Abstract
It has been proposed that multiple sperm storage organs (spermathecae) could allow polyandrous females to control paternity. There is little conclusive evidence for this since insemination of individual spermathecae is generally not experimentally manipulable. Here, we examined sperm use patterns in the Australian redback spider (Latrodectus hasselti), which has paired, independent spermathecae. We assessed paternity when two rivals were forced to inseminate a single storage organ or opposite storage organs. When males inseminated a single spermatheca, mean paternity of the female's first mate was 79.8% (median 89.4%), and 38% of first mates achieved 100% paternity. In contrast, when males inseminated opposite organs, the mean paternity of the first mate was 49.3% (median 49.9%), only 10% of males achieved complete precedence, and paternity was normally distributed, suggesting sperm mixing. Males responded to this difference by avoiding previously inseminated female reproductive tracts. Complete sperm precedence can only be achieved if females permit males to copulate with both reproductive tracts. Females often cannibalize smaller males during their first copulation, thus limiting their paternity to 50%. These data show that multiple sperm storage organs can increase female control of paternity.
Keywords: paternity, sperm precedence, insemination pattern, cryptic female choice, multiple sperm storage organs, redback spiders
1. Introduction
Sperm competition (Parker 1970), the competition between the sperm of multiple males for fertilization of a limited number of ova, explains the evolution of male reproductive strategies and morphologies across taxa (Birkhead & Moller 1998; Simmons 2002). More controversial is whether selection on females to control fertilization success of rival males (‘post-copulatory choice’) has significant effects on female or male traits (Kokko et al. 2002, 2003; Cameron et al. 2003). Selection on males for success in sperm competition may be more intense than selection on females to control paternity because variance in male fitness as a function of fertilization success is typically much higher than variance in female fitness as a function of genetic traits of their mates (e.g. Andersson 1994)—the only possible source of benefit for post-copulatory choice. Thus, female post-copulatory choice could have a relatively small effect on male reproductive success and impose only weak selection on male traits (e.g. Kirkpatrick 1996; Cameron et al. 2003). Evidence of female mechanisms to control paternity would contradict this view (e.g. Eberhard 1996), but conclusive tests of female control are difficult because it is often impossible to disentangle male- from female-based effects (e.g. Birkhead 1998; Simmons 2002).
In some arthropods, sperm are stored for long periods in specialized storage organs (spermathecae). Characteristics of the spermathecae may influence sperm competition and paternity, thus providing some female control (Eberhard 1996; Simmons 2002). Here, we test whether multiple sperm storage organs could allow polyandrous females to control paternity (e.g. Eberhard 1985, 1996; Stockley 1997; Hellriegel & Ward 1998). Multiple spermathecae could allow female control if (i) female-controlled patterns of insemination across the organs result in predictable variation in paternity, or (ii) sperm of competing males are held in separate organs and used selectively at fertilization (Eberhard 1985; Uhl 2002).
Testing whether multiple organs increase female control of paternity is difficult because insemination pattern across organs is often not experimentally manipulable (e.g. Hellriegel & Bernasconi 2000). We examine paternity as a function of insemination pattern in the Australian redback spider (Latrodectus hasselti)—a species with paired, independent spermathecae, each of which is inseminated by one of the male's paired copulatory organs (palps) following a separate courtship. These features allowed us to compare sperm use patterns after we forced rival males to inseminate the same or opposite spermathecae. Redback females sometimes prevent males from achieving two copulations by premature cannibalism or aggression (Andrade 1996, 1998) and then mate with a rival (Andrade 2000). Our treatments allowed us to determine whether this behaviour significantly affects paternity. We also tested for male phenotypic correlates of paternity when sperm were in direct competition (same-spermatheca) or not (opposite-spermathecae). If females selectively use the sperm of preferred rivals when ejaculates are separated, we predicted a bimodal distribution of paternity in the opposite-spermathecae treatment, whereas we expected a normal distribution if sperm use from each spermatheca was equal.
While there are a growing number of studies of paternity in spiders (e.g. Elgar 1998; Elgar et al. 2000; Schäfer & Uhl 2002; Uhl 2002), few specify insemination pattern across spermathecae by rival males, and, to our knowledge, none has manipulated insemination pattern. Thus, this examination of the effect of the paired spermathecae of spiders provides a unique examination of the potential effect of female reproductive morphology on paternity.
2. Material and Methods
Spiders were from an outbred laboratory population of L. hasselti (parents from Perth, Western Australia: 1999, 2001; New South Wales, Australia: 2002). Spiders were reared on a 12 : 12 light : dark cycle and held separately to ensure virginity (see Snow & Andrade 2004). Redbacks are nocturnal, so all mating trials were conducted in a dark cycle under red lights.
(a) Treatments
Virgin females were mated to two virgin males, each of which was allowed one palp insertion. Females were randomly placed in a same-side treatment (rival males insert in the same spermatheca) or an opposite-side treatment (insertions in opposite spermathecae). All males were manipulated to ensure the assigned insemination pattern by removing the embolus (apical portion of the palp; see Bhatnagar & Rempel 1962) from the palp that was not to be used. Males were anaesthetized with CO2 and both had the left or right embolus removed (same-side treatment); or one male had the left and one male the right embolus removed (opposite-side treatment). Males with manipulated palps performed all normal courtship behaviours in a typical sequence (see Forster 1995).
Females had 48 h to build webs in separate arenas (see Andrade & Banta 2002). A trial began when the first male was introduced to a female's web. This male was removed after one copulation; the second male was introduced the following day. A double-mating trial ended after the second male completed one copulation. We noted courtship and copulation durations for both males.
Redback males facilitate sexual cannibalism when they shift their abdomen above the female's fangs during copulation (Forster 1992). Cannibalism may affect sperm transfer and fertilization success (Andrade 1996; Snow & Andrade 2004), so we classified cannibalistic damage to mated males as (i) minimal cannibalism (no/slight abdominal surface damage, males typically survive) or (ii) substantial cannibalism (abdomen punctured, contents partially or completely consumed).
For all spiders, we measured mass ±0.1 mg (Ohaus Explorer balance), age (days since final moult) and size (mean patella–tibia length of front legs, Nikon Simple PCI measurement software). Condition was scored as residuals from a regression of mass on average leg length (Jakob et al. 1996), which correlates with survivorship under starvation in redbacks (Andrade 2000).
(b) Paternity
We assessed paternity using the sterile male technique, where doubly mated females copulate with one normal (N) and one irradiated (R) male. Eggs fertilized by R males do not develop as a result of deleterious mutations carried by the sperm, whereas eggs fertilized by N males develop normally (Boorman & Parker 1976). We exposed R males to 9 krad of gamma irradiation at 0.82 krad min−1 (from a Cs 137 source). Presentation of N and R males was randomized and reciprocated within treatments to control for potential effects of irradiation on sperm.
For each female, one male was randomly assigned to each sperm treatment (N or R). Doubly mated females were fed twice a week until four egg sacs were produced or the female died. Approximately 15 days after deposition, the translucent eggs were counted and classified as developed (spiderling hatching imminent) or undeveloped. Forty-five females were mated in the opposite-side treatment (22 NR; 23 RN), 40 of which produced offspring (20 NR, 20 RN); 33 females were mated in the same-side treatment (16 NR, 17 RN), and 32 of these produced offspring (16 NR, 16 RN).
To estimate paternity of N males in experimental matings (RN or NR), we controlled for the proportion of eggs that do not develop after matings with pairs of normal males (12 NN, normal control) and the proportion that do develop after matings with pairs of irradiated males (8 RR, sterility control; see Boorman & Parker 1976 for calculation). Control males had one insertion each with unmanipulated palps.
(c) Statistical analyses
Variables were tested for normality (p>0.05, Lilliefors (Kolmogorov–Smirnov) test) and transformed where necessary. Non-parametric procedures were used if transformed data were non-normal. Summary statistics are back-transformed means with 95% CI for transformed P2 data. For multiple t-tests, results were sequential bonferroni-corrected (pB). We used Systat 10.2 (SYSTAT Software Inc. 2002) and report mean±s.d.
Sample sizes vary for some tests because observations were occasionally missed. Analyses of changes in paternity across multiple egg sacs include only females that produced at least four egg sacs (controls: 6 NN, 4 RR; NR and RN combined: 20 same-side, 20 opposite-side). Other analyses use average P2 across all sacs produced.
3. Results
Irradiation sterilized males, but did not affect male behaviour or female fecundity (table 1 in the Electronic Appendix). Spiderling development did not change over the first four sacs in the NN treatment (Friedman test statistic=4.429, p=0.110, n=6) and the number of eggs in the first four egg sacs in NN and RR treatments did not decrease over time (repeated measures ANOVA, within subjects F3,27=1.757, p=0.179). Thus, declining offspring viability or fecundity over successive sacs did not confound P2 estimates.
A two-factor ANOVA (first factor: insemination pattern (same-side/opposite-side); second factor: mating order (NR/RN)) showed a significant effect of insemination pattern on paternity (F1,68=8.913, p=0.004), but no effect of mating order (p=0.109) and no interaction (p=0.643). Most matings in the same-side treatment showed first male sperm precedence (median P2=10.6%, 95% CI: 8.12–38.6%) with P2 values falling under 20% in 65.6% (21/32) of trials (figure 1). In contrast, most opposite-side matings showed mixed paternity (mean P2=50.7±27.8%) with P2 values falling between 20 and 80% in 67.5% (27/40) of trials (figure 1).
Figure 1.
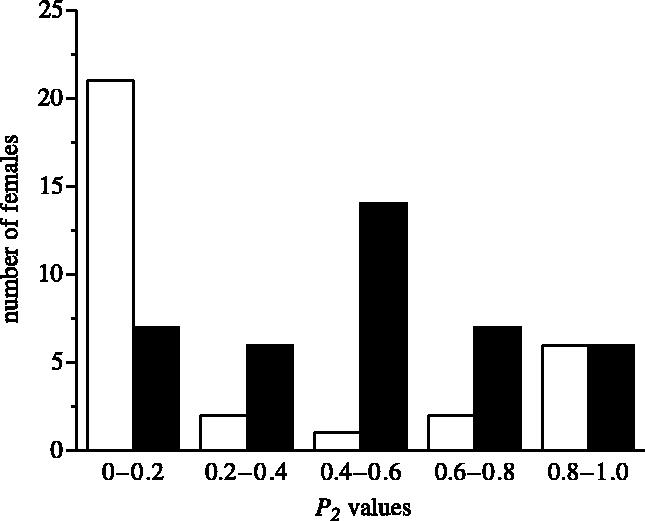
Frequency distribution of untransformed average P2 values grouped into five classes for the same-side treatment (open bars, n=32) and opposite-side treatment (filled bars, n=40).
Paternity did not change across the first four egg sacs (same-side: Friedman test statistic=0.260, p=0.967, n=20; opposite-side: repeated measures ANOVA, F3,57=0.326, p=0.806, n=20). This suggests no sperm stratification in the same-side treatment (Siva-Jothy & Tsubaki 1989), and no change in relative sperm use from the spermathecae in the opposite-side treatment. We analysed possible correlates of paternity separately within each treatment.
(a) Same-side treatment
The distribution of paternity was significantly non-normal in the same-side treatment (Lilliefors p<0.001, n=32). Although most matings resulted in first male precedence (P2=0–0.2), 6/32 (18.8%) matings showed last male precedence (P2=0.80–1.0), but there were few intermediate values (figure 1). P2 increased as the difference between the copulation duration of the second and first male increased (rs=+0.553, n=31, pB<0.022). This relationship may be driven by the extremes of the distribution. The second male copulated longer in five of the six cases of second male precedence (83%), whereas the second male copulated longer in only 6/21 (29%) cases of first male precedence (Fisher's exact test, p=0.027). There was no relationship between P2 and the relative value (second male–first male) of other male traits we measured (p>0.10: size rs=−0.249, n=29; condition rs=0.132, n=24; age rs=−0.236, n=32; courtship duration rs=−0.106, n=32).
(b) Opposite-side treatment
Paternity followed a normal distribution (Lilliefors p=0.540, n=40) in the opposite-side treatment, with few cases of first or last male precedence (figure 1). Variation in P2 was not explained by relative copulation duration (R2=0.025, p=0.634, n=39), size (R2=0.060, p=0.311, n=37), condition (R2=0.021, p=0.554, n=59), age (R2=0.036, p=0.503, n=40) or courtship duration (R2=0.010, p=0.837, n=40).
(c) Cannibalism
There was no difference in the frequency of cannibalism on first (36/71) compared with second males (35/71) overall, and the pattern of insemination did not affect cannibalism rate (second male cannibalized: same-side 20/38, opposite-side 17/35, , p=0.729). However, females copulated longer with males they cannibalized in same-side (pooled t74=−2.874, pB=0.035) and opposite-side treatments (pooled t69=−2.993, pB=0.032; table 2 in the Electronic Appendix).
Cannibalism of the second male was not significantly associated with higher P2 as a function of cannibalism of the first male in either treatment (figure 2; general linear model (GLM): same-side, cannibalism of first male, F1,24=0.022, p=0.883; cannibalism of second male, F1,24=0.588, p=0.451; interaction: F1,24=0.709, p=0.408; opposite-side, cannibalism of first male, F1,34=0.277, p=0.602; cannibalism of second male, F1,34=0.138, p=0.712, interaction: F1,34=0.086, p=0.771). The power of these tests was low because of small sample sizes and relatively high variance (power: same-side=0.15; opposite-side=0.10; effect sizes calculated using within-group means and variances; Faul & Erdfelder 1992).
Figure 2.
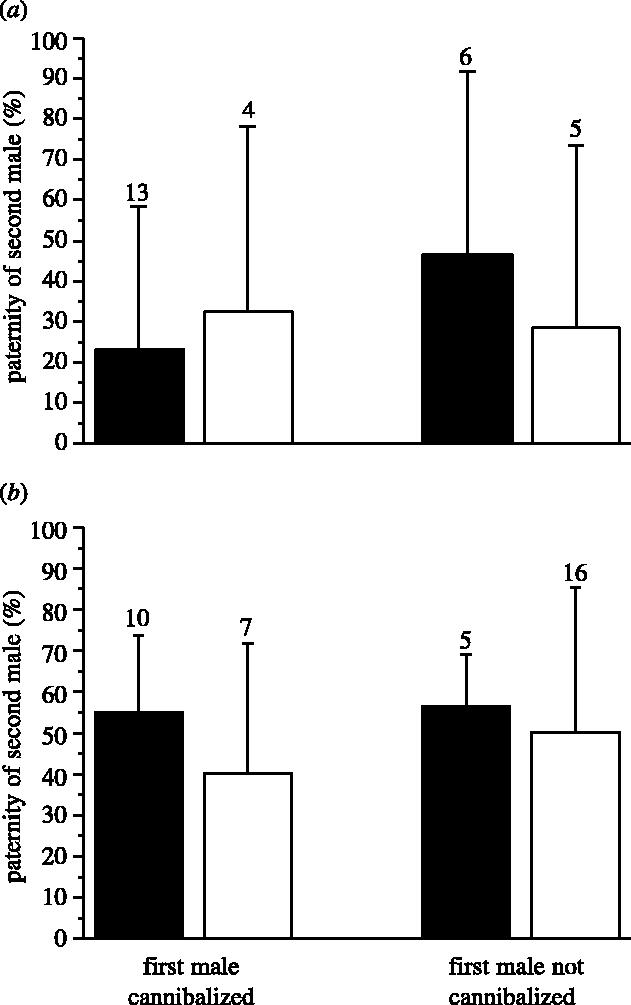
Comparison of paternity of second males that are cannibalized (filled bars) or not cannibalized (open bars) as a function of whether or not the female cannibalized her first mate. (a) Rival males inserted in the same spermatheca or (b) opposite spermathecae. Error bars are standard deviation; numbers above bars are sample sizes.
We found no difference in the condition of females that were cannibalistic in at least one mating (−3.59±68.85) and their non-cannibalistic counter-parts (2.93±80.85, t59=0.320, p=0.750). Cannibalized and non-cannibalized males did not differ in courtship duration (first males: t74=−0.913, p=0.364; second males: t71=0.902, p=0.37), age (first males: t74=−1.741, p=0.086; second males: t71=−0.884, p=0.379) or condition (first males: t71=1.719, p=0.090; second males: t65=−0.196, p=0.846, table 2 in the Electronic Appendix). However, at the first mating, cannibalized males were significantly smaller than males that were not cannibalized (pooled t73=2.729, pB=0.032), whereas at the second mating, cannibalized males tended to be larger than males that were not cannibalized (pooled t68=2.517, pB=0.056, table 2 in the Electronic Appendix). Although 68% (48/71) of females exhibited the same cannibalistic behaviour at both matings (; p=0.003), within females there was a tendency to cannibalize relatively larger males at the second rather than the first copulation. Cannibalized second males were larger than the female's first mate (mean size difference: +0.234±0.405 mm), whereas non-cannibalized second males were smaller (mean size difference: −0.056±0.306 mm; pooled t65=−3.323, pB=0.009).
(d) Male copulatory behaviour
Ninety-five percent (19/20) of unmanipulated second males (NN and RR controls) inserted in the tract opposite to the one inseminated by the first male (χ2=15.211, p<0.000 1). In the same-side treatment, second males often attempted to insert manipulated palps rather than using an intact palp in the same tract as their rival. Thus, 75.2% (100/133) of second males in the same-side treatment did not copulate within the 8 h mating trial, compared with only 38.4% (28/73) of second males in the opposite-side treatment (Fisher's exact test, p<0.0001). Manipulated males that mated successfully did so in typical courtship durations (control matings versus first male's courtship, GLM F1,97=0.00, p=0.985; second male's courtship, GLM F1,97=1.35, p=0.248).
4. Discussion
Male reproductive success is significantly influenced by how sperm are stored across female storage organs in the Australian redback spider (L. hasselti). First male sperm precedence (P2<20%) was the predominant pattern when two males inseminated the same reproductive tract, whereas mixed paternity (20%<P2<80%) was most common when opposite tracts were inseminated. While males were permitted only single insertions in this experiment, this result is likely to predict paternity across a variety of insemination patterns. If a male inseminates only one spermatheca, he will share paternity if the female remates; whereas the first male to inseminate a given spermatheca will have a high expectation of sperm precedence in that organ, or complete precedence if he copulates twice (figure 1). Clearly, separated sperm storage allows females to effectively influence paternity by limiting the number of copulations achieved by a given male. For example, if a female permits her first mate only one copulation, she creates the potential for a rival male to inseminate the empty organ and sire at least 50% of her offspring (figure 1). Male mating behaviour is apparently tuned to the fertilization payoffs of these insemination patterns. Males preferentially attempt to insert in a virgin tract (even if their corresponding copulatory organ is damaged).
Although males choose which spermatheca they will try to inseminate, females determine whether copulation will occur. Redback females are much larger than males (Andrade 1996) and can prevent copulation at little cost. In the field, some males are killed by females immediately after their first mating (12.5%; Andrade 1998). Our results show the timing of female cannibalistic behaviour can differentially affect fertilization success. Females were more likely to kill smaller rather than larger males after their first copulation. During the second copulation, however, female behaviour switched, and cannibalism of males that were larger relative to the first mate was likely. In nature, a female's second copulation is usually with the same male that achieved the first insemination (Andrade 1998). Thus, this pattern suggests female behaviour restricts the opportunity for smaller males to copulate twice, but permits two copulations from larger males. Behaviourally limiting male insertion number is a mechanism by which females influence paternity, and this mechanism critically depends on paired spermathecae.
In addition to subverting sperm precedence, multiple spermathecae could allow direct selection of sire via sperm selection (Siva-Jothy & Hooper 1996; Ontronen 1997; Hellriegel & Bernasconi 2000; Ward 2000) if there is differential release from the spermathecae at fertilization (e.g. Uhl 2002). In this study, despite significant variation in paternity (0–100%) when sperm were in separate spermathecae, none of the male traits we measured predicted fertilization success. If females select sperm, first and last male precedence should occur often and at similar frequencies in the opposite-side treatment (favoured males having been randomly assigned to the first or second mating). We found no evidence for this (figure 1). Instead, sperm mixing probably determines paternity when rival ejaculates are in opposite spermathecae in redback spiders. Although the sperm are stored separately, they are presumably released simultaneously at fertilization to a common duct (e.g. Foelix 1996). Paternity could reflect the outcome of a numerical raffle where the relative number of sperm inseminated by two males predicts success (Parker et al. 1990). The lack of an effect of copulation duration is not inconsistent with this mechanism as male redbacks transfer most of their sperm within 5 min (Snow & Andrade 2004). Variation in paternity (figure 1) may be explained by the wide variation in the number of sperm ejaculated by virgin males (Snow & Andrade 2004).
While the opposite-side treatment was useful to test for sperm selection, the outcome of competition when males inseminate the same spermatheca tells us more about the dynamics of sperm competition in nature, where many males achieve two copulations (Andrade 1998). The high frequency of first male sperm precedence in redbacks (and perhaps other Latrodectus spiders) is caused by the deposition of a sclerite sperm plug (hardened tip of the embolus; Snow 2003). The plug is deposited at the entrance to a female's spermatheca at copulation (Bhatnagar & Rempel 1962; Kaston 1970; Berendonck & Greven 2002; Snow 2003). Relative copulation duration predicted paternity only when both males inseminated one spermatheca, perhaps because longer copulations facilitated successful plug deposition by first males and/or longer copulations by second males facilitated circumvention of an existing plug. This would predict occasional plug failures leading to second male precedence and that this would be most common when second males have relatively longer copulations, as we found here.
We limited females to single insertions from two males, but it is likely the plug-mediated precedence effect would be similar regardless of the number of mates or time since first mating. First, plugs are long-lived, having been found in spermathecae of alcohol-preserved females (M. C. B. Andrade, personal observation). Second, dissections of multiply mated females show plugs lodged in the entrance to the spermatheca with others in the insemination tubules (Snow 2003). Dissections of field-caught females suggest that many mate with a maximum of two males (Andrade 1996), and so experience conditions similar to our trials. Third, there was no change in P2 over four egg sacs. This comprises a significant proportion of female reproductive lifespan in nature, where a mean of six sacs are produced (Andrade & Banta 2002), and shows that sperm mixing does not change over time in the opposite-side treatment.
Although premature cannibalism has a negative effect on total paternity, partial cannibalism during each copulation may yield paternity benefits within each tract (e.g. Andrade 1996). We found no direct evidence for such an effect in either treatment, but low sample sizes and large standard error precluded meaningful tests. There are several indirect indications that cannibalism affects paternity. There is a robust association between cannibalism and prolonged copulation (§3; Andrade 1996; Snow & Andrade 2004), and relative copulation duration can predict paternity (§3; Andrade 1996). Further work is necessary to determine whether cannibalism-mediated increases in copulation duration directly translate into higher paternity. However, cannibalism is also associated with increased sperm transfer (Snow & Andrade 2004), decreased female receptivity (Andrade 1996) and female cannibalistic behaviour (with timing favouring relatively larger males (§3)) is an important determinant of male success. Thus our data suggest sequential, cannibalism-mediated female choice (see Elgar et al. 2000).
While first male precedence is mediated by female behaviour or physiology in some systems, suggesting benefits of this paternity pattern for females (e.g. Eberhard & Huber 1998; Knoflach 1998), first male precedence mediated by male adaptations to sperm competition may also constrain female reproductive strategies. First male precedence reduces the ability of females to (i) ‘trade up’ to higher quality or more compatible sires by remating after their first copulation (Clark et al. 1999; Mack et al. 2002; Pitcher et al. 2003), (ii) maximize genetic diversity of offspring (e.g. Watson 1991) and (iii) use relative copulation duration to control paternity through raffle competition (e.g. Eberhard 1996; Simmons 2002). Such constraints on fertilization strategies might be costly to females if there is significant variance in female fitness as a function of the genetic diversity or genetic traits of her offspring. Female traits for overcoming or limiting sperm precedence suggest sustained selection for female control of paternity, and thus genetic benefits of choosiness. There is evidence of female strategies to overcome direct costs imposed by competing males in other systems (e.g. Hosken & Stockley 2004; Wigby & Chapman 2004); our study is, to our knowledge, unique in showing that female behaviour and morphology could interact to minimize indirect costs of male manipulation of paternity.
Acknowledgments
We thank W. G. Eberhard, D. O. Elias, A. C. Mason, J. C. Johnson, M. M. Kasumovic and a reviewer for helpful comments, undergraduate assistants for rearing spiders, and NSERC, CFI and OIT for funding (to M.C.B.A.).
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Supplementary Material
References
- Andersson M. Princeton University Press; 1994. Sexual selection. [Google Scholar]
- Andrade M.C.B. Sexual selection for male sacrifice in the Australian redback spider. Science. 1996;271:70–72. [Google Scholar]
- Andrade M.C.B. Female hunger can explain variation in cannibalistic behavior despite male sacrifice in redback spiders. Behav. Ecol. 1998;9:33–42. [Google Scholar]
- Andrade, M. C. B. 2000 Sexual selection and male mating behavior in a cannibalistic spider. Ph.D. thesis, Division of Neurobiology & Behavior, Cornell University, Ithaca, NY.
- Andrade M.C.B, Banta E.M. Value of male remating and functional sterility in redback spiders. Anim. Behav. 2002;63:857–870. [Google Scholar]
- Berendonck B, Greven H. Morphology of female and male genitalia in Latrodectus revivensis Shulov, 1948 (Araneae, Theridiidae) with regard to sperm priority patterns. In: Toft S, Scharff N, editors. European arachnology. Aarhus University Press; Aarhus, Denmark: 2002. pp. 157–167. [Google Scholar]
- Bhatnagar R.D.S, Rempel J.G. The structure, function, and postembryonic development of the male and female copulatory organs of the black widow spider Latrodectus curacaviensis (Muller) Can. J. Zool. 1962;40:465–510. [Google Scholar]
- Birkhead T.R. Cryptic female choice: criteria for establishing female sperm choice. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Moller A.P. Sperm competition, sexual selection, and different routes to fitness. In: Birkhead T.R, Moller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 759–779. [Google Scholar]
- Boorman E, Parker G.A. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol. Entomol. 1976;1:145–155. [Google Scholar]
- Cameron E, Day T, Rowe L. Sexual conflict and indirect benefits. J. Evol. Biol. 2003;16:1055–1060. doi: 10.1046/j.1420-9101.2003.00584.x. [DOI] [PubMed] [Google Scholar]
- Clark A.G, Begun D.J, Prout T. Female×male interactions in Drosophila sperm competition. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, MA: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Eberhard W.G. Princeton University Press; 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Eberhard W.G, Huber B.A. Courtship, copulation, and sperm transfer in Leucauge mariana (Araneae, Tetragnathidae) J. Arachnol. 1998;26:342–368. [Google Scholar]
- Elgar M.A. Sperm competition and sexual selection in spiders and other arachnids. In: Birkhead T.R, Moller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 759–779. [Google Scholar]
- Elgar M.A, Schneider J.M, Herberstein M.E. Female control of paternity in the sexually cannibalistic spider Argiope keyserlingi. Proc. R. Soc. B. 2000;267:2439–2443. doi: 10.1098/rspb.2000.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E. Bonn University, Dept of Psychology; Bonn: 1992. GPOWER: A priori, post-hoc and compromise power analyses for MS-DOS [Computer program] [Google Scholar]
- Foelix R. 2nd edn. Oxford University Press; Oxford: 1996. Biology of spiders. [Google Scholar]
- Forster L.M. The stereotyped behavior of sexual cannibalism in Latrodectus hasselti Thorell (Araneae: Theridiidae) the Australian redback spider. Aust. J. Zool. 1992;40:1–11. [Google Scholar]
- Forster L.M. The behavioral ecology of Latrodectus hasselti (Thorell), the Australian redback spider (Araneae: Theridiidae): a review. Rec. West. Aust. Mus. 1995;52:13–24. [Google Scholar]
- Hellriegel B, Bernasconi G. Female-mediated differential sperm storage in a fly with complex spermathecae Scatophaga stercoraria. Anim. Behav. 2000;59:311–317. doi: 10.1006/anbe.1999.1308. [DOI] [PubMed] [Google Scholar]
- Hellriegel B, Ward P.I. Complex female reproductive tract morphology: its possible use in postcopulatory female choice. J. Theor. Biol. 1998;190:179–186. [Google Scholar]
- Hosken D.J, Stockley P. Sexual selection and genital evolution. Trends Ecol. Evol. 2004;19:87–93. doi: 10.1016/j.tree.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Jakob E.M, Marshall S.D, Uetz G.W. Estimating fitness: a comparison of body condition indices. Oikos. 1996;77:61–67. [Google Scholar]
- Kaston B.J. Comparative biology of American black widow spiders. San Diego Soc. Nat. Hist. Trans. 1970;16:33–82. [Google Scholar]
- Kirkpatrick M. Good genes and direct selection in the evolution of mating preferences. Evolution. 1996;50:2125–2140. doi: 10.1111/j.1558-5646.1996.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Knoflach B. Mating in Theridion varians Hahn and related species (Araneae: Theridiidae) J. Nat. Hist. 1998;32:545–604. [Google Scholar]
- Kokko H, Brooks R, McNamara J.M, Houston A.I. The sexual selection continuum. Proc. R. Soc. B. 2002;269:1333–1340. doi: 10.1098/rspb.2002.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack P.D, Hammock B.A, Promislaw D.E.L. Sperm competitive ability and genetic relatedness in Drosophila melanogaster: similarity breeds contempt. Evolution. 2002;56:1789–1795. doi: 10.1111/j.0014-3820.2002.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Ontronen M. Sperm numbers, their storage and usage in the fly Dryomyza anilis. Proc. R. Soc. B. 1997;264:777–782. [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A, Simmons L.W, Kirk H. Analysing sperm competition data: simple models for predicting mechanisms. Behav. Ecol. Sociobiol. 1990;27:55–65. [Google Scholar]
- Pitcher T.E, Neff B.D, Rodd F.H, Rowe L. Multiple mating and sequential mate choice in guppies: females trade up. Proc. R. Soc. B. 2003;270:1623–1629. doi: 10.1098/rspb.2002.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M, Uhl G. Determinants of paternity success in the cellar spider Pholcus phalangiodes (Araneae: Pholcidae): the role of male and female mating behavior. Behav. Ecol. Sociobiol. 2002;51:368–377. [Google Scholar]
- Simmons L.W. Princeton University Press; 2002. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Siva-Jothy M.T, Hooper R.E. Differential use of stored sperm during oviposition in the damselfly Caloperyx splendens xanthostoma (Charpentier) Behav. Ecol. Sociobiol. 1996;39:389–393. [Google Scholar]
- Siva-Jothy M.T, Tsubaki Y. Variation in copulation duration in Mnais pruinosa pruinosa Selys (Odonata: Calopterygidae) 1. Alternative mate-securing tactics and sperm precedence. Behav. Ecol. Sociobiol. 1989;24:39–45. [Google Scholar]
- Snow, L. S. E. 2003 Postcopulatory sexual selection in Australian redback spiders, (Latrodectus hasselti Thorell). MSc thesis, Department of Zoology, University of Toronto.
- Snow L. S. E, Andrade M. C. B. Pattern of sperm transfer in redback spiders: implications for sperm competition and male sacrifice. Behav. Ecol. 2004;15:785–792. [Google Scholar]
- Stockley P. Sexual conflict resulting from adaptations to sperm competition. Trends Ecol. Evol. 1997;12:154–159. doi: 10.1016/s0169-5347(97)01000-8. [DOI] [PubMed] [Google Scholar]
- Uhl G. Female genital morphology and sperm priority patterns in spiders (Araneae) In: Toft S, Scharff N, editors. European arachnology. Aarhus University Press; Aarhus, Denmark: 2002. pp. 145–156. [Google Scholar]
- Ward P.I. Cryptic female choice in the yellow dung fly Scathophaga stercoraria (L.) Evolution. 2000;54:1680–1686. doi: 10.1111/j.0014-3820.2000.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Watson P.J. Multiple paternity as genetic bet-hedging in female sierra dome spiders Linyphia litigiosa (Linyphiidae) Anim. Behav. 1991;41:343–360. [Google Scholar]
- Wigby S, Chapman T. Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution. 2004;58:1028–1037. doi: 10.1111/j.0014-3820.2004.tb00436.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


