Abstract
The evolution of migration in birds remains an outstanding, unresolved question in evolutionary ecology. A particularly intriguing question is why individuals in some species have been selected to migrate, whereas in other species they have been selected to be sedentary. In this paper, we suggest that this diverging selection might partially result from differences among species in the behavioural flexibility of their responses to seasonal changes in the environment. This hypothesis is supported in a comparative analysis of Palaearctic passerines. First, resident species tend to rely more on innovative feeding behaviours in winter, when food is harder to find, than in other seasons. Second, species with larger brains, relative to their body size, and a higher propensity for innovative behaviours tend to be resident, while less flexible species tend to be migratory. Residence also appears to be less likely in species that occur in more northerly regions, exploit temporally available food sources, inhabit non-buffered habitats and have smaller bodies. Yet, the role of behavioural flexibility as a response to seasonal environments is largely independent of these other factors. Therefore, species with greater foraging flexibility seem to be able to cope with seasonal environments better, while less flexible species are forced to become migratory.
Keywords: seasonal environments, animal movement, phenotypic flexibility, foraging ecology, conservation
1. Introduction
Faced with similar seasonal changes in the environment, some birds migrate to less severe regions for the winter while others remain in the same region during the whole year. Discovering why birds have adopted such different strategies remains a challenging task, but one that is critical to fully understanding the ecology and evolution of bird migration. Explanations proposed in previous studies include variation among species in dependence on temporally and spatially variable food resources (Levey & Stiles 1992; Newton 1995; Chesser and Levey 1998), competitive ability (Cox 1985; Pérez-Tris & Tellería 2002) and life history traits (Greenberg 1982). In this paper, we suggest that behavioural flexibility—the ability of individuals to express distinct behaviours in different contexts through innovation and learning processes (Klopfer 1962; Piersma & Drent 2003; Lefebvre et al. 2004)—might also influence whether birds develop either migratory or resident behaviour in environments with sharp seasonal changes.
The ability to produce flexible behavioural responses has been shown to enhance survival when birds are exposed to novel or altered environmental situations (Sol et al. 2005). Following a similar logic, behavioural flexibility should also provide important fitness benefits in animals facing seasonal environments (Reader & MacDonald 2003; Sol 2003). Given that change in foraging conditions is one of the main problems birds must confront among seasons (Cox 1985; Rappole 1995; Newton & Dale 1996a,b), flexibility in foraging behaviour should be particularly relevant in this context. Birds may improve their efficiency at exploiting seasonal food resources through a number of behavioural adjustments (Morse 1980), for example, by shifting to different feeding sites or food types between seasons (Grubb & Waite 1990). The greater ability of flexible species to modify or invent new foraging behaviours may also allow them to diversify their trophic niche (Greenberg 1990; Lefebvre et al. 1997, 2004), increasing their probability of surviving under the stressful winter conditions. One may thus expect that individuals with greater foraging flexibility would be better able to cope with seasonal changes in foraging conditions than less flexible species. This implies in turn that less flexible species should be the ones more likely to abandon their breeding areas during winter. This idea will be termed the behavioural flexibility–migratory precursor hypothesis.
We explore the role of behavioural flexibility as an adaptation to seasonal environments with a comparative analysis of passerines (order Passeriformes), a group of birds that is relatively uniform in terms of general ecology (Chesser and Levey 1998) but variable in foraging flexibility (Sol 2003) and migratory behaviour (Winkler & Leisler 2004). We focus on species from the Western Palaearctic region because this is the part of the world with the most abundant comparative data on foraging flexibility. We test two major predictions of the behavioural flexibility–migratory precursor hypothesis.
First, if behavioural flexibility helps to deal with foraging stress during winter, resident passerines should express flexible behaviours more often during this season than in the rest of the year. We use a recently proposed measure of flexibility, the propensity for innovative feeding (Lefebvre et al. 1997; Reader & Laland 2002), to test this prediction. Innovation rate is a cognitive measure based on an exhaustive frequency count of food types and foraging techniques that ornithologists consider to be novel or opportunistic (Lefebvre et al. 1997; see §2). In birds, it has proven to be useful in the study of cognition, ecology and evolution (see Sol 2003; Lefebvre et al. 2004), testing many of the predictions on the ecological implications of behavioural flexibility anticipated by Peter Klopfer several decades ago (e.g. Klopfer & MacArthur 1960; Klopfer 1962; see Lefebvre & Bolhuis 2003 for a historical review).
Second, if foraging flexibility is an important adaptation to deal with seasonal environments, we also predict that resident species should not only be more innovative than migratory species but they also should have larger brains. The size of the brain, relative to the size of the body, limits the capacity of animals to process and store more information and thus affects the ability of individuals to produce flexible responses to environmental challenges (Jerison 1973; Lefebvre et al. 1997; Allman 2002; Reader & Laland 2002). In both birds and primates, innovation rate is positively linked with the relative size of the brain and, specifically, with its association areas (Timmermans et al. 2000; Reader & Laland 2002). Nevertheless, our use of two correlated measures of flexibility (structural and behavioural) has some advantages. One is that identifying consistent patterns with two conceptually different measures would increase our confidence that we are observing a robust pattern. In addition, foraging innovations and brain size seem to measure different aspects of flexibility (Sol 2003). The frequency with which animals innovate depends upon the specific ecological context in which individuals live, as suggested by the low taxonomic levels (i.e. species; Sol 2003) at which most of the variance is concentrated. Conversely, variation in brain size is mostly concentrated at higher taxonomic levels (Bennett & Harvey 1985; Nealen & Ricklefs 2001; Sol 2003) and thus appears to describe differences in neural substrates that evolved early in the diversification of avian lineages. The two measures also carry different potential sources of error, with the structural measure of brain size being much less affected by observational error than innovation rate. Thus, our use of both measures allows a broader test of the ecological significance of behavioural flexibility on the response of birds to seasonal environments.
2. Methods
(a) Species data
We used all Passerine species breeding in the Western Palaearctic region (i.e. 134 species belonging to 16 families, according to Sibley & Monroe 1990). Each species was classified either as a long-distance (LD) migrant (wintering south of Sahara), a short-distance (SD) migrant (wintering south of its breeding range but north of Sahara) or a resident, based on information provided by Cramp et al. (1988–1994). Most species categorized as SD migrants were also partial migrants, that is, species in which the whole or parts of some populations migrate (Berthold 1993). We refer to these three categories as ‘seasonal behavioural strategies’.
(b) Measuring foraging flexibility
(i) Foraging innovations
Foraging innovations were gathered from 24 European journals. The short note sections of these journals were exhaustively reviewed over the 1970–2002 period by readers that were most often blind to the hypothesis. The readers looked for key words like ‘never reported’, ‘not seen before’, ‘first report’, ‘unusual’ or ‘novel’ and noted all cases of new food types or feeding behaviours observed by ornithologists. Examples of innovations include Turdus merula using twigs to clear away snow while searching for food or Pyrrhula pyrrhula eating flesh from chicken and duck carcasses (see Lefebvre et al. 1997 for further examples and details of the method). We excluded Mediterranean species because this area was poorly covered by the journals we surveyed and were left with a total of 123 species. Our dataset for this study included 298 innovations and is available upon request.
The frequency of foraging innovations may suffer from a bias caused by some species being investigated more than others. This may be a problem because while residents can be observed all year long, long-distant migrants leave the breeding area during the winter. We dealt with this problem in two ways. First, we searched for the number of papers published per species in the online version of Zoological Record (from 1993 to 2002) and included this variable as a covariate in the models comparing migratory habits with innovation rate. Second, we ran a more restrictive test only using innovations reported outside winter, so individuals from migratory and non-migratory species had similar opportunities to be observed. This analysis was restricted to innovations reported in passerines that are common breeders in the British Isles (categories 1, 2 and 3 in Mullarney et al. 2000; n=68 species). The British Isles are a particularly good region to focus on, given the high number of innovations reported by British ornithologists (60% of the European data base; see Lefebvre & Bolhuis 2003). We only used innovations recorded from 1 March to 1 October, the period delimited by the two regional peaks in bird migration (Cramp et al. 1988–1994); we ran a second analysis (not presented) with a cut-off point of May 1 for winter, which yielded similar conclusions. In these analyses, research effort was restricted to papers published outside the winter by including key words like ‘breeding’, ‘reproduction’ or ‘summer’ in the online search.
We used the information on British birds to investigate whether species tend to innovate more in winter than in other seasons. This analysis was limited to 31 species for which at least one foraging innovation was reported. We calculated the residuals of a regression of innovation rate against research effort to account for the fact that some species may be more investigated than others. This latter variable was estimated separately for winter and the other seasons.
(ii) Brain size
In birds, the size of the mesopallium and nidopallium are the best neural predictors of variation in foraging flexibility (Timmermans et al. 2000), but data on these brain areas are available for only 32 species. However, because these association areas represent up to 55% of the avian brain, their relative size can be closely predicted (95% of the explained variance; Timmermans et al. 2000) from the size of the whole brain, data which are available for many more species. We consequently used brain size as a surrogate for these brain areas. Data on brain size, taken from Mlíkovský (1989a,b,c, 1990), DeVoogd et al. (1993), Székely et al. (1996), Garamszegi et al. (2002) and Iwaniuk (2003), were available for a total of 105 Western Palaearctic species. We used brain mass, when available, but we also included cranial endocast measures converted to mass by multiplying the reported value by 1.036 g ml−1 (density of fresh brain tissue). In four cases, we used telencephalon volumes reported in DeVoogd et al. (1993), which were transformed to brain mass using a linear regression. Brain measurements are significantly repeatable across methods (Iwaniuk & Nelson 2002) and literature sources (Garamszegi et al. 2002). To deal with the fact that larger species tend to have larger brains, we estimated the residuals of log–log least-squares regression of brain mass against body mass (Bennett & Harvey 1985). These residuals (hereafter called relative brain size) were then used to test for an association between brain size and seasonal behavioural strategies.
(c) Alternative explanations
Based on information from Cramp et al. (1988–1994), we quantified several factors that could inflate or obscure the predicted link between foraging flexibility and migratory habits: (i) mid-latitude of the distribution range (Newton 1995), measured as the midpoint between N and S latitudinal degrees of each species' distribution, (ii) occurrence in buffered habitats (Chesser and Levey 1998), categorized as whether or not the species is primarily a conifer specialist (Alerstam 1991), (iii) use of temporally variable diet types, quantified as whether the species is mostly insectivorous or is mostly a ground forager, (iv) clutch size, measured as mean number of eggs in first clutches, (v) food storing, categorized as the presence or absence of this strategy in the species and (vi) body mass, measured in grams and restricted to female values (when available) to avoid biases in sexually selected species.
(d) Statistical methods
(i) Assumptions and exploratory analyses
We assume throughout the paper that SD migrants are an intermediate category between residents and LD migrants. Although this is suggested in the finding that the amount and incidence of migratory activity are genetically correlated (Pulido et al. 1996), the assumption still awaits general comparative evidence. Migratory behaviour can change over short time-intervals and, thus, reconstructing its ancestral states would run considerable risk. As an alternative, the assumption that SD migrants represent a bridge between residents and LD migrants may be evaluated with the maximum likelihood approach proposed by Pagel (1994). This method estimates the rate of changes in a character without the need to reconstruct ancestral character states. We used the software Multi-state to assess whether some evolutionary transitions between residence, SD and LD migration were more likely than others. This analysis supported our assumption. Thus, while the transitions from residence to LD migration and vice versa were non-significant, all those linking each category with SD migration were significantly different from zero (figure 1).
Figure 1.
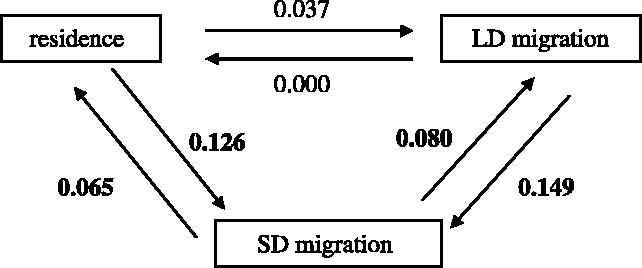
The likelihood of evolutionary transitions between seasonal behavioural strategies as estimated by maximum likelihood techniques (see §2). Significant parameters (p<0.05) are shown in bold.
We next used a variance component analysis (Harvey & Pagel 1991; Cassey et al. 2004) to examine how variation in migratory habits is partitioned throughout different taxonomic levels (species, genus, family, superfamily and parvorder; following Sibley & Monroe 1990). Most of the variation in migratory habits was distributed among the lowest taxonomic levels (species–genus levels, 46.4%) and the family level (49%; table 1, Electronic Appendix). Following Bennett and Owens (2002), we studied how variation in behavioural flexibility relates to variation in migratory patterns at both the species and family levels (see below).
(ii) Species-level analyses
The predictions that relative brain size and innovation rates vary between seasonal behavioural strategies were tested with an ANOVA. In addition, innovation rates were examined with an ANCOVA that included research effort as a covariate. Relative brain size was normally distributed, but innovation rate was not. As a precaution, we repeated the analyses of innovation rates using randomization tests (Software RT, Manly 1997), which yielded the same conclusions as the ANOVA. These latter analyses are not reported in the text.
Significance tests derived from conventional tests cannot always be trusted because the hierarchical structure of phylogenetic descent may lead to similarities due to common ancestry rather than independent evolution (Harvey & Pagel 1991; Garland et al. 1993). We dealt with this problem using empirically scaled computer simulations of continuous traits evolving along a phylogenetic hypothesis, which allowed us to obtain null distributions of F statistics for ANOVA and ANCOVA (Garland et al. 1993). These null distributions define critical values for hypothesis testing that account for non-independence of species due to common ancestry. Our phylogenetic hypothesis was based on the molecular phylogeny of Sibley & Ahlquist (1990), complemented with information from other sources (Blondel et al. 1996; Helbig et al. 1996; Kvist et al. 1996; Martin & Clobert 1996; Mönkkönen & Orell 1997; Cibois & Pasquet 1999; Helbig & Seibold 1999; Omland 2000; Alonso et al. 2001; Voelker 2001; Ericson & Johansson 2003; Klicka et al. 2003; Chubb 2004; Spicer & Dunipace 2004; Voelker & Spellman 2004). Based on our phylogenetic hypothesis, we ran the simulations under two evolutionary models (gradual and speciational Brownian motion; see Garland et al. 1993); both models provided consistent results and in the text we only report results based on the speciational model. The analyses were performed with the phenotypic diversity analysis programme developed by T. Garland Jr. and coworkers, and described in Garland et al. (1993).
Finally, we tested our predictions adjusting for the potential influence of confounding variables. Migratory status is an ordinal variable. Thus, the different categories can be ranged from lowest (resident) to highest (LD migratory) tendency for seasonal movements but the intervals between adjacent categories are unknown. This type of response variables cannot be analysed with traditional multiple regressions, which assume that the variable must be continuous and normally distributed. Moreover, none of the usual phylogenetic-based methods can deal with a discrete response variable and a combination of continuous and categorical predictors. Generalized linear mixed models (GLMMs) provide an alternative framework for analysing categorical data in which observations are likely to be correlated due to common ancestry (Goldstein 1995; Littell et al. 1996; Blackburn & Duncan 2001; Duncan et al. 2002; Cassey et al. 2004). We used models in which the taxonomic variable ‘family’ was included as a random effect, which allowed us to ensure that the significance tests for the fixed-effect predictors were not biased by the non-independence of species belonging to the same family (Duncan et al. 2002). Because migratory status is an ordinal variable, we generally adopted models in which the error structure was defined as ordinal multinomial. In the analyses of innovation frequency, however, we pooled SDM and residents in the multivariate models, as they showed no differences in innovation rate and implemented models with binomial error. All the analyses were conducted with SAS 8.2.
(iii) Family-level analyses
The size of the brain, relative to body size, is known to vary significantly at the highest taxonomic levels (Bennett & Harvey 1985; Nealen & Ricklefs 2001; Sol 2003), a conclusion that also applies to the species studied here (table 1, Electronic Appendix). Hence, we also tested the predicted association between relative brain size and seasonal behavioural strategies at the family level. For all species of Western Palaearctic Passeriformes, we estimated the proportion (arcsine transformed) of species in each family that has evolved migratory behaviour. This proportion was then compared with the average relative brain size of each family using a least-squares regression. We repeated the analysis using the method of independent contrasts (ICs) to deal with phylogenetic effects (Felsenstein 1985). The phylogenetic hypothesis for our 16 families was based on DNA hybridization (Sibley & Ahlquist 1990), using genetic distances to estimate branch lengths. The ICs were calculated with the software PDAP (Garland et al. 1993) and compared with an ordinary linear regression forced through the origin.
3. Results
(a) Do passerines innovate more during winter than in other seasons?
Feeding innovations in resident British passerines were observed more often in winter (55) than outside winter (40), even though 76.6% of observations reported in the short note sections were carried out in seasons other than winter. The finding that species tend to innovate more often in winter was confirmed when comparing the innovation frequency between winter and other seasons within species, controlling for seasonal research effort (Pair-wise t-test: t30=2.84, p=0.0079; figure 2).
Figure 2.
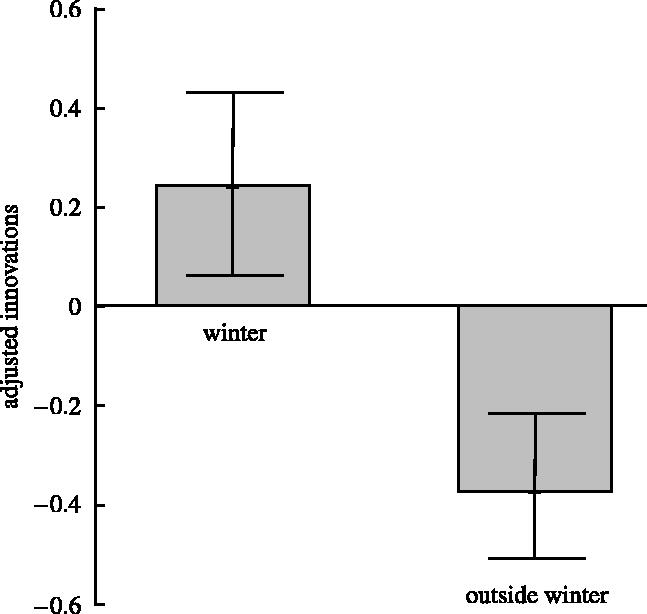
Frequency of feeding innovations per species adjusted for differences in reporting bias for winter and for the rest of the year.
(b) Are resident species more innovative than migratory species?
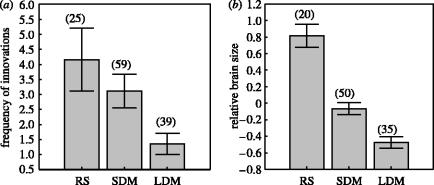
Foraging innovation rates differed significantly among seasonal behavioural strategies (F2,120=4.36, p=0.014; figure 3a). The LD migrants tended to be less innovative than both residents and SD migrants (Scheffe post hoc test, p=0.009, p=0.018, respectively); SD migrants had values similar to those seen in residents (p=0.435). These differences still remained significant when foraging innovations were adjusted for interspecific variation in research effort (F2,119=5.51, p=0.005), indicating that they were not merely the result of some species being more observed than others. The fact that passerines tend to innovate more often in winter than in the other seasons does not explain the pattern either. Among birds breeding in the British Isles, migratory species also showed significantly lower rates of feeding innovations in seasons other than winter than resident species, regardless of whether (F1,66=5.65, p=0.02) or not (F1,67=4.76, p=0.03) research effort was statistically controlled for.
Figure 3.
Differences (mean±s.e.m.) in (a) foraging innovation rates and (b) relative brain size between residents, SD migrants and LD migrants. The number of species appears between brackets.
The difference in innovation rate between LD migrants and the rest of the species still held when applying phylogenetically controlled ANOVAs, regardless of whether (F1,118=9.81, p=0.01) or not (F1,118=7.94, p=0.02) we adjusted for research effort. The picture did not even change when potential confounding effects were accommodated in a multivariate model (table 2, Electronic Appendix), despite the fact that some of these effects were significantly associated with migratory behaviour. Compared with residents and SD migrants, LD migrants tended to live in more northerly regions (t101=2.43, p=0.016), avoid conifer forests (t101=−2.87, p=0.005), have smaller body sizes (t101=−2.89, p=0.005) and rely primarily on insects (t101=2.38, p=0.019) and food gleaned from the ground (t101=−2.63, p=0.009). Latitude and insectivory were non-significant under more conservative Bonferroni standards.
(c) Do resident birds have larger brains than migratory ones?
The size of the brain, relative to body size, differed significantly among seasonal behavioural strategies (F2,102=42.68, p<0.0001; phylogenetically informed test, p<0.0001; figure 3b). Resident species had larger brains than SD migrants (Scheffe post hoc test, p<0.0001) and these in turn had smaller brains than LD migrants (p=0.002). These differences remained after adjusting for potential confounding effects (z=3.36, p=0.0008). In the minimum adequate model (table 3, Electronic Appendix), a higher migratory tendency was associated with an insectivorous diet (z=4.48, p<0.0001), avoidance of conifer forests (z=−2.19, p=0.02) and reduced clutches (z=1.98, p=0.04), although the latter two variables were non-significant under Bonferroni standards.
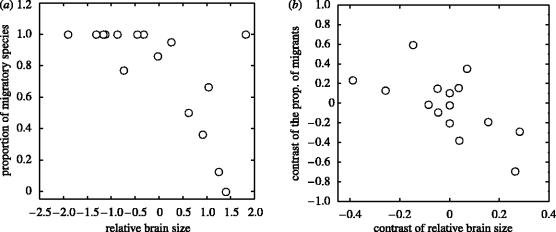
At the family level, relative brain size was negatively associated with the proportion of migratory species: large-brained families tended to contain fewer migratory species than small-brained families (F1,14=11.25, p=0.005; figure 4a). The result was similar when ICs were used to correct for phylogenetic inertia (F1,14=9.23, p=0.009; figure 4b).
Figure 4.
Relationship between mean relative brain size and proportion of migratory species per family in Western Palaearctic Passeriformes: (a) non-controlling and (b) controlling for the phylogeny. Phylogenetic effects are controlled with the method of the independent contrasts (ICs).
4. Discussion
Two main conclusions may be drawn from our study. First, flexible behaviours appear to be part of the adaptive arsenal with which birds avoid winter starvation, as suggested by the fact that resident passerines performed innovative feeding behaviours more often in winter than in the rest of the year. Second, resident species appear to be more flexible in behaviour than migratory species. Thus, LD migrants showed a lower tendency for innovative behaviours than SD migrants and residents, a pattern that held when we controlled for phylogenetic effects and biases known to affect innovation measures. Moreover, migration was more common in small-brained birds at both the species and family level, independently of phylogeny and other correlates of seasonal behavioural strategies. This latter result confirms and extends that of Winkler & Leisler (2004), who reported a negative relationship between migratory behaviour and the relative size of the forebrain in almost 30 songbird species. Behavioural flexibility thus appears to play an important role in the adaptive response of birds to seasonal environments, at least in the Palaearctic region, which is consistent with the behavioural flexibility–migratory precursor hypothesis.
Behavioural flexibility is not the only factor that has been suggested to explain why some species are migratory whereas others are resident (Greenberg 1982; Cox 1985; Levey & Stiles 1992; Newton 1995; Chesser and Levey 1998; Pérez-Tris & Tellería 2002). Perhaps the most obvious alternative explanation is the fact that species are exposed to different environmental conditions (Rappole 1995). The selective pressures favouring migratory behaviour are expected to be stronger in higher latitudes, as seasonal variation in the environmental conditions in these regions is more dramatic (Alerstam et al. 2003). Indeed, the proportion of migratory species increases with latitude (Newton & Dale 1996a,b), a pattern also detected in our analyses. The characteristics of the habitat have also been suggested to influence seasonal behavioural strategies. Migratory behaviour is predicted to be more likely in lineages that occur in non-buffered habitats (Levey & Stiles 1992; Chesser and Levey 1998), where resources are expected to be more variable and require high mobility to be tracked. Conversely, residence is more likely in habitats less subject to seasonal changes. Our findings support this hypothesis, indicating that conifer forests constitute relatively buffered habitats where birds might have entirely sedentary habits (Alerstam 1991).
Among the environmental factors that change among seasons, food supply is the most often implicated in bird migration (Newton 1995; Rappole 1995; Alerstam et al. 2003). Migration is generally considered an adaptation for exploiting seasonal peaks of resource abundance and avoiding seasonal resource depression (Alerstam et al. 2003). In northern temperate regions, food availability is particularly crucial to the winter survival of birds as food is in short supply, temperatures are low and the days are short (Jansson et al. 1981; Berthold & Terrill 1991; Newton & Dale 1996b). One well-known adaptation of northerly species to winter food shortages is caching, which reduces the variance in food supply between seasons (Grubb & Waite 1990). Yet, caching came out as non-significant in our models. This might in part be because specialized caching occurs in relatively few species. In addition, caching species often rely on conifer seeds (Cramp et al. 1988–1994), a factor that is significantly associated with seasonal behavioural strategies and which may have obscured any relationship between caching and migration in the multivariate models. The importance of food in determining migratory movements is nonetheless supported in two of our other results. First, birds that exploit temporally limited food sources, such as flying insects or food obtained from the ground, tend to be migratory (Levey & Stiles 1992; Chesser and Levey 1998). Thus, some species seem to be forced to migrate because their food sources are no longer available during the winter. Similarly, Newton (1995) showed that European songbird species that eat insects, which are less abundant in winter, are more likely to migrate than those that eat seeds. Second, LD migrants were smaller in body size than both SD migrants and residents, a result that may also be interpreted in terms of foraging ecology. Pérez-Tris & Tellería (2002) have recently shown that residents are larger than migrants in the partially migrant blackcap (Sylvia atricapilla), which they relate to contest competition for resources. Smaller birds also have disproportionally higher energetic needs than larger birds and are constrained to handle small resource items (see Brändle et al. 2002).
Even though the foraging ecology of the species and the seasonality of the environment where it occurs have long been suspected to affect whether a bird either moves or stays in its breeding area during winter (e.g. Herrera 1978; Alerstam 1991; Berthold 1993; Newton 1995), flexibility in foraging behaviour has rarely been considered in discussions on the ecology of bird migration. Adverse winter conditions can be alleviated to some extent by adaptations for improved feeding efficiency and energy conservation (hypothermia, food hoarding or structural adaptations), but adaptations to current conditions in fluctuating environments may not be wholly appropriate for future demands. In this context, flexible behaviours might provide substantial benefits to individuals such as new resources or more efficient ways to exploit the environment. Several case studies highlight the role of cognition in coping with sharp seasonal changes, even in tropical areas. Tebbich et al. (2002) have shown that tool use in Darwin's finches only replaces the more usual gleaning technique in habitats where seasonal droughts drive insects away from foliage and into crevices. Grant & Grant (1989) reported that the only finches that survived a seasonal drought in the Galapagos Islands were those who switched their foraging techniques. Thus, natural selection may favour the evolution of enhanced behavioural flexibility in species that must face sharp seasonal changes in the environment.
Directional selection for reduced behavioural flexibility in migrants is another plausible explanation for the observed differences in flexibility between migratory and resident species (Berthold & Terrill 1991). The information gathered by migrants as they travel through novel environments may only be useful for short periods and information relevant to one environment may also expose individuals to risks (e.g. novel predators) in another. Thus, in migratory species learning and innovating may have more costs than benefits and genetic programmes may be favoured over flexible behaviours (see Berthold & Terrill 1991). Moreover, large brains are energetically expensive to produce and maintain and may thus be too costly for migrants forced to travel LDs (Winkler & Leisler 2004).
While the observed differences in behavioural flexibility between migratory and resident species may be in part a consequence of using these distinct strategies, the behavioural flexibility–migratory precursor hypothesis explicitly suggests that some differences in flexibility preceded and influenced the evolution of such strategies. The idea here is that ancestral differences in brain size may, via increased behavioural flexibility, have pre-adapted some avian lineages to cope with seasonal changes, allowing them to be residents. Conversely, less flexible species would be less capable of dealing with these changes, driving them to abandon the breeding areas during the winter. Because of the difficulty of precisely estimating the ancestral states of seasonal behavioural strategies (Zink 2002), it is not currently possible to pinpoint the extent to which relative brain size is a precursor or a consequence of seasonal behavioural strategies. The alternative of using likelihood methods (Pagel 1994) proved useful in demonstrating that SD migration is an intermediate evolutionary step between residence and LD migration, but it does not contribute to clarifying whether transitions between seasonal behavioural strategies preceded or followed changes in brain size (D. Sol, unpublished).
Yet, it is hard to imagine that most differences in relative brain size between migratory and resident species are a mere consequence of their different strategies for dealing with seasonal environments. Experimental and field evidence indicates that the key features of the migratory syndrome are genetically based and can be rapidly altered through selection (Berthold et al. 1992; Pulido et al. 1996; Able & Belthoff 1998; Zink 2002; Winkler & Leisler 2004). This suggests that whether or not a species expresses migratory behaviour largely depends on the selective pressures that prevail in its current environment. Because much of the difference in brain size between lineages evolved early in the evolutionary history of birds (Bennett & Harvey 1985; Nealen & Ricklefs 2001; Sol 2003; present study), long before the current migratory systems became established (see Blondel and Mourer-Chauvire 1998), it is quite conceivable that the ancestors of both migratory and resident species already showed significant differences in behavioural flexibility when exposed to seasonal selection pressures. While this does not exclude the possibility that differences in behavioural flexibility have been accentuated by divergent selection in resident and migratory species, it suggests that behavioural flexibility, at least in the Palaearctic region, may have predisposed some lineages for migratory behaviour. Testing if this is true in other migration systems may be an interesting avenue for future research.
One potential implication of this study is that, because of their reduced behavioural flexibility, migratory species could have a limited capacity to respond behaviourally to environmental changes, such as habitat destruction or global warming. Indeed, many migratory populations have suffered a marked decline in numbers in the last few decades (Robbins et al. 1989; Maurer & Heywood 1993; Rappole & McDonald 1994). The possibility that migratory species might have difficulty coping with environmental changes may be one of the factors implicated in this decline (Maurer & Heywood 1993; Veltman et al. 1996; Sol et al. 2005). Because behavioural responses may seriously affect the capacity of animals to deal with new environmental challenges (Sol et al. 2005), behavioural flexibility should be explicitly considered in models aimed at predicting the response of migratory species to future threats.
Acknowledgements
We are grateful to S.M. Reader, J. Pérez-Tris, F. Pulido, J. Morand-Ferron, D. Kramer, C. Hall, J. Bond, E. Dolgin, N. Sager and two anonymous referees for reviewing the manuscript, to A.N. Iwaniuk for sending us his database of brain measurements, to T. Garland and M. Pagel for making their software for phylogenetic-based analyses available to us and to B. Leisler for sending us their papers in press. This project was supported by a Québec Ministry of Education Postdoctoral fellowship (Canada) and a Ramón y Cajal grant from the Ministerio de Ciencia y Tecnología (Spain) to D.S., and a NSERC (Canada) grant to L.L.
Supplementary Material
References
- Able K.P, Belthoff J.R. Rapid ‘evolution’ of migratory behaviour in the introduced house finch of eastern North America. Proc. R. Soc. B. 1998;265:2063–2071. 10.1098/rspb.1998.0541 [Google Scholar]
- Alerstam T. Cambridge University Press; Cambridge: 1991. Bird migration. [Google Scholar]
- Alerstam T, Hedenström A, Akesson S. Long-distance migration: evolution and determinants. Oikos. 2003;103:247–260. [Google Scholar]
- Allman J. Scientific American Library; New York: 2002. Evolving brains. [Google Scholar]
- Alonso J.A, Allende L.M, Rubio I, Ruiz del Valle V, Guillen J, Martinez-Laso J, Lowy E, Varela P, Zamora J, Arnaiz-Villena J. The old world sparrows (genus passer) phylogeography and their relative abundance of nuclear mtDNA pseudogenes. J. Mol. Evol. 2001;53:144–154. doi: 10.1007/s002390010202. [DOI] [PubMed] [Google Scholar]
- Bennett P.M, Harvey P.H. Relative brain size and ecology in birds. J. Zool. Lond. 1985;207:151–169. [Google Scholar]
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford: 2002. Evolutionary ecology of birds. [Google Scholar]
- Berthold P. Oxford University Press; Oxford: 1993. Bird migration: a general survey. [Google Scholar]
- Berthold P, Terrill S.B. Recent advances in studies of bird migration. Annu. Rev. Ecol. Syst. 1991;22:357–378. [Google Scholar]
- Berthold P, Helbig A.J, Mohr G, Querner U. Rapid microevolution of migratory behaviour in a wild bird species. Nature. 1992;360:668–669. [Google Scholar]
- Blackburn T.M, Duncan R.P. Determinants of establishment success in introduced birds. Nature. 2001;414:195–197. doi: 10.1038/35102557. [DOI] [PubMed] [Google Scholar]
- Blondel J, Mourer-Chauviré C. Evolution and history of the western Palaeartic avifauna. Trends Ecol. Evol. 1998;13:488–492. doi: 10.1016/s0169-5347(98)01461-x. [DOI] [PubMed] [Google Scholar]
- Blondel J, Catzeflis F, Perret P. Molecular phylogeny and the historical geography of the warblers of the genus Sylvia (Aves) J. Evol. Biol. 1996;9:871–891. [Google Scholar]
- Brändle M, Prinzing A, Pfeifer R, Brandl R. Dietary niche breadth for Central European birds: correlations with species-specific traits. Evol. Ecol. Res. 2002;4:643–657. [Google Scholar]
- Cassey P, Blackburn T.M, Sol D, Duncan R.P, Lockwood J.L. Global patterns of introduction effort and establishment success in birds. Proc. R. Soc. B. 2004;271(Suppl. 6):S405–S408. doi: 10.1098/rsbl.2004.0199. 10.1098/rsbl.2004.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser R.T, Levey D.L. Austral migrants and the evolution of migration in the New World birds: diet, habitat, and migration revisited. Am. Nat. 1998;152:311–319. doi: 10.1086/286171. [DOI] [PubMed] [Google Scholar]
- Chubb A.L. Nuclear corroboration of DNA–DNA hybridization in deep phylogenies of hummingbirds, swifts, and passerines: the phylogenetic utility of ZENK (ii) Mol. Phylogenet. Evol. 2004;30:128–139. doi: 10.1016/s1055-7903(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Cibois A, Pasquet E. Molecular analysis of the phylogeny of 11 genera of the Corvidae. Ibis. 1999;141:297–306. [Google Scholar]
- Cox G.W. The evolution of avian migration systems between temperate and tropical regions of the New World. Am. Nat. 1985;126:451–474. [Google Scholar]
- Cramp S.K, Simmons E.L, Perrins C.M. Handbook of the birds of Europe, the Middle East and North Africa. vol. 5. 1988–1994. [Google Scholar]
- DeVoogd T.J, Krebs J.R, Healy S.D, Purvis A. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc. R. Soc. B. 1993;254:75–82. doi: 10.1098/rspb.1993.0129. [DOI] [PubMed] [Google Scholar]
- Duncan N, Blackburn T.M, Worthy T.H. Prehistoric bird extinctions and human hunting. Proc. R. Soc. B. 2002;269:517–521. doi: 10.1098/rspb.2001.1918. 10.1098/rspb.2001.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson G.P, Johansson U.S. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Mol. Phylogenet. Evol. 2003;29:126–138. doi: 10.1016/s1055-7903(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z, Møller A.P, Erritzøe J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. B. 2002;269:961–967. doi: 10.1098/rspb.2002.1967. 10.1098/rspb.2002.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr., Dickerman A.W, Janis C.M, Jones J.A. Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 1993;42:265–292. [Google Scholar]
- Goldstein H. Edward Arnold; Oxford: 1995. Multilevel statistical models. [Google Scholar]
- Grant B.R, Grant P.R. Natural selection in a population of Darwin's finches. Am. Nat. 1989;133:377–393. [Google Scholar]
- Greenberg R.S. Demographic aspects of long-distance migration. In: Keast A, Morton E.S, editors. Migrant birds in the neotropics: ecology, behavior, distribution, and conservation. Smithsonian Institutions Press; Washington, DC: 1982. pp. 493–504. [Google Scholar]
- Greenberg R.S. Ecological plasticity, neophobia, and resource use in birds. Stud. Avian Biol. 1990;13:431–437. [Google Scholar]
- Grubb T.C.J, Waite T.A. Behavioral strategies for coping with winter. SQEBC Special Publication; Montreal: 1990. Behavioural responses to winter in north-temperate and boreal bark-foraging birds: a review. pp. 116–149. [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; Oxford: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Helbig A.J, Seibold I. Molecular phylogeny of Palearctic–African Acrocephalus and Hippolais warblers (Aves: Sylviidae) Mol. Phylogenet. Evol. 1999;11:246–260. doi: 10.1006/mpev.1998.0571. [DOI] [PubMed] [Google Scholar]
- Helbig A.J, Martens J, Seibold I, Henning F, Schottler B, Wink M. Phylogenetic and species limits in the Palearctic chiffchaff Phylloscopus collybita complex: mitochondrial genetic differentiation and bioacoustic evidence. Ibis. 1996;138:650–666. [Google Scholar]
- Herrera C. Ecological correlates of residence and non-residence in a Mediterranean passerine bird community. J. Anim. Ecol. 1978;47:871–890. [Google Scholar]
- Iwaniuk, A. N. 2003 The evolution of brain size and structure in birds. Ph.D. thesis, Monash University, Clayton.
- Iwaniuk A.N, Nelson J.E. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 2002;80:16–23. [Google Scholar]
- Jansson C, Ekman J, von Brömssen A. Winter mortality and food supply in tits Parus spp. Oikos. 1981;37:313–322. [Google Scholar]
- Jerison H.J. Academic Press; New York: 1973. Evolution of the brain and intelligence. [Google Scholar]
- Klicka J, Zink R.M, Winker K. Longspurs and snow buntings: phylogeny and biogeography of a high-latitude clade (Calcarius) Mol. Phylogenet. Evol. 2003;26:165–175. doi: 10.1016/s1055-7903(02)00360-3. [DOI] [PubMed] [Google Scholar]
- Klopfer P.H. Prentice Hall International Inc; London: 1962. Behavioral aspects of ecology. [Google Scholar]
- Klopfer P.H, MacArthur R.H. Niche size and faunal diversity. Am. Nat. 1960;94:293–300. [Google Scholar]
- Kvist L, Ruokonen M, Orell M, Lumme J. Evolutionary patterns and phylogeny of tits and chickadees (genus Parus) based on the sequence of the mitochondrial cytochrome b gene. Ornis Fennica. 1996;73:145–156. [Google Scholar]
- Lefebvre L, Bolhuis J.J. Positive and negative correlates of feeding innovations in birds: evidence for limited modularity. In: Laland K, Reader S.M, editors. Animal innovation. Oxford University Press; Oxford: 2003. pp. 39–62. [Google Scholar]
- Lefebvre L, Whittle P, Lascaris E, Finkelstein A. Feeding innovations and forebrain size in birds. Anim. Behav. 1997;53:549–560. [Google Scholar]
- Lefebvre L, Reader S.M, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 2004;63:233–246. doi: 10.1159/000076784. [DOI] [PubMed] [Google Scholar]
- Levey D.L, Stiles F.G. Evolutionary precursors of long-distance migration: resource availability and movement patterns in neotropical landbirds. Am. Nat. 1992;140:447–476. [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Cary: 1996. SAS system for mixed models. [Google Scholar]
- Manly B.F.J. CASM; Dunedin, New Zealand: 1997. RT: a program for randomization testing [Google Scholar]
- Martin T.E, Clobert J. Nest predation and avian life-history evolution in Europe versus North America: a possible role of humans? Am. Nat. 1996;147:1028–1046. [Google Scholar]
- Maurer B.A, Heywood S.G. Geographic range fragmentation and abundance in neotropical migrant birds. Conserv. Biol. 1993;7:501–509. [Google Scholar]
- Mlíkovský J. Brain size in birds: 1. Tinamiformes through Ciconiiformes. Vést. cs. Spolec. Zool. 1989a;53:33–45. [Google Scholar]
- Mlíkovský J. Brain size in birds: 2. Falconiformes through Gaviiformes. Vést. cs. Spolec. Zool. 1989b;53:200–213. [Google Scholar]
- Mlíkovský J. Brain size in birds: 3. Columbiformes through Piciformes. Vést. cs. Spolec. Zool. 1989c;53:252–264. [Google Scholar]
- Mlíkovský J. Brain size in birds: 4. Passeriformes. Acta Soc. Zool. Bohemoslo. 1990;54:27–37. [Google Scholar]
- Mönkkönen M, Orell M. Clutch size and cavity excavation in parids (Paridae): the limited breeding opportunities hypothesis tested. Am. Nat. 1997;149:1164–1174. doi: 10.1086/286045. [DOI] [PubMed] [Google Scholar]
- Morse D.H. Harvard University Press; Cambridge: 1980. Behavioral mechanisms in ecology. [Google Scholar]
- Mullarney K, Svensson L, Grant P.J. Princeton University; Princeton: 2000. Birds of Europe. [Google Scholar]
- Nealen P.M, Ricklefs R.E. Early diversification of the avian brain: body relationship. J. Zool. Lond. 2001;253:391–404. [Google Scholar]
- Newton I. The contribution of some recent research on birds to ecological understanding. J. Anim. Ecol. 1995;64:675–696. [Google Scholar]
- Newton I, Dale L.C. Bird migration at different latitudes in eastern North America. Auk. 1996a;113:626–635. [Google Scholar]
- Newton I, Dale L.C. Relationship between migration and latitude among west European birds. J. Anim. Ecol. 1996b;65:137–146. [Google Scholar]
- Omland K.E. Cryptic genetic variation and paraphyly in ravens. Proc. R. Soc. B. 2000;267:2475–2482. doi: 10.1098/rspb.2000.1308. 10.1098/rspb.2000.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 1994;255:37–45. [Google Scholar]
- Pérez-Tris J, Tellería J.L. Migratory and sedentary blackcaps in sympatric non-breeding grounds: implications for the evolution of avian migration. J. Anim. Ecol. 2002;71:211–224. [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003;18:228–233. [Google Scholar]
- Pulido F, Berthold P, Van Noordwijk A. Frequency of migrants and migratory activity are genetically correlated in a bird population: evolutionary implications. Proc. Natl Acad. Sci. USA. 1996;93:14642–14647. doi: 10.1073/pnas.93.25.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappole J.H. Smithsonian Institution Press; Washington: 1995. The ecology of migrant birds. [Google Scholar]
- Rappole J.H, McDonald M.V. Cause and effect in population declines of migratory birds. Auk. 1994;111:652–660. [Google Scholar]
- Reader S, Laland K. Social intelligence, innovation and enhanced brain size in primate. Proc. Natl Acad. Sci. USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader S.M, MacDonald K. Environmental variability and primate behavioural flexibility. In: Laland K, Reader S.M, editors. Animal innovation. Oxford University Press; Oxford: 2003. pp. 83–116. [Google Scholar]
- Robbins C.S, Sauer J.R, Greenberg R.S, Droege S. Population declines in North American birds that migrate to the neotropics. Proc. Natl Acad. Sci. USA. 1989;86:7658–7662. doi: 10.1073/pnas.86.19.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds: a study in molecular evolution. [Google Scholar]
- Sibley C.G, Monroe B. Yale University Press; New Haven, CT: 1990. Distribution and taxonomy of birds of the world. [Google Scholar]
- Sol D. Behavioural flexibility: a neglected issue in the ecological and evolutionary literature? In: Laland K, Reader S.M, editors. Animal innovation. Oxford University Press; Oxford: 2003. pp. 62–82. [Google Scholar]
- Sol, D., Duncan, R. P., Blackburn, T. M., Cassey, P. & Lefebvre, L. 2005 Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA102, 5460–5465. [DOI] [PMC free article] [PubMed]
- Spicer G.S, Dunipace L. Molecular phylogeny of songbirds (Passeriformes) inferred from mitochondrial 16S ribosomal RNA gene sequences. Mol. Phylgenet. Evol. 2004;30:325–335. doi: 10.1016/s1055-7903(03)00193-3. [DOI] [PubMed] [Google Scholar]
- Székely T, Catchpole C.K, DeVoogd A, Marchl Z, DeVoogd T.J. Evolutionary changes in a song control area of the brain (HVC) are associated with evolutionary changes in song repertoire among European warblers (Sylvidae) Proc. R. Soc. B. 1996;263:607–610. [Google Scholar]
- Tebbich S, Taborsky M, Fessl B, Dvorak M. The ecology of tool use in the woodpecker finch (Cactospiza pallida) Ecol. Lett. 2002;5:656–664. [Google Scholar]
- Timmermans S, Lefebvre L, Boire D, Basu P. Relative size of the hyperstriatum ventrale is the best predictor of feeding innovation rate in birds. Brain Behav. Evol. 2000;56:196–203. doi: 10.1159/000047204. [DOI] [PubMed] [Google Scholar]
- Veltman C.J, Nee S, Crawley M.J. Correlates of introduction success in exotic New Zealand birds. Am. Nat. 1996;147:542–557. [Google Scholar]
- Voelker G. Molecular evolutionary relationships in the avian genus Anthus (Pipits: Motacillidae) Mol. Phylogenet. Evol. 2001;11:84–94. doi: 10.1006/mpev.1998.0555. [DOI] [PubMed] [Google Scholar]
- Voelker G, Spellman G.M. Nuclear and mitochondrial DNA evidence of polyphyly in the avian superfamily Muscicapoidea. Mol. Phylogenet. Evol. 2004;30:386–394. doi: 10.1016/s1055-7903(03)00191-x. [DOI] [PubMed] [Google Scholar]
- Winkler H, Leisler B. To be a migrant: ecomorphological burdens and chances. In: Greenberg R.S, Marra P.P, editors. Birds of two worlds. Smithsonian Institute Press; Washington: 2004. pp. 79–86. [Google Scholar]
- Zink R.M. Towards a framework for understanding the evolution of avian migration. J. Avian Biol. 2002;33:433–436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.