Abstract
Vertebrate alarm calls can contain information about the type of predator and the degree of danger, but young animals often respond to alarm calls differently from adults. The distinct behaviour of young may reflect an imperfect stage in the gradual development of the adult response, or a response adapted to specific risks faced by young. In this study, we tested whether nestling white-browed scrubwrens, Sericornis frontalis, responded to different alarm calls according to their specific risks of predation. As predators on the ground pose a danger to scrubwren nestlings, whereas flying predators do not, we predicted that they would respond to ground alarm calls but not to aerial alarm calls. In a field playback experiment, we tested the response of young to aerial and ground alarm calls, each presented in a shorter (less urgent) and longer (more urgent) form. We found that both 5- and 11-day-old nestlings responded to ground alarm calls, and did so more strongly to the more urgent playback. By contrast, the response to aerial alarm calls started to develop only towards the end of the nestling stage. Thus, scrubwren nestlings can distinguish between different types of alarm calls and react more strongly to calls warning of a predator posing greater danger, appropriate to the nestling stage of development. Furthermore, they use the length of ground alarm calls as an indicator of the degree of danger.
Keywords: vocal communication, alarm calls, age-dependent risk, predation, nestling vocalization, non-begging vocalization
1. Introduction
The alarm calls of vertebrates can encode information about the type of predator, the urgency of the response, or both (Macedonia & Evans 1993). Whether alarm calls carry information about urgency or predator class is likely to be adapted to the hunting strategies of each species' predators and the potential for anti-predator responses (Macedonia & Evans 1993; Evans 1997; Fichtel & Kappeler 2002). As the response to alarm calls is potentially costly in terms of time and energy (Curio 1993), and a wrong response might be fatal, the correct identification of type and immediacy of the threat is critical.
The correct response to and identification of alarm calls are particularly important for infants and juveniles. Especially in the first weeks of their lives, the young of most vertebrates are not able to perform fast and efficient escape manoeuvres, and are often incapable of correctly identifying predators themselves (Seyfarth & Cheney 1980; Mateo 1996a). For example, young vervet monkeys (Cercopithecus aethiops), although able to respond appropriately to different adult alarm calls, show limited innate abilities to identify predators correctly, and frequently give alarm calls to non-predatory birds (Seyfarth & Cheney 1980). Many infants thus have to rely on adults to identify predators and to assess the degree of danger that they pose. The early development of an appropriate response to adult alarm calls is therefore likely to be under strong selection. Studies on primates and rodents have shown an innate predisposition to respond to species-specific alarm calls (Herzog & Hopf 1984; Mateo 1996b; McCowan et al. 2001), but also marked differences between adult and juvenile reactions to alarm calls. The development of an adult-like alarm-call response takes 6–12 months and may depend on social learning (Hauser 1988; Mateo 1996a; Ramakrishnan & Coss 2000; McCowan et al. 2001).
The differences between adult and infant reactions to alarm calls are not necessarily only a sign of an incomplete development, but could also be a stage-dependent adaptation to differing predation risks and living conditions (Owings & Loughry 1985; Miller & Hicinbothom 1991; Hersek & Owings 1994; Ramakrishnan & Coss 2000; Hanson & Coss 2001). The young of California ground squirrels, Spermophilus beecheyi, react more strongly to alarm calls given to terrestrial predators than to alarm calls given to aerial predators, which is the opposite to adult behaviour, possibly owing to a high infanticide risk (Hanson & Coss 2001). Similar age-dependent changes in the response to alarm calls are likely to occur in other vertebrate species that experience changing risks during their developmental stages.
The onset of the development of alarm-call reactions in birds is potentially adapted to the different developmental histories of precocial young (those that leave the nest shortly after hatching) and altricial young (those that remain initially in the nest, totally dependent on their parents). The young of precocial and semi-precocial birds respond to alarm calls from the first day post-hatching onwards, by freezing and suppressing their vocalizations (Impekoven 1976a,b; Miller & Blaich 1986). Contrary to the early development in precocial young, studies on species with altricial young indicate a later onset of the alarm-call response. Ten-day-old great tit, Parus major, nestlings do not react to an adult ‘seet’ alarm call, normally given to aerial predators (Ryden 1980), while 16- to 18-day-old nestlings suppress their begging vocalizations after playbacks of the same call (Ryden 1978). By contrast, 5-day-old pied flycatcher, Ficedula hypoleuca, nestlings suppress their vocalizations when hearing alarm calls that are given to ground as well as aerial predators (Khayutin 1985; Alexandrov et al. 2001). These studies suggest that the timing of development of the reaction to aerial and ground alarm calls varies depending on the risk posed by the respective predators. Aerial alarm calls might not be relevant for altricial nestlings, so that the reaction develops later than the reaction towards ground alarm calls, whereas both alarm calls might be important for precocial young, and so the reaction develops before hatching. To our knowledge, no study on birds has so far tested whether the development of the reaction to alarm calls elicited by different types of predators is adapted to the threat they pose.
The white-browed scrubwren, Sericornis frontalis, provides an ideal opportunity to test the adaptiveness of the nestling alarm-call response. Scrubwrens lay their eggs in well-concealed, domed nests on the ground, so that predators on the ground represent a much more imminent danger for nestlings than flying predators. Predation on scrubwren nests is common, and most broods are taken by pied currawongs, Strepera graculina, an omnivorous bird that is well known for predation on passerine nests (Wood 1998). Currawongs hunt by sight and sound, and while on the ground, react to playbacks of scrubwren nestling calls with search behaviour (D. Platzen, unpublished data). When encountering a predator, adult scrubwrens give two types of alarm call: ground alarm (‘buzz’) calls when a predator is on the ground or perched nearby, and aerial alarm (‘trill’) calls when a predator flies overhead (Higgins & Peter 2002). The number of elements in an aerial alarm call communicates the response urgency to other adults: a one-element call leads to increased vigilance, while a four-element call prompts immediate flight (Leavesley & Magrath in press). In an earlier study, we found that scrubwren nestlings react with almost complete silence to parental ground alarm calls when only 5 days old, and show no change in their response up to 11 days old (Platzen & Magrath 2004), indicating that the onset of this behaviour happens early in nestling development. Furthermore, scrubwren nests are usually buried deep inside the leaf litter or inside grass tussocks so that nestlings have a limited view outside the nest. Experience of predator encounters is therefore probably restricted to aural stimuli, which limits the opportunity to learn about predators.
We used a playback experiment to test whether scrubwren nestlings respond specifically to ground but not aerial alarm calls, an adaptive difference to the response found in adults, given their vulnerability specifically to predators on the ground. We also tested whether an accurate urgency response is present during the nestling stage by playing back long and short alarm calls. Furthermore, to ensure that the nestlings responded to the structural differences of the two call types, and not simply to the length of the call playbacks, we matched the length of short ground and long aerial alarm-call playbacks, while keeping the amplitude constant. Our predictions were that: (i) ground alarm calls suppress nestling vocalization, and to a greater extent after hearing a more urgent call; (ii) aerial alarm calls do not suppress vocalization, regardless of the urgency of the call; and (iii) any response to aerial alarm calls would occur only late in the nestling period.
2. Material and methods
(a) Study species
Scrubwren trill calls are aerial alarm calls consisting of high-frequency, narrow-band elements (duration, 60–100 ms; frequency range, 7–10 kHz; peak frequency, 7.5–7.8 kHz; figure 1a). We will refer to them as aerial alarm calls throughout this paper. Buzz calls near the nest are mainly given in the presence of predators on the ground, and we will refer to them as ground alarm calls. Ground alarm-call elements are generally longer than aerial alarm-call elements and have their energy spread over a wide frequency range (duration, 120–180 ms; frequency range, 3–12 kHz; peak frequency, 6.5–8.0 kHz; figure 1b). Calling can last up to several minutes, depending on how long the danger is present, and can attract other group members (Higgins & Peter 2002).
Figure 1.
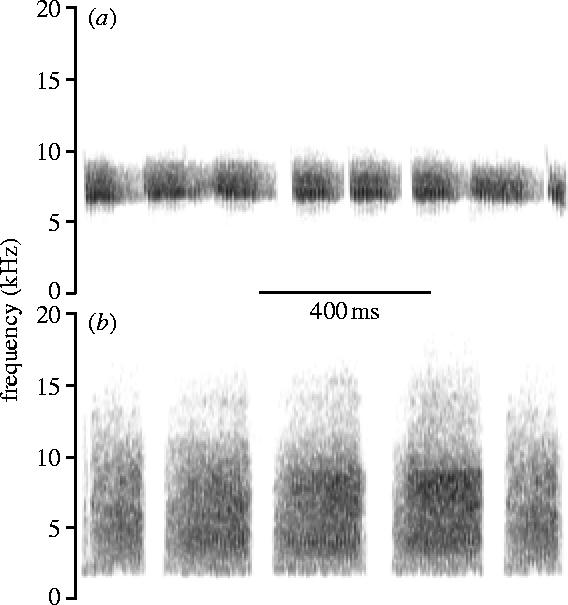
Sonograms of experimental playback sounds: (a) a long aerial alarm call (‘trill’), and (b) a short ground alarm call (‘buzz’). The short aerial alarm call and long ground alarm-call playbacks are not illustrated, but differed only in duration and not element type (§2). Spectrograms were created using a filter bandwidth of 699.4 Hz, frame length of 256 points, and the grid resolution set to 21.53 Hz and 1.45 ms.
In addition to begging when parents arrive with food, nestlings regularly give short ‘peep’ calls in the absence of parents (Maurer et al. 2003; see below). In the laboratory, nestlings peep at a higher rate when hungrier (Maurer et al. 2003), and broods in the wild have silent and vocal periods, with rates varying among nests, presumably reflecting hunger. Calling activity also increases with nestling age (D. Platzen, unpublished data). The average call rate during active periods is 1.05 calls s−1±0.06 s.e.m. (n=98 in this study), but call rates in a brood of three nestlings can be as high as 3 calls s−1 (D. Platzen, unpublished data).
(b) Playback experiment
The experiment was designed to test differences in the reaction of nestlings towards short and long versions of aerial and ground alarm calls. We therefore used four different playback sequences, short and long aerial alarm calls as well as short and long ground alarm calls, for each brood. (i) The short aerial alarm-call playback consisted of a three-element trill call that was 337±66 ms (mean±s.d.) in length. (ii) The long aerial alarm-call playback consisted of eight elements and was 1037±104 ms in length (figure 1a). (iii) To enable a direct comparison of aerial and ground alarm calls, independent of duration and sound output, we designed the short ground alarm-call playbacks to be of the same duration as the long aerial alarm-call playback (mean±s.d.: 1050±119 ms; 5.5±1.1 elements; figure 1b). The number of elements for long aerial and short ground alarm-call playbacks could not be kept constant without changing the natural call rate. (iv) The long ground alarm-call playback consisted of 10 s of ground alarm calls. All alarm-call playbacks had the same average amplitude. To control for our experimental set-up, and to make the four playbacks as similar as possible, we added a recording of background noise at the beginning of each of the first three alarm-call playbacks so that the overall length of all playbacks was 10 s. We took the background noise from the original recordings of the alarm calls and edited and amplified it in the same manner as the alarm calls.
We used calls from each brood's own parents to prepare the playbacks to avoid effects of pseudoreplication (Kroodsma 1998) or parent–offspring recognition (Medvin et al. 1992). To gain aerial alarm calls, we ran a stuffed currawong that was mounted in a flying position down a fishing line above the nest while an adult scrubwren was perching close to the nest. All vocalizations of the focal adult were recorded onto a Sony TCD-D100 DAT recorder at 44.1 kHz sampling frequency, with two Audio Technica ATM15a condenser lapel microphones attached to the vegetation around the nest. In 13 out of 16 trials the adult reacted by giving aerial alarm calls with 5.8±3.0 elements. We recorded ground alarm calls by placing a mounted currawong close to the nest, similar to the method described in Platzen & Magrath (2004). Recordings were digitally transferred onto a Macintosh computer, filtered to remove sound below 2 kHz, and edited for playback using Canary v.1.2.4. (Charif et al. 1995).
The experiments were conducted between October and December 2002 at 13 different nests when nestlings were 5 and 11 days old. Playbacks were performed following the methods used in Platzen & Magrath (2004), but with a Sony CD Walkman D-EJ751 and a Response Dome Tweeter speaker (1.5–20 kHz) to play back the alarm calls. The order of playbacks was randomized between, as well as within nests, by using a Latin square design with permutations in a random order. The experience of playbacks when 5 days old is extremely unlikely to affect the response of 11-day-old young, as both types of alarm call are given frequently near the nest, and an extra 13 s of calls would have little effect on the rate over nearly a week. We recorded the vocalizations of each focal brood with an ATM15a microphone placed 16 cm from the nest entrance, connected by a 15 m cable to a DAT recorder. Recordings started 1 min before the start of the playback and ended 1 min after the end of each playback. Playback amplitudes were kept similar within and between nests: aerial alarm calls (mean±s.d.) 54.7 dB±4.2; ground alarm calls 54.1 dB±3.5 (paired t-test: t=1.6, p=0.12; all amplitudes measured at the nests). These amplitudes lie within the natural range of both calls. The amplitude of background playbacks was 35.8 dB±4.8 following the editing and amplification of alarm calls. Decibel values are 1 pW m−2. Recordings were calibrated in Canary using a reference file of known sound pressure level, measured with a Bruel and Kjaer-type 2205 sound-level meter.
The recordings of the experiments were transferred digitally to Raven v.1.0 (Charif & Alberg 2003). We measured all calls given by the nestlings from 15 s before the 10 s playback started to 15 s after the end of the playback (40 s for all playbacks). Spectrograms were created using a filter bandwidth of 124 Hz, frame length 512 points, and the grid resolution set to 86.1 Hz and 5.8 ms. We counted the number of calls before, during and after our playbacks, and measured the start time for each call. Call rates during the 9 s before the start of each playback that contained background noise were not statistically different from the call rates during the 9 s of background noise playback (mean before playback (mean±s.e.m.): 1.03 calls s−1±0.07; during: 1.04±0.08; paired t-test: n=73, t=−0.2, p=0.84). We therefore concluded that our playback equipment and the background noises of the alarm-call sequences had no influence on the nestling response.
(c) Statistical analysis
We performed experiments at 13 nests, but lost one brood around day 10, resulting in 25 playbacks with four treatments. We could not analyse one short ground alarm and one long ground alarm (both on day 5) because of technical difficulties, and analysed only partly, one long aerial alarm on day 11 and one short ground alarm on day 5, following interference by either the parents or environmental noise. Owing to the unbalanced nature of the data, we used a Linear Mixed Model approach with restricted maximum-likelihood estimation (REML) in Genstat 5 (release 4.2, Genstat-Committee 2000). Our models contained ‘age’ (5 or 11 days), ‘type’ of playback (aerial, ground), ‘duration’ of playback (short, long) and all interactions as fixed effects. As random factors, we included a nest identifier, to control for variance between nests, and a blocking factor nested within nests that encoded for all treatments at a nest (1–8), to control for variance between treatments at each nest. To assess the significance of the fixed effects, we used the sub-model routine in Genstat (Genstat-Committee 2000), and calculated the change in deviance caused by dropping a fixed effect from a full model that contained all significant effects. The resulting change in deviance approximates a χ2 distribution. In none of our models did the residuals deviate significantly from a normal distribution.
We derived three variables from our initial measurements of the nestling calls as response variates. (i) The call change ratio is the number of calls given during the 15 s after each playback, divided by the number of calls given during the 15 s before each playback (a continuous variable with 0=complete suppression and 1=no change). (ii) The duration of suppression is the time from the end of the alarm-call playback until resumption of calling. As these two variables were tightly correlated (log correlation: n=96, r=−0.76, p<0.01), we also analysed (iii) the first factor ‘PC1’ of a principal component analysis of change ratio and suppression as a combined measure of the intensity of nestling response. Higher values of PC1 indicate a stronger response, with a greater duration of suppression and fewer calls. The first factor explained 86.5% of the variance between these two variables with loadings of 0.93 for suppression and −0.93 for change ratio.
3. Results
(a) Discrimination between aerial and ground alarm calls
Nestlings reacted more strongly to ground alarm calls than to aerial alarm calls (response=PC1, effect=type: χ12=35.49, p<0.001; figure 2a). After hearing the ground alarm playbacks they called less (change ratio, type: χ12=34.13, p<0.001; figure 2b) and resumed calling after a longer duration of suppression (suppression, type: χ12=26.36, p<0.001; figure 2c). This response was, over all four playbacks, independent of the age of the nestlings (PC1, type×age: χ12=2.2, p=0.14; change ratio, type×age: χ12<0.01, p=0.96; latency, type×age: χ12=2.03, p=0.15).
Figure 2.
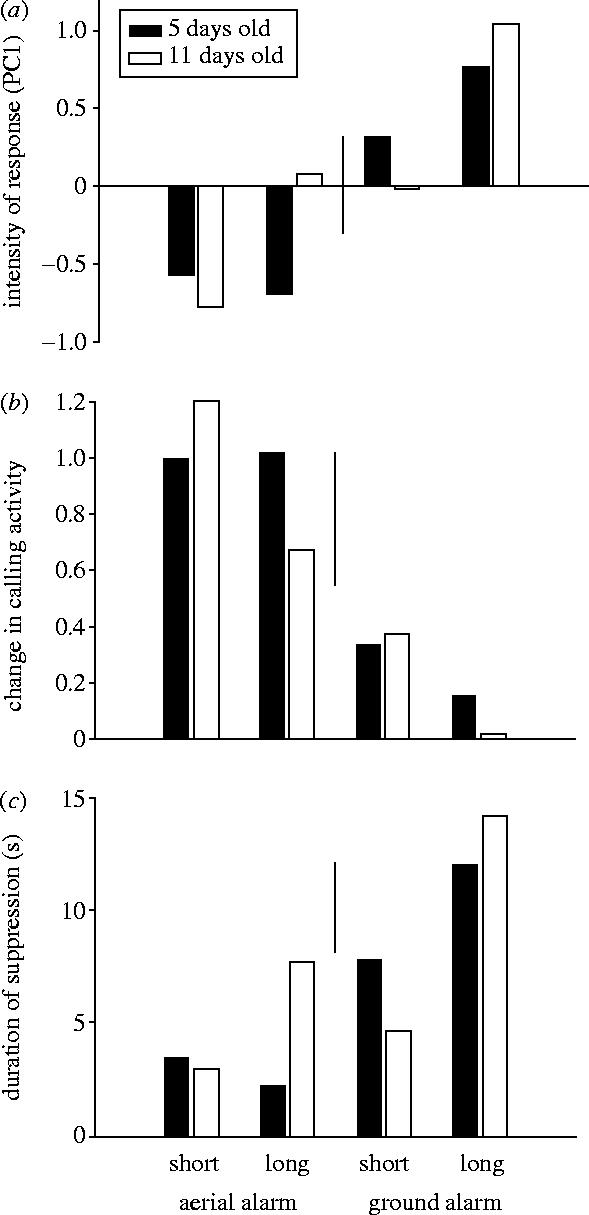
Response of 5- and 11-day-old nestlings to playback of ground (‘buzz’) and aerial (‘trill’) alarm calls. Bars show estimated means of the restricted maximum-likelihood analyses; lines between bars show the least significant difference (two times the standard error of the difference). (a) Intensity of the response shown as the first factor of a principal component analysis of ‘number of calls’ and ‘latency’; more positive values indicate a greater suppression of vocalization. (b) Change in vocal activity shown as the ratio of the number of calls given during the 15 s interval after the experimental playbacks divided by the number of calls given in the 15 s interval before the playbacks. A value of 0 indicates complete suppression of vocalization and a value of 1 indicates no change at all. (c) Duration of suppression after the end of an alarm-call playback; greater values indicate greater suppression of vocalization.
Restricting the analysis to only long aerial and short ground alarm calls, the two playbacks of similar duration showed a difference in response according to nestling age. Five-day-old nestlings did not react to long aerial alarm calls, but responded to short ground alarm calls. By contrast, 11-day-old nestlings showed only a small difference in the response to the two playbacks. Compared with 5-day-old nestlings, they increased their reaction to long aerial alarm calls and reduced their reaction to short ground alarm calls (PC1, type×age: χ12=4.63, p=0.03; figure 2a). The duration of suppression increased in 11-day-old nestlings in response to long aerial alarm calls and decreased less strongly after short ground alarm calls compared with 5-day-old nestlings (suppression, type×age: χ12=7.15, p<0.01; figure 2c). There was no detectable effect on the number of calls (change ratio, type×age: χ12=1.05, p=0.31; figure 2b). Overall, these age differences show that on day 11, nestlings had started to react to aerial alarm calls.
(b) Effect of call length on nestling reaction
Nestlings responded more intensely to playbacks of long alarm calls than playbacks of short alarm calls (PC1, duration: χ12=10.91, p<0.001; figure 2a). They reduced their calling activity significantly more after hearing long alarm calls (change ratio, duration: χ12=4.62, p=0.03; figure 2b) and increased the time during which calling was suppressed (suppression, duration: χ12=14.66, p<0.001; figure 2c). This distinct response to short and long calls was significantly stronger in old nestlings than in young nestlings (PC1, duration×age: χ12=6.63, p=0.01; figure 2a) and old nestlings responded with longer suppression times compared with young nestlings after hearing long alarm calls (suppression, duration×age: χ12=14.66, p<0.001; figure 2c). The different reaction of young and old nestlings appeared only as a non-significant trend in the data for the number of calls (change ratio, duration×age: χ12=2.53, p=0.11; figure 2b). The change in sensitivity towards longer alarm calls in 11-day-old nestlings appeared to be mainly influenced by an intensified reaction towards long aerial alarm calls that did not appear in 5-day-old nestlings. The reaction towards ground alarm calls of different duration was similar in young and old nestlings, but with a more pronounced distinction between short and long calls in old nestlings.
4. Discussion
Scrubwren nestlings showed a much more intense response to ground than to aerial alarm calls throughout the nestling period. Playbacks of ground alarm calls suppressed the vocalization of 5- and 11-day-old nestlings, whereas there was no response to aerial alarm-call playbacks in 5-day-old nestlings and only partial suppression of calls in 11-day-old nestlings. In contrast to nestlings, adults respond to multi-element aerial alarm calls with immediate flight (Leavesley & Magrath in press). As predators on the ground pose a far greater threat to the nestlings than aerial predators, while the opposite is likely to be true for adults, our data suggest that the alarm-call response of scrubwren nestlings is adapted to their developmental stage, rather than being an imperfect version of the adult reaction to alarm calls. The nestlings furthermore developed an urgency response towards ground alarm calls by suppressing their vocalization for longer, and calling less after hearing long alarm calls compared with short alarm calls. These findings show that the ability to respond to alarm calls carrying information about the type of threat and the urgency of the response can develop early in life.
The differentiation between parental alarm calls signalling different types of threat might be a common adaptation in young birds. Similar to our first study (Platzen & Magrath 2004), nestlings showed a consistent, strong response to the 10 s ground alarm-call playback that was fully developed in 5-day-old nestlings. In a natural situation, ground alarm calls are given for as long as a predator is near the nest, which can be up to several minutes (D. Platzen, unpublished data). The suppression of nestling vocalization in the presence of a searching predator is thus likely to be very efficient.
In contrast to the response to the ground alarm call, a response to the aerial alarm playbacks was only apparent in 11-day-old nestlings, and even then, only when the playback was representative of a very urgent alarm signal. The threat that flying predators pose to scrubwren nestlings does not change over the course of the nestling stage, so that the observed response to aerial alarm calls in older nestlings probably foreshadows the change in risk after fledging. We predict that the response to aerial alarm calls increases over the last days before fledging, and that fledglings respond more strongly to aerial alarm calls than to ground alarm calls, as the threat from flying predators is stronger for fledglings than for nestlings. This prediction is consistent with the findings of the limited number of studies on other bird species. Pied flycatchers start to respond to parental alarm calls that are given to predators that are a threat to the nest when only 5 days old, well before fledging (Khayutin 1985; Alexandrov et al. 2001). By contrast, great tit nestlings only develop a response to aerial alarm calls late in the nestling period (Ryden 1978, 1980). Further experimental work on altricial species with differing nesting ecology and warning-call systems is needed to test this hypothesis.
The differential response to aerial and ground alarm calls in scrubwren nestlings was not caused by an inability to hear high frequencies, despite the fact that in some species, young nestlings may be able to hear only lower frequencies (e.g. pied flycatcher; Khayutin 1985). In general, a species' hearing sensitivity is correlated with the highest frequencies used in the vocal repertoire (Dooling 1982), and the hearing ranges of young in chickens, Gallus domesticus, and mallard ducks, Anas platyrhynchos, match the frequencies of parental food and contact calls (Saunders et al. 1974). A commonly used call during parental feeding visits in scrubwrens is the ‘chip’ call that has its main energy in a frequency range similar to the aerial alarm calls (7–9 kHz; Higgins & Peter 2002), so we examined the response of 5-day-old nestlings to these calls. During 38 trials from 2001 to 2003, we played back chips with a mean peak frequency of 8.2 kHz±1.0 s.d. to scrubwren nestlings on day 5, often following alarm-call playbacks (Platzen & Magrath 2004). We found that peep-call rates increased during the chip playbacks compared with the 15 s beforehand, indicating that the nestlings responded to the playbacks by resuming normal call rates (mean before: 0.52 calls s−1±0.09 s.e.m, after: 1.04±0.18 s.e.m.; paired t-test: t=−3.4, p=0.002). It seems unlikely that another parental call of similar frequency cannot be heard. Thus, behavioural distinction rather than developmental constraints appears to have led to the different responses to aerial and ground alarm calls in scrubwren nestlings.
Nestlings responded to alarm calls of different duration with different levels of vocal inhibition. This response was independent of the context of the calls because other parental behaviour or cues from a predator were not present. The greater response to longer calls seems adaptive if longer calls signal a predator remaining near the nest for longer. We have not yet tested whether ground alarm calls encode information about the distance of a predator.
The idea that the response to alarm calls in infants and juveniles is the subject of developmental adaptation has been formulated for mammals (Owings & Loughry 1985; Miller & Hicinbothom 1991; Hersek & Owings 1994; Hanson & Coss 2001) and precocial birds (Miller & Hicinbothom 1991), and seems likely to be widely applicable. The difference in the reaction to aerial and ground alarm calls in scrubwren nestlings and adults shows intriguing similarities to the alarm-call response and production of juvenile and adult California ground squirrels (Hanson & Coss 1997, 2001). These parallels indicate that predation risk leads not only to similar signalling systems across a wide range of vertebrates (Macedonia & Evans 1993; Evans 1997), but also to similar developmental histories. Social learning appears to be important for mammals to develop a functional alarm-call response (Hauser 1988; Mateo 1996a; Ramakrishnan & Coss 2000; McCowan et al. 2001). In scrubwrens it is more likely that internal developmental processes, combined with the exposure to acoustic environmental stimuli, are sufficient to develop a functional response. Visual cues from the environment are extremely restricted during the nestling stage, and the response to ground alarm calls is fully developed when nestlings are only 5 days old. That learning is not necessary for the development of an alarm-call response has been shown for precocial bird species (Impekoven 1976b; Miller & Blaich 1986). Similarly, two species of altricial nestlings responded to their own species' alarm calls even if raised by another species, although their response was modified slightly by learning (Davies et al. 2004). A comparative approach to developmental processes in avian and mammalian alarm-call systems seems promising to further our understanding of the evolutionary processes shaping alarm-call behaviour.
Acknowledgments
We thank Stuart Rae and Adam Leavesley for mounting the flying currawong. Alan Muir and Bob Phillips helped with technical problems and Judith Scarl, Geoff Kay and Cathrine Martin helped in the field. Comments by Elsie Krebs, Chris Evans, Junko Kondo, Adam Leavesley and three anonymous referees greatly improved the manuscript. This study was financed by an Australian Research Council grant to R.D.M. The research was conducted under permits from the Australian Bird and Bat Banding Scheme, Australian National Botanic Gardens, Environment ACT and the Australian National University Ethics Committee.
References
- Alexandrov L.I, Korneeva E.V, Golubeva T.B. Increasing selectivity of defense behavior in the ontogeny of pied flycatcher nestlings. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova. 2001;51:110–113. [PubMed] [Google Scholar]
- Charif R.A, Alberg W. Cornell Laboratory of Ornitholgy; Ithaca, NY: 2003. Raven 1.0 user's manual. [Google Scholar]
- Charif R.A, Mitchell S, Clark C.W. Cornell Laboratory of Ornithology; Ithaca, NY: 1995. Canary 1.2 user's manual. [Google Scholar]
- Curio E. Proximate and developmental aspects of antipredator behavior. Adv. Study Behav. 1993;22:135–238. [Google Scholar]
- Davies N.B, Madden J.R, Butchart S.H.M. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. B. 2004;271:2297–2304. doi: 10.1098/rspb.2004.2835. doi:10.1098/rspb.2004.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling R.J. Auditory perception in birds. In: Kroodsma D.E, Miller E.H, editors. Acoustic communication in birds. vol. 1. Academic Press; New York: 1982. pp. 95–130. [Google Scholar]
- Evans C.S. Referential signals. Perspect. Ethol. 1997;12:99–143. [Google Scholar]
- Fichtel C, Kappeler P.M. Anti-predator behavior of group-living Malagasy primates: mixed evidence for a referential alarm call system. Behav. Ecol. Sociobiol. 2002;51:262–275. [Google Scholar]
- Genstat-Committee. Clarendon Press; Oxford: 2000. Genstat 5 release 4.2 reference manual. [Google Scholar]
- Hanson M.T, Coss R.G. Age differences in the response of California ground squirrels (Spermophilus beecheyi) to avian and mammalian predators. J. Comp. Psychol. 1997;111:174–184. doi: 10.1037/0735-7036.111.2.174. [DOI] [PubMed] [Google Scholar]
- Hanson M.T, Coss R.G. Age differences in the response of California ground squirrels (Spermophilus beecheyi) to conspecific alarm calls. Ethology. 2001;107:259–275. [Google Scholar]
- Hauser M.D. How infant vervet monkeys learn to recognize starling alarm calls—the role of experience. Behaviour. 1988;105:187–201. [Google Scholar]
- Hersek M.J, Owings D.H. Tail flagging by young California ground-squirrels, Spermophilus beecheyi—age specific participation in a tonic communicative system. Anim. Behav. 1994;48:803–811. [Google Scholar]
- Herzog M, Hopf S. Behavioral responses to species-specific warning calls in infant squirrel monkeys reared in social isolation. Am. J. Primatol. 1984;7:99–106. doi: 10.1002/ajp.1350070204. [DOI] [PubMed] [Google Scholar]
- Higgins P.J, Peter J.M. Oxford University Press; Melbourne: 2002. Handbook of Australian, New Zealand & Antarctic birds. [Google Scholar]
- Impekoven M. Prenatal parent–young interactions in birds and their long term effects. Adv. Study Behav. 1976;7:201–253. [Google Scholar]
- Impekoven M. Responses of laughing gull chicks (Larus atricilla) to parental attraction calls and alarm calls, and effects of prenatal auditory experience on responsiveness to such calls. Behaviour. 1976;56:250–278. [Google Scholar]
- Khayutin S.N. Sensory factors in the behavioral ontogeny of altricial birds. Adv. Study Behav. 1985;15:105–152. [Google Scholar]
- Kroodsma D.E. Suggested experimental designs for song playbacks. Anim. Behav. 1998;37:600–609. [Google Scholar]
- Leavesley, A. & Magrath, R. D. In press Communication about danger: urgency alarm calling in a bird. Anim. Behav.
- McCowan B, Franceschini N.V, Vicino G.A. Age differences and developmental trends in alarm peep responses by squirrel monkeys (Saimiri sciureus) Am. J. Primatol. 2001;53:19–31. doi: 10.1002/1098-2345(200101)53:1<19::AID-AJP2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Macedonia J.M, Evans C.S. Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology. 1993;93:177–197. [Google Scholar]
- Mateo J.M. The development of alarm call response behaviour in free living juvenile Belding's ground squirrels. Anim. Behav. 1996;52:489–505. [Google Scholar]
- Mateo J.M. Early auditory experience and the ontogeny of alarm call discrimination in Belding's ground squirrels (Spermophilus beldingi) J. Comp. Psychol. 1996;110:115–124. doi: 10.1037/0735-7036.110.2.115. [DOI] [PubMed] [Google Scholar]
- Maurer G, Magrath R.D, Leonard M.L, Horn A.G, Donnelly C. Begging to differ: scrubwren nestlings beg to alarm calls and vocalize when parents are absent. Anim. Behav. 2003;65:1045–1055. [Google Scholar]
- Medvin M.B, Stoddard P.K, Beecher M.D. Signals for parent offspring recognition—strong sib–sib call similarity in cliff swallows but not barn swallows. Ethology. 1992;90:17–28. [Google Scholar]
- Miller D.B, Blaich C.F. Alarm call responsivity of mallard ducklings 3. Acoustic features affecting behavioral inhibition. Dev. Psychobiol. 1986;19:291–301. doi: 10.1002/dev.420190402. [DOI] [PubMed] [Google Scholar]
- Miller D.B, Hicinbothom G. Alarm call responsivity of mallard ducklings 10. Ontogenic adaptation or artifact of arousal. Bird Behav. 1991;9:114–120. [Google Scholar]
- Owings D.H, Loughry W.J. Variation in snake-elicited jump-yapping by black-tailed prairie dogs—ontogeny and snake specificity. Z. Tierpsychol. 1985;70:177–200. [Google Scholar]
- Platzen D, Magrath R.D. Parental alarm calls suppress nestling vocalization. Proc. R. Soc. B. 2004;271:1271–1276. doi: 10.1098/rspb.2004.2716. doi:10.1098/rspb.2004.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan U, Coss R.G. Age differences in the responses to adult and juvenile alarm calls by bonnet macaques (Macaca radiata) Ethology. 2000;106:131–144. [Google Scholar]
- Ryden O. Differential responsiveness of great tit nestlings, Parus major, to natural auditory stimuli—response strength as related to stimulus significance and previous individual exposure. Z. Tierpsychol. 1978;47:236–253. [Google Scholar]
- Ryden O. Heart rate response in great tit nestlings (Parus major) to an alarm call. J. Comp. Physiol. Psychol. 1980;94:426–435. doi: 10.1037/h0077680. [DOI] [PubMed] [Google Scholar]
- Saunders J.C, Gates G.R, Coles R.B. Brain stem evoked responses as an index of hearing thresholds in one day old chicks and ducklings. J. Comp. Physiol. Psychol. 1974;86:426–431. doi: 10.1037/h0036132. [DOI] [PubMed] [Google Scholar]
- Seyfarth R.M, Cheney D.L. The ontogeny of vervet monkey alarm calling behavior—a preliminary report. Z. Tierpsychol. 1980;54:37–56. [Google Scholar]
- Wood K.A. Seasonal changes in diet of pied currawongs Strepera graculina at Wollongong, New South Wales. Emu. 1998;98:157–170. [Google Scholar]


