Abstract
The release of hatchery-reared fishes for restoring threatened and endangered populations is one of the most controversial issues in applied ecology. A central issue has been to determine whether releases cause extinction of local wild populations. This may arise either through domesticated or non-local fishes hybridizing with wild fishes, or through inappropriate behavioural interactions; for example, many hatchery fishes show exaggerated aggressive and competitive behaviour and out-compete wild counterparts. The impact of the impoverished hatchery environment in shaping behaviour is only now receiving attention. Attempts to counteract hatchery-related behavioural deficiencies have utilized intensive training programmes shortly before the fishes are released. However, we show here that simple exposure to variable spatial and foraging cues in the standard hatchery environment generates fishes with enhanced behavioural traits that are probably associated with improved survival in the wild. It appears that fishes need to experience a varying and changeable environment to learn and develop flexible behaviour. Using variable hatchery rearing environments to generate suitable phenotypes in combination with a knowledge of appropriate local genotypes, rehabilitation of wild fishes is likely to succeed, where to date it has largely failed.
Keywords: cod, development of behaviour, restocking, environmental heterogeneity
1. Introduction
The last century has seen dramatic declines in marine fish species (Dulvy 2003). Populations can be lost through poor management (Pauly et al. 2002), and recovery from these collapses is slow, or does not occur at all (Hutchings 2000). Restocking has been commonly used in attempts to counter the effects of over-fishing, environmental degradation and recruitment failures. Yet it remains a controversial technique, both in terms of its consequences for wild populations, and with respect to its ability to successfully increase fish biomass (Nordeide et al. 1994; Olla et al. 1998; Salvanes 2001; Myers et al. 2004). The effects of the relaxed selection pressures in hatcheries and the release of fishes with divergent genetic backgrounds have played a central role in the debate, with fears for the consequences of the likely hybridization between released and wild fishes (Einum & Fleming 1997; Fleming & Einum 1997; McGinnity et al. 1997, 2003; Myers et al. 2004; Sundstrøm et al. 2004). Other concerns focus on the altered behaviour of hatchery fishes. On the one hand, aggressive released individuals appear to out-compete resident wild fishes (e.g. Sundstrøm et al. 2003), but, on the other hand, the vast majority of these released fishes typically die before they can affect the population biomass (Olla et al. 1998; Brown & Laland 2001). Attempts at improving post-release survival have recently used intensive pre-release training programmes to compensate for behavioural deficiencies. However, we show here that such deficiencies can largely be overcome by exposing hatchery fishes to variability in their early-rearing environment.
Growing and developing in a conventional hatchery does little to prepare fishes for the transition to a natural environment. A typical hatchery provides safe and constant housing. Fishes receive a plentiful supply of nutritious pellets, so there is no need actively to search for food, although the fishes may compete for access to it (Olla et al. 1998). However, after release the fishes must learn to feed on live prey, a task that many fail to do (Ersbak & Haase 1983; Ellis et al. 2002). In some cases, released fishes are so poor at this dietary transition they consume small pebbles and stones which resemble the food pellets they were reared on (Ellis et al. 2002). Additionally, hatchery-rearing tanks provide excellent protection from predators, so it is unsurprising that a large proportion of post-release mortality occurs through predation (Olla et al. 1994, 1998). Thus, although the hatchery environment is very successful in terms of rearing large numbers of fishes, it does little to generate fishes that have an ability to behave flexibly and adjust to life in the variable, natural world. Despite the clear behavioural differences between wild and hatchery-reared fishes, the majority of hatchery managers continue to release large numbers of naive fishes in the hope that some will survive.
Recently, researchers have devised various ways of training hatchery fishes to prepare them for release (Olla et al. 1998). For example, they are fed live prey in an effort to improve their ability to transfer from an artificial diet to a natural one. However, these studies report mixed results and the hatchery fishes are often slower than wild fishes at switching to new prey items as they become abundant (Olla et al. 1994), or they never reach the same feeding efficiency as wild fishes (Sundstrøm & Johnsson 2001). Others have shown that experience of live food in enriched, small glass aquaria generates fishes with better foraging skills than fishes reared on pellets in large black plastic tanks without enrichment (Brown et al. 2003). Similarly, a number of studies have begun to investigate ways to train fishes about the risks of predation (Berejikian 1995; Brown & Laland 2001). Again, these approaches have had variable success: some studies showing an effect (Brown & Smith 1998; Mirza & Chivers 2000; Hossain et al. 2002) and others finding none (Johnsson & Abrahams 1991; Vilhunen & Hirvonen 2003). One possible reason for the contradictory effects of training may lie in the fact that an ability to forage or avoid a predator often requires more than just an ability to recognize food or a threat. Rather, it requires the fishes to respond in a flexible manner and to show a propensity to learn and adapt to new situations.
Work with other captive vertebrates such as birds and mammals has already illustrated how increasing environmental complexity, sometimes referred to as environmental enrichment, can increase behavioural and neuronal plasticity, improve cognitive performance and increase survival in reintroduced species (Hunter et al. 2002; Kempermann et al. 2002; Bredy et al. 2003; Rabin 2003). Similarly, with fishes, resource predictability and distribution can influence behaviour and social interactions (McLaughlin et al. 1992; Grand & Grant 1994; Ryer & Olla 1996, 1997). Furthermore, an ability to learn and generate adaptive behaviour is most apparent in species that experience environmental heterogeneity (Papaj 1986; Odling-Smee & Braithwaite 2003). We therefore hypothesized that providing hatchery fishes with a variable environment should promote an ability to learn and, ultimately, would produce fishes that are behaviourally more versatile and more likely to survive when released into the wild. To test this, we devised four different rearing environments that varied in their levels of heterogeneity. Juvenile cod were reared in these different conditions and then compared across a range of behavioural trials to quantify behaviours that are likely to be associated with post-release survival.
2. Material and methods
To control for genetic background, we used juvenile, hatchery-bred North Sea cod from the same brood stock of (100) wild-caught 8–10 kg cod; juveniles were spawned on the same day. Newly hatched larvae were initially housed for eight weeks in 7000 l aquaria before being moved, post-larvae, into holding tanks (95×95×60 cm) and maintained on a diet of commercially produced fish pellets. For each of the experiments (1 and 2), we started with 400 individuals that were divided randomly among four types of rearing environment (i.e. 100 fishes per treatment). The holding tanks were similar to the type found in fish hatcheries, but these were manipulated to provide different experiences of spatial heterogeneity and food availability. Variable visual cues were created by the addition of cobble and kelp to the tank, representing typical habitat structures that wild juvenile cod interact with while foraging (Tupper & Boutilier 1995). Pellet food was used throughout this rearing phase but, given that prey encounter rate is variable in nature, the distribution and availability of the pellets were varied in some treatments.
The four rearing treatments were:
‘hatchery’—this was identical to a normal hatchery tank in that it provided constant food and no spatial cues.
‘F’—fish experienced variation in food availability but had no spatial cues.
‘S’—fish had variable spatial cues but constant food input.
‘S+F’—fish experienced both variable food and spatial cues.
Each holding tank was supplied with aerated, flowing seawater (approximately 10±1 °C) at a depth of 40 cm. Pebbles and rocks (cobble) and a plastic model of kelp on the base of the holding tank provided spatial cues. The position of these was randomly changed once a week while the tanks were cleaned. Cod reared with no spatial cues were reared in plain tanks but were cleaned at the same time as the landmarks were moved in the ‘S’ and ‘S+F’ tanks to control for this disturbance. Food variability was created by a variable feeding regime. Food could be presented in four possible 2 h intervals across the day. A pseudorandom sequence provided variable schedules: fish could receive food in one meal in the first 2 h, or this could be spread across two, three or four feeding intervals. Feeding regimes varied between days and weeks, and ran over a four weekly cycle. In addition, the position at which food was introduced also changed on a daily basis. Fish reared with constant food cues were fed small amounts of food pellets continuously between 08.00 and 16.00 hours at a fixed position that remained constant throughout rearing. All rearing tanks received the same total quantity of food each day.
In experiment 1, fish weight was measured once at the end of the behavioural assays. In experiment 2, we took weight measurements at two different time-points, two months before and after the assays, and used these to calculate a measure of growth rate .
We ran two separate experiments in which behavioural skills, likely to be associated with post-release survival, were quantified. In experiment 1, cod were reared in the four rearing environments for 14 weeks. In experiment 2, four further groups were reared for 20 weeks. However, owing to a technical problem, the fish in the ‘hatchery’ condition perished just prior to the behavioural screening. Growth and size data were collected for this group, and the behavioural data from the remaining three rearing treatments were collected.
Three behavioural assays were used to determine how rearing environment affected juvenile behaviour. The observer was naive to fish background throughout the assays. All trials were run in 70 ×40×40 cm test aquaria behind a black plastic curtain. The first assay quantified the motivation of the cod to move into and explore a novel area containing a restrained conspecific (n=12, experiment 1; n=10, experiment 2, per treatment). Individual test fish were placed in a grey PVC start-box (16×16 cm) and a second ‘stimulus’ fish was housed in a clear perspex restraining area (16×16 cm) at the opposite end of the tank. Ten minutes after the fish were put into the tank, the start-box was opened remotely and the latency of the fish to leave this area to enter the brightly illuminated tank and interact with the ‘stimulus’ fish was measured.
The second assay investigated how quickly fish recovered after a mild stressor was applied. Cod, like many species of fishes, typically show an elevated respiratory rate and a freezing response when they are threatened or stressed. A fish can remain motionless on the substrate as part of this freezing response for prolonged periods of time (Godin 1997). To create a stressful experience, individual cod were chased with a 6×6 cm green hand-net for 30 s to simulate a chase by a predator or a conspecific (n=9, experiment 1; n=10, experiment 2, per treatment). Recovery from the stressor was monitored over 45 min. Opercula beat rate and activity, in terms of swimming activity, were assessed at 5 min intervals and compared with background pre-stressor measures (taken 5 min before the stressor was applied).
Finally, in experiment 1, individual fish were released into a tank with a glass cylinder containing a single, live mysid shrimp to investigate attraction to live prey. Fish were observed to determine the time-point at which the cod swam in a directed fashion to within one body length of the cylinder. Seven fish from each treatment were tested (a total of 28 trials). In experiment 2, transfer from pellet to live prey was investigated in pairs of fish from the same rearing environment. Each pair was treated as a single data point. Pairs were given experience of prey (two-spotted gobies, Gobiusculus flavescens, a natural prey for juvenile cod; Salvanes & Nordeide 1993) the night before the experiment. Prey could find refuge sites within the test aquaria around the water inlet/outlet tubes and behind the air stone. Any remaining prey were removed in the morning and three new prey added. Fish were then observed at hourly intervals to determine how many prey were consumed. Trials terminated after five observations. Seven pairs of fish from each treatment were tested (a total of 21 trials).
Parametric analyses were used when data were normally distributed or could be transformed to meet the assumptions of analyses of variance (ANOVA). Initially, we used a separate one-way ANOVA for each of the behavioural assays to determine whether rearing treatment had an effect on (i) latency to enter a novel area, (ii) recovery of opercula beat rate to a basic level, and recovery of swimming activity and (iii) reaction to novel prey (experiment 1). A repeated measures ANOVA was used to compare the cumulative number of prey consumed in experiment 2.
Owing to the loss of the ‘hatchery’ treatment group in experiment 2, we ran a further set of analyses to determine whether it would be appropriate to compare the behavioural data collected in experiment 2 with those of the ‘hatchery’ group in experiment 1. To determine the validity of this, we compared the datasets (i), (ii) and (iii) for the three treatment groups ‘S’, ‘F’ and ‘S+F’ in a two-way ANOVA. As there were no significant interactions between experiment number and treatment for any of the comparisons, we ran a further series of two-way ANOVAs for each behavioural assay. These combined the data from experiments 1 and 2, but this time included the ‘hatchery’ group from experiment 1; however, we made the assumption here that there were no interactions.
Proportional data were arcsine transformed. Student–Newman–Keuls (SNK) post hoc tests determined which rearing treatments generated significant effects (Underwood 1997). Kruskal–Wallis tests were used when the data could not be transformed to meet the assumptions of parametric analyses (Siegal & Castellan 1988).
3. Results
(a) Motivation to enter a novel area to join a conspecific
A one-way ANOVA with latency to enter a novel area as the dependent variable showed a significant difference between rearing treatments in experiment 1 (F3,44=9.48, p<0.001; figure 1a). An SNK post hoc test revealed that differences arise because the cod reared in the ‘hatchery’ environment were slower at entering the novel environment than fish reared in the other treatment groups (figure 1a).
Figure 1.
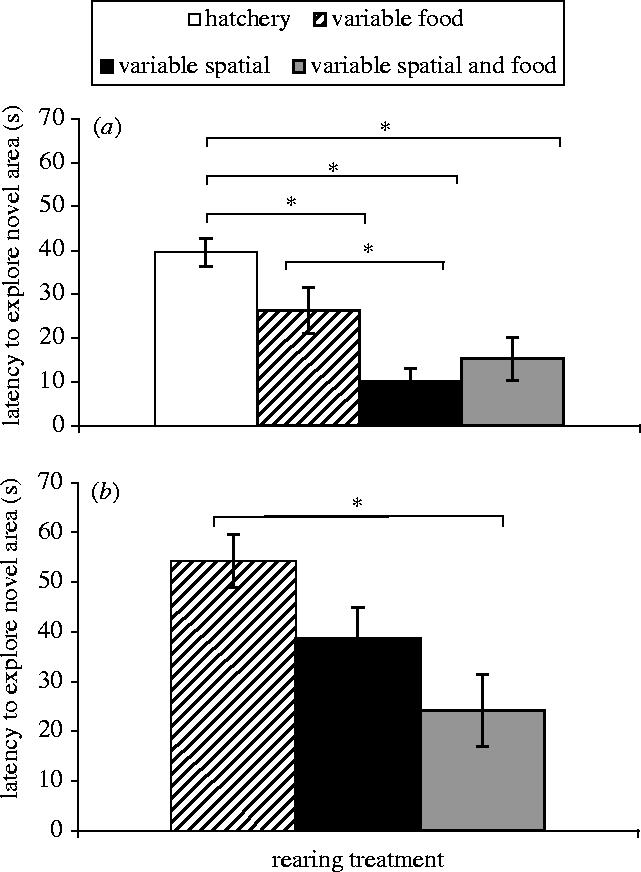
Latency±s.e. to leave a sheltered start-box to access a novel area that contained a stimulus fish. Each bar represents a different type of environmental background. (a) Cod that were exposed to different rearing treatments for 14 weeks. (b) Cod exposed to different rearing treatments for 20 weeks. Significant SNK post hoc test results are shown by the lines and asterisks above the bars.
In experiment 2, there was also a significant effect of rearing treatment (F2,27=5.58, p=0.009; figure 1b). A post hoc test determined that this was due to fish with experience of variable spatial and food cues (‘S+F’) being faster at entering the novel area than fish reared in a plain environment with variable food availability (‘F’; figure 1b).
Comparing treatments across experiments 1 and 2 in a combined two-way ANOVA (making the assumption there are no interactions, see §2 for more detail) reveals similar patterns in behaviour. Cod raised with experience of variable spatial cues either alone or in combination with variable food availability (‘S’ and ‘S+F’) were fastest at leaving an enclosure to move into the novel area (F3,73=10.56, p<0.001). There were, however, some effects specific to the duration of exposure to rearing treatment. Cod exposed to the rearing treatments for 20 weeks (experiment 2) were slower at moving into and exploring a novel environment (F1,73=15.80, p<0.001) than fish reared in the same types of environment for 14 weeks (experiment 1).
(b) Reaction to a simulated attack by a predator
Ability to recover from a stressful experience was analysed using the time to resume background opercula beat rate, and the time to resume background swimming activity as dependent variables in two separate one-way ANOVAs.
Looking at the recovery of opercula beat rate first, we found significant effects of rearing environment in both experiments: experiment 1, F3,32=14.96, p<0.001, figure 2a; experiment 2, F2,27=5.28, p=0.01, figure 2b. In experiment 1, cod that had experience of spatial heterogeneity (‘S’ and ‘S+F’) were faster at resuming their pre-stressor gill-beat rate (figure 2a). In experiment 2, only those reared in the most variable environment (‘S+F’) were faster at resuming their pre-stressor gill-beat rate (figure 2b).
Figure 2.
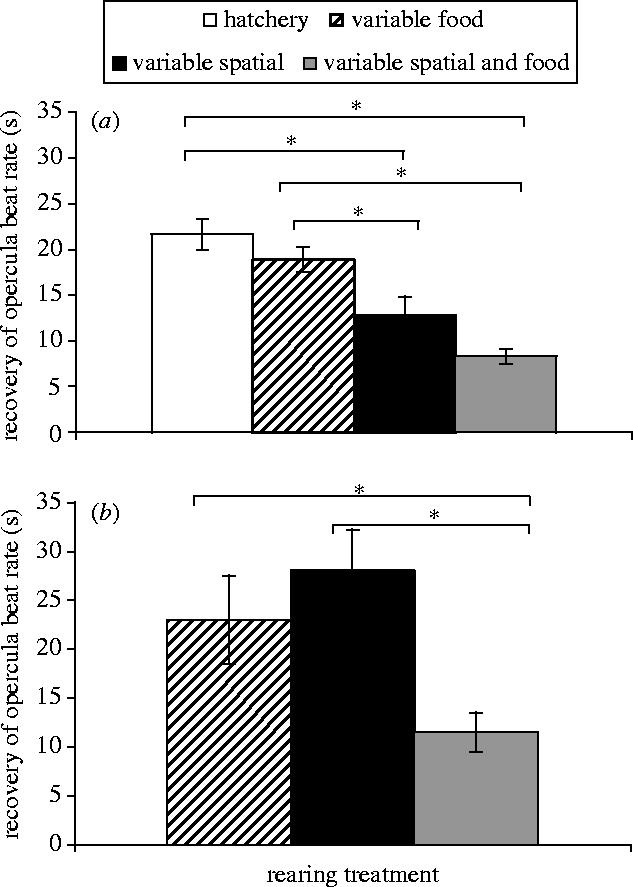
Recovery from a simulated attack by a predator measured as time taken to resume background pre-stressor opercula beat rate±s.e plotted for fish from each type of environmental background. (a) Cod that had experienced the different rearing treatments for 14 weeks. (b) Cod reared for 20 weeks in the different rearing treatments. Significant SNK post hoc tests are illustrated by the connecting lines and asterisks over the different bars.
There were also differences in how quickly the cod resumed their swimming activity. Significant effects of rearing treatment were found in both experiments: experiment 1, F3,32=4.45, p=0.01; experiment 2, F2,27=4.74, p=0.02. In experiment 1, differences arise because fish that experienced variability in feeding regime (‘F’ and ‘S+F’) were more active within the test tank as they recovered from the stressor (SNK post hoc test, p<0.05). In experiment 2, the cod that had experienced the most variable environment, ‘S+F’, were quickest to recover their background swimming activity levels (SNK post hoc test, p<0.05).
A combined analysis across experiments 1 and 2 revealed that fish that had experienced variable food and spatial cues (‘S+F’) were always the first to resume their background opercula beat rate and that they were faster at recovering their pre-stressor swimming activity levels (F3,61=8.15, p<0.01; F3,61=6.62, p<0.01). There was some effect of duration of exposure to rearing treatment during recovery; cod in experiment 2 were slower at recovering their opercula beat rate (F1,61=9.86, p=0.03), but there were no differences in the amount of swimming activity observed between experiments.
(c) Response to live prey
Time taken for the cod in experiment 1 to respond to a live mysid shrimp was used as the dependent variable in a one-way ANOVA. There was a significant treatment effect (F3,32=5.26, p=0.005, figure 3a) with fish with the most experience of environmental variability (‘S+F’) showing least latency (i.e. they were the fastest) at responding to the live shrimp.
Figure 3.
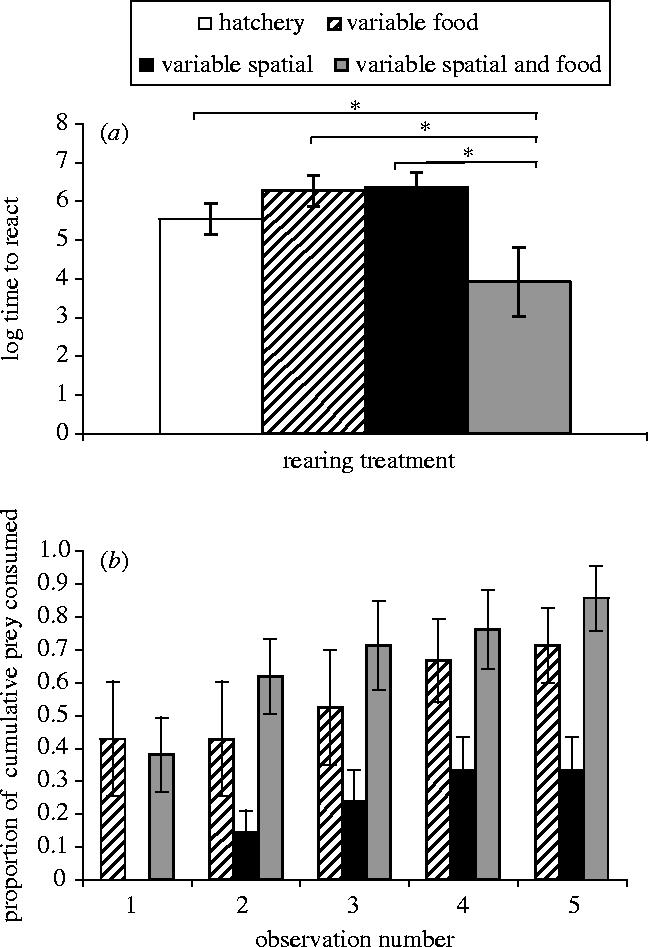
Response to live prey. (a) Time±s.e. taken to respond to a live mysid shrimp restrained in a glass cylinder (cod reared for 14 weeks). Significant SNK post hoc tests are illustrated by the connecting lines and asterisks over the different bars. (b) Cumulative number of live prey consumed by juvenile cod±s.e. (reared for 20 weeks) as a function of time since encounter.
Fish reared in experiment 2 were given access to live prey and a repeated measures ANOVA was used to compare the cumulative proportion of prey consumed. Cod with experience of variable food availability (‘F’ and ‘S+F’) were faster at transferring from a diet of pellet food to live prey (F2,18=4.98, p=0.02, figure 3b).
(d) Size and growth data
A Kruskal–Wallis test comparing fish weights across rearing treatments in experiment 1 found significant differences between groups, with ‘hatchery’ fish showing the largest weight (H=20.78, p<0.001). We also found a significant effect of growth rate in experiment 2 (H=196.10, p<0.001), with the fish from the ‘hatchery’ condition again found to have the fastest growth rate.
4. Discussion
Together, these results reveal a complex interaction between experience of different types of environmental heterogeneity and subsequent behavioural responses. These results, in line with enrichment experiments on other higher vertebrates (Gomez-Pinilla et al. 1998; Sacett et al. 1999; Allen et al. 2003), reveal that it is possible to alter cod behaviour by manipulating the rearing environment. Cod in experiment 1, reared in conditions that most closely resemble a plain hatchery tank, were found to behave poorly across all of the behavioural assays. In contrast, fish reared with experience of different levels of environmental variability appeared to have more flexible behavioural repertoires. Across experiments 1 and 2, early experience with both variable spatial and food cues consistently produced cod that were faster in terms of their attraction (experiment 1), their consumption (experiment 2) of live prey, their speed of exploration of a new environment and their recovery from a stressful experience.
Fish in both experiments 1 and 2 that received experience of spatial heterogeneity, either on its own (‘S’) or in combination with variable food (‘S+F’), were faster at leaving the enclosed start-box to enter and explore a novel area containing a stimulus fish. Although it is not possible to determine the precise motivation for entering the novel area, exploration or attraction towards the stimulus fish, this assay can be considered to quantify boldness and exploratory behavioural traits. Thus, fish exposed to spatial heterogeneity during rearing exhibit bolder, more curiosity driven behaviour. An animal that shows exploratory behaviour and a tendency to be bold may well cope better in a changeable environment where food patches vary in their productivity and location, or where the fish moves from one area to another during its lifetime (habitat shift). However, it is clearly not always advantageous to be bold: while some level of boldness will be adaptive, bolder individuals may put themselves at more risk (Sih et al. 2004). It should also be noted that our assay quantified boldness in an environment where there was no risk. It would be interesting to know whether the ‘S’ and ‘S+F’ cod would behave in the same way if they were able to perceive some form of threat in the novel environment. Furthermore, a more recent experiment using similarly reared fish has demonstrated that, although fish exposed to spatial heterogeneity during rearing are bolder, they were also faster at seeking shelter than fish reared without spatial cues (Salvanes & Braithwaite submitted).
An ability to recover quickly from a stressful experience could have a number of positive effects on fishes in the wild. Faster recovery would, for example, lead to faster resumption of foraging, which is important in fishes of small size, or when food is limited and there is competition (Krause et al. 1998). Furthermore, stress responses are physiologically demanding and prolonged exposure to stressful conditions can adversely affect the health of fishes (Pickering & Pottinger 1989). Our results demonstrate that fish that experienced the most heterogeneity, ‘S+F’ in both experiments, and variable spatial cues on their own, ‘S’ in experiment 1, were fastest at recovering their pre-stressor opercula beat rate, a measure that has previously been used to quantify the reaction to a stressful or noxious stimulation in fishes (Sneddon et al. 2003a,b). In addition to the increased respiratory rate, most cod exhibited a freezing response after being chased with a net. The cod that were consistently first to resume their swimming activity were, again, the ‘S+F’ fish. Cod exposed to variable food cues alone, ‘F’ in experiment 1, were also found to recover their swimming activity faster than the ‘hatchery’ or ‘S’ fish.
The combined analyses comparing fish in experiments 1 and 2 revealed some differences in the behaviours of fish exposed to the rearing environments for either 14 or 20 weeks. In terms of boldness, fish in experiment 2 (exposed for 20 weeks) were slower at leaving the start-boxes compared with cod in experiment 1 (exposed for 14 weeks). Similarly, although there were no differences in how quickly fish from experiments 1 and 2 resumed their swimming activity after the stressor was applied, there were differences in their opercula beat rate recovery. As with the boldness measure, the older fish in experiment 2 took longer to recover their opercula beat rate than the fish in experiment 1. It is not clear why these differences between experiments 1 and 2 arise, however, they may be related to the fact that the fish in experiment 2 are older and larger, which may affect both motivation and physiology. Further experiments are needed to determine whether it is age, size or duration of exposure to the rearing treatments that underpins these observations.
Owing to space constraints, both experiments 1 and 2 had one rearing tank per treatment. There are a number of reasons to consider the fish in each treatment as independent. First, in each experiment 400 individuals were randomly distributed among the four rearing tanks, and we propose that, under these circumstances, it can reasonably be argued that fish of differing competitive and learning potential are therefore likely to be equally represented in all the tanks. Second, considerable care was taken to ensure that the only factors that differed between the rearing tanks were the treatments themselves (e.g. the tanks were housed in the same area, received identical lighting and were kept at the same temperature). Finally, experiments 1 and 2, which were run independently and used different rearing tanks, show broadly similar results to one another, suggesting that the differences between treatments are repeatable.
Our data show that, with regard to changing from an artificial to a natural diet, variable food presentation had a significant effect, but we found no effect from just spatial variation experience during the same rearing period. Cod that have experienced changes in where and when food is available were faster at reacting and moving towards a live prey item and, in experiment 2, were faster at consuming live prey compared with fish that were provided with an unchanging food input point. These differences in ability to switch to a natural diet arise even though these cod have been reared on pellet food until the behavioural screening. Therefore, one way to prepare hatchery-reared fishes for the transition to foraging in the natural environment would be to rear them with a varying supply of food pellets. Using these types of rearing environment, possibly in combination with a short phase exposing fishes to live prey prior to release, may significantly improve the foraging capacity of hatchery-reared fishes, and therefore improve post-release survival.
The fish that consistently performed well in each of the three assays were those that had previous experience with both variable food and spatial cues. While encountering predators, cod may reduce their risk of mortality by rapid escape to shelter in cobble and kelp. Survival in wild fishes is already known to depend on the complexity of the sea floor (Tupper & Boutilier 1995). Rapid recovery from stress in fishes with experience of environmental variability will enable these fishes to resume their search for prey faster, once the risk of mortality has decreased. Our results therefore indicate that hatchery fishes can be prepared for heterogeneous natural habitats by rearing them in facilities that, in addition to having a variable food supply, contain cues, such as cobble and kelp, representing the typical type of spatial landscape found in fishes' nursery areas.
In both experiments, fish reared in the ‘hatchery’ environments grew larger than fish in the remaining treatments. It is not clear how access to variable cues in the environment leads to a decreased growth rate. It is possible these slower growing fish are more active and use more energy than the fish with the constant supply of food in the plain tank. Ryer & Olla (1997) found, for juvenile walleye pollock, that changing spatial and temporal aspects of food distribution changes energy expenditure during foraging and that this influences growth. When food was clumped, the walleye grew more slowly owing to higher swimming activity probably representing an increase in time spent searching for food. An alternative explanation could be that fishes reared in enriched environments pay more attention to other tasks than feeding, with the cost of a lower growth rate. This result suggests that there may be a trade-off between fast growth and behavioural flexibility.
Overall, our results demonstrate that exposing marine fishes to variable rearing habitats promotes diet transfer, flexibility in space use and activity for hatchery-reared fishes. The work indicates that simple changes to traditional rearing techniques could have considerable positive effects on the survival of released fishes. Despite the poor success of releasing fishes for rehabilitation of marine and fresh water stocks all over the world, it is surprising that the application of behaviour to aquatic conservation has been neglected (Shumway 1999). Our results indicate that hatchery fishes need not only food, but also the opportunity to experience environmental variability to promote flexible behaviour.
Acknowledgments
This work was funded by the EU-project Bergen Marine Food Chain Research Infrastructure and the Research Council of Norway (project 130192/140), and the Leverhulme Trust (F/00158/N). We thank Dag Aksnes, Nick Colegrave, Emma Cunningham, Jarl Giske, Sean Nee, Andrew Read and Lynne Sneddon for discussion and two anonymous referees for constructive critique. We also thank Saga Fjord Sea Farm A/S for providing the fish, and Mette Hordnes, Graeme Mckenzie, Frank Midtøy, Julie Skadal and George Steedman for technical assistance.
Footnotes
Both authors contributed equally to this work.
References
- Allen C.B, Celikel T, Feldman D.E. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Berejikian B.A. The effects of hatchery and wild ancestry and experience on the relative ability of steelhead trout fry (Oncorhynchus mykiss) to avoid a benthic predator. Can. J. Fish. Aquat. Sci. 1995;52:2476–2482. [Google Scholar]
- Bredy T.W, Humpartzoomian R.A, Cain D.P, Meaney M.J. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Brown C, Laland K. Social learning and life skills training for hatchery reared fish. J. Fish Biol. 2001;59:471–493. [Google Scholar]
- Brown G.E, Smith R.J.F. Acquired predator recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery-reared fish to recognize chemical cues of a predator. Can. J. Fish Aquat. Sci. 1998;55:611–617. [Google Scholar]
- Brown C, Davidson T, Laland K. Environmental enrichment and prior experience improve foraging behaviour in hatchery-reared Atlantic salmon. J. Fish Biol. 2003;63(Suppl. 1):187–196. [Google Scholar]
- Dulvy N.K. Extinction vulnerability in marine populations. Fish Fish. 2003;4:25–64. [Google Scholar]
- Einum S, Fleming I.A. Genetic divergence and interactions in the wild among native, farmed and hybrid Atlantic salmon. J. Fish Biol. 1997;50:634–651. [Google Scholar]
- Ellis T, Hughes R.N, Howell B.R. Artificial dietary regime may impair subsequent foraging behaviour of hatchery-reared turbot released into natural environment. J. Fish Biol. 2002;61:252–264. [Google Scholar]
- Ersbak K, Haase B.L. Nutritional deprivation after stocking as a possible mechanism leading to mortality in stream-stocked brook trout. North Am. J. Fish. Manage. 1983;3:142–151. [Google Scholar]
- Fleming I.A, Einum S. Experimental tests of genetic divergence of farmed from wild Atlantic salmon due to domestication. ICES J. Mar. Sci. 1997;54:1051–1063. [Google Scholar]
- Godin J.-G. Evading predators. In: Godin J.-G, editor. Behavioural ecology of teleost fish. Oxford University Press; Oxford: 1997. pp. 191–236. [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak J.P. Spatial learning & physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- Grand T.C, Grant J.W.A. Spatial predictability of resources and the ideal free distribution in convict cichlids, Cichlasoma nigrofasciatum. Anim. Behav. 1994;48:909–919. [Google Scholar]
- Hossain M.A.R, Tanaka M, Masuda R. Predator–prey interaction between hatchery-reared Japanese flounder juvenile, Paralichthys olivaceus, and sandy shore crab, Matuta lunaris: daily rhythms, anti-predator conditioning and starvation. J. Exp. Mar. Biol. Ecol. 2002;267:1–14. [Google Scholar]
- Hunter S.A, Bay M.S, Martin M.L, Hatfield J.S. Behavioural effects of environmental enrichment on harbour seals (Phoca vitulina concolor) and grey seals (Halichoerus grypus) Zool. Biol. 2002;21:375–387. [Google Scholar]
- Hutchings J.A. Collapse and recovery of marine fishes. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- Johnsson J.I, Abrahams M.V. Interbreeding with domestic strain increases foraging under threat of predation in juvenile steelhead trout (Oncorhynchus mykiss)—an experimental study. Can. J. Fish. Aquat. Sci. 1991;48:243–247. [Google Scholar]
- Kempermann G, Gast D, Gage F.H. Neuroplasticity in old age: sustained five-fold induction of hippocampal neurogenesis by long term environmental enrichment. Ann. Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Krause J, Loader S.P, McDermott J, Ruxton G.D. Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proc. R. Soc. B. 1998;265:2373–2379. [Google Scholar]
- McGinnity P, Stone C, Taggart J.B, Cooke D, Cotter D, Hynes R, McCamley C, Cross T, Ferguson A. Genetic impact of escaped farmed Atlantic salmon (Salmo salar) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural environment. ICES J. Mar. Sci. 1997;54:998–1008. [Google Scholar]
- McGinnity P, et al. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc. R. Soc. B. 2003;270:2443–2450. doi: 10.1098/rspb.2003.2520. doi:10.1098/rspb.2003.2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R.L, Grant J.W.A, Kramer D.L. Individual variation and alternative patterns of foraging movements in recently emerged brook charr (Salvelinus fontinalis) Behaviour. 1992;120:286–301. [Google Scholar]
- Mirza R.S, Chivers D.P. Predator-recognition training enhances survival of brook trout: evidence from laboratory and field-enclosure studies. Can. J. Zool. 2000;78:2198–2208. [Google Scholar]
- Myers R.A, Levin S.A, Lande R, James F, Murdoch W.W, Paine R.T. Hatcheries and endangered salmon. Science. 2004;303:1980. doi: 10.1126/science.1095410. [DOI] [PubMed] [Google Scholar]
- Nordeide J.T, Fosså J.H, Salvanes A.G.V, Smedstad O.M. Testing if year-class strength of coastal cod, Gadus morhua L., can be determined at the juvenile stage. Aquat. Fish. Manage. 1994;25(Suppl. 1):101–116. [Google Scholar]
- Odling-Smee L, Braithwaite V.A. The influence of habitat stability on landmark use during spatial learning in the three-spined stickleback. Anim. Behav. 2003;65:701–707. [Google Scholar]
- Olla B.L, Davis M.W, Ryer C.H. Behavioural deficits in hatchery-reared fish: potential effects on survival following release. Aquat. Fish. Manage. 1994;25(Suppl. 1):19–34. [Google Scholar]
- Olla B.L, Davis M.W, Ryer C.H. Understanding how the hatchery environment represses or promotes the development of behavioural survival skills. Bull. Mar. Sci. 1998;62:531–550. [Google Scholar]
- Papaj D.R. Interpopulation differences in host preference and the evolution of learning in the butterfly, Battus philenor. Evolution. 1986;40:518–530. doi: 10.1111/j.1558-5646.1986.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Guénette S, Pitcher T, Sumaila U.R, Walters C.J, Watson R, Zeller D. Towards sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- Pickering A.D, Pottinger T.G. Stress response and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiol. Biochem. 1989;7:253–258. doi: 10.1007/BF00004714. [DOI] [PubMed] [Google Scholar]
- Rabin L.A. Maintaining behavioural diversity in captivity for conservation: natural behaviour management. Anim. Welf. 2003;12:85–94. [Google Scholar]
- Ryer C.H, Olla B.L. Growth depensation and aggression in laboratory reared coho salmon: the effect of food distribution and ration size. J. Fish Biol. 1996;48:686–694. [Google Scholar]
- Ryer C.H, Olla B.L. Altered search speed and growth: social versus independent foraging in two pelagic juvenile fishes. Mar. Ecol. Prog. Ser. 1997;153:273–281. [Google Scholar]
- Sacett G.P, Novak M.F.S.X, Kroeker R. Early experience effects on adaptive behavior: theory revisited. Ment. Retard. Dev. Disabil. Res. Rev. 1999;5:30–40. [Google Scholar]
- Salvanes A.G.V. Ocean ranching. In: Steele J, Thorpe S, Turekian K, editors. Encyclopedia of ocean sciences. Academic Press; London: 2001. pp. 1973–1982. [Google Scholar]
- Salvanes, A. G. V. & Braithwaite, V. A. Submitted. Exposure to variable spatial information in the early rearing environment generates asymmetries in social interactions in cod (Gadus morhua). Behav. Ecol. Sociobiol.
- Salvanes A.G.V, Nordeide J. Dominating sublittoral fish species in a west Norwegian fjord and their trophic links to cod (Gadus morhua L.) Sarsia. 1993;78:221–234. [Google Scholar]
- Siegal S, Castellan N.J. 2nd edn. McGraw-Hill Book Company; London: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Shumway C.A. A neglected science: applying behaviour to aquatic conservation. Environ. Biol. Fish. 1999;55:183–201. [Google Scholar]
- Sih A, Bell A, Chadwick Johnson J. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sneddon L.U, Braithwaite V.A, Gentle M.J. Do fish have nociceptors: evidence for the evolution of a vertebrate sensory system. Proc. R. Soc. B. 2003a;270:1115–1121. doi: 10.1098/rspb.2003.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon L.U, Braithwaite V.A, Gentle M.J. Novel object test: examining nociception and fear in the rainbow trout. J. Pain. 2003b;4:431–440. doi: 10.1067/s1526-5900(03)00717-x. [DOI] [PubMed] [Google Scholar]
- Sundstrøm L.F, Johnsson J.I.J. Experience and social environment influence the ability of young brown trout to forage on live novel prey. Anim. Behav. 2001;61:249–255. doi: 10.1006/anbe.2000.1593. [DOI] [PubMed] [Google Scholar]
- Sundstrøm L.F, Lohmus M, Johnsson J.I. Investment in territorial defence depends on rearing environment in brown trout (Salmo trutta) Behav. Ecol. Sociobiol. 2003;54:249–255. [Google Scholar]
- Sundstrøm L.F, Lohmus M, Johnsson J, Devlin R.H. Growth hormone transgenic salmon pay for growth potential with increased predation mortality. Proc. R. Soc. B. 2004;271(Suppl.):S350–S352. doi: 10.1098/rsbl.2004.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper M, Boutilier R.G. Effects of habitat on settlement, growth, and postsettlement survival of Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 1995;52:1834–1841. [Google Scholar]
- Underwood A.J. Cambridge University Press; Cambridge: 1997. Experiments in ecology. [Google Scholar]
- Vilhunen S, Hirvonen H. Innate antipredator responses of Arctic char (Salvelinus alpinus) depend on predator species and their diet. Behav. Ecol. Sociobiol. 2003;55:1–10. [Google Scholar]


