |
| 60 (F) | 22~40 | 78~116 | 94 | 14 | 93 |
| 70 (M) | 41~54 | 88~201 | 145 | 23 | 148 |
| 37 (F) | 41~54 | 101~191 | 129 | 16 | 126 |
M: Male; F: Female
Fig. 1.
Correlation of blood-Pb with age in normotensive
Table 4 compares the determined concentration of Pb in blood samples of normal Pakistani adults with the reported values from other countries. The mean concentration of Pb in whole blood samples of our normal subjects (139 μg/L) is lower than the reported normal level for India (212 μg/L) (Bhardwaj et al., 1991), and is comparable to the value from Belgium (140 μg/L) (Louekari et al., 1991). Our value lies within the range given by FRG (82~148 μg/L) (Brockhaus et al., 1983) and is higher than that reported by USA (92 μg/L) (Louekari et al., 1991), Japan (49 μg/L) (Louekari et al., 1991) and UK (122 μg/L) (Subramanian and Meranger, 1983).
Table 4.
Blood lead levels (µg/L) in normal subjects from different populations
Blood samples of 244 (128 male and 116 female) clinically diagnosed hypertensive adults with no sign of occupational exposure were also analyzed for blood-Pb levels with the results summarized in Table 5 in the form of mean, median and standard deviation (SD). In the 244-hypertensive subjects the systolic pressure varied between 170~250 mmHg mean 209 mmHg and diastolic pressure ranged between 105~140 mmHg (mean of 117 mmHg). The determined concentration range of Pb was 166~497 μg/L (mean=255, median=237) in hypertensive adults. In order to study whether the apparent difference between the determined mean blood concentration of lead in males and females is statistically significant, student t-test was applied at 95% confidence levels to the dataset of the hypertensive adults. The out put of t-test shows that for Pb the t-statistical value (5.27) is greater than t-critical value (1.96), indicating significant differences between the two population groups.
Table 5.
Blood lead levels (µg/L) in male and female hypertensive adults
| N (sex) | Age range (years) | Concentrationrange (µg/L) | Mean (µg/L) | (±)SD (µg/L) | Median(µg/L) |
| 244 (M+F) | 43~66 | 166~497 | 255 | 57 | 237 |
| 128 (M) | 43~66 | 211~497 | 272 | 56 | 251 |
| 116 (F) | 43~66 | 166~443 | 235 | 52 | 219 |
M: Male; F: Female
All the hypertensive adults were in the age group of 43~66 years, so three adult sub groups with respect to age were made. Correlation between blood lead levels in these subgroups with respect to age, systolic and diastolic blood pressure is shown in Fig.2. It was evident that significant higher blood-Pb levels existed in the hypertensive older age group. Similar observation was found in Venezuela that a relationship exists between the whole blood-Pb levels and hypertension (Granadillo et al., 1995). The higher levels of Pb in blood samples of hypertensive patients could be attributed to higher intake of these metals through dietary items, environment or due to malfunction in the metabolism of Pb which ultimately developed hypertension. In this study the levels of blood lead were found to be lower in females than in normal male subjects.
Fig. 2.
Correlation of blood-Pb concentration with age, systolic and diastolic blood pressure (SBP and DBP) in hypertensive adults
Various biochemical parameters such as total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) and triglyceride were studied in normal subjects as well as in hypertensive adults. Results are summarized in Table 6. The determined levels of various biochemical parameters were well within the range of normal specified limits (Stephen, 2001). The correlation of these biochemical parameters with metal concentration reveals that blood-Pb shows close association with high TC and LDL-C. There exists evidence indicating that exposure to certain pesticides might affect lipoprotein metabolism in humans (Kurppa et al., 1984).
Table 6.
Determined levels of biochemical parameters studied in normotensive and hypertensive adults
| No. | Lipid profile | Concentration levels (mg/dl) |
|
| Normotensive adults | Hypertensive adults | ||
| 1 | Total cholesterol | 140~200 | 190~265 |
| 2 | LDL cholesterol | 100~132 | 118~170 |
| 3 | HDL cholesterol | 35~55 | 15~47 |
| 4 | Triglyceride | 40~150 | 44~178 |
Our data belong to the general population with significantly higher measured levels of blood lead measured in persons not previously believed to have significant lead exposure. The prospective nature of this study regarding the lead (a globally distributed toxin) levels in blood is a major contribution to hypertension, which in turn is becoming a major risk factor for heart attack and stroke (Cheng et al., 2001).
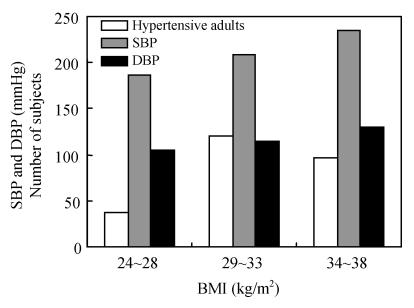
Blood pressure, body height and weight in 244 adults were measured. Body mass index (BMI) decreased with age while prevalence of hypertension increased with age. Meanwhile the prevalence of hypertension increased with age and with BMI. Among hypertensive adults occurrence of blood pressure increased progressively with increasing BMI from 15% at a BMI of 24~28 kg/m2 to 38% at a BMI of 34~38 kg/m2 (Fig.3). Mean blood total cholesterol levels increased from 190~265 mg/dl with increased BMI from 24~38 kg/m2. It was noted that distribution of BMI in the elderly was different from that of youths with our findings suggesting that it is important to control the BMI among the elderly hypertensive.
Fig. 3.
Correlation of BMI with systolic and diastolic blood pressure (SBP and DBP) in hypertensive adults
CONCLUSION
This study provided evidence for an association between blood lead and blood pressure at measured levels observed in the general population of Pakistani adults with no sign of occupational exposure to lead. Behavioral and environmental factors that explain hypertension must be identified to develop effective prevention policies.
Acknowledgments
The authors wish to acknowledge the cooperation of medical staff of Federal Government Services Hospital and Shifa International Hospital, Islamabad.
References
- 1.Bargman RF. Dietary factors in essential hypertension. Prog Food Nutr Sci. 1985;9(1-2):109–147. [PubMed] [Google Scholar]
- 2.Batuman V, Landy E, Marcsaka JK, Wedeen R. Contribution of lead to hypertension with renal impairment. N Engl J Med. 1983;309:17–21. doi: 10.1056/NEJM198307073090104. [DOI] [PubMed] [Google Scholar]
- 3.Behne D. Sources of error in sampling and sample preparation for trace element analysis in medicine. J Clin Chem Clin Biochem. 1981;19:115–120. [PubMed] [Google Scholar]
- 4.Bhardwaj S, Chandra O, Khan AS. Serum and urinary lead levels in hypertension. Indian J Pharmac. 1991;23:69–77. [Google Scholar]
- 5.Brainina K, Schafer H, Ivanova A, Khanina R. Determination of copper, lead and cadmium in whole blood by stripping voltametry with the use of graphite electrode. Anal Chim Acta. 1996;330(2-3):175–181. doi: 10.1016/0003-2670(96)00181-X. [DOI] [Google Scholar]
- 6.Brockhaus A, Freier I, Ewers U, Jermann E, Dolgner R. Levels of cadmium and lead in blood in relation to smoking, sex, occupation, and other factors in an adult population of FRG. Int Arch Occup Environ Health. 1983;52(2):167–175. doi: 10.1007/BF00405420. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension the normative aging study. American J Epidemiol. 2001;153(2):164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 8.Granadillo VA, Tahan JE, Salgado O. The influence of the blood levels of lead, aluminum and vanadium upon the arterial hypertension. Clin Chim Acta. 1995;233(1-2):47–59. doi: 10.1016/0009-8981(94)05966-V. [DOI] [PubMed] [Google Scholar]
- 9.Harlan WR. The relationship of blood lead levels to blood pressure in the US population. Environ Health Perspect. 1998;78:9–14. doi: 10.1289/ehp.88789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurppa K, Hietanen E, Klockars M, Partinen M, Rantannen J, Ronnemaa T, Viikari J. Chemical exposures at work and cardiovascular morbidity. Atherosclerosis, ischemic heart disease, hypertension, cardiomyopathy and arrhythmias. Scand J Work Environ Health. 1984;10(6):381–388. doi: 10.5271/sjweh.2316. [DOI] [PubMed] [Google Scholar]
- 11.Landgrin PJ, Baker E, Whitworth R, et al. Biochemistry of Ultra Trace Elements. In: Needleman HL, editor. Low Lead Exposure, the Clinical Implementation of Current Research. New York and London: Plenum Press; 1980. p. 17. [Google Scholar]
- 12.Louekari K, Valkonen S, Pousi S, Virtanen L. Estimated dietary intake of lead and their concentration in blood. Sci Total Environ. 1991;105:87–99. doi: 10.1016/0048-9697(91)90331-8. [DOI] [PubMed] [Google Scholar]
- 13.McMichael AJ, Johnson HM. Long-term mortality profile of heavily exposed lead smelter workers. J Occup Med. 1982;24:375–378. doi: 10.1097/00043764-198205000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population, results from NHANES III. Kidney Int. 2003;63(3):1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 15.Nixon DE. Routine clinical determination of lead, arsenic, cadmium and thallium in urine and whole blood by inductively coupled plasma mass spectrometry. Spectrochim Acta. 1996;51B(1):13–25. [Google Scholar]
- 16.Pocock SJ, Shaper AG, Ashby D, Delves I. Blood lead concentration, blood pressure, and renal function. Br Med J. 1984;289:872–874. doi: 10.1136/bmj.289.6449.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rifai N, Bachorik PS, Albers J, et al. Textbook of Clinical Chemistry. 3rd Ed. Philadelphia: W.B. Saunders Company; 1999. pp. 809–861. [Google Scholar]
- 18.Shang S, Hang W. Flame atomic absorption spectrometry using micro volume injection technique for the determination of Cu, Zn, Ca, Mg and Fe in whole blood from healthy infant and mother ears. Fresenius J Anal Chem. 1997;357(7):997–999. doi: 10.1007/s002160050290. [DOI] [Google Scholar]
- 19.Stephen M. Current Medical Diagnosis and Treatment. 40th Ed. USA: Lawrence Tierney, McGraw Hill Company; 2001. [Google Scholar]
- 20.Subramanian KS, Meranger JC. Blood levels of cadmium, copper, lead and zinc in children in a British Columbia community. Sci Total Environ. 1983;30:231–244. doi: 10.1016/0048-9697(83)90015-3. [DOI] [PubMed] [Google Scholar]
- 21.Xilei L, Renterghem V, Cornelis R, Mees L. Radiochemical neutron activation analysis for thirteen trace metals in human blood serum by using inorganic ion exchange. Anal Chim Acta. 1988;211(1):231–241. doi: 10.1016/S0003-2670(00)83683-1. [DOI] [Google Scholar]